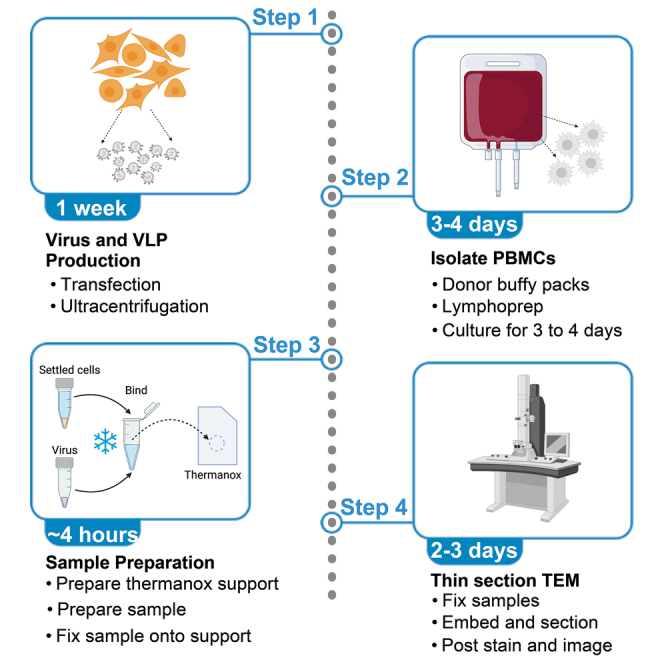
Summary
Glycan-glycan interactions between viral particles and host cells may lengthen the dwell time of the virus on the cell surface to facilitate cellular receptor engagement. Here, we present a protocol for visualizing glycan-mediated binding between virus or virus-like-particles (VLPs) and human peripheral blood mononuclear cells using transmission electron microscopy (TEM). We describe steps for virus and VLP production, isolation of human peripheral blood mononuclear cells, and sample preparation. We then detail procedures for thin-section TEM.
For complete details on the use and execution of this protocol, please refer to Spillings et al.1
Subject areas: Cell Biology, Microbiology, Microscopy
Graphical abstract
Highlights
-
•
Steps for investigating glycan-mediated interactions
-
•
Single-cell-level visualization of glycan interactions using electron microscopy
-
•
Maintenance of cellular glycocalyx structure by eliminating centrifugation
-
•
Visualization of glycan interactions between glycosylated substrates
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Glycan-glycan interactions between viral particles and host cells may lengthen the dwell time of the virus on the cell surface to facilitate cellular receptor engagement. Here, we present a protocol for visualizing glycan-mediated binding between virus or virus-like-particles (VLPs) and human peripheral blood mononuclear cells using transmission electron microscopy (TEM). We describe steps for virus and VLP production, isolation of human peripheral blood mononuclear cells, and sample preparation. We then detail procedures for thin-section TEM.
Before you begin
Introduction
Glycobiology and the importance of glycans as signaling and attachment molecules is underappreciated in most fields of biological research. Glycans are utilized by pathogens as cell surface attachment factors to facilitate binding and entry into target host cells and tissues. The most well described interactions are between glycans and cellular lectins,2,3,4 however these are sugar-protein-based interactions. More recently researchers have uncovered direct glycan-glycan interactions between pathogens (bacteria and viruses) and their target host cells5,6,7 and have shown that interference with these interactions can result in decreased pathogen binding and entry.
Although high affinity glycan-glycan interactions can be investigated with biophysical tools such as glycan arrays, SPR and ITC, these studies are limited since they do not represent the total sum of interactions within the complex biological context of whole pathogens and cells during interactions. Furthermore, challenges in visualizing the cellular and viral glycocalyxes is confounded since most experimental practices are damaging to the delicate glycan structures. By analogy, the cellular glycocalyx can be likened to floating sea-grass anchored to the sea bed. During centrifugation of cells, the glycocalyx is subjected to significantly large g-forces which may cause temporary compression and collapse of the cellular glycocalyx.
