Abstract
A randomized, double-blind, placebo-controlled trial was conducted to confirm whether collagen peptide supplementation for 12 week has a beneficial effect on body fat control in older adults at a daily physical activity level. Participants were assigned to either the collagen group (15 g/day of collagen peptide) or the placebo group (placebo drink). Body composition was measured by bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DEXA). In total, 74 participants (collagen group, n = 37; placebo group, n = 37) were included in the final analysis. The collagen group showed a significant reduction in total body fat mass compared with the placebo group, as evidenced by both BIA (p = 0.021) and DEXA (p = 0.041) measurements. Body fat mass and percent body fat of the whole body and trunk reduced at 12 weeks compared with baseline only in the collagen group (whole body: body fat mass, p = 0.002; percent body fat, p = 0.002; trunk: body fat mass, p = 0.001; percent body fat, p = 0.000). Total fat mass change (%) (collagen group, −0.49 ± 3.39; placebo group, 2.23 ± 4.20) showed a significant difference between the two groups (p = 0.041). Physical activity, dietary intake, and biochemical parameters showed no significant difference between the groups. The results confirmed that collagen peptide supplementation had a beneficial effect on body fat reduction in older adults aged ≥ 50 years with daily physical activity level. Thus, collagen peptide supplementation has a positive effect on age-related changes.
Keywords: Collagen peptide, Proteins, Fat body, Adipose tissue
INTRODUCTION
Adipose tissue is a metabolically dynamic and endocrine organ and is well known for its function in energy storage and mobilization according to nutrient availability and body needs [1]. It is the largest endocrine organ in the human body, can secrete hormones and inflammatory molecules, and has an important effect on multiple processes such as adipogenesis, metabolism, and chronic inflammation [1,2]. Therefore, the expansion of adipose tissues, particularly the visceral, causes low-grade inflammation, which contributes to insulin resistance and dyslipidemia [2,3]. Normal aging involves important changes in the body composition, including decreased muscle mass and increased fat mass [4]. With aging, the body’s muscle mass steadily decreases by 1%–2% annually after the age of 50, and the resulting decrease in the basal metabolic rate induces body fat mass and weight gain [5,6]. Age-related changes contribute to age-related diseases such as cardiovascular events, diabetes mellitus, hypertension, and several types of cancer [7]. As body fat mass peaks at the age of 65–70 years [4], materials with anti-adipogenic effect must be developed and discovered from naturally available sources for the middle-aged population.
Collagen is the most representative protein in the human body, accounting for 30% of body protein [8]. Specifically, it is the primary structural protein of connective tissues and is ubiquitous within the extracellular matrix of skeletal muscles and tendons [9]. Recently, many products, such as cosmetics or food, containing collagen or denatured collagen, have been widely used for various purposes [10]. Collagen peptides, which are hydrolyzed from natural collagen, have a high absorption rate and are used as a bioavailable food supplement without any side effects [11,12]. Improving skin quality, including hydration, elasticity, and visibility of wrinkles, is one of the best known benefits of collagen supplementation [13]. Recently, benefits from hydrolyzed collagen were also reported on lesion prevention, tissue repair, weight loss, and increase in fat-free mass (FFM). [14]. In randomized controlled trials, Zdzieblik et al. [7] announced that 15 g of collagen peptide supplementation reduced the body weight and body fat mass in older sarcopenic men. Then, they [15] reported that the intake of specific bioactive collagen peptides (15 g/day) significantly decreased the body fat mass, body weight, and waist circumference following resistance training in middle-aged, untrained men. Jendricke et al. [16] found that specific collagen peptides (15 g/day) in combination with resistance training improve body fat mass in premenopausal women. However, collagen supplementation further enhances the positive effects of resistance training and does not show the effect of collagen supplementation alone.
Those who are not accustomed to exercise training may need more management of body fat mass. However, resistance training in untrained older adults is difficult and increases the risk of falls and fall-related injuries [17]. Several preclinical studies have reported the anti-obesity effect of collagen protein to the reduction of adipogenic differentiation by regulating peroxisome proliferator-activated receptor-γ (Pparγ) and CCAAT/enhancer binding protein-alpha (C/EBPα) expression [18,19]. Tak et al. [20] reported that the anti-obesity effect of collagen intake was evident even without resistance training. Moreover, intake of low-molecular collagen peptides for 12 weeks reduced the percent body fat in overweight adults; however, no difference in body weight, body mass index (BMI), or fat mass was found. Although they confirmed the effect of 2 g of collagen intake per day, many studies [7,15,16] have reported that consuming at least 15 g of collagen per day has clinical effects.
This clinical trial was conducted to confirm whether collagen peptide supplementation has a beneficial effect on body fat control in older adults with daily physical activity level. This study aimed to evaluate the effect of collagen peptide supplementation (15 g/day) for 12 weeks on body fat in older adults aged ≥ 50 years.
MATERIALS AND METHODS
Participants
The clinical trial was conducted from November 2021 to July 2022 at Kyung Hee University, complied with the Declaration of Helsinki, and was approved by the Institutional Review Board (IRB No. KHGIRB-21-508) of the Bioethics Review Committee of Kyung Hee University. In total, 84 older adults aged ≥ 50 years participated in the study. Written informed consent following a fully detailed description of the study protocol was obtained from all participants before their enrollment. The following selection criteria were used: 1) a BMI between 23 and 32 kg/m2, 2) non-intake of any other protein supplementation in the past 6 months, 3) agreement to the take collagen peptide supplement for 12 weeks and evaluate the effects (survey, body measurement, etc.).
