Abstract
octurnal animals use their vision and acute hearing to adapt to the nighttime environment. Light pollution has become a serious problem for nocturnal animals in coastal areas, especially nesting sea turtles and sea turtle hatchlings. Hatchlings use visual clues to find the ocean. However, when the artificial light is stronger than the natural light, hatchlings become either misoriented, disoriented or both. Due to rapid tourism development on Lanyu Island, new sources of light pollution, especially streetlights, pose a serious threat to sea turtle hatchlings. In this study, we used a portable lamp constructed by Liteon Inc. on a circular area of a turtle nesting beach to see how artificial light sources could affect green turtle hatchlings’ sea finding behavior. In the experiments, we tested hatchling behavior under different lamp settings (strong or weak light intensity; white or yellow light; lamp shield presence or absence) and moon visibilities (moonlit or moonless). The hatchlings’ crawling tracks and locations at the end of the trials were recorded. Results showed that the light intensity had no effect on hatchling sea finding behavior. White light had a stronger impact on hatchling sea finding behavior than yellow light. When the lamp shield was installed on moonlit nights, more hatchings were able to find the sea under both white and yellow lights. Thus, it is recommended that light shields be installed on the streetlights of Lanyu Island in order to protect the sea turtle hatchlings effectively.
Keywords: Lanyu Island, Green sea turtle hatchling, Artificial lights, Lamp shield, White and yellow lights
BACKGROUND
Natural light comes from the sun, moon and stars and is important to many animals because it is a source of energy and information. It can regulate animals’ physiological features, such as biological clocks, and is important to the survival of many organisms (Kramer and Birney 2001; Verutes et al. 2014). Nocturnal animals use their vision and acute hearing to adapt to the nighttime environment and to survive and forage in order to maintain their physiologies, adjust their metabolisms, growth and other relevant behaviors (Silva et al. 2017).
The population of humans living on the coast is increasing at a higher rate than anywhere else (Nicholls 1995). This results in coastal areas becoming one of the most vulneable areas to anthropogenic stresses (Bird et al. 2004). Sea turtles are one of the most affected species of wildlife in coastal areas (Salmon et al. 2000; Salmon 2003). Many nesting beaches are close to residental or developed areas, such as malls, cities, factories and power plants. With the increasing population, development and light emission, light pollution becomes a serious problem. Light pollution occurs when artificial lights impact the physiology (e.g., foraging, reproduction, metabolism, orientation, migration) and behaviors of animals. It increases threats to their survival and decreases their ability to adapt (Perry and Fisher 2006). Skyglow from nearby cities can also affect sea turtles negatively (Garrett et al. 2019). Coastal artificial lights can deter female turtles from nesting, decrease nesting success, concentrate nests, and increase egg predation (Pilcher et al. 2000; Salmon et al. 2000; Salmon 2006; Wyneken et al. 2000). They can also disturb the sea finding behavior of hatchlings (Berry et al. 2013; Hamann et al. 2007; Salmon 2006).
Sea turtle hatchlings use their vision to search for the brighter horizon when crawling towards the ocean (Limpus and Kamrowski 2013; Limpus 1971). However, nearby artificial lights disturb their ability to search for the ocean and decrease their chances of survival (Tuxbury and Salmon 2005; Witherington and Martin 2000). Artificial lights can create two kinds of disturbances: misorientation, where hatchlings move in the a circular motion or remain motionless without a clear direction; and disorientation, where hatchlings crawl in the opposite direction of the ocean and move towards the artifical lights (Salmon and Witherington 1995; Verheijen 1985).
The degree to which hatchlings are disturbed by artificial light depends on its intensity and wavelength (Witherington 1991). Green turtle hatchlings show positive phototaxis towards short-wave blue light with a weaker response to long-wave red light within the 350 to 540 nm wavelength range (Levenson et al. 2004; Mrosovsky 1972). An ERG (electroretinogram) study showed that long-wave red light has to be approximately 600 times stronger than the blue light before hatchling green turtles will have the same response behavior (Mrosovsky 1972). Both adult and hatchling green turtles can be influenced by light wavelengths between 440 to 700 nm, but hatchlings are particularly sensitive to the 350 to 450 nm wavelength range (Horch et al. 2008; Witherington and Bjorndal 1991; Witherington and Martin 2000).
There are two main nesting sites in Taiwan: Wanan Island of Penghu Archipelago and Lanyu Island of Taitung County. Long-term studies have shown that the nesting population on Lanyu Island has increased since 1997 and become the major nesting island in Taiwan (Cheng et al. 2008 2018). However, the rapid development of tourism and the accompanying facilities, such as bars, restaurants and streetlights by the beach, create a major threat to nesting female and hatchling sea turtles.
Lanyu Island is located in the Pacific Ocean, approximately 145 km southeast of Taiwan. The size of the island is 45.7 square kilometers (Fig. 1). The number of tourists visiting the island per year in 2016 was 110,000, which then increased to approximately 140,000 in 2019 and reached 160,000 in 2020 (Taitung County Report 2020). Due to COVID-19, most tourists visited domestic islands instead of traveling abroad. This resulted in the rapid increase of tourists to this island. In order to create safer roads and attract tourists, many of the bars and restaurants were built near the nesting beaches. Most streetlights around the island that face the beaches use bright LED lighting. This resulted in more artificial light spilling onto the nesting beaches. Ko (2020)found that nearly 90% of the green turtle hatchlings on this island failed to conduct sea finding behavior, even under the full moon. The streetlights thus pose serious threat to the hatchlings on this island.