We have successfully overcome the limitations of biological assays to detect and quantify the effect of these glycan mediated interactions within biologically relevant, in vitro systems. Herein we describe a technique for visualizing glycan interactions within biological samples using thin section transmission electron microscopy (TEM).
Institutional permissions (if applicable)
We have used these glycan binding assays to determine the relative binding of both infectious HIV virus and non-infectious VLPs to peripheral blood mononuclear cells (PBMCs).
All infectious virus work should be undertaken in a laboratory which has the appropriate approvals for your state and country. All infectious HIV work undertaken for this work was conducted within a certified PC3 laboratory (pursuant to section 90 of the Australian Gene Technology Act, 2000, and in compliance with the relevant Australian/New Zealand standards).
Handling of untested, deidentified donor blood products (sourced from Australian Red Cross Lifeblood, Brisbane) was performed using universal precautions, assuming all components may be infective. When processing blood products, ensure that the procedures are carried out in accordance with your local health and safety guidelines.
Virus and VLP production
Timing: 1 week
Prior to starting the assay, ensure that viral or VLP stocks are sufficient for the experiment. If necessary, prepare fresh stocks by transfection.
-
1.
For the production of both infectious HIV and non-infectious VLPs, plasmid DNA is introduced to HEK293T cells by polyethylenimine (PEI MAX) transient transfection. Seed the cells at a density of 7.5 × 106 cells per T175 flask (Corning) 18–24 h prior to transfection, in 30 mL fresh culture media. HEK293T cells are maintained in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% (v/v) fetal calf serum and penicillin/streptomycin solution (Thermo Scientific).
-
2.
Generate infectious HIV-1 viral stocks and non-infectious VLPs for TEM imaging by transfecting the plasmids pNL4-3 or pNL4-3 ΔRTΔINΔVifΔVpr without any additional marker plasmids. Transfect a total of 12 μg plasmid DNA per T175 flask of HEK293T cells.
-
3.
Collect the culture supernatants 48 h post transfection and clarify by centrifugation (1500 × g, for 10 min at room temperature (20°C–25°C)) followed by passing through a 0.2–0.45 μm syringe filter, twice.
-
4.
Pellet the virus and VLPs through a 5 mL 20% sucrose cushion using ultracentrifugation at 100 000 × g, 4°C for approximately 1 h (SW-32 Ti rotor; total ω2t 2.7 × 1010, L-90 ultracentrifuge, Beckman Coulter). The resulting pellet is resuspended in DPBS, for 16–24 h at 4°C.
-
5.
Since the virus or VLPs are to be used for TEM imaging, an additional purification step through an OptiPrep gradient is necessary. Prepare a step gradient from 4% to 16% OptiPrep.
-
6.
Layer the viral or VLP supernatant on top of the gradient and ultracentrifuge at 100 000 × g, 4°C for approximately 1 h (SW-32.1 Ti rotor; total ω2t 2.7 × 1010, L-90 ultracentrifuge, Beckman Coulter).
-
7.
Using underlighting to visualize the viral/VLP band carefully remove this layer to a fresh screwcap tube then aliquot and place the tubes directly into freezer boxes for storage at ‒80°C until needed.
-
8.
The concentration of virus and VLP produced is determined using an enzyme-linked immunosorbent assay (ELISA) p24CA, following manufacturer’s recommendations (Xpress Bio).
Note: The transfection supernatant must be clarified by filtering twice (step 3).
Isolation of peripheral blood mononuclear cells (PBMCs) from donor buffy coats
Timing: 3–4 days
We have only used freshly isolated PBMCs for the glycan binding assays. If frozen PBMCs are to be used, culture conditions may need to be optimized to ensure the cells are healthy.
-
9.
Freshly isolated PBMCs that have been iL-2 stimulated and PHA activated are used as the host cell in this assay. Typically, three to four days prior to the experiment PBMCs are isolated from donor buffy coats sourced from healthy HIV-1 seronegative blood donors (Australian Red Cross Lifeblood, Brisbane). The PBMCs are purified by density gradient centrifugation over lymphoprep (StemCell Technologies).
-
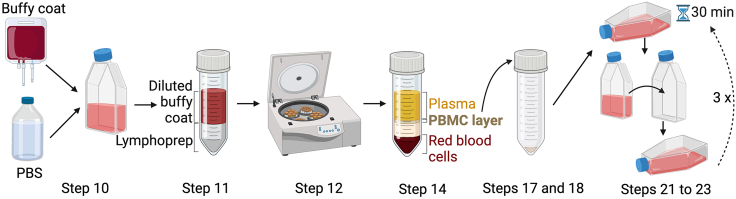
10.
Buffy coat is diluted 1: 1.5 with room temperature (20°C–25°C) Mg2+ and Ca2+ free PBS (pH 7.4) in a sterile tissue culture flask (Figure 1).
-
11.Transfer approximately 25 mL of diluted buffy coat to a sterile 50 mL screw-cap centrifugation tube.
-
a.Slowly underlay the buffy:PBS mix with 12–13 mL lymphoprep (StemCell Technologies), taking care not to mix the denser lymphoprep layer with the diluted buffy coat (Figure 1).
-
a.
Note: Depending on the number of cells required for the binding assay, it may be necessary to process more than one centrifuge tube of diluted buffy coat.
-
12.
Centrifuge the density gradient preparations at 800 × g, room temperature (20°C–25°C) for 20 min (Figure 1). Set the centrifuge brake to low to avoid disturbing the resulting layers.
-
13.
Use a serological pipette to slowly pipette off the upper buffer/serum layer and discard.
-
14.
Use a sterile, disposable transfer pipette, carefully remove the PBMC layer just above the lower red blood cell layer (Figure 1) and transfer into a new 50 mL centrifugation tube.
-
15.
Collect all the PBMC layers from one donor into one 50 mL tube.
-
16.
Add room temperature (20°C–25°C) PBS (Mg2+ and Ca2+ free) to the collected PBMC layers such that the final volume of the centrifuge tube is approximately 40 mL.
-
17.
Wash the PBMC layer twice with 30–40 mL PBS (Mg2+ and Ca2+ free) per wash, with centrifugation at 320 × g, 10 min, 4°C. After each wash gently resuspend the PBMC pellet in cold PBS (Figure 1).
-
18.
Perform two final washes with PBS (Mg2+ and Ca2+ free) and centrifugation at 130 × g, 10 min, 4°C. After each wash gently resuspend the PBMC pellet in cold PBS.
-
19.
After the final wash, resuspend the pellet in complete RPMI containing 10% FBS, 2 mM L-glutamine and 100 U/mL penicillin/streptomycin antibiotic.
-
20.
Since the cells are to be used for TEM imaging it is recommended that they are macrophage depleted.
-
21.
Transfer the PBMCs, resuspended in 30 mL complete RPMI, into a T175 flask (Corning). Lay the flask flat in a 37°C incubator, 5% CO2. Macrophages will attach to the plastic flask whereas the peripheral blood leukocytes will remain in suspension.
-
22.
After 30 min stand the flask upright and allow the media and unattached cells to drain to the base of the flask. Without disturbing the attached macrophages, collect the cells remaining in suspension and transfer to a new T175 flask.
-
23.
Repeat the incubation and transfer at least three times (Figure 1). In between transfers visualize the attached cells under a microscope. A visible reduction in the quantity of attached cells should be evident with each successive macrophage attachment cycle.
-
24.
After the final macrophage depletion, use a hemocytometer to count the cells and seed them into complete RPMI media in a culture flask at the cell density of 2 × 106 cells/mL.
-
25.
The resulting PBMCs are maintained in complete RPMI supplemented with iL-2 (50 U/mL) and PHA (10 μg/mL). On the day of the binding experiment, collect the PBMCs into a 50 mL screw-cap centrifugation tube. Gently pellet the cells at 150–300 g, for 10 min at room temperature (20°C–25°C). Gently resuspend the pelleted cells in fresh complete RPMI at 1 × 106 cells/mL.
Note: Within our laboratory we have noticed variations in the efficiency of PBMC isolation, based on the length of time between blood draw and buffy pack processing. Where possible we limit the time between blood draw to the start of PBMC processing to 18–24 hours. Buffy packs older than 24 hours old are avoided.
Note: All buffers, tubes, serological pipettes and tips should be sterile. DPBS and PBS used for PBMC related work is always Mg2+ and Ca2+ free.
CRITICAL: When working with blood products, universal precautions should be followed to ensure the health and safety of personnel.
Figure 1.
Cartoon schematic of PBMC isolation from donor buffy coats for use in TEM
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Buffy coat | Australian Red Cross Lifeblood | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine (PEI-MAX) | Polysciences | Cat # 24765 |
| DMEM | Thermo Fisher Scientific | Cat # 11995073 |
| RPMI | Thermo Fisher Scientific | Cat # 21870092 |
| FBS, qualified, Australian origin | Thermo Fisher Scientific | Cat # 10099141 |
| L-glutamine | Thermo Fisher Scientific | Cat # 25030081 |
| Penicillin-streptomycin (10 000U/mL) | Thermo Fisher Scientific | Cat # 15140122 |
| 10 x PBS | Thermo Fisher Scientific | Cat # 70011044 |
| DPBS | Thermo Fisher Scientific | Cat # 14190144 |
| Phytohemagglutinin (PHA) | Thermo Fisher Scientific | Cat #R30852701 |
| Interleukin-2 (iL-2) | Sigma-Aldrich | Cat # 11147528001 |
| Lymphoprep | STEMCELL Technologies | Cat # 07851 |
| OptiPrep Density Gradient Medium | Sigma-Aldrich | Cat #D1556 |
| Sucrose | Sigma-Aldrich | Cat #S0389 |
| PNGase F, recombinant | New England Biosciences | Cat #P0708L |
| 25% Glutaraldehyde, aqueous, EM grade | ProSciTech | Cat #C001 |
| Thermanox, plastic support | ProSciTech | Cat # GL082 |
| 1.5 mm biopsy punch | ProSciTech | Cat # BIPP04756 |
| BEEM Embedding Capsules, Size 3, PE | ProSciTech | Cat # RB004 |
| Poly-L-lysine | Sigma-Aldrich | Cat #P8920 |
| 4% Osmium tetroxide | ProSciTech | Cat #C011 |
| LX112 resin | ProSciTech | Cat # 21310 |
| NMA | ProSciTech | Cat #C046 |
| DDSA | ProSciTech | Cat #C044 |
| DMP-30 | ProSciTech | Cat #C053 |
| Uranyl acetate | ProSciTech | Cat #C079 |
| Lead citrate | ProSciTech | Cat #C073 |
| Critical commercial assays | ||
| p24CA ELISA | Xpress Bio | Cat # XB-1010 |
| Experimental models: Cell lines | ||
| HEK293T: human, female, embryonic kidney derived cell line that is epithelial-like in morphology, hypotriploid and contains the SV40 T-antigen. | ATCC | Cat # CRL-3216 |
| Recombinant DNA | ||
| Human Immunodeficiency Virus 1 (HIV-1), Strain NL4-3 Infectious Molecular Clone (pNL4-3), ARP-2852, contributed by Dr. M. Martin | NIH AIDS Reagent Program | https://www.hivreagentprogram.org/Catalog/Clones/ARP-114.aspx |
| pNL4-3 ΔRTΔINΔVifΔVpr | Mak Laboratory | Spillings et al.1 |
| Software and algorithms | ||
| Fiji Cell Counter | N/A | ImageJ Cell Counter Plugin: https://imagej.nih.gov/ij/plugins/cell-counter.html |
| GraphPad Prism Software, v 9.1.1 | N/A | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Falcon tissue culture flasks, T175, vented | Corning | Cat # 353112 |
| Centrifugation tube, 50 mL screw-cap | Sarstedt | Cat # 62.547.254 |
| Costar 24-well plates, sterile | Corning | Cat # 3524 |
| Costar 12-well plates, sterile | Corning | Cat # 3513 |
| Microtube, 1.5 mL, screw cap with internal o-ring | SSI | Cat # SSIB2231-S0S |
| 38.5 mL, open-top, thinwall ultraclear tube | Beckman Coulter | Cat # 344058 |
| 17 mL, open-top, thinwall ultraclear tube | Beckman Coulter | Cat # 344061 |
| MiniSart CA Syringe Filter, 0.22 μm (Blue) | Sartorius | Cat# S6534 |
| MiniSart CA Syringe Filter, 0.45 μm (Yellow) | Sartorius | Cat #S6555 |
Materials and equipment
LX112
| Reagent | Quantity | Resulting solution |
|---|---|---|
| DDSA | 61.8 g | Mix for 5 min to result in Mix A |
| LX112 resin | 48 g | |
| NMA | 51.6 g | Mix for 5 min to result in Mix B |
| LX112 resin | 60 g | |
| Mix A | 100 g | Mix for 5 min to result in Mix C |
| Mix B | 100 g | |
| Mix C | 200 g | Mix for 5 min, aliquot, label and freeze as final LX112 mixture |
| DMP-30 | 2.8 g |
CRITICAL: Many of the stains and fixatives used for sample preparation in electron microscopy are hazardous. Glutaraldehyde, LX112, DDSA, NMA, DMP-30, osmium tetroxide, lead citrate and uranyl acetate are all classed as dangerous goods. Uranyl acetate is radioactive. Please ensure that the relevant laboratory approvals are in place to store these products and that personnel are suitably trained for the handling, use and disposal thereof.
Step-by-step method details
Thin section, transmission electron microscopy
Timing: >3 days
OptiPrep purified virus and VLPs are bound to target host cells at entry non-permissive temperatures. For HIV, temperatures below 22°C do not support efficient fusion and cell entry. Samples are immobilized on a plastic support (Thermanox) and fixed for EM imaging.
-
1.Place 10–15 mL of cell suspension into a 15 mL tube.
-
a.Stand the tube in the incubator and allow cells to settle by gravity.Note: This should take approximately 45–60 minutes.
-
b.Carefully remove the media and unsettled cells.
-
c.Resuspend the cell pellet very gently with 5 mL prewarmed DPBS and allow to settle by gravity.
-
d.Carefully remove the DPBS and unsettled cells and resuspend the cell pellet in 1 mL prewarmed DPBS.
-
e.Transfer the cells to a 1.5 mL conical screw cap tube.
-
f.Place at 37°C until needed, allowing the cells to settle during this period.
-
a.
-
2.
Whilst the cells are settling in the 1.5 mL tube, prepare the virus or VLPs for the assay. This may include a PNGase F treated control.
Note: PNGase F cleaves N-linked glycans from glycoproteins between the innermost N-Acetyl-glucosamine (GlcNAc) and asparagine residues of high mannose, hybrid, and complex oligosaccharides. Other glycosidase enzymes may be appropriate for your experiment.
-
3.
In a total reaction volume of 10 μL, treat with 450 U PNGase F enzyme in the manufacturer’s recommended glycobuffer 2.
-
4.
Incubate the PNGase F treated samples at 37°C for 1 h. For the untreated control, set up the equivalent reaction in glycobuffer 2, without the addition of enzyme.
-
5.Whilst the cells are settling and virus/VLP samples are being treated with PNGase F freshly prepare the Thermanox supports.
-
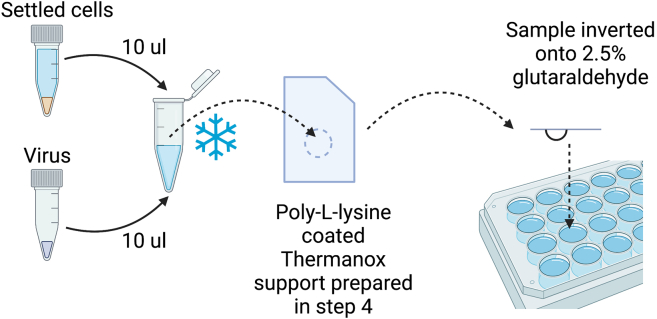
a.Using clean scissors, cut the Thermanox supports into rectangle shapes with the top right-hand corner clipped (similar in shape and size of small mobile phone sim-card). When working with the supports, position the cut corner in the top right position to assist in orientation of the support and sample in downstream processes (Figure 2).
-
b.Using a 1.5 mm biopsy punch, gently score a circle shape into the surface of the support.
-
c.Using forceps, clean the Thermanox supports by submerging into 100% ethanol and allowing to airdry.
-
d.Place the clean and dry supports onto filter paper inside a petri dish, scored side up. Pipette a 5–10 μL droplet of poly-L-lysine (1 mg/mL) into each scored circle and allow to sit for 30 min. Monitor the droplets to ensure that they do not evaporate too quickly.
-
e.Using lint free tissues (such as Kimwipes (Kimtech Science)) wick off the excess poly-L-lysine and allow the supports to air dry at room temperature (20°C–25°C) for 20–30 min.
-
a.
Note: Thermanox supports can be made up to 24 hours in advance and stored in a closed disposable petri dish at 4°C. Always store the supports, poly-L-lysine side up.
-
6.
Prior to combining the cells with virus or VLPs, incubate both tubes at 4°C for at least 10–15 min. From this point forward, place all tubes on ice to maintain entry non-permissive temperatures.
-
7.
Prepare a 24-well tissue culture plate by pipetting ∼ 1.5 mL 2.5% glutaraldehyde in PBS into the appropriate number of wells.
-
8.
Carefully remove all but 100 μL of the DPBS from above the settled cells from step 5. Very gently pipette the cell pellet to resuspend the cells.
-
9.
Transfer 10 μL of cell suspension to the chilled virus or VLP tubes. Very gently mix the cells and virus/VLP by pipetting the mixture 5 times.
-
10.
Immediately transfer approximately 3–5 μL of the mixture to the poly-L-lysine coated region of a Thermanox support (Figure 2).
-
11.
Allow the sample to bind to the poly-L-lysine for 5–10 min, monitoring the droplets to ensure that they do not dry by evaporation.
-
12.
Gently invert the Thermanox supports onto the 2.5% glutaraldehyde in PBS (step 6) to fix the samples. Each Thermanox support should be fixed in it’s own well on the 24-well plate (Figure 2). Samples are stored at 4°C until they can be processed for EM.
-
13.
To process the Thermanox supports for EM, remove the supports from the glutaradehyde solution and place them sample side up in a 12-well culture dish. Using a Pelco Biowave, process the supports by washing in PBS twice for 40 s at 150 W, without vacuum.
-
14.
Post-fix the Thermanox supports in 1% osmium tetroxide for 12 min (2 min power on, 2 min power off, 2 min power on, then repeat the series) at 80 W under vacuum.
-
15.
Dehydrate the samples through an ethanol series (30%, 50%, 70%, 90% and 100%) for 40 s at 150 W, without vacuum. Repeat the 100% ethanol step.
-
16.
After the final 100% ethanol step, infiltrate the sample with LX112 resin (50% ethanol: 50% resin x 2) for 3 min at 250 W, under vacuum.
Note: The prepared LX112 resin mixture detailed in the materials and equipment section above can be stored indefinitely at ‒80°C. If the mixture is stored at ‒20°C, it should be stored for a maximum of 2 weeks.
-
17.
Fill BEEM capsules (size 3) with resin and invert over marked sample area.
-
18.
Once polymerized, snap the BEEM capsules off the dish using pliers. Samples should now be at the surface of the resin block, with the scored circular imprint visible. Remove the BEEM capsules from the resin block prior to sectioning the samples.
-
19.
Trim the resin block to the region of the sample, as marked by the scored imprint.
-
20.
Section the blocks at 60–70 nm using an ultramicrotome with diamond knife.
-
21.
Collect the sections onto specimen grids.
-
22.
Post stain with 5% uranyl acetate in 50% ethanol for 90 s. Rinse the grids three times in pure water, wicking the excess water away using filter paper.
-
23.
Stain with Reynold’s lead citrate for 45 s followed by three washes with pure water, wicking the excess water away using filter paper.
-
24.
Allow the grids to air dry and then store in a grid box until the grids can be imaged.
-
25.
Image the grids using a TEM operated at 80 kV.
Figure 2.
Cartoon schematic of sample preparation for TEM
Expected outcomes
In the context of visualizing glycan mediated interactions through TEM, PNGase F treatment of virion particles removes N-linked glycans from the virion surface glycoproteins (Figure 3). TEM images were analyzed by an individual blinded to the experiment. Viral particles within 0–500 nm of the cell surface were counted manually using Fiji Cell Counter.8 Only viruses that displayed an electron dense core were counted in the analysis.
Figure 3.
TEM images of untreated and PNGase F treated virus combined with PBMCs at entry non-permissive temperatures
(A) Cartoon schematic of a whole PBMC with untreated HIV virions (left) and with PNGaseF treated virions (right) (B) Representative TEM images of PBMCs. Whole cell scale bar = 2 μm; selected cell region scale bar = 500 ηm.
When visualized under TEM, cells mixed with PNGase F treated virus has a significantly reduced number of viral particles associated with the cellular surface. This demonstrates that the removal of viral N-linked glycans negatively impacts viral-cell associations (see Spillings et al.1).
Limitations
This assay should be optimized for each laboratory and cell/virus system for which it is being used. An important consideration is the need to ensure that the glycocalyx is not damaged prior to fixation in glutaraldehyde.
Troubleshooting
Problem 1
-
•
Visualizing the cell-virus glycan-glycan interactions by electron microscopy fails (thin section, transmission electron microscopy assay, Step 1 through to 19).
Potential solution
When working with cells, centripetal force during the concentration of cells by centrifugation may compress or damage the surface glycan structures. We have overcome this by allowing the cells to sediment by gravity without the use of centrifugation. This process is slow and hence the cells are placed in a 37°C incubator to maintain their viability.
Problem 2
-
•
LX112 resin is viscous and difficult to use during sample infiltration (thin section, transmission electron microscopy assay, Step 13).
Potential solution
Make up fresh resin prior to processing and store at ‒20°C for a maximum of 2 weeks.
Problem 3
-
•
Staining artefacts such as electron dense deposits are visible during TEM imaging (thin section, transmission electron microscopy assay, Step 21).
Potential solution
Ensure all equipment and surfaces are clean and free of dust. Centrifuge the tubes containing the stains immediately prior to use to pellet stain precipitates (steps 11 and 19). Cover grids during the staining process and place sodium hydroxide pellets adjacent to droplets of stain to absorb carbon dioxide which can lead to the precipitation of lead carbonate (step 19).
Problem 4
-
•
No cells are seen in the sections when imaged (thin section, transmission electron microscopy assay, Step 21).
Potential solution
The concentration of cells added to the Thermanox coverslip may have been too low. Ensure that the first sections cut from the blockface are picked on grids and viewed in the TEM. The cells will be right at the surface and it is easy to section through them (steps 17 and 18).
Problem 5
-
•
PNGaseF is a suitable negative control for gross removals of N-glycans but not for O-glycans or removal of specific glycan components (thin section, transmission electron microscopy assay, Steps 2 and 3).
Potential solution
When investigating glycan-based interactions, a suitable negative control should be chosen. For our study we specifically investigated glycan-glycan based interactions between cells and virus. In order to remove a majority of the surface glycan structures, we chose to utilize the glycosidase enzyme PNGase F. This enzyme cleaves N-linked glycans between the innermost GlcNAc and asparagine residues on high mannose, hybrid and complex oligosaccharides. We have successfully used this enzyme to treat both PBMCs as well as VLPs and virus. Other glycosidase enzymes include neuraminidases (for the removal of sialic acid), galactosidases (for the removal of galactosidase), fucosidases (for the removal of fucose) and mannosidases (for the removal of mannose). These alternative glycosidases could be applied to investigate specific glycan-based interactions. For example, studies investigating proteinaceous lectin and complex N-linked glycan interactions may elect to utilize sialidase enzyme treatments as their negative control.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Johnson Mak (j.mak@griffith.edu.au).
Materials availability
Plasmids generated in this study will be provided, upon request to the lead contact, and may require fulfillment of an MTA.
Acknowledgments
1) This work was funded by the Australian Research Council (grant FT100100297 to J.M.), the Australian Centre for HIV and Hepatitis (to J.M.), and the Australian National Health and Medical Research Council Project Grant (1121697 to J.M.). 2) We are grateful for the assistance given to us by the staff of the Centre for Microscopy and Microanalysis, The University of Queensland (Brisbane, Australia), with respect to the sample preparation and imaging of PBMCs by TEM. 3) Figures were created using BioRender.com.
Author contributions
Conceptualization, B.LS., J.M.; Methodology and Investigation, B.L.S., K.G., E.L.; Writing – Original Draft, B.L.S.; Writing – Review and Editing, B.L.S., K.G., E.L., R.W., J.M.; Supervision, R.W., J.M.; Funding Acquisition, J.M. All authors have reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Belinda L. Spillings, Email: b.devilliers@griffith.edu.au.
Johnson Mak, Email: j.mak@griffith.edu.au.
Data and code availability
This study did not generate/analyse datasets or code.
References
- 1.Spillings B.L., Day C.J., Garcia-Minambres A., Aggarwal A., Condon N.D., Haselhorst T., Purcell D.F.J., Turville S.G., Stow J.L., Jennings M.P., Mak J. Host glycocalyx HIV proximal to the cell surface via oligomannose-GlcNAc glycan-glycan interactions to support viral entry. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110296. [DOI] [PubMed] [Google Scholar]
- 2.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Bobardt M.D., Saphire A.C.S., Hung H.C., Yu X., Van der Schueren B., Zhang Z., David G., Gallay P.A. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 4.Turville S.G., Cameron P.U., Handley A., Lin G., Pöhlmann S., Doms R.W., Cunningham A.L. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 5.Belotserkovsky I., Brunner K., Pinaud L., Rouvinski A., Dellarole M., Baron B., Dubey G., Samassa F., Parsot C., Sansonetti P., Phalipon A. Glycan-Glycan Interaction Determines Shigella Tropism toward Human T Lymphocytes. mBio. 2018;9 doi: 10.1128/mBio.02309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day C.J., Tran E.N., Semchenko E.A., Tram G., Hartley-Tassell L.E., Ng P.S.K., King R.M., Ulanovsky R., McAtamney S., Apicella M.A., et al. Glycan:glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells. Proc. Natl. Acad. Sci. USA. 2015;112:E7266–E7275. doi: 10.1073/pnas.1421082112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole J., Day C.J., von Itzstein M., Paton J.C., Jennings M.P. Glycointeractions in bacterial pathogenesis. Nat. Rev. Microbiol. 2018;16:440–452. doi: 10.1038/s41579-018-0007-2. [DOI] [PubMed] [Google Scholar]
- 8.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyse datasets or code.