The exclusion criteria for the clinical trial participants were as follows: 1) difficulties in performing normal daily activities, 2) changed more than ± 5% of the usual weight within the past 3 months, 3) participating in other drug clinical trials within 3 months, 4) pregnant, lactating, or planning to become pregnant, 5) regular involvement in high intensity resistance exercise, 6) alcoholics, 7) fractures within 1 year, 8) other health problems (these problems include acute or chronic diseases, uncontrolled hypertension or diabetes, mental illness, severe gastrointestinal symptoms or a history of gastrointestinal surgery, asthma, or lung disease). In total, 10 out of 84 eligible participants dropped out from the clinical trial because of reasons such as discontinued intervention, non-appearance at examination dates, and non-compliance with the study protocol.
Study design
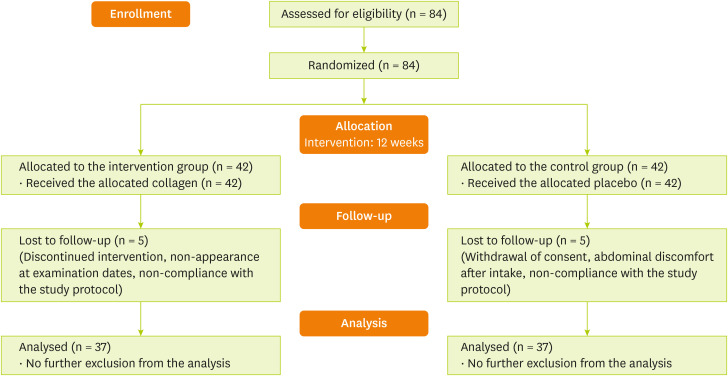
The randomized, double-blind, placebo-controlled trial was conducted to evaluate the effect of 15 g/day of collagen peptide supplementation for 12 weeks on body fat changes in older adults aged ≥ 50 years. Flow diagram of the participants is presented in Figure 1. Before the visit, telephone interviews were conducted to ensure that both the selection and exclusion criteria were met. After baseline measurements, they received random numbers and given identification numbers on recruitment: collagen group (n = 42), which received 15 g/day of collagen peptide as a beverage (liquid formulation), or placebo group (n = 42; placebo drink). Those who were responsible for deciding on study eligibility and conducting the measurements were kept unaware of the results of the randomization throughout the study process. Compliance was assessed by counting the remaining products and the compliance calendar weekly and < 80% of the taken number of products was considered to have dropped out from the study. All participants were instructed to maintain their usual dietary patterns and physical activity during the study period. The intake of protein supplementation, other than the products provided in this study, was limited.
Figure 1. Consolidated standards of reporting trials flow diagram of the participants through the study.
The body composition, biochemical indices, and survey of all participants were measured before (week 0, pre-test) and after (week 12, post-test) the intervention. At weeks 0 and 12, participants were instructed to fast for at least 12 hours from 9:00 and 12:00. The measurements at baseline and 12 week were as follows: 1) questionnaires about general characteristics (only in baseline) and international physical activity questionnaire (IPAQ), 2) assessments for physical examination include bioelectrical impedance analysis (BIA) and dual-energy X-ray absorptiometry (DEXA), 3) dietary intake (3-day food record), 4) blood test with total cholesterol, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein- cholesterol (HDL-C), triglyceride (TG), and serum glucose.
Study product
The collagen peptide product was provided by COSMAX NS Inc The collagen peptide supplementation contained 15 g of BODYBALANCE®P (GELITA AG, Eberbach, Germany), a type with low-molecular weight of approximately 3 kDa collagen, the dose at which significant clinical effects have been demonstrated [7,14,15]. BODYBALANCE®P is a functional peptide extracted from porcine raw materials using thermal or chemical hydrolysis, followed by specific and controlled enzymatic hydrolysis. The ratio of amino acid composition of collagen peptides is presented in Table 1. Collagen peptides and placebo products were in liquid form, contained 60 mL, and were packaged in opaque white containers. The placebo product was manufactured to have the same color, flavor, and taste as the collagen peptide product, the purple drink tastes and flavor like grapes. Xanthan gum and purified water were used in the placebo products to ensure that all conditions, such as taste and texture, were comparable.
Table 1. Amino acid composition of the collagen peptides (per g collagen).
| Amino acid | Weight (%) |
|---|---|
| Glycine | 22.2 |
| Proline | 12.7 |
| Hydroxyproline | 11.9 |
| Glutamic acid | 10.2 |
| Alanine | 8.6 |
| Arginine | 7.3 |
| Aspartic acid | 5.8 |
| Serine | 3.2 |
| Leucine | 2.7 |
| Valine | 2.4 |
| Phenylalanine | 2.1 |
| Threonine | 1.8 |
| Hydroxylysine | 1.6 |
| Isoleucine | 1.4 |
| Histidine | 1.0 |
| Methionine | 0.9 |
| Tyrosine | 0.8 |
Body composition
Body composition was measured using the Inbody 970 body composition analyzer (InBody 970; Biospace, Seoul, Korea) and DEXA (Hologic QDR-4500W; Hologic, Marlborough, MA, USA) at baseline and 12 weeks. DEXA scans were conducted by the same trained and qualified investigator and calibrated in accordance with the manufacturer’s instructions before each measurement by a phantom scan. Body weight, BMI, waist–hip ratio (WHR), body fat mass, and skeletal muscle mass (SMM) were measured. The scanner can accurately measure the fat mass of each part. Height was measured using a digital scale (BSM330; Inbody, Seoul, Korea). Participants fasted for > 12 hours the day before the test.
Dietary intake and physical activity
At baseline and week 12 of the clinical trial, the participants were asked to answer the questionnaires on dietary intake and physical activities. Dietary intake was investigated by the 3-day food records. All participants received a food record booklet, and a trained dietitian instructed them how to record meals at the baseline visit. The documentation included three consecutive days, including 1 day on the weekend and 2 days during the week. Study participants were instructed to maintain their usual diet. Intentionally reducing meal size or increasing protein intake additionally for weight loss was not recommended. Nutrient intake from food records was analyzed using the web-based Computer Nutrition Analysis Program (Can-Pro) version 5.0 (The Korean Nutrition Association, Seoul, Korea).
IPAQ was used to evaluate the physical activity level of the participants. The questionnaire is based on the amount of activity 7 days before filling out. The “total physical activity” score used in this study was calculated as follows: “walking activity + moderate activity + high intensity activity.” Each activity refers to what has been performed for at least 10 min. The calculation method is as follows: 1) walking activity = minutes of activity/day × days per week × 3.3, 2) moderate activities = minutes of activity/day × days per week × 4.0, 3) high intensity activity = minutes of activity/day × days per week × 8.0. Metabolic equivalent tasks (Met) were calculated from the date obtained.
Biochemical parameters
Blood samples were collected early in the morning after at least 12 hours of fasting, and participants were asked to avoid greasy meals and overeating. The samples were analyzed at GC (Green Cross Labs), clinical laboratory at the baseline and week 12 of the trial [21]. Plasma glucose (mg/dL) was analyzed by UV spectrophotometry, whereas total cholesterol (mg/dL), HDL-C (mg/dL), LDL-C (mg/dL), and TG (mg/dL) were analyzed by enzymatic colorimetric assay. Each participant’s blood sample was collected after at least 12 hours of fasting, and they were asked to avoid greasy meals and overeating through advance text messages. After the test, the remaining samples were immediately discarded.
Statistical analysis
Data were analyzed using a statistical analysis program IBM SPSS version 26.0 (IBM, Seoul, Korea). Data normality was checked through the Shapiro–Wilk test at a 5% significance level. Between-group differences were evaluated using an independent t-test or Mann–Whitney U test for continuous variables, and a χ2 test for discrete variables. Changes between baseline and after 12 weeks were analyzed using the paired t-test or Wilcoxon signed rank test. In addition, only participants who completed all follow-up studies (n = 74) were analyzed. The p values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics of the study population
Participant characteristics are presented in Table 2. Neither the collagen nor the placebo groups differed significantly in age, sex, body weight, BMI, WHR, education level, marital status, smoking rate, energy intake, physical activity, or menopause rate.
Table 2. Baseline characteristics of the participants.
| Variable | Placebo (n =37) | Collagen (n = 37) | p value* | |
|---|---|---|---|---|
| Age (yr) | 60.5 ± 6.1 | 62.2 ± 6.5 | 0.243 | |
| 50–64 | 30 (81) | 27 (73) | 1.000 | |
| ≥ 65 | 7 (19) | 10 (27) | ||
| Sex (M/F) | 8 (22)/29 (78) | 8 (22)/29 (78) | 0.407 | |
| Body weight (kg) | 65.1 ± 8.1 | 64.3 ± 8.1 | 0.647 | |
| BMI (kg/m2) | 25.7 ± 2.3 | 25.9 ± 2.0 | 0.767 | |
| WHR | 0.91 ± 0.05 | 0.92 ± 0.04 | 0.313 | |
| Education level | 0.263 | |||
| ≤ Middle school | 3 (8) | 8 (22) | ||
| High school | 22 (59) | 19 (51) | ||
| ≥ University | 12 (32) | 10 (27) | ||
| Marital status | 0.314 | |||
| Married | 37 (100) | 36 (97) | ||
| Single | 0 (0) | 1 (3) | ||
| Smoker | 3 (8) | 3 (8) | 0.898 | |
| Energy intake (kcal/day) | 1,568.7 ± 398.0 | 1,655.5 ± 399.0 | 0.352 | |
| IPAQ (METs)† | 2,828.9 ± 2,001.7 | 3,086.6 ± 2,809.1 | 0.651 | |
| Menopausal women | 27 (93) | 28 (97) | 0.839 | |
Data are presented as means ± standard deviations or number (%).
The participants take the collagen peptide supplement (15 g/day) or placebo once daily during the 12-week intervention period.
No statistically significant differences in age, sex, body weight, BMI, WHR, educational level, marital status, smoking status, energy intake, physical activity, and menopausal status were determined the between placebo and collagen groups at study baseline.
F, female; M, male; BMI, body mass index; WHR, waist–hip ratio; IPAQ, international physical activity questionnaire; MET, metabolic equivalent task; n, number of subjects.