Fig. 1.
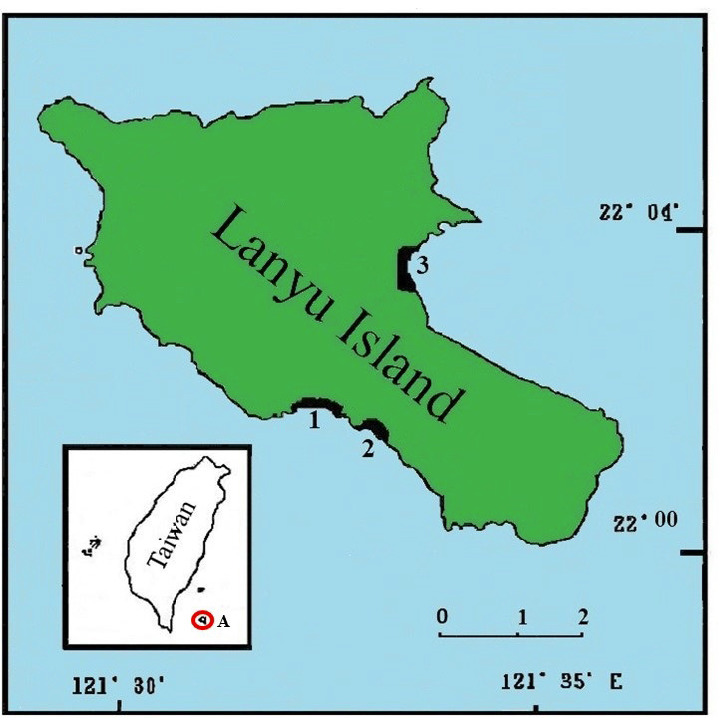
Map of Lanyu Island with marked nesting beaches, 1: Big Bai-Dai Beach, 2: Small Bai-Dai Beach, 3: Donchin Beach. “A” and the red circle on the inset map on the lower left indicate Lanyu Island.
In order to understand the impact of streetlights on the sea finding behavior of green turtle hatchlings, as well as possible solutions, we cooperated with the Liteon Inc. to construct a portable lamp to conduct experiments on the nesting beach. There are two purposes of this study: first, to determine how the sea finding behavior of the hatchlings is affected under the lamp with strong and weak light intensities, with and without the lamp shield installed and on moonlit or moonless nights, and second, to determine the sea finding behavior of hatchlings under long-wave yellow light (440 to 700 nm) and short-wave white light (350 to 540 nm).
MATERIALS AND METHODS
Study site and period
There are three nesting beaches on Lanyu Island: Big Ba-Dai, Ba-Dai and Donchin (Fig. 1). Ba-Dai hosts the most turtle nests, Big Ba-Dai receives the most artificial light, and Donchin is a relatively large and undisturbed beach. We moved the nests from the other beaches to Donchin, since it offered the most space and fewest disturbances. The experiments lasted from midJune to mid-September of 2019 and 2020.
Nest and hatchling collection
Beaches were patrolled every two hours from 7 pm to 3 am each night. In order to prevent nests from overcrowding the main nesting beach and protect them from light pollution, nests were relocated from Big Ba-Dai and Ba-Dai beaches to Donchin Beach and reburied at the undisturbed site within three hours of depositing the eggs. According to a previous study, the nest incubation periods on the island ranges from 50 to 55 days (Cheng et al. 2009). A wired cage was installed from 7 pm to 7 am daily starting at day 45 of incubation to collect the emerged hatchlings. Caged nests were visited according to the beach patrol schedule, every two hours from 7 pm to 3 am.
Hatchlings were collected if they had naturally emerged from the nests and avoided if they were stunted, weak or yet to be hatched (Pendoley and Kamrowski 2016). The collected hatchlings were stored in a ventilated light-tight insulation box until the experiment began.
Design of the portable lamp
Based on the new design of a turtle-friendly light proposed by Robertson et al. (2016),a portable lamp was constructed by Lite-On Inc. The lamp was compliant with Taiwan Road Lighting Specifications. Experiments were designed to mimic the light pollution of streetlights and its effect on hatchling crawling behavior by exposing the hatchlings to different light sources, intensities, installed light shield or uninstalled light shield, and different levels of moonlight. Due to the fact that hatchlings have a strong phototaxis to short-wave light, the second set of experiments were designed to determine hatchling crawling behavior under yellow and white light. The lamp was 2 meters tall and composed of 40 LED light bulbs which could be replaced depending on the color of light needed for the experiment. In order to prevent any vibration created by the generator, the lamp used lifespan (Fig. 2).
Fig. 2.

The portable lamp installed on the Donchin nesting beach of Lanyu Island.
Lamp shield and light intensity determination
The lamp shields were designed to shield light from the front and sides of the lamp. The light reached up to 12 meters from the source without a shield, while it only reached up to 6 m with the shield installed. Light intensity was determined using a portable spectroscopic spectrometer (Chunyua Scientific Technology, model MK350N Premium) 9 meters from the light source. Light intensity reached 37.45 lx with the strong white light without the shield and decreased to 4.153 lx with the shield.
