Abstract
Objective: To analyze the association between transperineal pelvic floor ultrasound findings and posterior pelvic injury and prolapse in postpartum women. Methods: A total of 108 postpartum women received treatment from January 2020 and December 2022 were divided into 2 groups, with 53 cases in a pelvic floor disorder (PFD) group and 55 cases in the no PFD group according to whether they developed PFD after delivery. The relationship between ultrasound data and the Pelvic Floor Distress Inventory (PFDI-20) scores was analyzed by Pearson correlation. The diagnostic value of transperineal pelvic floor ultrasound for PFD was analyzed by using the receiver operating characteristic curve, and the relationship between transperineal pelvic floor ultrasound parameters and PFD was analyzed by using the RR hazard ratio. Results: The distance from the bladder neck to the posterior inferior border of the pubic symphysis, the distance from the cervix to the posterior inferior border of the pubic symphysis, and the shortening rate during retraction were shorter or lower in the PFD group than those in the no PFD group. Additionally, bladder descent, cervical subluxation, urethral rotation, anterior and posterior diameters of the static levator ani muscle (LAM), anterior and posterior diameters of the retracted LAM, anterior and posterior diameters of the LAM in the maximal Valsalva maneuver, and PFDI-20 scores in the PFD group were longer or higher than those of the no PFD group (P<0.01). Shortening rate during retraction, bladder descent, cervical subluxation, urethral rotation, and elongation at maximal Valsalva maneuver were positively correlated with the PFDI-20 score (R = 0.027, 0.053, 0.102, 0.002, 0.011, 0.123, respectively, all P<0.05). Conclusions: The degree of bladder descent, cervical subluxation, urethral rotation, shortening rate during retraction, and elongation at maximal Valsalva maneuver are closely related to the PFD I-20 score.
Keywords: Transperineal pelvic floor ultrasound, posterior pelvic injury, prolapse, postpartum women, pelvic floor function
Introduction
Pelvic floor disorders (PFDs) are caused by congenital degeneration of the pelvic floor tissue or acquired anatomic alterations of the pelvic organs [1], and represent a spectrum of changes associated with pelvic floor abnormalities, such as stress urinary incontinence (SUI), pelvic injuries, and pelvic organ prolapse (POP) [2,3]. Epidemiologic surveys have shown that 25%-30% of adult women have PFDs [4], with a global prevalence of POP ranges from 2%-50% [5] and a median global SUI prevalence of 27.6% [6].
The most commonly used screening method for PFDs is the Pelvic Organ Prolapse Quantification (POP-Q) System [7]. The gold standard for the diagnosis of PFDs is based on pelvic magnetic resonance imaging (MRI) [8]. However, MRI is expensive and time-consuming, which limits its clinical use. Pelvic floor ultrasound is emerging as a diagnostic method for women with PFDs because it is affordable, convenient, and noninvasive. With the use of pelvic floor ultrasound, it is feasible to assess pelvic floor tissue status in postpartum women while verifying the impact of pregnancy on pelvic floor structures. Relevant studies have shown the reliability of three-dimensional (3D) pelvic floor ultrasound in detecting the functional status of the pelvic floor in postpartum women [9]. It has been suggested [10] that the structure and function of the anterior pelvic tissues, such as the bladder and urethra, can be assessed by examining the pelvic diaphragmatic fissure through 3D ultrasound and subsequently measuring and post-processing various parameters related to it. Transperineal pelvic floor ultrasound can be used to acquire images of postpartum pelvic diaphragmatic fissures, thereby providing more comprehensive information for the diagnosis of PFDs [11].
The use of transperineal pelvic floor ultrasound has been increasing over the past few years. Many studies, both in obstetrics and gynecology, have demonstrated that transperineal pelvic floor ultrasound can provide valuable information for clinicians. In obstetrics, transperineal pelvic floor ultrasound is a reliable and replicable method for both static and dynamic assessments of pelvic floor morphology and biometry [12]. Before and during labor, this ultrasound serves as a useful complementary tool in specific clinical scenarios. Recently, several studies have identified a correlation between pelvic floor dimensions and the coactivation of levator ani muscle (LAM) at term and the labor outcome [13,14]. However, the value of transperineal pelvic floor ultrasound in postpartum women with posterior pelvic injury and prolapse has not been assessed.
Therefore, in this study, we explored the diagnostic value of transperineal pelvic floor ultrasound in predicting posterior pelvic injury and prolapse in postpartum women. Our study aimed to provide a methodological reference for the proactive prevention of posterior pelvic injury and prolapse following childbirth.
Materials and methods
Study design and ethics
The study was a retrospective analysis. The subjects were admitted to Pudong New Area Peoples’ Hospital from January 2020 and December 2022. This study has been reviewed and approved by the medical ethics committee of Pudong New Area Peoples’ Hospital. Flow diagram detailing the selection of patients is shown in the Figure 1.
Figure 1.
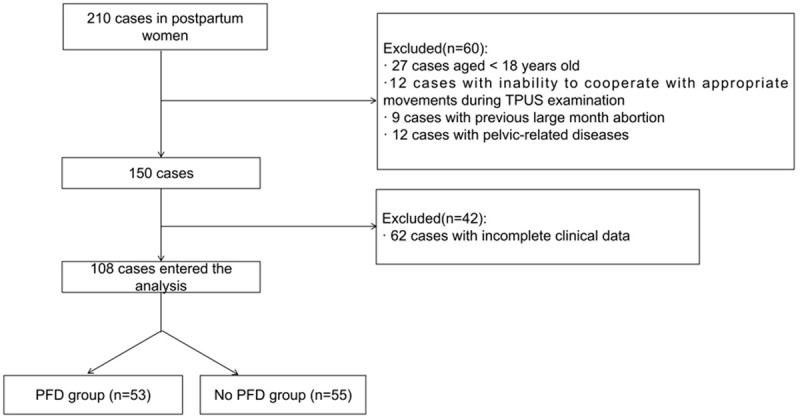
Flow chart of patient recruitment.
Inclusion criteria
(1) Patients who received the first postpartum pelvic floor ultrasound 42-60 days after delivery; (2) Patients with singleton delivery; (3) Patients with a delivery history of fewer or equal to 2 times; (4) Patients who underwent the same delivery mode for both deliveries; (5) Patients without a history of pelvic surgery; (6) Patients who met the criteria for the diagnosis of PFDs; (7) Patients with complete medical records.
Exclusion criteria
(1) Patients with missing general information or clinical data; (2) Patients who were unable to cooperate with the transperineal ultrasound examinations; (3) Patients with a history of late abortion; (4) Patients with a history of pelvic-related diseases.
Diagnosis of PFDs
A thorough patient history and a physical examination are essential for evaluating prolapse. Before performing the history and physical, assessment, it is advisable to employ any pertinent standardized questionnaires to gauge the extent of distress or bother experienced by the patient. Questions regarding bulge and pressure, as well as experiences of splinting or the need to apply pressure on the vagina or rectum to facilitate urination or defecation, are highly indicative of prolapse. If these symptoms are affirmed, they are likely to indicate an objective prolapse. A thorough obstetric and gynecologic history includes number of pregnancies, route of delivery, and age of menopause. Prolapse can frequently have a genetic component, and patients may often have a family history of a connective tissue disorders or recount instances where their mothers or aunts underwent hysterectomy due to prolapse.
Materials
PFD grouping
According to the 9th edition of Obstetrics and Gynecology [15], the postpartum pelvic floor function was determined as follows: (1) uterine prolapse ≥ I degree; (2) patients with SUI; (3) bladder, rectum, or urethral bulge. PFDs were determined by one or more of the above, and patients with PFDs were included in a PFD group (n = 53), while those without were included in a no PFD group (n = 55).
Transperineal pelvic floor ultrasound
The transperineal pelvic floor ultrasound images were acquired using an ultrasound detector (Sequoia Paraon, Siemens, Germany, RAB-6 convex array 3D volumetric transducer, 6.0 MHz). During the examination, each patient was in a lithotomy position after urination, and the probe was placed close to the perineum with the indication point facing upward. The patient was instructed to perform the Valsalva maneuver (holding the breath and exerting downward abdominal pressure) for at least 6 seconds, and three standard median sagittal sections of the pelvic floor were acquired for dynamic images to be archived and analyzed. The horizontal line passing through the posterior inferior border of the pubic symphysis was used as a reference line. U indicates the lowest point of the base of the bladder, CX indicates the lowest point of the cervix, and R indicates the lowest point of the anterior wall of the rectum. Transperineal pelvic floor ultrasound was performed at 8 weeks postpartum.
Pelvic floor distress inventory-20 (PFDI-20) scores
The PFDI-20 score was evaluated at 8 weeks postpartum. The questionnaire consisted of 3 subscales: anorectal disease, POP, and genitourinary disease, with a total of 20 questions, and scores for asymptomatic, symptomatic, mild, moderate, and severe ranged from 0 to 4. Each subscale score (0-100) = (sum of scores of each item/number of items) × 25. The PFDI-20 score (0-300) is the sum of the three subscales. Higher PFDI-20 scores indicate severer PFD symptoms.
Data collection and measurement
The main indications were ultrasound parameters, including the distance from the bladder neck to the posterior inferior border of the pubic symphysis, bladder descent, distance from the cervix to the posterior inferior border of the pubic symphysis, cervical subluxation, urethral rotation, anterior and posterior diameters of the static LAM, anterior and posterior diameters of the retracted LAM, anterior and posterior diameters of the LAM in the maximal Valsalva maneuver, the shortening rate during retraction, and the elongation at maximal Valsalva maneuver.
Secondary indicators were demographic data and clinical characteristics, including body mass index (BMI), age, weeks of labor, neonatal weight, neonatal head size, presence of scoliosis, use of a midwife, painless delivery, and presence of vaginal tears. In addition, PFDI-20 scores were collected.
Statistical analysis
SPSS 20.0 software (Chicago SPSS Co., Ltd.) was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation. The independent t-test was used for comparison between the two groups, and the paired t-test was used for comparison of the same group at different time points. The correlation analysis between ultrasound parameters and PFDI-20 scores was performed using Pearson’s correlation. The diagnostic value of ultrasound data for PFDs was analyzed by receiver operating characteristic (ROC) curve. Delong test was used to compare the area under the curve (AUC) of bladder descent, cervical subluxation, urethral rotation, shortening rate during retraction, and the elongation at maximal Valsalva maneuver. α = 0.05 was used as the test level for significance.
Results
Comparison of demographic data and clinical characteristics
There were no significant differences between the two groups in terms of BMI, age, week of labor, neonatal weight, neonatal head diameter, presence of scoliosis, use of a midwife, and painless delivery (P>0.05). However, there were more cases with vaginal tear in the PFD group than in the no PFD group (P<0.01) (Table 1).
Table 1.
Comparison of demographic data and clinical characteristics of the two groups
| Indicator | PFD group (n = 53) | No PFD group (n = 55) | χ2/t | P |
|---|---|---|---|---|
| Body mass index | 25.44±1.11 | 24.57±1.13 | 0.554 | 0.581 |
| Age | 29.45±2.67 | 28.96±2.45 | 0.842 | 0.321 |
| Gestational week of delivery | 37.82±1.21 | 38.03±1.01 | 0.695 | 0.467 |
| Neonatal body weight | 2894.32±358.53 | 2899.96±343.12 | 0.356 | 0.712 |
| Neonatal head diameter | 34.12±1.21 | 33.99±1.14 | 0.856 | 0.402 |
| Perineal scoliosis | 0.480 | 0.384 | ||
| Yes | 6 (11.32%) | 9 (16.36%) | ||
| No | 47 (88.68%) | 46 (83.64%) | ||
| Use of midwife | 0.801 | 0.369 | ||
| Yes | 7 (13.21%) | 5 (9.09%) | ||
| No | 46 (86.79%) | 50 (90.91%) | ||
| Painless delivery | 0.603 | 0.504 | ||
| Yes | 11 (20.75%) | 16 (29.09%) | ||
| No | 42 (79.25%) | 39 (70.91%) | ||
| Vaginal tear | 16.996 | <0.001 | ||
| No | 13 (24.53%) | 30 (54.55%) | ||
| 1 degree | 24 (45.28%) | 17 (30.91%) | ||
| 2 degrees | 16 (30.19%) | 8 (14.54%) |
Note: PFD is pelvic floor disorder.
Comparison of ultrasound findings
The distance from the bladder neck to the posterior inferior border of the pubic symphysis, the distance from the cervix to the posterior inferior border of the pubic symphysis, and the shortening rate during retraction were shorter or lower in the PFD group than those in the no PFD group. The bladder descent, cervical subluxation, urethral rotation, anterior, and posterior diameters of the static LAM, anterior and posterior diameters of the retracted LAM, anterior and posterior diameters of the LAM in maximal Valsalva maneuver, and the elongation at maximal Valsalva maneuver were longer or higher in the PFD group than those in the no PFD group (P<0.01). See Table 2.
Table 2.
Comparison of ultrasound measurements between the two groups
| PFD group (n = 53) | No PFD group (n = 55) | P | |
|---|---|---|---|
| Distance from the bladder neck to the posterior inferior border of the pubic symphysis | 19.86±4.64 | 24.98±5.02 | 0.01 |
| Bladder descent | 28.98±6.43 | 19.04±6.53 | 0.02 |
| Distance from the cervix to the posterior inferior border of the symphysis pubis | 32.45±10.34 | 38.43±9.67 | 0.01 |
| Cervical descent | 26.21±5.97 | 23.12±6.89 | 0.02 |
| Urethral rotation | 59.53±12.12 | 35.01±9.18 | 0.003 |
| Anterior and posterior diameters of resting anal fissure | 46.94±5.35 | 41.53±5.20 | 0.01 |
| Anterior and posterior diameters of retracted anal fissure | 38.98±7.84 | 27.73±3.84 | 0.003 |
| Anterior and posterior diameters of the anal fissure during Maximal Valsalva maneuver | 52.24±7.45 | 42.45±4.78 | 0006 |
| Shortening rate during retraction | 18.03±4.24 | 32.44±6.02 | 0.002 |
| Elongation at Maximal Valsalva maneuver | 10.21±2.12 | 4.56±2.31 | 0.001 |
Note: PFD is pelvic floor disorder; compared with the no PFD group.
Relationship between ultrasound data and PFDI-20 scores
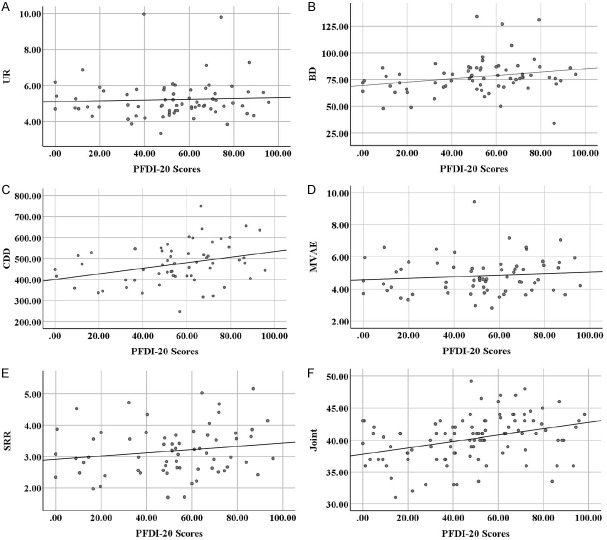
The PFDI-20 score was higher in the PFD group (188.82±25.77) than in the no PFD group (56.35±13.24) (P<0.01). Pearson’s correlation analysis demonstrated that shortening rate during retraction, bladder descent, cervical subluxation, urethral rotation, and elongation at maximal Valsalva maneuver were positively correlated with the PFDI-20 scores (R = 0.027, 0.053, 0.102, 0.002, 0.011, 0.123, respectively, all P<0.05) (Figure 2).
Figure 2.
Relationship between ultrasound parameters and PFDI-20 scores. UR: Urethral rotation; BD: Bladder descent; CDD: Cervical subluxation; MVAE: Elongation at maximum Valsalva maneuver; SRR: Shortening rate during retraction.
ROC curve analysis of ultrasound values for PFD diagnosis
The ROC curves, with the PFD group as positive samples and the absence of PFD as negative samples, showed that when the cut-off value was 0.47, the AUC for the diagnosis of PFD exceeded 0.7 for bladder descent, cervical subluxation, urethral rotation, shortening rate during retraction, and elongation at maximal Valsalva maneuver (Table 3 and Figure 3).
Table 3.
ROC curve analysis of ultrasound measurements for the diagnosis of PFDs
| Indicator | AUC | 95% CI | P | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Bladder descent | 0.801 | 0.712, 0.852 | <0.001 | 29.33 mm | 62.00 | 91.68 |
| Cervical subluxation | 0.765 | 0.697, 0.843 | <0.001 | 25.04 mm | 78.00 | 69.01 |
| Urethral rotation | 0.732 | 0.665, 0.832 | <0.001 | 55.21 | 66.00 | 69.78 |
| Shortening rate during retraction | 0.786 | 0.698, 0.853 | <0.001 | 16.97% | 70.00 | 76.02 |
| Elongation at maximum Valsalva maneuver | 0.778 | 0.721, 0.874 | <0.001 | 9.47% | 60.00 | 86.13 |
| Joint | 0.943 | 0.872, 0.961 | <0.001 | - | 84.00 | 88.32 |
Note: PFD is pelvic floor disorder, ROC is receiver operating characteristics, and AUC is area under the curve.
Figure 3.
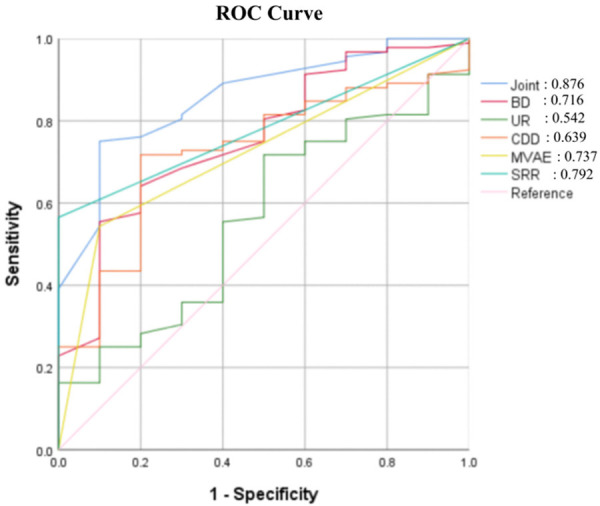
ROC curves of ultrasound parameters for the diagnosis of posterior pelvic injury and prolapse in postpartum women. UR: Urethral rotation; BD: Bladder descent; CDD: Cervical subluxation; MVAE: Elongation at maximum Valsalva maneuver; SRR: Shortening rate during retraction.
The difference between the AUCs was analyzed by Delong test. It was found that the AUC of cervical subluxation was statistically greater than that of the shortening rate during retraction (P<0.05). However, there was no significant difference in the AUCs between bladder descent and elongation at maximal Valsalva maneuver (P>0.05) (Table 4).
Table 4.
Delong test
| Z | P | 95% CI | |
|---|---|---|---|
| Cervical subluxation vs. shortening rate during retraction | -3.624 | <0.001 | (0.02, 0.07) |
| Bladder descent vs. Elongation at maximum Valsalva maneuver | -0.012 | 0.066 | (-0.001, 0.001) |
Relationship between ultrasound parameters and PFD
Using the significant values in the ROC curve analysis as the thresholds, the results showed that bladder descent, cervical subluxation, urethral rotation, shortening rate during retraction, and elongation at Valsalva maneuver resulted in RR values of 3.511, 3.642, 2.401, 0.401, and 2.998, respectively (Table 5).
Table 5.
Relationship between ultrasound measurements and PFDs
| Indicator | Cases | NO PDF | RR | 95% CI | P | |
|---|---|---|---|---|---|---|
| Bladder descent | 3.511 | 2.589, 5.844 | <0.001 | |||
| ≤29.09 mm | 76 | 25 (32.89%) | 51 (67.11%) | |||
| >29.09 mm | 32 | 28 (87.5%) | 4 (12.5%) | |||
| Cervical subluxation | 3.642 | 2.328, 6.896 | <0.001 | |||
| ≤24.94 mm | 50 | 14 (28%) | 36 (72%) | |||
| >24.94 mm | 58 | 39 (67.24%) | 19 (32.76%) | |||
| Urethral rotation | 2.401 | 1.653, 4.426 | <0.001 | |||
| ≤54.92° | 61 | 15 (24.59%) | 46 (75.41%) | |||
| >54.92° | 47 | 38 (80.85%) | 19 (19.15%) | |||
| Shortening rate at retraction | 0.401 | 0.223, 0.497 | <0.001 | |||
| ≤17.25% | 48 | 35 (72.92%) | 13 (17.08%) | |||
| >17.25% | 60 | 18 (30%) | 42 (70%) | |||
| Elongation at maximum Valsalva maneuver | 2.998 | 1.988, 4.679 | <0.001 | |||
| ≤9.82% | 70 | 24 (34.29%) | 46 (65.71%) | |||
| >9.82% | 38 | 29 (76.32%) | 9 (23.68%) |
Note: PFD is pelvic floor disorder.
Multivariable logistic regression model derivation and development
The multivariable analysis model included five covariates. Using these five variables (Table 5), a scoring system was developed to identify patients with PFDs. The risk score for individual patient was calculated using the following formula: xβ = -3.147 + (1.678 × bladder descent) + (1.338 × cervical subluxation) + (0.676 × urethral rotation) + (0.465 × shortening rate during retraction) + (0.874 × elongation at Valsalva maneuver). The probability of PFD was calculated using the following formula (ŷ = 1/[1 + exp. (-xβ)]): ŷ = 1/[1 + exp. (-3.147 + (1.678 × bladder descent) + (1.338 × cervical subluxation) + (0.676 × urethral rotation) + (0.465 × shortening rate during retraction) + (0.874 × elongation at maximal Valsalva maneuver))].
Discussion
From an anatomical point of view, the pelvic floor system functions as a cohesive unit, where the tissues interact and interconnect to maintain the structural and functional integrity of the pelvic floor. Any structural damage or functional degradation within the pelvic floor system may disrupt the balance of the system [16]. Throughout pregnancy, the weight of the uterus gradually increases, causing chronic strain on the anterior pelvic muscles, ligaments, and nerves. Additionally, during delivery, the load on the supportive tissues of the pelvic floor increases, which may lead to changes in the positioning of the urethra, bladder, rectum, and other pelvic floor organs after vaginal delivery, subsequently elevating the risk of PFDs [17-21]. In this study, we found that the distance from the cervix to the posterior inferior border of the pubic symphysis, the degree of bladder descent, and the anterior and posterior diameters of the LAM during maximal Valsalva maneuver differed significantly between the groups with or without PFDs. These results suggest that pelvic floor ultrasound is of high clinical value for PFDs, because it can provide clear and effective information about the structural and morphologic changes in pelvic floor, benefiting clinical diagnosis [22-25]. The reason is that pelvic floor ultrasound can dynamically assess the state of the pelvic floor cavities at rest, contraction and maximal Valsalva maneuver. Through the measurement of various ultrasound data in the bladder and anterior pelvis, it can assess changes in the neck of the bladder, as well as the middle and posterior pelvic cavities in real time, which helps physicians understand the morphologic function of the cervix and the LAM, further clarifying the possible etiology of PFDs [26-28].
Transperineal pelvic floor ultrasound has unique advantages in displaying the anatomy of the pelvic floor. In particular, the combined application of transperineal 2D and 3D ultrasound can not only clearly display the anterior pelvic anatomy and functional status, but also quantify various reference indicators. Many scholars believe that indicators, such as urethral funnel, pelvic diaphragmatic fissure, and anal raphe can well reflect the function of the anterior pelvis [29]. The anatomic structure of the pelvic floor plays an important role in maintaining the normal position of the pelvic floor organs. The degree of urethral rotation and bladder descent can reflect the mobility of the urethra and bladder. Structural defects and hypermobility of the urethra and bladder neck, combined with weak or damaged support structures of the pelvic floor, may lead to PFDs and trigger POP [30-32]. In this study, we found that vaginal tears were more severe and PFDI-20 scores were higher in the PFD group than those in the group without PFD. These results may be related to anal fissures and changes in pelvic floor muscles. These alterations are affected by various physiological changes during pregnancy, including hormone levels, which increase gravitational and abdominal loads on the pelvic organs, alter collagen metabolism within pelvic floor tissues, and subsequently compromise the support structures and functional integrity of the pelvic floor. Pelvic floor muscles gradually relax during pregnancy due to continuous pressure, and overstretching of the pelvic floor muscles during vaginal delivery can lead to muscle tears, leading to PFDs [33,34]. In this study, we analyzed the relationship between ultrasound findings and the severity of PFDs. It was found that the reduction rate was negatively correlated with the PFDI-20 score, whereas bladder descent, cervical subluxation, urethral rotation, and elongation at maximal Valsalva maneuver were positively correlated with the PFDI-20 score. This suggests a correlation between post-partum ultrasound parameters, indicators of pelvic floor musculature, and the PFDI-20 score. Pelvic floor dynamic ultrasound can accurately observe the bladder, muscles, vaginal wall and other tissues and organs of the pelvic floor through multilevel tomographic 3D imaging, which provides a reliable basis for the diagnosis of PFDs after vaginal delivery [35-37]. In this study, the AUC of bladder descent, cervical subluxation, urethral rotation, shortening rate during retraction, and elongation at maximal Valsalva maneuver was 0.916, and the RR values of each measures were 3.643, 3.742, 2.511, 0.308, and 3.144, respectively. These measures were all closely related to PFD. Thus, transperineal pelvic floor ultrasound holds significant value in both the diagnosis and the further understanding of the pathogenesis of PFDs.
However, there are some limitations to this study. First, we used a retrospective research design, which has its inherent flaws. Second, we did not obtain detailed obstetric-related data during the labor and delivery process, such as the duration of the first and second stage of labor. Third, we included only women in the early postpartum period, so changes in pelvic floor structure and function in the later postpartum period could not be observed. In future studies, we will conduct prospective observational studies to investigate the structural and functional changes in the pelvic floor during different postpartum periods with long-term follow-up.
In conclusion, transperineal pelvic floor ultrasound can assess posterior pelvic injury and prolapse in postpartum women.
Acknowledgements
This work was supported by Youth project of Shanghai Pudong New Area Municipal Health Bureau (PW2022B-15).
Disclosure of conflict of interest
None.
References
- 1.Lanigan LG, Russell DS, Woolard KD, Pardo ID, Godfrey V, Jortner BS, Butt MT, Bolon B. Comparative pathology of the peripheral nervous system. Vet Pathol. 2021;58:10–33. doi: 10.1177/0300985820959231. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P, Andersson KE, Apostolidis A, Birder L, Bliss D, Brubaker L, Cardozo L, Castro-Diaz D, O’Connell PR, Cottenden A, Cotterill N, de Ridder D, Dmochowski R, Dumoulin C, Fader M, Fry C, Goldman H, Hanno P, Homma Y, Khullar V, Maher C, Milsom I, Newman D, Nijman RJM, Rademakers K, Robinson D, Rosier P, Rovner E, Salvatore S, Takeda M, Wagg A, Wagner T, Wein A members of the committees. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol Urodyn. 2018;37:2271–2272. doi: 10.1002/nau.23551. [DOI] [PubMed] [Google Scholar]
- 3.Nygaard IE, Shaw JM. Physical activity and the pelvic floor. Am J Obstet Gynecol. 2016;214:164–171. doi: 10.1016/j.ajog.2015.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parden AM, Griffin RL, Hoover K, Ellington DR, Gleason JL, Burgio KL, Richter HE. Prevalence, awareness, and understanding of pelvic floor disorders in adolescent and young women. Female Pelvic Med Reconstr Surg. 2016;22:346–54. doi: 10.1097/SPV.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown HW, Hegde A, Huebner M, Neels H, Barnes HC, Marquini GV, Mukhtarova N, Mbwele B, Tailor V, Kocjancic E, Trowbridge E, Hayward L. International urogynecology consultation chapter 1 committee 2: epidemiology of pelvic organ prolapse: prevalence, incidence, natural history, and service needs. Int Urogynecol J. 2022;33:173–187. doi: 10.1007/s00192-021-05018-z. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shaikh G, Syed S, Osman S, Bogis A, Al-Badr A. Pessary use in stress urinary incontinence: a review of advantages, complications, patient satisfaction, and quality of life. Int J Womens Health. 2018;10:195–201. doi: 10.2147/IJWH.S152616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teleman P, Laurikainen E, Kinne I, Pogosean R, Jakobsson U, Rudnicki M. Relationship between the pelvic organ prolapse quantification system (POP-Q), the pelvic floor impact questionnaire (PFIQ-7), and the pelvic floor distress inventory (PFDI-20) before and after anterior vaginal wall prolapse surgery. Int Urogynecol J. 2015;26:195–200. doi: 10.1007/s00192-014-2434-6. [DOI] [PubMed] [Google Scholar]
- 8.Augusto KL, Bezerra LRPS, Murad-Regadas SM, Vasconcelos Neto JA, Vasconcelos CTM, Karbage SAL, Bilhar APM, Regadas FSP. Defecatory dysfunction and fecal incontinence in women with or without posterior vaginal wall prolapse as measured by pelvic organ prolapse quantification (POP-Q) Eur J Obstet Gynecol Reprod Biol. 2017;214:50–55. doi: 10.1016/j.ejogrb.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Dietz HP. Pelvic floor ultrasound: a review. Clin Obstet Gynecol. 2017;60:58–81. doi: 10.1097/GRF.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 10.El Sayed RF, Alt CD, Maccioni F, Meissnitzer M, Masselli G, Manganaro L, Vinci V, Weishaupt D ESUR and ESGAR Pelvic Floor Working Group. Magnetic resonance imaging of pelvic floor dysfunction-joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017;27:2067–2085. doi: 10.1007/s00330-016-4471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinzaki M, Yamada Y, Nagura T, Nakahara T, Yokoyama Y, Narita K, Ogihara N, Yamada M. Development of upright computed tomography with area detector for whole-body scans: phantom study, efficacy on workflow, effect of gravity on human body, and potential clinical impact. Invest Radiol. 2020;55:73–83. doi: 10.1097/RLI.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao L, Li F, Wang D, Sheng S. Evaluation of acupuncture treatments of postpartum female pelvic floor dysfunction by four-dimensional transperineal pelvic floor ultrasound. Medicine (Baltimore) 2021;100:e27236. doi: 10.1097/MD.0000000000027236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coblentz AC, Teixeira SR, Mirsky DM, Johnson AM, Feygin T, Victoria T. How to read a fetal magnetic resonance image 101. Pediatr Radiol. 2020;50:1810–1829. doi: 10.1007/s00247-020-04768-0. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein I, Komisaruk BR, Pukall CF, Kim NN, Goldstein AT, Goldstein SW, Hartzell-Cushanick R, Kellogg-Spadt S, Kim CW, Jackowich RA, Parish SJ, Patterson A, Peters KM, Pfaus JG. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) J Sex Med. 2021;18:665–697. doi: 10.1016/j.jsxm.2021.01.172. [DOI] [PubMed] [Google Scholar]
- 15.Kotaska A. Postpartum venous thromboembolism prophylaxis may cause more harm than benefit: a critical analysis of international guidelines through an evidence-based lens. BJOG. 2018;125:1109–1116. doi: 10.1111/1471-0528.15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tim S, Mazur-Bialy AI. The most common functional disorders and factors affecting female pelvic floor. Life (Basel) 2021;11:1397. doi: 10.3390/life11121397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross E, Scott F. The Female Golfer. Strength and Conditioning for Golf. Routledge; 2022. pp. 75–100. [Google Scholar]
- 18.Shoseyov O, Kam D, Ben ST, Shtein Z, Vinkler S, Posen Y. Nanocellulose composite biomaterials in industry and medicine. Extracellular Sugar-Based Biopolymers Matrices. 2019:693–784. [Google Scholar]
- 19.Kennedy C, Bargman JM. Noninfectious complications of peritoneal dialysis. Nolph and Gokal’s Textbook of Peritoneal Dialysis. 2020:1–44. [Google Scholar]
- 20.Lawrence DJ. Peer-reviewer acknowledgements for the Association of Chiropractic Colleges Research Agenda Conference 2018. JCE. 2018;32:50. [Google Scholar]
- 21.Rafi R, Nguyen T. CPC-EM full text issue. Clin Pract Cases Emerg Med. 2022;6:14–17. [Google Scholar]
- 22.Gao Y, Zhao Z, Yang Y, Zhang M, Wu J, Miao Y. Diagnostic value of pelvic floor ultrasonography for diagnosis of pelvic organ prolapse: a systematic review. Int Urogynecol J. 2020;31:15–33. doi: 10.1007/s00192-019-04066-w. [DOI] [PubMed] [Google Scholar]
- 23.Taithongchai A, Sultan AH, Wieczorek PA, Thakar R. Clinical application of 2D and 3D pelvic floor ultrasound of mid-urethral slings and vaginal wall mesh. Int Urogynecol J. 2019;30:1401–1411. doi: 10.1007/s00192-019-03973-2. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Li F, Wang D, Sheng S. Evaluation of acupuncture treatments of postpartum female pelvic floor dysfunction by four-dimensional transperineal pelvic floor ultrasound. Medicine (Baltimore) 2021;100:e27236. doi: 10.1097/MD.0000000000027236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frawley H, Shelly B, Morin M, Bernard S, Bø K, Digesu GA, Dickinson T, Goonewardene S, McClurg D, Rahnama’i MS, Schizas A, Slieker-Ten Hove M, Takahashi S, Voelkl Guevara J. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol Urodyn. 2021;40:1217–1260. doi: 10.1002/nau.24658. [DOI] [PubMed] [Google Scholar]
- 26.Hodges PW, Stafford RE, Hall L, Neumann P, Morrison S, Frawley H, Doorbar-Baptist S, Nahon I, Crow J, Thompson J, Cameron AP. Reconsideration of pelvic floor muscle training to prevent and treat incontinence after radical prostatectomy. Urol Oncol. 2020;38:354–371. doi: 10.1016/j.urolonc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Novak J, Jacisko J, Stverakova T, Juehring D, Sembera M, Kolar P, Kobesova A. The significance of intra-abdominal pressure on postural stabilization: a low back pain case report. Slovak Journal of Sport Science. 2021;7:3–18. [Google Scholar]
- 28.Petrone P, Asensio JA, Marini CP. Diaphragmatic injuries and post-traumatic diaphragmatic hernias. Curr Probl Surg. 2017;54:11–32. doi: 10.1067/j.cpsurg.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Stanojevic M. Caring for the normal newborn. Perinatolo. 2022:1041–1109. [Google Scholar]
- 30.Quaghebeur J, Petros P, Wyndaele JJ, De Wachter S. Pelvic-floor function, dysfunction, and treatment. Eur J Obstet Gynecol Reprod Biol. 2021;265:143–149. doi: 10.1016/j.ejogrb.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Soave I, Scarani S, Mallozzi M, Nobili F, Marci R, Caserta D. Pelvic floor muscle training for prevention and treatment of urinary incontinence during pregnancy and after childbirth and its effect on urinary system and supportive structures assessed by objective measurement techniques. Arch Gynecol Obstet. 2019;299:609–623. doi: 10.1007/s00404-018-5036-6. [DOI] [PubMed] [Google Scholar]
- 32.Ji R, He B, Wu J. Application of transperineal ultrasound combined with shear wave elastography in pelvic floor function assessment after hysterectomy. Medicine (Baltimore) 2023;102:e32611. doi: 10.1097/MD.0000000000032611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Geelen H, Ostergard D, Sand P. A review of the impact of pregnancy and childbirth on pelvic floor function as assessed by objective measurement techniques. Int Urogynecol J. 2018;29:327–338. doi: 10.1007/s00192-017-3540-z. [DOI] [PubMed] [Google Scholar]
- 34.Stansfield E, Kumar K, Mitteroecker P, Grunstra NDS. Biomechanical trade-offs in the pelvic floor constrain the evolution of the human birth canal. Proc Natl Acad Sci U S A. 2021;118:e2022159118. doi: 10.1073/pnas.2022159118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Gruting IMA, Stankiewicz A, Van Delft KWM, Doumouchtsis SK, Inthout J, Sultan AH, Thakar R. Diagnostic test accuracy of magnetic resonance imaging and pelvic floor ultrasound for diagnosis of levator ani muscle avulsion. Ultrasound Obstet Gynecol. 2022;60:559–569. doi: 10.1002/uog.24955. [DOI] [PubMed] [Google Scholar]
- 36.Santoro GA, Wieczorek AP, Shobeiri SA, Stankiewicz A. Endovaginal ultrasonography: methodology and normal pelvic floor anatomy. Pelvic Floor Disorders: A Multidisciplinary Textbook. 2021:111–131. [Google Scholar]
- 37.Paul K, Darzi S, Werkmeister JA, Gargett CE, Mukherjee S. Emerging nano/micro-structured degradable polymeric meshes for pelvic floor reconstruction. Nanomaterials (Basel) 2020;10:1120. doi: 10.3390/nano10061120. [DOI] [PMC free article] [PubMed] [Google Scholar]