*p value obtained by the χ2 test or Student’s t-test; †The score is calculated using the IPAQ, with a higher score indicating greater physical activity.
Changes in body composition measured using BIA system
Changes in body composition using BIA are shown in Table 3. Body weight (p = 0.048), BMI (p = 0.022), WHR (p = 0.002), and visceral fat area (p = 0.000) were significantly decreased only in the collagen group at 12 week compared to baseline. Body fat mass and percent body fat of the whole body and trunk also reduced at 12 weeks compared with baseline only in the collagen group (whole body: body fat mass, p = 0.002; percent body fat, p = 0.002; trunk: body fat mass, p = 0.001; percent body fat, p = 0.000). FFM and SMM were not significantly different within and between groups.
Table 3. Changes in the body composition measured by bioelectrical impedance analysis system.
| Variable | Placebo (n = 37) | Collagen (n = 37) | p value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Changes | p value* | Baseline | 12 weeks | Changes | p value* | ||||
| Weight (kg) | 65.1 ± 8.1 | 65.0 ± 8.5 | −0.1 ± 1.5 | 0.739 | 64.3 ± 8.1 | 63.8 ± 8.0 | −0.5 ± 1.4 | 0.048‡ | 0.260 | ||
| BMI (kg/m2) | 25.7 ± 2.3 | 25.7 ± 2.3 | −0.1 ± 0.6 | 0.647 | 25.9 ± 2.0 | 25.6 ± 2.0 | −0.2 ± 0.6 | 0.022‡ | 0.204 | ||
| WHR | 0.91 ± 0.05 | 0.90 ± 0.04 | −0.00 ± 0.02 | 0.170 | 0.92 ± 0.04 | 0.91 ± 0.04 | −0.01 ± 0.02 | 0.002§ | 0.146 | ||
| Visceral fat area (m2) | 110.5 ± 29.3 | 108.1 ± 27.6 | −2.4 ± 8.3 | 0.084 | 116.3 ± 32.3 | 110.6 ± 29.2 | −5.7 ± 8.7 | 0.000∥ | 0.106 | ||
| Fat-free mass (kg) | 42.5 ± 7.0 | 42.7 ± 7.1 | 0.2 ± 0.9 | 0.246 | 41.2 ± 7.0 | 41.4 ± 6.9 | 0.2 ± 1.95 | 0.131 | 0.753 | ||
| Skeletal muscle mass (kg) | 23.2 ± 4.2 | 23.3 ± 4.3 | 0.1 ± 0.5 | 0.350 | 22.4 ± 4.3 | 22.6 ± 4.2 | 0.1 ± 0.6 | 0.147 | 0.643 | ||
| Fat mass | |||||||||||
| Whole body | |||||||||||
| Body fat mass (kg) | 22.5 ± 4.6 | 22.5 ± 4.5 | −0.0 ± 1.2 | 0.881 | 23.1 ± 4.9 | 22.4 ± 4.5 | −0.7 ± 1.2 | 0.002§ | 0.021‡ | ||
| Percent body fat (%) | 34.8 ± 5.6 | 34.4 ± 5.2 | −0.3 ± 1.3 | 0.121 | 36.0 ± 5.9 | 35.2 ± 5.6 | −0.8 ± 1.5 | 0.002§ | 0.148 | ||
| Trunk | |||||||||||
| Body fat mass (kg) | 11.6 ± 2.4 | 11.4 ± 2.3 | −0.2 ± 0.7 | 0.159 | 11.9 ± 2.3 | 11.4 ± 2.1 | −0.4 ± 0.7 | 0.001§ | 0.100 | ||
| Percent body fat (%) | 249.9 ± 52.9 | 247.2 ± 53.3 | −2.7 ± 15.2 | 0.285 | 260.9 ± 47.2 | 251.6 ± 44.5 | −9.3 ± 14.7 | 0.000∥ | 0.064 | ||
Data are presented as means ± standard deviations.
The participants take the collagen peptide supplement (15 g/day) or placebo once daily during the 12-week intervention period.
BMI, body mass index; WHR, waist–hip ratio.
*Significance was determined by the paired t-test, p < 0.05; †Significance was determined by the independent t-test, p < 0.05; ‡p < 0.05; §p < 0.01; ∥p < 0.001.
Changes in body composition measured using DEXA
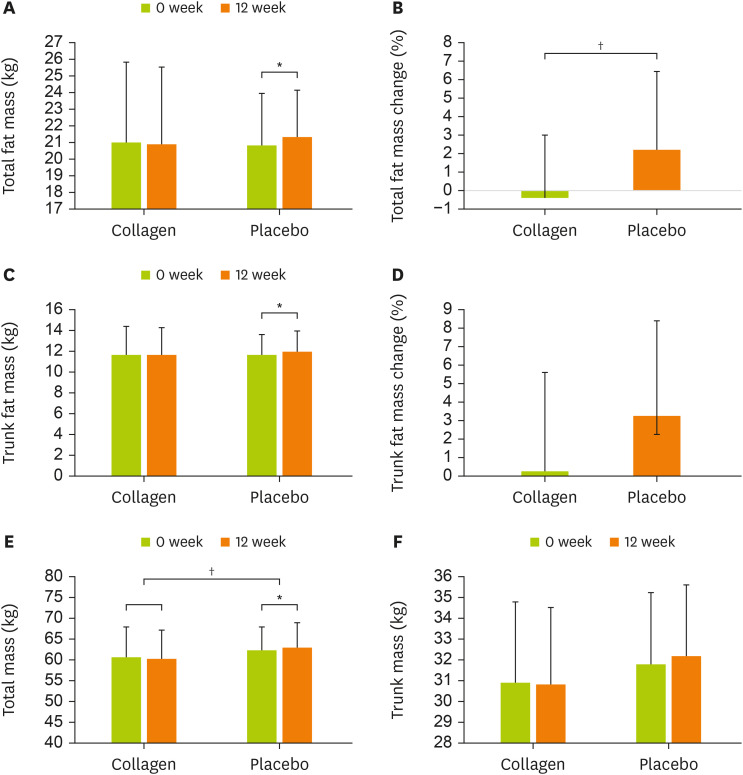
Body composition changes according to collagen peptide supplementation are presented in Figure 2. Total fat and trunk fat significantly increased at 12 weeks compared with baseline in the placebo group (total fat, p = 0.044; trunk fat, p = 0.018). However, no significant difference was observed in the collagen group. Total fat mass change (%) (collagen group, −0.49 ± 3.39; placebo group, 2.23 ± 4.20) showed a significant difference between the two groups (p = 0.041). Total trunk mass change (%) (collagen group, 0.27 ± 5.92; placebo group, 3.25 ± 5.10) showed no significant difference.
Figure 2. Changes in the body composition measured by dual-energy X-ray absorptiometry. (A) Total fat mass (kg), (B) Total fat mass change (%), (C) Trunk fat mass (kg), (D) Trunk fat mass change (%), (E) Total mass (kg), and (F) Trunk mass (kg). Data are presented as below: placebo, participants ingested the placebo product; collagen, participants ingested 15 g/day of the collagen peptide supplement.
*Significantly different at p < 0.05 by the paired t-test or the Wilcoxon signed rank test, *p < 0.05; †Significantly different at p < 0.05 by the independent t-test, †p < 0.05.
Changes in biochemical parameters and physical activity level
Changes in the participant’s biochemical parameters and physical activity level (IPAQ) are presented in Table 4. Biochemical parameters and IPAQ levels were not different at 12 weeks from baseline in the collagen and placebo groups.
Table 4. Changes in the biochemical parameters and physical activity level.
| Variables | Placebo (n = 37) | Collagen (n = 37) | p value† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Changes | p value* | Baseline | 12 weeks | Changes | p value* | |||
| Biochemical parameters | ||||||||||
| Total cholesterol (mg/dL) | 200.4 ± 40.6 | 200.9 ± 42.5 | 0.5 ± 22.0 | 0.882 | 191.7 ± 44.0 | 190.2 ± 42.2 | −1.5 ± 24.6 | 0.710 | 0.706 | |
| Serum glucose (mg/dL) | 102.8 ± 22.2 | 107.2 ± 24.4 | 4.4 ± 25.7 | 0.307 | 103.7 ± 16.2 | 102.8 ± 13.5 | −0.9 ± 11.9 | 0.660 | 0.263 | |
| HDL cholesterol (mg/dL) | 57.0 ± 14.5 | 57.1 ± 14.8 | 0.1 ± 8.4 | 0.938 | 58.0 ± 11.4 | 57.4 ± 12.1 | −0.6 ± 6.8 | 0.613 | 0.705 | |
| LDL cholesterol (mg/dL) | 123.2 ± 37.8 | 123.8 ± 39.2 | 0.7 ± 18.6 | 0.834 | 117.8 ± 39.6 | 116.3 ± 39.2 | −1.5 ± 21.7 | 0.685 | 0.655 | |
| Triglyceride (mg/dL) | 133.2 ± 71.3 | 127.7 ± 74.2 | −5.6 ± 38.6 | 0.383 | 118.7 ± 56.2 | 113.4 ± 49.3 | −5.3 ± 44.2 | 0.468 | 0.978 | |
| Physical activity level | ||||||||||
| IPAQ (METs)‡ | 2,828.9 ± 2,001.7 | 3,191.8 ± 2,455.7 | 362.9 ± 2,144.4 | 0.310 | 3,086.6 ± 2,809.1 | 3,432.9 ± 2,319.0 | 346.3 ± 2,568.7 | 0.418 | 0.976 | |
Date are presented as means ± standard deviations.
The participants take a collagen peptide supplement (15 g/day) or a placebo once daily during the 12-week intervention period.
No statistically significant differences in biochemical parameters and physical activity level were found between the collagen and placebo groups.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; IPAQ, international physical activity questionnaire; MET, metabolic equivalent task.
*No significant difference by the paired t-test at p < 0.05; †No significant difference by the independent t-test at p < 0.05; ‡The score is calculated using the IPAQ, with a higher score indicating greater physical activity.
Changes in dietary intake
The dietary intake of the participants is shown in Table 5. No difference in energy, carbohydrate, and fat intake was found before and after the clinical trial in the collagen and placebo groups. Only the collagen group showed an increase in protein intake of approximately 10g from 66.8 ± 21.9g to 76.1 ± 17.1. It is thought to be due to the increase in protein intake by the collagen peptide supplement including 15 g of collagen peptide protein. No difference in protein intake was found before and after the clinical trial in the placebo group.
Table 5. Changes in dietary intake including collagen supplementation.
| Variables | Placebo (n = 37) | Collagen (n = 37) | p value† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Changes | p value* | Baseline | 12 weeks | Changes | p value* | ||
| Energy (kcal) | 1,568.7 ± 398.0 | 1,542.4 ± 419.5 | −26.4 ± 428.2 | 0.710 | 1,655.5 ± 399.0 | 1,561.9 ± 356.7 | −93.6 ± 384.6 | 0.147 | 0.480 |
| Carbohydrate (g) | 218.2 ± 55.0 | 209.0 ± 63.1 | −9.2 ± 56.5 | 0.327 | 234.4 ± 58.2 | 214.7 ± 54.8 | −19.7 ± 59.7 | 0.052 | 0.440 |
| Protein (g) | 62.4 ± 16.1 | 64.8 ± 20.0 | 2.5 ± 24.2 | 0.541 | 66.8 ± 21.9 | 76.1 ± 17.1 | 9.3 ± 21.4 | 0.012* | 0.197 |
| Fat (g) | 49.1 ± 19.5 | 49.2 ± 19.6 | 0.1 ± 26.1 | 0.987 | 49.8 ± 15.2 | 45.3 ± 16.0 | −4.5 ± 17.8 | 0.136 | 0.386 |
Data are presented as means ± standard deviations.
The participants take a collagen peptide supplement (15 g/day) or product once daily during the 12-week intervention period.
*Significance was determined by the paired t-test, at p < 0.05, *p < 0.05; †No significant difference by the independent t-test at p < 0.05.
DISCUSSION
The clinical trial evaluated whether collagen peptide supplementation has a beneficial effect on the changes in the body fat of older adults without resistance training. The results confirmed that collagen peptide intake (15 g/day) for 12 weeks had a beneficial effect on body fat reduction, including abdominal fat, in older adults aged ≥ 50 years with daily physical activity level.
Collagen has a relatively low biological value, mainly due to the low amounts of branched chain amino acids and lysine [22]. However, it has an excellent amino acid composition and contains relatively high amounts of arginine and glycine, which are important for the synthesis of creatine in the human body; thus, interest in collagen supplementation has recently drawn attention to the possibility of body composition control [7,14]. Several recent randomized controlled trials [7,15,16] have reported that collagen peptide supplementation reduces body fat mass. However, these are not the only effects of collagen supplementation by consuming collagen peptides intake after resistance exercise training during the intervention period. Zdzieblik et al. [7] reported that collagen peptide supplementation (15 g/day) in combination with resistance training increased FFM and reduced body fat mass in older people with sarcopenia. They [15] also announced that specific bioactive collagen peptides (15 g/day) combined with resistance exercise training significantly improved FFM and SMM and decreased the body fat mass, body weight, and waist circumference in middle-aged men. Jendricke et al. [16] found that specific collagen peptides (15 g/day] in combination with resistance training improve FFM and reduced body fat mass in premenopausal women. That is, FFM and SMM was increased with the intake of collagen, a protein, after resistance exercise, and the body fat mass was decreased together.
Recent preclinical studies have found the anti-obesity effect of collagen peptides through the suppression of body fat accumulation and regulation of lipid metabolism [18,23]. Astre et al. [19] reported an anti-adipogenic effect of collagen peptides on in vitro and in vivo models. They found that collagen peptide intake prevents lipid accumulation in adipocytes by suppressing the expression of adipogenic master transcription factors such as Pparγ, C/EBPα and adipocyte protein 2 genes, which mainly regulate the differentiation and maintenance of adipocytes [19,23]. The collagen utilized in the clinical trial was a hydrolyzed low-molecular collagen peptide extracted from porcine, which closely resembles human collagen [22] and comprises functional peptides that demonstrate physiological activity. To date, only one clinical trial evaluated the effect of collagen intake on body composition changes, particularly body fat mass without resistance training. The trial evaluated the effect of 2 g low-molecular collagen intake derived from skate skin for 12 weeks on body fat changes in obese adults [20]. The results revealed that collagen supplementation significantly lowered body fat, but did not affect on body weight, BMI, or sagittal abdomen distance. The intake of 2 g/day of collagen may be insufficient to induce clinical effects; thus, this clinical trial was conducted with 15 g, which is the concentration currently used in many collagen studies [7,14,15]. As a result, significant decreases were shown in body weight, BMI, WHR, and abdominal fat mass in older adults aged ≥ 50 years with daily physical activity level.
Changes in body composition are well-known representative age-related changes [4]. With age, FFM and SMM decrease, leading to lower basal metabolic rate, which increased body fat [5,6]. These changes are a major cause of the increasing incidence of cardiovascular diseases, type 2 diabetes, metabolic syndrome, and various cancers in older adults [7]. They are very interested in reducing body fat for health, but have a high risk of injury, so they cannot rely on exercise to change body composition. Older adults prefer to maintain familiar meals and find it difficult to change their eating habits [24]. Therefore, even if for health, there may be limitations in adapting an unfamiliar 'healthy diet' and maintaining intake. Older adults need a safe and convenient way to manage body fat mass while maintaining their usual physical activity and diet, therefore products must be developed and their effectiveness need to be verified. The collagen peptides used in the clinical trial is a safety-secured product, and it influences body fat reduction in the older adults without exercise. This can be a safe and effective method for older people who want to lose body fat. A decrease in food intake is another change with aging, which is often accompanied by decreased appetite and ability to eat [24,25]. Poor appetite, dysphagia, or chewing difficulty is a very prevalent condition in older adults, affecting approximately 30% of older people. With aging, the food intake of older adults decreases [26]; however, the recommended amount of protein increases [27,28]. At this time, protein supplementation can be a good strategy. In the results, the collagen group showed an increase of approximately 10 g in protein intake at 12 weeks compared with the baseline, and no difference in the placebo group was found before and after the test. This means that collagen peptide supplementation is effective not only in managing body fat but also in meeting recommended protein intake in older adults.
Arginine and glycine, amino acids that play important roles as precursors for creatine synthesis in the body, are abundant in collagen [7]. Creatine is present in high concentrations in skeletal and cardiac muscles [29], which can be synthesized through the metabolism of glycine, arginine, and methionine [30]. With age, intake of protein (amino acids) tends to decrease, and people may experience vitamin deficiencies [31]. This means that the creatine synthesis may be a particular burden for older adults [30]. Thus, collagen, which is rich in arginine and glycine, could be a more suitable form of protein supplementation for older people. The collagen utilized in the study was a hydrolyzed low-molecular collagen peptide extracted from porcine, which closely resembles human collagen [32]. The beneficial effects of consuming hydrolyzed collagen are closely linked to metabolic peptides that have undergone degradation in the digestive tract [33]. Collagen peptides with low-molecular size are rapidly absorbed from the gastrointestinal tract also in peptide form. In addition, collagen peptides could act as signal messengers in anabolic cellular processes cartilage, tendons, and ligaments, which might help improve painful symptoms of degenerative diseases in older people.
This study has several limitations. The intake time of collagen supplements by the participants was different. The timing of protein supplementation is crucial for FFM synthesis, and the daily protein intake must be effectively distributed evenly between meals rather than consuming it after meals. The subjects were instructed to take the supplements between meals, however, subjects just used their most convenient time to consume supplements daily. Therefore, the timing of supplement intake may have been insufficient for FFM synthesis. Also, we assumed that the body fat changes were attributed to the collagen consumption, but, we could not exclude the possibility that the effect was merely the effect of the extra protein consumption.
Despite these limitations, this study has several advantages. First, this is one of the few studies that have investigated the effects of collagen peptide supplementation alone, without altering participants’ physical activity levels or eating habits. Let alone, we found a positive effect on body fat mass reduction. Second, the participants of this study showed high intake compliance (> 90%). Therefore, with this, we may suggest taking a collagen drink can be a simple method that have a positive effect on changes in body composition after middle age, when body fat increases and physical activity tends to decrease.
In conclusion, this clinical trial evaluated whether collagen peptide supplementation (15 g/day) for 12 weeks has a beneficial effect on body fat changes in older adults even without resistance training. The results confirmed that collagen peptide intake had a beneficial effect on body fat reduction, including abdominal fat, in older adults aged ≥ 50 years with daily physical activity level. This finding might suggest that collagen peptide supplementation has a positive effect on age-related changes by body fat mass reduction.
ACKNOWLEDGEMENTS
The authors would like to thank the BK21 program “AgeTech-Service Convergence Major” through the National Research Foundation by the Ministry of Education of Korea [5120200313836].
Footnotes
Funding: This research was supported by Cosmax Co., Ltd., and Korea Institute for Advancement of Technology grant funded by the Korea Government (MOTIE) (P0014276, Industry Innovation Foundation Building Program).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Park J, Ahn H, Park YK.
- Data curation: Park J, Kim M.
- Formal analysis: Park J, Park YK.
- Funding acquisition: Shin H.
- Writing - original draft: Park J, Ahn H, Park YK.
- Writing - review & editing: Park J, Kim M, Shin H, Ahn H, Park YK.
References
- 1.Parra-Peralbo E, Talamillo A, Barrio R. Origin and development of the adipose tissue, a key organ in physiology and disease. Front Cell Dev Biol. 2021;9:786129. doi: 10.3389/fcell.2021.786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giglio BM, Schincaglia RM, da Silva AS, Fazani ICS, Monteiro PA, Mota JF, Cunha JP, Pichard C, Pimentel GD. Whey protein supplementation compared to collagen increases blood Nesfatin concentrations and decreases android fat in overweight women: a randomized double-blind study. Nutrients. 2019;11:2051. doi: 10.3390/nu11092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SJ, Cho YG, Kim DH, Hwang YH. Effect of Lactobacillus sakei OK67 in reducing body and visceral fat in lifestyle-modified overweight individuals: a 12-week, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2023;15:3074. doi: 10.3390/nu15133074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzini A, Monti D, Santoro A. Editorial: adipose tissue: which role in aging and longevity? Front Endocrinol (Lausanne) 2020;11:583. doi: 10.3389/fendo.2020.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzeo RS, Tanaka H. Exercise prescription for the elderly: current recommendations. Sports Med. 2001;31:809–818. doi: 10.2165/00007256-200131110-00003. [DOI] [PubMed] [Google Scholar]
- 6.Frestedt JL, Zenk JL, Kuskowski MA, Ward LS, Bastian ED. A whey-protein supplement increases fat loss and spares lean muscle in obese subjects: a randomized human clinical study. Nutr Metab (Lond) 2008;5:8. doi: 10.1186/1743-7075-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. 2015;114:1237–1245. doi: 10.1017/S0007114515002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short KR, Nair KS. The effect of age on protein metabolism. Curr Opin Clin Nutr Metab Care. 2000;3:39–44. doi: 10.1097/00075197-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am. 2003;32:805–822. doi: 10.1016/s0889-8529(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 10.Ito N, Seki S, Ueda F. Effects of composite supplement containing collagen peptide and ornithine on skin conditions and plasma IGF-1 levels-A randomized, double-blind, placebo-controlled trial. Mar Drugs. 2018;16:482. doi: 10.3390/md16120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemati M, Oveisi MR, Abdollahi H, Sabzevari O. Differentiation of bovine and porcine gelatins using principal component analysis. J Pharm Biomed Anal. 2004;34:485–492. doi: 10.1016/s0731-7085(03)00574-0. [DOI] [PubMed] [Google Scholar]
- 12.Oesser S, Adam M, Babel W, Seifert J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL) J Nutr. 1999;129:1891–1895. doi: 10.1093/jn/129.10.1891. [DOI] [PubMed] [Google Scholar]
- 13.Reilly DM, Lozano J. Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesthet Res. 2021;8:2. [Google Scholar]
- 14.Schunck M, Zague V, Oesser S, Proksch E. Dietary supplementation with specific collagen peptides has a body mass index-dependent beneficial effect on cellulite morphology. J Med Food. 2015;18:1340–1348. doi: 10.1089/jmf.2015.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zdzieblik D, Brame J, Oesser S, Gollhofer A, König D. The influence of specific bioactive collagen peptides on knee joint discomfort in young physically active adults: a randomized controlled trial. Nutrients. 2021;13:523. doi: 10.3390/nu13020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jendricke P, Centner C, Zdzieblik D, Gollhofer A, König D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11:892. doi: 10.3390/nu11040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques DL, Neiva HP, Marinho DA, Marques MC. Manipulating the resistance training volume in middle-aged and older adults: a systematic review with meta-analysis of the effects on muscle strength and size, muscle quality, and functional capacity. Sports Med. 2023;53:503–518. doi: 10.1007/s40279-022-01769-x. [DOI] [PubMed] [Google Scholar]
- 18.Daskalaki MG, Axarlis K, Tsoureki A, Michailidou S, Efraimoglou C, Lapi I, Kolliniati O, Dermitzaki E, Venihaki M, Kousoulaki K, Argiriou A, Tsatsanis C. Fish-derived protein hydrolysates increase insulin sensitivity and alter intestinal microbiome in high-fat-induced obese mice. Mar Drugs. 2023;21:343. doi: 10.3390/md21060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astre G, Deleruyelle S, Dortignac A, Bonnet C, Valet P, Dray C. Diet-induced obesity and associated disorders are prevented by natural bioactive type 1 fish collagen peptides (Naticol®) treatment. J Physiol Biochem. 2018;74:647–654. doi: 10.1007/s13105-018-0650-0. [DOI] [PubMed] [Google Scholar]
- 20.Tak YJ, Kim YJ, Lee JG, Yi YH, Cho YH, Kang GH, Lee SY. Effect of oral ingestion of low-molecular collagen peptides derived from skate (Raja Kenojei) skin on body fat in overweight adults: a randomized, double-blind, placebo-controlled trial. Mar Drugs. 2019;17:157. doi: 10.3390/md17030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi R, Park MJ, Oh Y, Kim SH, Lee SG, Lee EH. Validation of multiple equations for estimating low-density lipoprotein cholesterol levels in Korean adults. Lipids Health Dis. 2021;20:111. doi: 10.1186/s12944-021-01525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33:969–976. doi: 10.2337/dc09-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodds MWJ, Haddou MB, Day JEL. The effect of gum chewing on xerostomia and salivary flow rate in elderly and medically compromised subjects: a systematic review and meta-analysis. BMC Oral Health. 2023;23:406. doi: 10.1186/s12903-023-03084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souto Barreto P, Cesari M, Morley JE, Roberts S, Landi F, Cederholm T, Rolland Y, Vellas B, Fielding R. Appetite loss and anorexia of aging in clinical care: an ICFSR task force report. J Frailty Aging. 2022;11:129–134. doi: 10.14283/jfa.2022.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- 27.Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60:501–509. doi: 10.1093/ajcn/60.4.501. [DOI] [PubMed] [Google Scholar]
- 28.Churchward-Venne TA, Breen L, Phillips SM. Alterations in human muscle protein metabolism with aging: protein and exercise as countermeasures to offset sarcopenia. Biofactors. 2014;40:199–205. doi: 10.1002/biof.1138. [DOI] [PubMed] [Google Scholar]
- 29.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 30.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- 31.Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008;29:S143–S172. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- 32.León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen-sources and applications. Molecules. 2019;24:24. doi: 10.3390/molecules24224031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto S, Deguchi K, Onuma M, Numata N, Sakai Y. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol Pharm Bull. 2016;39:428–434. doi: 10.1248/bpb.b15-00624. [DOI] [PubMed] [Google Scholar]