Liteon Inc. used a DC24V, 70W, 2.5A, 1-10V eight-segment current selector to determine the light intensity. Light intensity increased from segment 1 to 8. In this study, we used segment 1 for the weak light and segment 8 for the strong light intensity. Light intensity reached 14.53 lx with the strong yellow light without the shield and decreased to 2.187 lx with the shield.
Liteon Inc. used a DC24V, 70W, 2.5A, 1-10V eight-segment current selector to determine the light intensity. Light intensity increased from segment 1 to 8. In this study, we used segment 1 for the weak light and segment 8 for the strong light intensity.
Moon phase determination
No moon and new moon were defined as “moonless”. The other moon phases were defined as “moonlit night” (Witherington and Martin 2000).
Design of circular arena
The circular arena was designed based on Bertolotti and Salmon (2005). A circle with a diameter of 3 meters was drawn on the beach and divided into 12 sections. Among these sections, degrees 345 to 15 faced the ocean, and degrees 165 to 195 faced the lamp. The lamp was placed 9 meters from the center of the arena (Fig. 3).
Fig. 3.
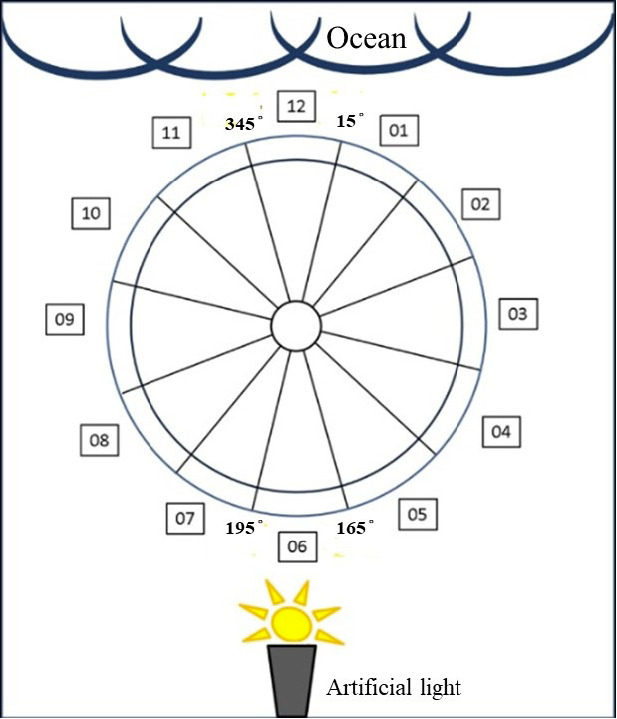
Design of the experimental arena.
Conducting the experiment
During the experiment, hatchlings were placed in a shallow depression about 10 cm deep in the center of the arena and covered with a light-proof box to protect them from the effects of the light. A 5 cm depression was made around the edge of the arena to indicate the end of the experiment. At least 10 hatchlings were used in each experiment. They were allowed to crawl for 4 minutes. For hatchlings that reached the edge of the arena within 4 minutes, their crawl tracks and final locations in degrees were recorded. Hatchlings that failed to reach the edge within 4 minutes were considered “incomplete” and removed from the test. All the hatchlings were released back to the ocean immediately after the experiment.
Four conditions were defined in the experiment: (1) hatchlings that crawled in a directionless, motionless or circular pattern and had end points located in the artificial light sections were defined as “disoriented”; (2) hatchlings that crawled towards artificial light or away from the ocean and had end points located in the artificial light sections were defined as “misoriented”; (3) hatchlings that crawled towards the ocean and had end points located in the ocean sections were defined as “sea finding”; (4) hatchlings that crawled towards neither light nor ocean sections and had end points located in sections other than the artificial light or ocean sections were defined as “other directions”.
Data analyses
Oriana 4 software was used to conduct a Rayleigh z test (Zar 1999) to determine whether the end points of the hatchlings in each experiment were distributed in a certain direction or randomly. In the analysis, r denotes the crawling direction of the hatchling (with high r values indicating that the hatchlings crawled in a similar dirction), and µ denotes the mean direction of the end point of crawling. A Watson-Williams F-test (Dimitriadis et al. 2018) was used to determine the differences between light intensities, use of lamp shield or not, moon visibilities, and lamp colors.
RESULTS
A total of 21 nests and 534 hatchlings were collected for the experiments. Among them, 22 hatchlings failed to crawl and were subsequently removed from the test. Thus, 512 hatchlings were used. In order to determine the effect of background light, crawl experiments were conducted with the lamp turned off during both moonlit and moonless nights prior to the experiments that used the lamp turned on.
Light turned off
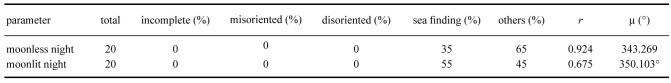
A Rayleigh test indicated that none of the hatchlings in the sample size used for these trials showed disoriented crawling, and that all behaved either “sea finding” or “other direction” crawling (Table 1, Fig. 4). The Watson-Willions F-test showed no difference between the moonlit and moonless nights (p > 0.05). Most hatchlings crawled towards the ocean.
Table 1.
Total hatchlings used and proportion of incomplete, disorieted, misoriented, correct sea finding, other directions, concentrated (r) and average degree with the lamp turned off
Fig. 4.
The end points of hatchlings with the lamp turned off on the (a) moonless night and (b) moonlit night. The circle is divided into 12 sections of 30 degrees each. The number on the exterior of the circle represent “degree” and correspond to the section number from degree 15 (faces the ocean) towards the right direction. One can determine in which direction the hatchling crawled from the number in the square. The diameter of the circle is 3 m. Hatchlings were released from the center point of the arena (circle).
Strong and weak white light (350 to 540 nm)
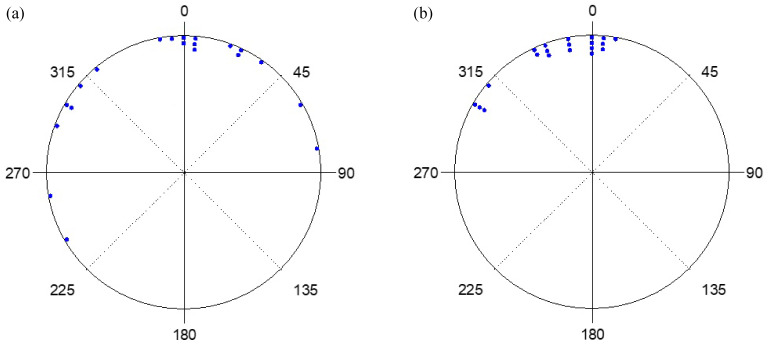
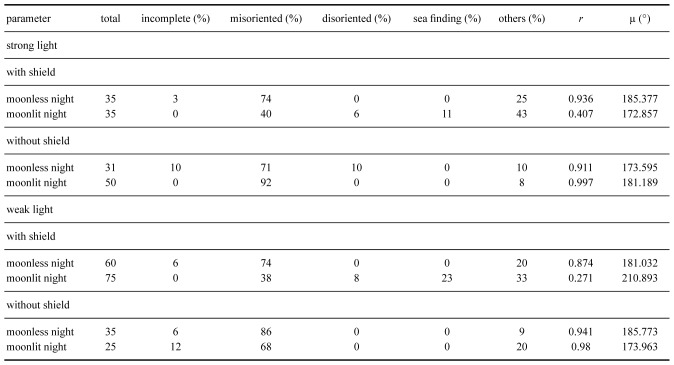
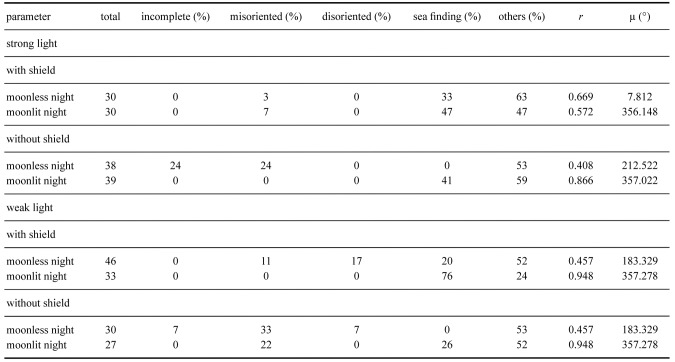
261 hatchlings were used for this part of the experiment. Among them, 11 did not complete the experiment, 13 exhibited sea finding crawling (5%), 173 exhibited misoriented crawling (69%), 56 crawled in other directions (22%) and 8 exhibited disoriented crawling (3%). The crawling behavior of hatchlings under the strong and weak white lights, with the lamp shield installed or uninstalled and on moonlit and moonless nights, along with statistical analyses, are shown in table 1. Results of the distribution of end points in strong and weak light, with and without lamp shield installed, and on moonlit and moonless nights are shown in figures 5 and 6. These results showed that more than 68% of hatchlings displayed misoriented crawling under both the strong and weak white light. Only in two cases, under both bright and weak light settings, with both the lamp shield installed and on moonlit nights, did a portion of hatchlings either crawl towards the sea or in other directions (Figs. 5 and 6; Table 2).
Fig. 5.
The end points of hatchlings when exposed to strong white light with (a) no lamp shield on a moonlit night, (b) no lamp shield on a moonless night, (c) lamp shield on a moonlit night, and (d) lamp shield on a moonless night.
Fig. 6.
The end points of hatchlings exposed to weak white light with (a) no lamp shield on a moonlit night, (b) no lamp shield on a moonless night, (c) lamp shield on a moonlit night, and (d) lamp shield on a moonless night.
Rayleigh tests showed that, under the strong light setting, the hatchlings crawled directionally in both moonlit and moonless nights, with and without the lamp shields installed (p < 0.05 in all cases). This test also showed that, under the weak light, the hatchlings crawled directionally without the lamp shield on both moonlit and moonless nights and with the lamp shield on moonless nights (p < 0.05 in all cases). When the lamp was set to the weak light setting and the shield was installed on moonlit nights, the hatchlings’ crawling direction was random (Z = 2.943, p = 0.052). The average r values were more than 0.87 on the moonless nights with both strong and weak intensities and with the lamp shield installed or uninstalled. The r values decreased to less than 0.41 in both strong and weak light settings with the lamp shield installed on moonlit nights. The average μ values were more than 170° in all cases (Table 2). These results suggest that most hatchlings crawled towards the artificial light. However, the moon did attract some hatchlings to crawl in the other directions (Table 2). The Watson-Williams F-test showed that, under the strong light, no difference was found between lamp shield installed or not, nor between moonlit and moonless nights. Also, no difference was found between moonlit and moonless nights without the lamp shield under the weak light setting (p > 0.05 in both cases; Figs. 5 and 6). Most hatchlings crawled towards the artificial light. However, the test found a significant difference between moonlit and moonless nights with the lamp shield installed under the weak light setting (p > 0.05). Some hatchlings crawled towards the ocean when the lamp shield was installed on moonlit nights (Fig. 5).
Table 2.
Total hatchlings used and proportion of incomplete, disoriented, misoriented, correct sea finding, other directions, concentrated (r) and average degree under the strong and weak white light
Strong and weak yellow light (593 nm)
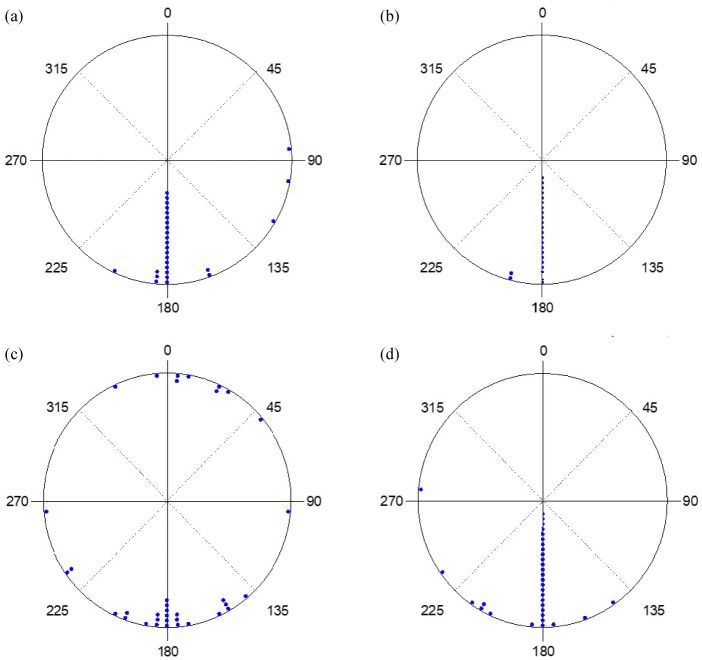
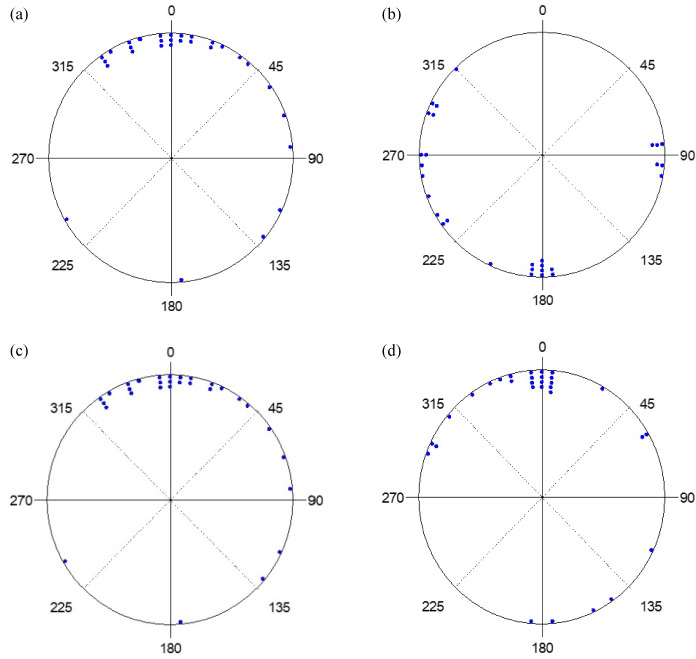
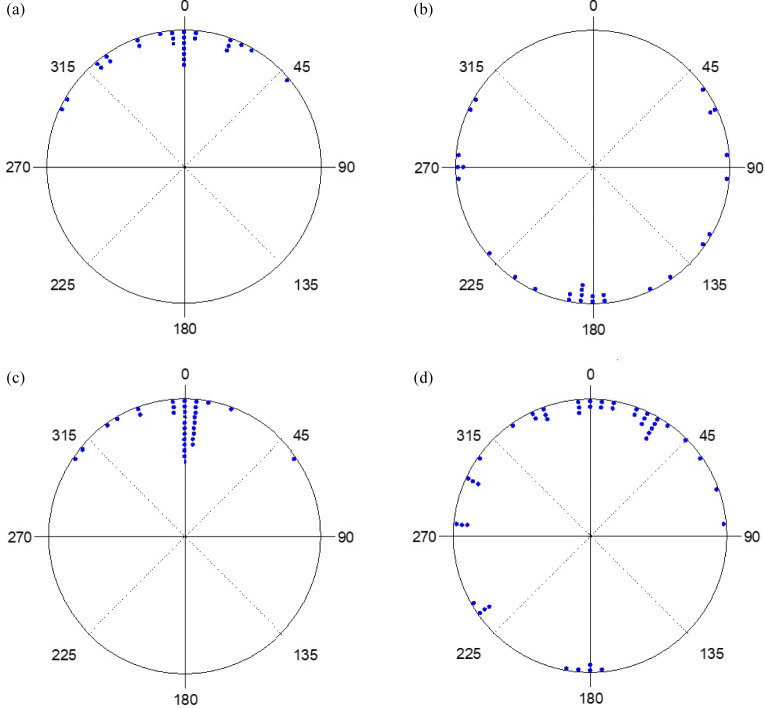
273 hatchlings were used. Among them, 11 did not complete the experiment, 81 displayed sea finding crawling (31%), 33 exhibited misoriented crawling (13%), 138 crawled in other directions (53%) and 10 demonstrated disoriented crawling (4%). The crawling behavior of hatchlings under the strong and weak yellow lights, with the lamp shield installed or not installed and on moonlit or moonless nights, along with statistical analyses, are shown in table 2. The distribution of end points under the strong and weak lights, with and without the lamp shield installed, and on moonlit and moonless nights are shown in figures 7 and 8. Results showed that, with the exception of when the lamp shield was not installed on moonless nights under both strong and weak light, more than 20% of the hatchlings crawled towards the ocean and in most cases, close to 50% or more crawled in the other directions (Table 3).
Table 3.
Total hatchlings used and proportion of incomplete, disoriented, misoriented, correct sea finding, other directions, concentrated (r) and average degree under the strong and weak yellow light
Rayleigh tests showed that the hatchlings all crawled in a particular direction on both moonlit and moonless nights, with and without the lamp shield installed, under both the strong and weak light setting (p < 0.05 in all cases). Comparisons showed that, except in the strong light with no lamp shield, the average r values were higher than 0.86 in all cases on moonlit nights. The values decreased to less than 0.67 on moonless nights. The average μ values were close to 360° in all cases on moonlit nights and decreased to less than 212° on moonless nights (Table 3). These results suggest that in the strong and weak yellow light, most hatchlings crawled towards the ocean on moonlit nights. However, in the absence of the moon, hatchlings crawled in random directions (Figs. 7 and 8). The Watson-Williams F-test showed that, under strong and weak light settings, a significant difference was found between moonlit and moonless nights with and without the lamp shield installed (p < 0.01 in both cases). Most hatchlings crawled towards the ocean on moonlit nights, while most hatchlings crawled towards the artificial light or in other directions on moonless nights (Figs. 7 and 8). This test showed that under both strong and weak light settings, hatchlings crawled towards the ocean in both moonlit and moonless nights when the lamp shield was installed (p > 0.05 in both cases).
Fig. 7.
The end points of hatchlings exposed to strong yellow light with (a) no lamp shield on a moonlit night, (b) no lamp shield on a moonless night, (c) lamp shield on a moonlit night, and (d) lamp shield on a moonless night.
Fig. 8.
The end points of hatchlings exposed to weak yellow light with (a) no lamp shield on a moonlit night, (b) no lamp shield on a moonless night, (c) lamp shield on a moonlit night, and (d) lamp shield on a moonless night.
DISCUSSION
This is the first study to use of a portable lamp that mimics real road conditions to determine the impact of streetlights on hatchling sea finding behavior. Due to the rapid development of tourism on Lanyu Island, hatchlings have been seriously impacted by artificial lights (Chang 2012; Ko 2020; Tsai 2016). Among the various sources of light pollution, streetlights are the most serious (Ko 2020). This study was designed to find a solution to decrease the disturbance of streetlights on hatchling sea finding behavior. Results found that under white light, 72% of hatchlings were misoriented and disoriented, which is similar to other studies (Lohmann et al. 2017; Salmon 2003; Tuxbury and Salmon 2005; Witherington and Martin 2000). Under yellow light, only 17% were misoriented and disoriented. Previous research supports the findings that decreasing artificial light increases the ability of hatchlings to crawl towards the sea under yellow light (Witherington and Bjorndal 1991). Before we conducted our lamp experiments, we tested hatchling sea finding behaviors with the lamp turned off. In these trials, all hatchlings crawled towards the ocean on both moonlit and moonless nights. Thus, we determined that the arena we selected was suitable for the artificial light experiments.
White light
We found that white light, either strong or weak in intensity and on moonlit and moonless nights, has a negative impact on hatchling sea finding behavior. In most cases, hatchlings crawled owards this artificial light. However, when the lamp shield was installed on the moonlit nights, some hatchlings crawled towards either the sea or in other directions. Pendoley and Kamrowski (2016) pointed out that the lamp shield can decrease the amount of artificial light radiating directly onto the hatchlings, and thus can decrease the chances of misoriention and disoriention. The impact of the artificial light decreased when there was a shield and there was moonlight.
The white light attracted the hatchlings because green turtle hatchlings are highly attracted to wavelengths of light from the 350 to 450 nm range (Witherington and Bjorndal 1991). The white light used in the experiment was composed of a combination of physical short-wave light sources (350 to 540 nm) and multi-color elements (designed by Liteon Inc.). We found in order from most to least, hatchlings exhibited misoriented crawling, then crawled in the other direction, and lastly exhibited disoriented crawling. Witherington and Martin (2000)pointed out that hatchlings may crawl away from the ocean in a misoriented and/or disoriented pattern if an artificial source is present. Hatchlings of olive ridley turtles (Karnad et al. 2009), hawksbill turtles (Horrocks 2001) and flatback turtles (Fritsches 2012; Pendoley 2005) all primarily crawled towards the short-wave light, which is similar to the findings from our study. This suggests that the short-wave white light does attract hatchlings, resulting in misoriented sea finding behavior.
The moon plays an important role in hatchling sea finding behavior. Hatchlings search for the ocean using their vision range of 0 to 180° horizonal and 0 to 30° vertical (Adamany et al. 1997; Kawamura et al. 2012; Witherington 1991). On a moonless night, artificial light can disturb the sea finding behavior of hatchlings (Berry et al. 2013; Salmon and Witherington 1995). The disturbance of artificial light decreases, however, on a night where the moon is visible (Berry et al. 2013; Salmon and Witherington 1995; Tuxbury and Salmon 2005; Witherington and Bjorndal 1991). However, in this study, even though the moon did attract some hatchlings and cause them to crawl towards the sea or other directions, most hatchlings were still misoriented and crawled towards the artificial light. This is because the artificial light can confuse the vision of hatchlings, making it difficult for them to discriminate it from natural light (Tuxbury and Salmon 2005). The presence of the moon can decrease the hatchlings’ attraction to artificial light (Berry et al. 2013). Thus,when the lamp shield was installed on a moonlit night, the impact of the lamp’s artificial light decreased.
Yellow light
Light wavelength plays a crucial role in hatchling sea finding behavior. Previous studies suggested that green turtle hatchlings were influenced by light wavelengths of 440 to 700 nm and that they were especially sensitive to 350 to 540 nm (Horch et al. 2008; Witherington and Bjorndal 1991; Witherington and Martin 2000). Our experiments using yellow light (ranging from 440–700 nm) showed that, compared to white light, more hatchlings exhibited appropriate sea finding behavior. In both the strong and weak light settings, hatchlings crawled towards the ocean on both moonlit and moonless nights when the lamp shield was installed. However, when the lamp shield was not installed on moonless nights, hatchlings exhibited misorientation and some even crawled towards the artificial light due to the fact that the lamp was the only light source.
The response of hatchlings to light intensity has been different among studies (Hirama et al. 2021; Pendoley and Kamrowski 2016; Rivas et al. 2015). Witherington and Bjorndal (1991) suggested that on a moonless night, strong, long-wave light can still attract hatchlings. Mrosovsky (1972) suggested that the light intensity of long-wave light must be 600 times stronger than that of short-wave light in order to have a similar impact on hatchling behavior. Robertson et al. (2016) suggested that the attraction of loggerhead hatchlings to artificial light increasing with increasing light intensity. In this study, the hatchlings had a similar response to strong and weak light intensity. Thus, intensity was not an influential factor.
The lamp shield can enhance hatchling sea finding behavior. Bertolotti and Salmon (2005) used recessed floor lamps to limit the range of light sources at Boca Raton, Florida and found that these lamps did not influence the sea finding behavior of loggerhead hatchlings. Dimitriadis et al. (2018) suggested that shielding direct and indirect visible lights on nesting beaches could improve hatchling sea finding behavior. They proposed that eliminating unnecessary light and reducing the lamp power to the minimum requirement for human safety can effectively decrease the interference on hatchling sea finding behavior. Pendoley and Kamrowski (2016) found that a lamp shield decreased the influence of artificial lights on Barrow Island, Australia on both green and flatback turtles. These results are similar to the findings of this study: a lamp shield can help decrease the misoriented crawling behavior of green turtle hatchlings.
Again, this study also found that the moon can reduce the influence of artificial light on hatchling sea finding behavior. Simiar results were found in the other studies (Berry et al. 2013; Kamrowski et al. 2014; Salmon and Witherington 1995). The moon can enhance the natural light and decrease the attractiveness of artificial light. When this occurs, hatchlings have a better ability to find the ocean (Salmon and Witherington 1995).
Synoptic discussion
In this study, light intensity was not found to be an influential factor under either the white or yellow light. White light had a stronger impact on hatchling sea finding behavior than yellow light. When the lamp shield was installed on a moonlit night, more hatchlings exhibited correct sea finding behavior under both white and yellow light. Thus, the presence of the moon and lamp shield are two factors that decrease the impact of artificial light on hatchling sea finding behavior.
CONCLUSIONS
Artificial light satisfies the basic needs of humans in the night. However, sea turtle hatchlings depend on natural light to find the ocean. Artificial light sources have a serious effect on the sea finding behavior of the hatchlings (current study; Lohmann et al. 2017; Witherington and Martin 2000; Witherington 1986 1991). For decades, human developments next to nesting beaches have posed serious threats to sea turtle populations (Carr and Ogren 1960; Kamrowski et al. 2012; McFarlane 1963). A study on Kelly’s Beach in Queensland, Australia found that 62% of loggerhead hatchlings failed to reach the oecan under the presence of artificial light (Berry et al. 2013). On Kalamaki and Marathonisi beaches in Greece, most green and loggerhead hatchlings crawled towards the brightest side of the beach. They displayed misoriention by crawling on the back of a sand dune or in irregular circles without direction (Dimitriadis et al. 2018). On St. George Island, Mexico, hatchlings from 21 out of 202 nests failed to locate the ocean in 2015. Increasing fail rates corresponded with higher light intensity on land (Price et al. 2018). In the Caribbean, the presence of artificial light decreased the breeding success of sea turtles and increased their mortality rate. These impacts were related to local economic development (Brei et al. 2016). Thus, it is important to find a balance of artificial light that can decrease the misorientation of sea turtles, along with effective management strategies to keep beaches suitable for nesting.
Long-term studies on Lanyu Island found that light pollution is the most serious threat to nesting green turtles and hatchling sea finding behavior. There is a serious spillover of streetlight onto the nesting beaches of Bai-Dai beach. Ko (2020) found that hatchlings failed to have sea finding behavior, even during moonlit nights, on this beach. This study suggests that the use of yellow light and installation of lamp shields on streetlights located on major roads can effectively improve hatchling sea finding behavior.
The increase of human population and activities results on the increase of threats caused by light pollution on sea turtles (Bourgeois et al. 2009; Gallaway et al. 2010; Kamrowski et al. 2014). On Lanyu Island, the increasing tourism activities on the coast threaten biodiveristy (Brei et al. 2016). Results of this study suggest that the use of yellow light (440 to 700 nm) along with the installation of lamp shields can improve the sea finding behavior of hatchlings on Lanyu Island.
Acknowledgments
The authors thank Ms. P-J Joe, Mr. S-W Hwang, J-Y Ko, and volunteers of the field station for their assistance on the experiments. This study is partly supported by a grant from Taitug County Government (Grant no. 111H32404). We also thank Lite-On Technology Corporation and Leotek Corporation for their constructing the portable lamp and provided partial surrport on the research.
Footnotes
Authors’ contributions: The first and second authors conducted the experiment and analyzed the data of the experiment. The third and fourth authors constructed the portable lamp. The last author is the corresponding author who designed the experiment and was responsible for editing the manuscript. The manuscript is supported by a grant from the Taitung County Government (Grant No. 111H32404) and a small grant from Lite-On Technology Corporation and Leotek Corporation to I-J Cheng.
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: The data and material is available upon request.
Consent for publication: The consent for publication is not applicable.
Ethics approval consent to participate: We have followed the ethical standards of the responsible committee on laboratory animal experimentation (MAC1070003527). All authors agree to publish this manuscript and follow the ethics of relevant agreements.
References
- Adamany SL, Salmon M, Witherington BE. 1997. Behavior of sea turtles at an urban beach III. Costs and benefits of nest caging as a management strategy. Florida Sci, pp. 239–253.
- Berry M, Booth DT, Limpus CJ. 2013. Artificial lighting and disrupted sea-finding behaviour in hatchling loggerhead turtles (Caretta caretta) on the Woongarra coast, south-east Queensland, Australia. Austra J Zool 61(2):137–145. doi:10.1071/ZO13028.
- Bertolotti L, Salmon M. 2005. Do embedded roadway lights protect sea turtles? Environ Manag 36(5):702–710. doi:10.1007/s00267004-0288-2. [DOI] [PubMed]
- Bird B, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv Biol18(5):1435–1439.
- Bourgeois S, Gilot-Fromont E, Viallefont A, Boussamba F, Deem SL. 2009. Influence of artificial lights, logs and erosion on leatherback sea turtle hatchling orientation at Pongara National Park, Gabon. Biol Conserv 142(1):85–93. doi:10.1016/J.BIOCON.2008.09. 028.
- Brei M, Pérez-Barahona A, Strobl E. 2016. Environmental pollution and biodiversity: Light pollution and sea turtles in the Caribbean. J Environ Econo Manag 77:95–116. doi:10.1016/J.JEEM.2016. 02.003.
- Carr AF, Ogren LH. 1960. The ecology and migrations of sea turtles. 4, The green turtle in the Caribbean Sea. Bulletin of the AMNH; v. 121, article 1.
- Chang C-M. 2012. Estimate the sea turtle protection area on Lanyu Island-cognition and attitude of stakeholders. MS thesis, Institute of Marine Affair and Resource Management, National Taiwan Ocean Univesity.
- Cheng I-J, Dutton PH, Chen C-L, Chen H-C, Chen Y-H, Shea J-W. 2008. Comparison of the Genetics and Nesting Ecology of Two Green Turtle Rookeries in Taiwan. J Zool 276(4):375–384. doi:10.1111/J.1469-7998.2008.00501.X.
- Cheng I-J, Huang C-T, Hung P-Y, Ke B-Z, Kuo C-W, Fong C. 2009. A ten year monitoring of the nesting ecology of the green turtle, Chelonia mydas, on Lanyu Island, Taiwan. Zool Stud 48(1):83–94.
- Cheng W-H, Chan Y-T, Cheng I-J. 2018. Geographically closed, yet so different: contrasting long-term trends at two adjacent sea turtle nesting populations in Taiwan due to different anthropogenic effects. PLoS ONE 13(7):e0200063. doi:10.1371/journal.pone. 0200063. [DOI] [PMC free article] [PubMed]
- Dimitriadis C, Fournari-Konstantinidou I, Sourbès L, Koutsoubas D, Mazaris AD. 2018. Reduction of sea turtle population recruitment caused by nightlight: evidence from the Mediterranean region. Ocean Coast Manag 153:108–115. doi:10.1016/J.OCECOAMAN.2017.12.013.
- Fritsches KA. 2012. Australian loggerhead sea turtle hatchlings do not avoid yellow. Mar Freshw Behav Physiol 45(2):79–89. doi:10.1 080/10236244.2012.690576.
- Gallaway T, Olsen RN, