Abstract
Previous studies have suggested that omega-3 polyunsaturated fatty acids, predominantly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have several health benefits. However, their effect on changes in skeletal muscle mass and strength has not been established, owing to differences in study designs. This systematic review aimed to investigate the recent evidence regarding the role of dietary EPA and DHA in muscle mass changes and their association with muscle strength. Databases including PubMed and Google Scholar were searched for randomized controlled trials and single-arm interventions that investigated the effects of omega-3 fatty acids on skeletal muscle mass, strength, and body composition in adults aged 18 years and older. A total of 18,521 studies were retrieved from the databases and manual searches; 21 studies were quality assessed, and the findings were summarized. Studies were categorized into 3 main categories according to the type of omega-3 fatty acid supplementation: pure compounds such as oil tablets, formulated forms with protein, leucine, and vitamin D, and ingredients added to enteral nutrition support products. Overall, the majority of the study results appeared to indicate that omega-3 fatty acids are beneficial for muscle health. However, meta-analysis was not conducted because of the heterogeneity of the study participants, evaluation method of muscle indices, and intervention periods among the studies. High-quality studies are required to validate our conclusions. However, this systematic review of the effects of EPA and DHA on skeletal muscle and body composition provides evidence that can be applied in both clinical and industrial settings.
Keywords: EPA, DHA, Skeletal muscle, Malnutrition
INTRODUCTION
During aging process, substantial changes occur in the body composition, including a decline in skeletal muscle and bone mass, weakened muscle strength, and deterioration of structural integrity [1]. Sarcopenia, defined as a gradual decrease in muscle mass and strength, leads to impaired overall health, including physical activity, cognitive function, and quality of life, as well as increased all-cause mortality in older adults [2,3]. Muscle gain and loss are also closely related to nutritional status, and decreased muscle mass is included in the criteria for assessing malnutrition in hospitalized patients [4,5]. Securing muscle mass and attenuating its loss are major health concerns, even in young populations.
Considerable scientific efforts have been made to examine the role and efficacy of certain bioactive compounds and nutrient supplements in maintaining or gaining skeletal muscle mass. Of the nutrients investigated, proteins, vitamin D, and omega-3 fatty acids, mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been of particular interest and have demonstrated positive relationships with skeletal muscle mass and enhanced skeletal muscle health in older adults, patients with various diseases, and healthy adults [6,7,8]. Although the effects of protein and vitamin D supplementation have been well studied, the roles of EPA and DHA in muscle health remain unclear. Clinical intervention studies have suggested that the consumption of EPA and DHA in the diet or pure form, has several health benefits in older adults [9] and patients with sarcopenic conditions [10,11], whereas some studies have reported no advantage of omega-3 on skeletal muscle health [12,13]. A well-known health advantage of EPA and DHA consumption is that they enhance cognitive function and attenuate cardiovascular and inflammation-related diseases [14,15]. Based on this evidence, DHA and EPA are consumed as functional food products and are added to several commercially available food products, such as foods for medical purposes and nutrient-fortified foods [16,17] which are usually supplied as alternatives to the main diet for off hospitalized patients or care-center-dwelling older adults [18].
To date, only a limited number of systematic reviews studies have been performed to test the effect of omega-3 fatty acids on muscle health, but the results of meta-analyses among those studies differed; one study reported no change in any indices of muscle mass and function [19], one study reported a positive effect only on muscle strength [20], and another study showed no effect, but the study subjects were limited to patients with cancer-associated cachexia [21]. In addition, these studies evaluated pure omega-3 fatty acids, although a substantial number of studies have been performed on omega-3 fatty acids formulated using proteins or other bioactive ingredients. Hence, the overall effect of omega-3 fatty acids on changes in skeletal muscle mass has not yet been conclusively proven, and the beneficial effects of omega-3 fatty acids as a diet or multi-ingredient form as well as a pure product in various populations including patients and young adults, need to be comprehensively investigated.
This study aimed to investigate the recent evidence on the roles of dietary EPA and DHA in skeletal muscle mass changes and their associations with muscle strength through a systematic review of pre-reported intervention studies.
SEARCH METHODS FOR THE IDENTIFICATION OF STUDIES
Data sources and literature searches
We systematically searched electronic databases following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. The PubMed and Google Scholar databases were searched for articles published between January 2003 and July 2023. The search terms, based on the objectives of this study, were used to investigate the effect of omega-3 fatty acid intake on skeletal muscle mass, lean body mass, and muscle strength in adults aged 18 years and older. Hence, the search category for main exposure was “omega-3 fatty acids,” and the search categories for main study outcomes were “skeletal muscle mass” and “skeletal muscle strength.” Each search category contained subsequent search terms, and the search terms are listed in Table 1. Medical Subject Headings (MeSH) terms or subject headings were used in coordination with the rules of the search engine.
Table 1. Search terms used for the literature search investigating the relationship between omega-3 fatty acid intake and muscle health.
| Variables | Key words | |
|---|---|---|
| Exposure | ||
| EPA or DHA | ("Fatty Acids, Omega-3"[Mesh]), ("Fatty Acids, Unsaturated"[Mesh]), ("Fish Oils"[Mesh]), ("Eicosapentaenoic Acid"[Mesh]), ("Docosahexaenoic Acids"[Mesh]) | |
| Health outcomes | ||
| Skeletal muscle mass | ("Body Composition"[Mesh]), ("cachexia"[Mesh]), | |
| ("Lean body mass"), ("sarcopenia"[Mesh]), | ||
| ("muscles"[MeSH Terms]), ("Muscle, Skeletal"[Mesh]) | ||
| Skeletal muscle strength | ("Hand Strength"[Mesh]) | |
Study selection based on inclusion and exclusion criteria
Intervention studies that evaluated the effects of omega-3 fatty acids on muscle mass and strength were included. The study population consisted of adults aged 18 years and older, regardless of sex, and the intervention was any omega-3 fatty acid support type. A comparison was made between the omega-3 fatty acid-supplemented group and the placebo group for randomized controlled trials (RCTs) and between baseline and post-intervention for single-arm trials. Animal or cellular studies, review papers, non-English language studies, unpublished data, and conference abstracts were excluded. In addition, studies that found that EPA or DHA was not the main intervention treatment, even though the study utilized EPA or DHA supplementation; studies that excluded muscle mass or strength as primary outcomes; and studies with invalid muscle mass measurements were excluded based on the article contents. After screening the literature based on the exclusion criteria, the remaining articles were retrieved for full-text review. After each search, 2 authors (MGK and SYB) independently extracted relevant reports based on their titles and abstracts. Full-text evaluations were performed by 2 independent authors (MGK and SYB), and disagreements on the evaluation results were primarily resolved through discussions and consultation with a third colleague.
Quality assessment and result summary
Quality assessment tools were applied to the systematic review to explore the effects of EPA or DHA consumption on skeletal muscle mass, body composition, and muscle strength based on the study design, study population, risk of bias, and result consistency. Risk of bias was assessed using a “revised Cochrane risk-of-bias tool for randomized trials (ROB2)” [23] for RCT studies and “risk of bias in non-randomized studies of interventions (ROBINS-I)’’ [24] for single-arm interventional studies. In conjunction with the quality assessment, study information, including the author, study area, study design, participants, intervention, and key outcomes, is briefly summarized in the tables. In the result Tables 2-4, “mg” values of EPA or DHA in reported articles were converted to “g,” and intervention periods were all expressed as “weeks (wk)”.
Table 2. Characteristics of the studies for pure omega-3 fatty acids.
| Author/Year/Country | Study design | Participants | Control treatment, No. of subjects (M, F) | Intervention, No. of subjects (M, F) | Duration of the intervention | Outcomes of interest | Risk of bias* |
|---|---|---|---|---|---|---|---|
| Xu et al. [28]/2022/China | RCT-parallel, double-blind | • Older Chinese adults aged ≥ 60 yr | n = 100 (42 M, 58 F) | n = 100 (42 M, 58 F) | 24 wk | • Skeletal muscle strength | Some concerns |
| • BMI range: 24.6–25.6 kg/m2 | 4 g/day of corn oil capsule (EPA + DHA < 0.05 g) | Omega-3 (1.34 g EPA, 1.07 g DHA) | • Handgrip strength | ||||
| Loss et al. [27]/2022/Brazil | RCT-parallel, double-blind | • Thirty healthy women | n = 15 (15 F) | n = 15 (15 F) | 4 days | • Skeletal muscle strength | Some concerns |
| • BMI range: 18.5–24.9 kg/m2 | Placebo (olive oil capsules) + after resistance exercise | Omega-3 (2.1 g EPA, 1.1 g DHA) + after resistance exercise | |||||
| Li et al. [26]/2021/China | RCT-parallel | • Community dwelling and hospitalized older Chinese adults aged 60 yr and diagnosed sarcopenia | n = 59 (12 M, 47 F) | n = 61 (22 M, 39 F) | 12 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 22.6–22.9 kg/m2 | Routine care without omega-3 or exercise | EPA 300 mg, DHA 200 mg | • Skeletal muscle strength | ||||
| 10 g of protein powder and 250 IU of vitamin D with aerobic and resistance exercise | • And fat mass | ||||||
| Rolland et al. [13]/2019/France and Monaco | RCT double-blind | • Community-dwelling, older people aged ≥ 70 yr | n = 420 | n = 417 | 144 wk | • Skeletal muscle strength (chair stand test, handgrip) | Some concerns |
| • BMI range: 26.0–26.3 kg/m2 | Placebo + the multi-domain intervention (physical activity, cognitive training, and nutritional counseling) | Omega-3 (800 mg DHA, 225 mg EPA) + the multi-domain intervention | • Walking speed | ||||
| McGlory et al. [29]/2019/Canada | RCT-parallel, double-blind | • Twenty healthy, recreationally active young women (age 19–31 yr) | n = 9 (9 F) | n = 11 (11 F) | 10 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 18–26 kg/m2 | Isoenergetic and volume equivalent sunflower oil | Consumed daily 20 mL | • Skeletal muscle strength | ||||
| Omega-3 (2.97 g EPA, 2.03 g DHA) | |||||||
| Cornish et al. [33]/2018/Canada | RCT-parallel | • Older men aged ≥ 65 yr | n = 12 (12 M) | n = 11 (11 M) | 12 wk | • Skeletal muscle mass | Some concerns |
| • Average BMI range: 27.5–27.7 kg/m2 | 3 g of a n-3-6-9 PUFAs blend + resistance training | Omega-3 (1.98 g EPA, 0.99 g DHA) + resistance training 3 times/wk | • Skeletal muscle strength | ||||
| 3 times/wk | • Functional ability | ||||||
| Wang et al. [30]/2017/China | RCT double-blind | • Type 2 diabetic patients with abdominal obesity | n = 50 (20 M, 30 F) | n = 49 (15 M, 34 F) | 24 wk | • Lean body mass | Some concerns |
| • Average BMI range: 25.4–25.9 kg/m2 | Corn oil | Omega-3 (1.34 g EPA, 1.07 g DHA) | • Skeletal muscle mass | ||||
| Da Boit et al. [31]/2017/UK | RCT | • Healthy older adults | n = 23 (13 M, 10 F) | n = 27 (14 M, 13 F) | 18 wk | • Lean body mass | Some concerns |
| • Average BMI range: 24.7–25.9 kg/m2 | 3 g safflower oil + resistance exercise training twice weekly | Omega-3 (2.1 g EPA, 0.6 g DHA) + resistance exercise training twice weekly | • Skeletal muscle strength | ||||
| Logan et al. [32]/2015/Canada | RCT | • Healthy, community-dwelling older women aged 60–76 yr | n = 12 (12 F) | n = 12 (12 F) | 12 wk | • Body composition | Some concerns |
| • Average BMI range: 26.3–27.9 kg/m2 | 3 g olive oil | Omega-3 (2 g EPA, 1 g DHA) | • Skeletal muscle strength | ||||
| • Physical function | |||||||
| Krzymińska-Siemaszko et al. [12]/2015/Poland | RCT-parallel | • Community dwelling older adults aged ≥ 60 yr of age with low muscle mass or at risk of low muscle mass | n = 20 (6 M, 14 F) | n = 30 (11 M, 19 F) | 12 wk | • Lean muscle mass | Some concerns |
| • Average BMI range: 22.9–23.4 kg/m2 | 1 drop of vitamin E solution (11 mg) daily | 1.3 g of omega-3 (660 mg EPA, 440 mg DHA + 200 mg other omega-3 fatty acids + 10 mg of vitamin E) | • Skeletal muscle mass | ||||
| • Skeletal muscle strength | |||||||
| • Physical performance | |||||||
| Smith et al. [11]/2015/USA | RCT-parallel, double-blind | • Older adults aged 60–85 yr | n = 15 (5 M, 10 F) | n = 29 (10 M, 19 F) | 24 wk | • Skeletal muscle mass | Some concerns |
| • Average BMI range: 18.5–34.9 kg/m2 | Placebo pills contained corn oil | Omega-3 (1.86 g EPA, 1.50 g DHA) | • Skeletal muscle strength | ||||
| Murphy et al. [10]/2011/Canada | RCT | • Patients with non-small cell lung cancer under chemotherapy | n = 24 (12 M, 12 F) | n = 16 (9 M, 7 F) | 6 wk | • Skeletal muscle mass | High |
| • Average BMI range: 26.2–27.3 kg/m2 | Standard medical care | Omega-3 (2.2 g EPA) | |||||
| Fearon et al. [25]/2006/UK | RCT-parallel, double-blind | • Patients with gastrointestinal and lung cancer between the ages of 18–80 yr | n = 171 (123 M, 48 F) | n = 175 (117 M, 58 F) | 8 wk | • Lean body mass | Some concerns |
| • Average BMI range: 20.9–21.4 kg/m2 | Placebo | 2 g EPA | |||||
| n = 172 (115 M, 57 F) | |||||||
| 4 g EPA |
RCT, randomized controlled trial; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BMI, body mass index.
*Risk of bias was assessed using a revised Cochrane risk-of-bias tool for randomized trials (ROB2).
Table 4. Characteristics of the studies for omega-3 fatty acid supplementation through enteral nutrition support.
| Author/Year/Country | Study design | Participants | Control treatment, No. of subjects (M, F) | Intervention, No. of subjects (M, F) | Duration of the intervention | Outcomes of interest | Risk of bias* |
|---|---|---|---|---|---|---|---|
| Abe et al. [40]/2018/Japan | Single-arm intervention | • Patients with pancreatic and bile duct cancer who underwent chemotherapy | n = 27 (16 M, 11 F) | 8 wk | • Skeletal muscle mass | Moderate | |
| • Average BMI range: 25.9 kg/m2 | Racol®, an enteral nutrient formulated with omega-3 (2–4 packs/200 kcal/300 mg of omega-3 fatty acids per pack) | ||||||
| Van der Meij et al. [38]/2012/The Netherland | RCT-parallel, double blind | • Patients with non-small cell lung cancer aged 18–80 yr | n = 20 (5 M, 15 F) | n = 20 (16 M, 4 F) | 5 wk | • Handgrip strength | Some concerns |
| • Average BMI range: 23.0-24.8 kg/m2 | Two packages per day of an iso-caloric control oral nutritional supplement (Ensure, Control) | Omega-3 (2.02 g EPA, 0.92 g DHA) added in same volume nutritional supplement (ProSure, Intervention) with control group | |||||
| Ryan et al. [37]/2009/Ireland | RCT-parallel | • Patients with esophageal cancer | n = 25 (M:F = 14:1) | n = 28 (M:F = 24:4) | 3 wk | • Lean body mass | Some concerns |
| • Average BMI range: 24.6-27.1 kg/m2 | Iso-caloric and iso-nitrogenous standard nutritional feed without EPA | Omega-3 (EPA 2.2 g/d + Enteral feed) | |||||
| Bauer et al. [39]/2005/Australia | Single-arm intervention | • Patients with pancreatic cancer, or non-small cell lung cancer | n = 7 (5 M, 2 F) | 8 wk | • Lean body mass | Moderate | |
| • Average BMI range: 26.8 kg/m2 | 16 g protein and 1.1 g EPA, as enriched oral nutritional supplement |
RCT, randomized controlled trial; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BMI, body mass index.
*Risk of bias was assessed using a revised Cochrane risk-of-bias tool for randomized trials (ROB2) for RCT studies and using a “risk of bias in non-randomized studies of interventions (ROBINS-I) for single-arm interventional studies.
RESULTS
Study identification and selection
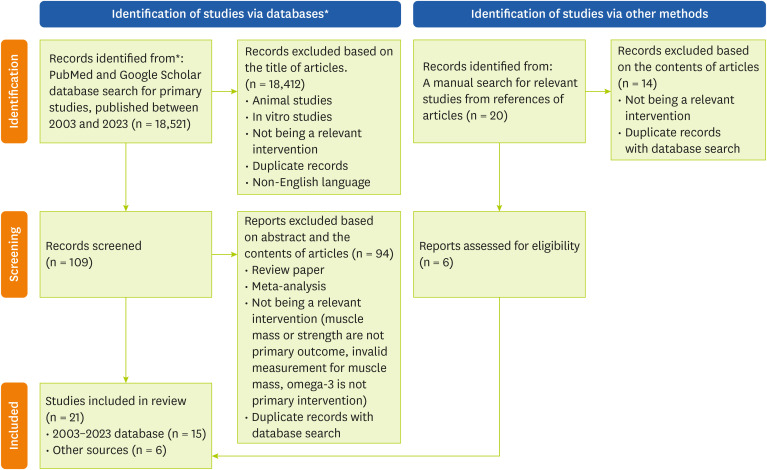
An initial database search of studies published between January 2003 and July 2023 identified 18,521 articles. After removing the duplicates, 18,412 articles were excluded. The main reasons for excluding articles from subsequent consideration were: irrelevant intervention, which included multifaceted interventions; interventions not providing omega-3 fatty acids to participants; interventions not aimed at measuring skeletal muscle mass; lean body mass, or muscle strength; or being cross-sectional in design without appropriate pre- and post-measures of indicators. In total, 15 articles fulfilled the inclusion criteria for quality assessment. During additional manual literature searches from reference lists in the database-searched articles, a total of 20 articles were newly identified, and of these, 6 articles were subjected to additional review. Finally, 21 articles were included in the analysis. The study selection process is illustrated in Figure 1.
Figure 1. Study selection flow chart for investigating the relationship between intake of omega-3 fatty acids and muscle health.
*The format of flow chart was adopted from The PRISMA 2020 statement.
Study characteristics
The results of the individual studies from the literature assessment are outlined in Tables 2-4 according to the main treatment type.
Intervention studies using pure omega-3 fatty acids
Thirteen studies used pure omega-3 fatty acids to examine their effect on skeletal muscle mass or strength, including hand grip strength. Seven studies were double-blind RCTs (Table 2) [11,25,26,27,28,29,30], whereas the others were RCTs that were not double-blinded or did not clearly note the blinding of participants or investigators [10,12,13,31,32,33]. The participant ages in 11 studies were in the range of 60 to 70 years, and 2 RCT studies investigated young adults aged 22 ± 3 years [27,29]. Of these, 2 studies [11,28] showed that intake of EPA and DHA in the range of 1–2 g/day enhanced skeletal muscle mass whereas one study [12] reported no significant effect of omega-3 fatty acid intake at doses of 0.6 g EPA and 0.44 g DHA. Another study reported that omega-3 fatty acids improved physical performance in cachectic patients with cancer; however, the doses of EPA and DHA were 2- to 4-fold higher than those used in the abovementioned studies. Omega-3 fatty acid supplementation improved physical mobilization and muscle strength in healthy young adults [25]. The other 6 studies were manually searched, described, and assessed in a previously performed systematic review and meta-analysis [19], but the characteristics of the study participants varied because the participants in 4 studies were older adults, one study included patients with cancer, and one study included patients with diabetes mellitus.
Intervention studies using omega-3 fatty acids with proteins and multi-ingredients
Of all studies, 4 were 2-arm RCTs (Table 3). The mean age of the study participants was between 63 and 77.4 years [9,34,35,36]. As studies have investigated the effect of omega-3 fatty acids in combination with other ingredients or foods (e.g., fruit juice), the control group for intervention in these studies corresponded to a formula or food without omega-3 fatty acids. All 4 studies suggested that combined supplementation of omega-3 fatty acids with other well-known health ingredients (e.g., whey protein, probiotics, vitamin D, and resveratrol) was beneficial to skeletal muscle health, but the dose of EPA, DHA and other ingredients varied among the studies.
Table 3. Characteristics of the studies for omega-3 fatty acids as multi-ingredient formulas.
| Author/Year/Country | Study design | Participants | Control treatment, No. of subjects (M, F) | Intervention, No. of subjects (M, F) | Duration of the intervention | Outcomes of interest | Risk of bias* |
|---|---|---|---|---|---|---|---|
| Rondanelli et al. [9]/2022/Italy | RCT-parallel, double-blind | • Sarcopenia patients aged ≥ 55 yr | n = 28 | n = 22 | 8 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 20–30 kg/m2 | Isocaloric placebo with the same flavor | Omega-3 (323.5 mg of EPA, 147.1 mg of DHA, 29.4 mg of the other types of omega-3), formulated with leucine (2.5 g), probiotic LPPS23 | • Skeletal muscle strength | ||||
| Murphy et al. [35]/2022/UK | RCT-parallel, double-blind | • Older adults aged ≥ 65 yr at risk of sarcopenia | n = 28 (13 M, 15 F) | n = 30 (15 M, 15 F) | 24 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 25.1–27.1 kg/m2 | 10.6 g Whey protein and 3.1 g Leucine | Omega-3 (0.8 g EPA, 1.1 g DHA) formulated with leucine and whey protein | • Skeletal muscle strength | ||||
| • Physical performance | |||||||
| Scotto di Palumbo et al. [36]/2022/UK | RCT-parallel, double-blind | • Irish older adults aged 70 yr and above | n = 16 (8 M, 8 F) | n = 21 (11 M, 10 F) | 24 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 20–30 kg/m2 | Placebo consisted of the fruit juice alone. contained whey protein isolate, vitamin D3, and resveratrol | Omega-3 (1.50 g EPA, 1.50 g DHA) in fruit juice | • Handgrip strength | ||||
| Nilsson et al. [34]/2020/USA | RCT-parallel | • ≥ 65 yr of age, male sex | n = 16 (16 M) | n = 16 (16 M) | 12 wk | • Skeletal muscle mass | Some concerns |
| • BMI range: 20–34.9 kg/m2 | Collagen protein 40 g/Sunflower oil 10 mL + HBRE | Omega-3 (1.51 g EPA, 0.95 g DHA) + HBRE | • Skeletal muscle strength |
RCT, randomized controlled trial; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BMI, body mass index; HBRE, home-based resistance exercise.
*Risk of bias was assessed using a revised Cochrane risk-of-bias tool for randomized trials (ROB2).
Intervention studies providing omega-3 fatty acids through enteral nutritional support
Of the 4 studies that provided omega-3 fatty acids through nutritional support products, 2 were 2-arm RCTs that provided nutritional formulas with EPA for intervention and only provided enteral nutritional support formulas for controls [37,38]. The other 2 studies were single-arm pre-post interventional studies supplementing omega-3 fatty acids in enteral nutritional formulas [39,40] (Table 4). The mean age of the participants was 55–75 years in all the 4 studies. Although patients’ conditions vary under nutritional support, studies have shown that omega-3 fatty acids supplementation through enteral nutrition is effective in enhancing muscle mass and function.
Risk of bias
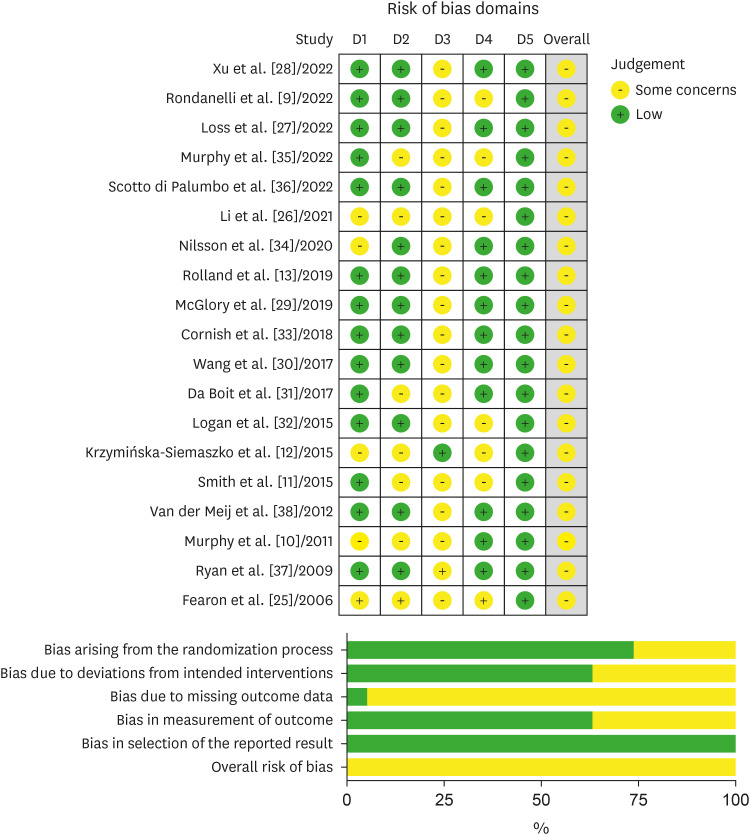
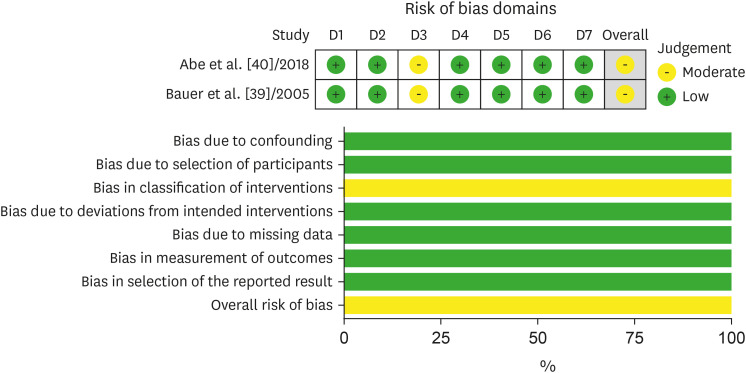
The results of the risk of bias assessment are shown in Figures 2 and 3. Most of the studies had a low risk of bias due to the “randomization process” and “deviations from intended intervention.” However, majority of studies had results of “some concerns” in the items of “bias in the measurement of the outcome” because many studies did not clearly indicate if the main treatment was concealed to study participants or investigators despite the title of the articles containing “double-blinded RCT.” The bias due to missing outcome data and the bias in the selection of the reported results was mostly low-risk, but some studies that did not provide the data values or outcomes of the study partially matched the main outcome of interest in this systematic review, presenting some concern or high risk. More than 90% of the studies had incomplete data outcomes, indicating that not all proposed analyses were presented.
Figure 2. Bias assessment of studies conducting a randomized controlled trial. The risk of bias was assessed by reviewing the author’s judgement according to the guidelines, and the percentage of studies belonging to a type of bias was presented. Risk of bias was assessed using a revised Cochrane risk-of-bias tool for randomized trials (ROB2) for randomized controlled trial studies.
Figure 3. Bias assessment of studies conducting a non-randomized controlled trial. The risk of bias was assessed by reviewing the author’s judgement according to the guidelines, and the percentage of studies belonging to a type of bias was presented. Risk of bias was assessed using a risk of bias in non-randomized studies of interventions (ROBINS-I) for single-arm interventional studies.
Subjective assessment of heterogeneity among studies
We further reviewed and assessed whether the searched articles were eligible for meta-analysis based on the characteristics of the study participants, the type of omega-3 intervention, and the type of outcome measured. Although the results of the “risk of bias” assessment indicated mostly “low” results, we subjectively evaluated that the conditions of study participants and type of interventions were widely heterogeneous among searched studies and not adequate for quantitative analysis. Based on our subjective assessment, a further meta-analysis was not performed.
DISCUSSION
In this systematic review, we searched for and examined studies investigating the effects of EPA and DHA on skeletal muscle mass and function. The outcomes reported in the literature can be divided into 3 categories according to the type of omega-3 fatty acid supplementation: omega-3 fatty acids with multi-ingredient supplementation, including proteins; omega-3 fatty acid supplementation through nutritional support; and pure omega-3 fatty acid supplementation. Although the intake of omega-3 fatty acids is beneficial for skeletal muscle in most studies, the results and related implications vary according to each literature category.
Many studies included in this systematic review investigated the effects of omega-3 fatty acid supplementation as a pure ingredient dispersed in oil, and 13 studies. Of these, one study with older males (n = 10) and females (n = 19) aged 60–85 years showed that a 6-month intake of EPA (1.86 g/d) and DHA (1.50 g/d) increased handgrip strength and thigh muscle volume without changing total body fat content [11], indicating that omega-3 fatty acids alone can induce favorable effects on skeletal muscles. Consumption of 1.34 g of EPA and 1.07 g of DHA for 6 months enhanced skeletal muscle mass compared to the 4.0 g corn oil supplemented control group, and significantly increased handgrip strength and physical performance, with p < 0.0001 in time and group interaction effect in older Chinese adults aged ≥ 60 years [28]. Although these two studies indicated the beneficial effect of omega-3 fatty acid supplementation, one study reported no significant effect of pure omega-3 fatty acid supplementation. In a study involving community-dwelling older adults in Poland aged 74.6 ± 8.0 years with low muscle mass, participants received the 12-week supplementation with a 1.3 g omega-3 fatty acid capsule (0.6 g EPA and 0.44 g DHA and 0.2 g of other types of omega-3 fatty acids) along with vitamin E. The study found that there were no changes in skeletal muscle mass, handgrip strength and physical activity, as assessed using the “Time Up and Go test” when compared to participants who were supplemented with only 1.1 g of vitamin E [12]. Similar to the results of studies reporting the effect of co-supplementation with proteins, the control group received beneficial compounds (e.g., vitamin E), which could lead to a decrease in the differences between the intervention and control groups. Another study conducted on cachectic patients with gastrointestinal and lung cancer showed that physical function improved by approximately 7%, and muscle weakness was delayed compared with placebo in patients receiving 2 or 4 g of EPA for 8 weeks [25]. Overall, these 4 studies were heterogeneous in terms of the consistency of results, dose of omega-3 fatty acids, timing of intervention, and participant status.
While most omega-3 fatty acid intervention studies have been conducted in older adults or patients with sarcopenia or cancer-bearing cachectic conditions, very few studies have reported the effect of omega-3 fatty acid supplementation in young adults. In this review, we identified 2 RCTs involving healthy young adults. One study investigated the effects of omega-3 fatty acids on muscle mass in healthy young females aged 19–31 years [29]. The study showed that daily supplementation of omega-3 fatty acids composed of 2.97 g EPA and 2.03 g DHA for 10 weeks attenuated 2 weeks of unilateral leg immobilization [29], suggesting that the muscle protective effect of omega-3 fatty acids was effective even in young adults. The other study also showed that, compared to the consumption of 3.2 g of olive oil control, the consumption of 2.1 g EPA and 1.1 g DHA for 4-consecutive days after resistance exercise significantly enhanced strength recovery assessed by muscle soreness and indices of muscle contraction (“maximal isometric torque” and “isokinetic voluntary torque”) in the lower limbs of healthy young females with a mean age of 22.5 years [27]. These 2 studies were comparable in that they both provided omega-3 fatty acids at similar doses; however, one study co-administered resistance exercise. Hence, the results of these 2 studies are implicated in the comparison of the effects of omega-3 fatty acids on muscle health between individuals with and without PA.
Manually searched 6 studies were previously reported in a study by Rondanelli et al. [19]. Patients who received 2.2 g of EPA/day exhibited maintained skeletal muscle mass at the level of baseline measurement, while patients in the reference and standard care groups who did not receive EPA supplementation lost muscle mass by 6.0% and 6.8%, respectively, during their first-ever chemotherapy [10]. Lean body mass and muscle mass was not altered by supplementation with omega-3 fatty acids containing 1.34 g of EPA and 1.07 g of DHA for 24 weeks in patients with type 2 diabetes mellitus and abdominal obesity [30]. In addition, the results of studies on older adults were inconsistent because one study reported a positive effect of omega-3 fatty acids only in females, and the other 3 studies reported no significant effect of omega-3 fatty acid supplementation on muscle mass or strength. Supplementation of 3.0 g/d of omega-3 fatty acids composed of 2.1 g EPA and 0.6 g DHA augments increases in muscle function and enhanced knee-extensor isometric strength per a unit cross-sectional muscle area in older females compared to those in the placebo group received 3.0 g safflower oil daily; however, those effects were not seen in male participants [31]. Daily intake of 3.0 g omega-3 fatty acids (1.98 g EPA and 0.99 g DHA) did not differ with resistance training for 12 weeks in older males aged ≥ 65 years [33]. Supplementation of 3.0 g/d omega-3 composed of 2.0 g EPA and 1.0 g DHA increased lean body mass by 1.9 g compared to baseline and enhanced results in the “Timed up and Go test” in healthy, community-dwelling older females aged 60–76 years, but no difference was found between the intervention group and the placebo group who received 3.0 g/d of olive oil instead of omega-3 fatty acids [32]. No significant effect was also found for the handgrip strength in a group of participants, 1.025 g of omega-3 fatty acids containing 0.8 g DHA and 0.225 g EPA in community-dwelling, older people aged ≥ 70 years [13]. Collectively, the results of studies investigating the effect of omega-3 as a pure compound are inconclusive.
All studies that treated omega-3 fatty acids with protein or as a multi-ingredient supplement were all conducted in older adults with sarcopenia [9,26,34,36]. In a study by Nilsson et al. [34], daily intake of 1.51 g EPA and 0.95 g DHA as a multi-ingredient supplement composed of whey protein, micellar casein, creatinine, and vitamin D with physical activity training for 12 weeks increased appendicular and total muscle mass by 3% and 2%, respectively, in free-living older males. In addition, the EPA and DHA intake groups showed improved lean mass-to-body fat ratios and an 8% increase in maximal hand grip strength compared to the placebo group, which consumed collagen and sunflower oil instead of EPA/DHA supplementation [34]. The effect of omega-3 supplementation on skeletal muscle mass and strength was investigated among Chinese adults aged 60 years and older who were diagnosed with sarcopenia but had normal cognitive functions [26]. Daily intake of 1.2 g EPA and 0.8 g DHA with whey protein, and 1,000 IU of vitamin D or their intake in parallel with aerobic and resistance training improved muscle mass (95% confidence interval, 1.86–3.36) compared to the control group, supplementation only group and supplementation with exercise group. Omega-3 supplementation increased grip strength by 3.614–9.118 fold in the control group and 3.44–8.907 fold in the control group [26]. In a 6-month trial, the consumption of fruit juice containing 1.5 g EPA, 1.5 g of DHA, 8 g of whey protein, 400 IU of vitamin D3, and 0.15 g of resveratrol did not induce beneficial changes in skeletal muscle mass compared to the consumption of placebo fruit juice which contained no nutrient supplements. However, it attenuated the time-induced decline in handgrip strength in older Irish adults with low skeletal muscle mass and low physical activity [36]. In an RCT conducted in Italy, daily intake of 0.5 g omega-3 fatty acids containing 0.32 g EPA and 0.15 g DHA, with 2.5 g leucine and probiotics as a form of water-dispensable powder for 8 weeks increased handgrip strength by 3.332 g in sarcopenia patients aged 79.7 ± 4.8 years [9]. These 3 studies utilized similar doses of whey protein supplementation (9.0–11.0 g) with omega-3 fatty acids (0.5–3.0 g), and showed enhanced handgrip strength in the intervention groups. The muscle enhancing effects of protein, leucine, vitamin D, and exercise as individual components or as a complex on skeletal muscle mass and function have been demonstrated in many studies [41,42,43]. However, the studies found in this systematic review further support the benefit of omega-3 fatty acids in a combined intervention of protein, vitamin D, and exercise.
While most studies utilizing protein supplementation with omega-3 are beneficial, one study reported no significant effects of EPA and DHA on skeletal muscle mass and strength. The study was conducted in a manner similar to the aforementioned studies with older adults aged ≥ 65 years [35] and defined sarcopenia or low handgrip strength according to the European Working Group on Sarcopenia in Older People 2010 (EWGSOP1) criteria [1]. A 10.6 g whey protein and 3.1 g of total leucine, or protein with omega-3 fatty acids containing 0.8 g EPA and 1.1 g DHA were supplied to the participants for 24 weeks. However, changes in appendicular muscle mass and composite leg strength in response to protein-only supplements or proteins with omega-3 fatty acids varied among individuals within the same group and were not significantly different between the intervention groups [35]. Such differences in outcomes may be due to differences in the control group, in which the same amount of protein was provided to participants, whereas no protein or beneficial component was supplied in the other 4 studies. Hence, the protein intake in the control group may have led to a decrease in the outcome gap between the omega-3 intervention and control groups. Nevertheless, EPA or DHA alone hardly showed a significant effect on muscle mass or strength and raised some concerns about stability or favorability [44]. The results of omega-3 fatty acid intervention together with multiple muscle-beneficial ingredients, could be implicated in the development of medical-purpose or care foods.
In this study, we further identified articles as a category of studies that provide omega-3 fatty acids through enteral nutrition support. Four studies were then identified and reviewed. Daily intake of 0.6–1.2 g of omega-3 fatty acids through nutritional support for 56 days significantly (p = 0.002) increased skeletal muscle mass by > 1.5 kg compared with the baseline value prior to the start of nutritional support in patients with biliary-pancreatic cancer [40]. A similar intervention study in cachectic patients with pancreatic and non-small-cell cancer showed that daily oral nutritional supplements enriched with 16 g of protein and 1.1 g EPA together with chemotherapy for 8 weeks resulted in significant improvements in weight and lean body mass along with improved nutritional status and functional capacity compared to baseline [39]. The results of these 2 studies indicate that treatment with omega-3 fatty acids is effective in enhancing the nutritional status and muscle function of patients; however, this study was a single-arm trial that had no control group and could not differentiate whether the outcome of the study was due to an intervention effect, time, or other factors. Patients with non-small cell lung cancer who received protein and energy supplements containing omega-3 fatty acids (2.02 g EPA and 0.92 g DHA) for 5 weeks exhibited enhanced cognitive function and physical activity compared to patients in the control group who received oral nutritional supplements without omega-3 fatty acids [38]. Administration of 2.2 g/d EPA through enteral nutrition for 3 weeks attenuated the loss of lean body mass after surgery in patients with esophageal cancer compared to the control group, which received only enteral nutrition without EPA supplementation [37]. Based on the study results, omega-3 fatty acid supplementation through nutritional support seems to be effective in preventing postoperative muscle loss in cancer patients, who usually experience cachexia; however, the primary aim of this study was to provide “nutritional support” for patients to secure energy and protein in the required amount range, and the independent effect of omega-3 fatty acid supplementation in the study setting seems difficult to assess. In addition, the characteristics of the patients and the method of outcome measurement varied among the studies. Further evidence is needed to confirm the beneficial effects of omega-3 fatty acid supplementation on enteral nutrition support.
Although many previous studies have suggested a positive effect of omega-3 fatty acids on skeletal muscle health, the effect of omega-3 fatty acids on muscle tissue seems to be indirect, based on the possible mechanism of EPA- or DHA-induced health benefits. Omega-3 fatty acids improve neurological and cognitive functions, leading to enhanced interactions between neuron-muscle receptors, pronounced physical activity, and muscle contraction [45]. Omega-3 fatty acids may enhance energy metabolism in skeletal muscles to produce and consume energy efficiently [32,46], resulting in skeletal muscle mass and activity. As omega-3 fatty acids are known to enhance blood flow [45], a fluent and sufficient supply of nutrients to the muscle could lead to an increase in muscle content and mass. Some of these aspects were reported in the literature found in this systematic review; however, the articles were limited in number, and the mechanisms of omega-3 fatty acids were only partially investigated in these studies.
Nevertheless, the results of the studies identified in the systematic review suggest that supplementation with omega-3 fatty acids is necessary to prevent sarcopenia and enhance the physical status of older adults or patients with cachexia. Based on these results, as a pure compound, high doses of EPA or DHA at more than 2.0 g/day, which is 5- to 8-folds higher than the recommended amount for heart health 250–500 mg/day [47], seem to be required to generate beneficial effects. The muscle favorable effects of this high dose of EPA and DHA are consistent with those of cross-sectional studies; however, these studies also suggest that low levels of EPA and DHA are beneficial [7,48]. Hence, the findings need to be validated in clinical studies performed with dose-dependent EPA and DHA interventions. In particular, omega-3 is required for nutritional support in cancer patients who mostly experience cachectic conditions during cancer treatment or hospital stay. Studies suggest that nutritional support with EPA idoes not have a harmful effect on food intake in patients with various cancers who usually have adverse gastrointestinal symptoms during cancer treatment. Supplementation with omega-3 fatty acids in combination with other ingredients could be a plausible option to cope with quality control and lower side effects in the pharmaceutical or nutraceutical industries that develop foods for medical purpose.
In this study, we could not determine the effect of omega-3 fatty acids on skeletal muscle mass or strength because of the varied results among studies. A meta-analysis could not be performed, due to the heterogeneity of study methods, study populations, study durations, or a combination of these factors. However, we conducted a comprehensive review of studies that investigated the effects of omega-3 fatty acids as multi-ingredient forms or nutritional supplements, whereas previous studies have mainly focused on pure compounds. The studies included in this systematic review have a low overall risk of bias, which may help adequately justify the existing evidence for the muscle-favorable effect of omega-3 fatty acids, without results of the meta-analysis. Further interventional studies designed to comprehensively investigate the underlying mechanisms of enhanced muscle gain and strength are necessary to address these issues.
CONCLUSION
This systematic review investigated the effects of omega-3 fatty acids on skeletal muscle mass and strength in RCTs and non-randomized single-arm interventions. Although the effects of omega-3 fatty acids on skeletal muscle mass and strength indices have not been clearly determined, a substantial number of individual studies included in this systematic review have suggested positive effects of omega-3 fatty acids on gaining or maintaining muscle mass in adults with sarcopenic conditions or cachexia. This literature review of the effects of EPA and DHA on skeletal muscle and body composition provides promising evidence that can be applied in clinical and industrial settings.
Footnotes
Funding: This research was supported by Daegu University (2021-0036).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Bu SY, Moon GK.
- Investigation: Bu SY, Moon GK.
- Methodology: Bu SY, Moon GK.
- Writing – original draft: Bu SY, Moon GK.
- Writing – review & editing: Bu SY, Moon GK.
References
- 1.Barazzoni R, Jensen GL, Correia MI, Gonzalez MC, Higashiguchi T, Shi HP, Bischoff SC, Boirie Y, Carrasco F, Cruz-Jentoft A, Fuchs-Tarlovsky V, Fukushima R, Heymsfield S, Mourtzakis M, Muscaritoli M, Norman K, Nyulasi I, Pisprasert V, Prado C, de van der Schuren M, Yoshida S, Yu Y, Cederholm T, Compher C. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr. 2022;41:1425–1433. doi: 10.1016/j.clnu.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. doi: 10.1016/j.metabol.2023.155533. [DOI] [PubMed] [Google Scholar]
- 3.Calvani R, Picca A, Coelho-Júnior HJ, Tosato M, Marzetti E, Landi F. Diet for the prevention and management of sarcopenia. Metabolism. 2023;146:155637. doi: 10.1016/j.metabol.2023.155637. [DOI] [PubMed] [Google Scholar]
- 4.Wobith M, Herbst C, Lurz M, Haberzettl D, Fischer M, Weimann A. Evaluation of malnutrition in patients undergoing major abdominal surgery using GLIM criteria and comparing CT and BIA for muscle mass measurement. Clin Nutr ESPEN. 2022;50:148–154. doi: 10.1016/j.clnesp.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Wiese ML, Gartner S, von Essen N, Doller J, Frost F, Tran QT, Weiss FU, Meyer F, Valentini L, Garbe LA, Metges CC, Bannert K, Sautter LF, Ehlers L, Jaster R, Lamprecht G, Steveling A, Lerch MM, Aghdassi AA. Malnutrition is highly prevalent in patients with chronic pancreatitis and characterized by loss of skeletal muscle mass but absence of impaired physical function. Front Nutr. 2022;9:889489. doi: 10.3389/fnut.2022.889489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossato LT, de Branco FM, Azeredo CM, Rinaldi AE, de Oliveira EP. Association between omega-3 fatty acids intake and muscle strength in older adults: a study from National Health and Nutrition Examination Survey (NHANES) 1999-2002. Clin Nutr. 2020;39:3434–3441. doi: 10.1016/j.clnu.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Isanejad M, Tajik B, McArdle A, Tuppurainen M, Sirola J, Kröger H, Rikkonen T, Erkkilä A. Dietary omega-3 polyunsaturated fatty acid and alpha-linolenic acid are associated with physical capacity measure but not muscle mass in older women 65-72 years. Eur J Nutr. 2022;61:1813–1821. doi: 10.1007/s00394-021-02773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M, Erkkilä AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr. 2016;115:1281–1291. doi: 10.1017/S000711451600012X. [DOI] [PubMed] [Google Scholar]
- 9.Rondanelli M, Gasparri C, Barrile GC, Battaglia S, Cavioni A, Giusti R, Mansueto F, Moroni A, Nannipieri F, Patelli Z, Razza C, Tartara A, Perna S. Effectiveness of a novel food composed of leucine, omega-3 fatty acids and probiotic Lactobacillus paracasei PS23 for the treatment of sarcopenia in elderly subjects: a 2-month randomized double-blind placebo-controlled trial. Nutrients. 2022;14:4566. doi: 10.3390/nu14214566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117:1775–1782. doi: 10.1002/cncr.25709. [DOI] [PubMed] [Google Scholar]
- 11.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–122. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krzymińska-Siemaszko R, Czepulis N, Lewandowicz M, Zasadzka E, Suwalska A, Witowski J, Wieczorowska-Tobis K. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J Environ Res Public Health. 2015;12:10558–10574. doi: 10.3390/ijerph120910558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolland Y, Barreto PS, Maltais M, Guyonnet S, Cantet C, Andrieu S, Vellas B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain lifestyle intervention on muscle strength in older adults: secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT) Nutrients. 2019;11:1931. doi: 10.3390/nu11081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Li W, Gao Y, Chen Y, Bai D, Weng J, Du Y, Ma F, Wang X, Liu H, Huang G. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double-blind, placebo-controlled trial. Eur J Nutr. 2021;60:1795–1808. doi: 10.1007/s00394-020-02373-3. [DOI] [PubMed] [Google Scholar]
- 15.Rist PM, Buring JE, Cook NR, Manson JE, Rexrode KM. Effect of vitamin D and/or omega-3 fatty acid supplementation on stroke outcomes: a randomized trial. Eur J Neurol. 2021;28:809–815. doi: 10.1111/ene.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane NE, Ivanova J, Emir B, Mobasheri A, Jensen MG. Characterization of individuals with osteoarthritis in the United States and their use of prescription and over-the-counter supplements. Maturitas. 2021;145:24–30. doi: 10.1016/j.maturitas.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Zargar A, Ito MK. Long chain omega-3 dietary supplements: a review of the National Library of Medicine Herbal Supplement Database. Metab Syndr Relat Disord. 2011;9:255–271. doi: 10.1089/met.2011.0004. [DOI] [PubMed] [Google Scholar]
- 18.Fialkow J. Omega-3 fatty acid formulations in cardiovascular disease: dietary supplements are not substitutes for prescription products. Am J Cardiovasc Drugs. 2016;16:229–239. doi: 10.1007/s40256-016-0170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondanelli M, Perna S, Riva A, Petrangolini G, Di Paolo E, Gasparri C. Effects of n-3 EPA and DHA supplementation on fat free mass and physical performance in elderly. A systematic review and meta-analysis of randomized clinical trial. Mech Ageing Dev. 2021;196:111476. doi: 10.1016/j.mad.2021.111476. [DOI] [PubMed] [Google Scholar]
- 20.Ma WJ, Li H, Zhang W, Zhai J, Li J, Liu H, Guo XF, Li D. Effect of n-3 polyunsaturated fatty acid supplementation on muscle mass and function with aging: a meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2021;165:102249. doi: 10.1016/j.plefa.2021.102249. [DOI] [PubMed] [Google Scholar]
- 21.de Castro GS, Andrade MF, Pinto FC, Faiad JZ, Seelaender M. Omega-3 fatty acid supplementation and its impact on systemic inflammation and body weight in patients with cancer cachexia-a systematic review and meta-analysis. Front Nutr. 2022;8:797513. doi: 10.3389/fnut.2021.797513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JP. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, Murray GD. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006;24:3401–3407. doi: 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Cui M, Yu K, Zhang XW, Li CW, Nie XD, Wang F. Effects of nutrition supplementation and physical exercise on muscle mass, muscle strength and fat mass among sarcopenic elderly: a randomized controlled trial. Appl Physiol Nutr Metab. 2021;46:494–500. doi: 10.1139/apnm-2020-0643. [DOI] [PubMed] [Google Scholar]
- 27.Loss LC, Benini D, de Lima-E-Silva FX, Möller GB, Friedrich LR, Meyer E, Baroni BM, Schneider CD. Effects of omega-3 supplementation on muscle damage after resistance exercise in young women: a randomized placebo-controlled trial. Nutr Health. 2022;28:425–432. doi: 10.1177/02601060211022266. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Lu Y, Yang X, Pan D, Wang Y, Yin S, Wang S, Sun G. Effects of fish oil-derived n-3 polyunsaturated fatty acid on body composition, muscle strength and physical performance in older people: a secondary analysis of a randomised, double-blind, placebo-controlled trial. Age Ageing. 2022;51:afac274. doi: 10.1093/ageing/afac274. [DOI] [PubMed] [Google Scholar]
- 29.McGlory C, Gorissen SH, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33:4586–4597. doi: 10.1096/fj.201801857RRR. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Wang Y, Zhu Y, Liu X, Xia H, Yang X, Sun G. Treatment for 6 months with fish oil-derived n-3 polyunsaturated fatty acids has neutral effects on glycemic control but improves dyslipidemia in type 2 diabetic patients with abdominal obesity: a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2017;56:2415–2422. doi: 10.1007/s00394-016-1352-4. [DOI] [PubMed] [Google Scholar]
- 31.Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, Thies F, Jeromson S, Hamilton DL, Speakman JR, Hambly C, Mangoni AA, Preston T, Gray SR. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr. 2017;105:151–158. doi: 10.3945/ajcn.116.140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan SL, Spriet LL. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS One. 2015;10:e0144828. doi: 10.1371/journal.pone.0144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornish SM, Myrie SB, Bugera EM, Chase JE, Turczyn D, Pinder M. Omega-3 supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men. Nutr Res. 2018;60:87–95. doi: 10.1016/j.nutres.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson MI, Mikhail A, Lan L, Di Carlo A, Hamilton B, Barnard K, Hettinga BP, Hatcher E, Tarnopolsky MG, Nederveen JP, Bujak AL, May L, Tarnopolsky MA. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. 2020;12:2391. doi: 10.3390/nu12082391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy CH, Connolly C, Flanagan EM, Mitchelson KA, de Marco Castro E, Egan B, Brennan L, Roche HM. Interindividual variability in response to protein and fish oil supplementation in older adults: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13:872–883. doi: 10.1002/jcsm.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scotto di Palumbo A, McSwiney FT, Hone M, McMorrow AM, Lynch G, De Vito G, Egan B Scotto di. Effects of a long chain n-3 polyunsaturated fatty acid-rich multi-ingredient nutrition supplement on body composition and physical function in older adults with low skeletal muscle mass. J Diet Suppl. 2022;19:499–514. doi: 10.1080/19390211.2021.1897057. [DOI] [PubMed] [Google Scholar]
- 37.Ryan AM, Reynolds JV, Healy L, Byrne M, Moore J, Brannelly N, McHugh A, McCormack D, Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–363. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 38.van der Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, Smit EF, van Leeuwen PA. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr. 2012;66:399–404. doi: 10.1038/ejcn.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy--a pilot study. Support Care Cancer. 2005;13:270–274. doi: 10.1007/s00520-004-0746-7. [DOI] [PubMed] [Google Scholar]
- 40.Abe K, Uwagawa T, Haruki K, Takano Y, Onda S, Sakamoto T, Gocho T, Yanaga K. Effects of ω-3 fatty acid supplementation in patients with bile duct or pancreatic cancer undergoing chemotherapy. Anticancer Res. 2018;38:2369–2375. doi: 10.21873/anticanres.12485. [DOI] [PubMed] [Google Scholar]
- 41.Bo Y, Liu C, Ji Z, Yang R, An Q, Zhang X, You J, Duan D, Sun Y, Zhu Y, Cui H, Lu Q. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: a double-blind randomized controlled trial. Clin Nutr. 2019;38:159–164. doi: 10.1016/j.clnu.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A, Phillips SM. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One. 2017;12:e0181387. doi: 10.1371/journal.pone.0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, Pouyet C, Hafnaoui N, Blot A, Cano N, Farigon N, Bongers A, Jourdan M, Luiking Y, Walrand S, Boirie Y. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147:2262–2271. doi: 10.3945/jn.117.252510. [DOI] [PubMed] [Google Scholar]
- 44.Hinriksdottir HH, Jonsdottir VL, Sveinsdottir K, Martinsdottir E, Ramel A. Bioavailability of long-chain n-3 fatty acids from enriched meals and from microencapsulated powder. Eur J Clin Nutr. 2015;69:344–348. doi: 10.1038/ejcn.2014.250. [DOI] [PubMed] [Google Scholar]
- 45.Fairbairn P, Tsofliou F, Johnson A, Dyall SC. Effects of a high-DHA multi-nutrient supplement and exercise on mobility and cognition in older women (MOBILE): a randomised semi-blinded placebo-controlled study. Br J Nutr. 2020;124:146–155. doi: 10.1017/S0007114520000719. [DOI] [PubMed] [Google Scholar]
- 46.Moradi S, Alivand M, KhajeBishak Y, AsghariJafarabadi M, Alipour M, Chilibeck PD, Alipour B. The effect of omega3 fatty acid supplementation on PPARγ and UCP2 expressions, resting energy expenditure, and appetite in athletes. BMC Sports Sci Med Rehabil. 2021;13:48. doi: 10.1186/s13102-021-00266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114:136–153. doi: 10.1016/j.jand.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Shin Y, Chang E. Increased intake of omega-3 polyunsaturated fatty acids is associated with reduced odds of low hand grip strength in Korean adults. Nutrients. 2023;15:321. doi: 10.3390/nu15020321. [DOI] [PMC free article] [PubMed] [Google Scholar]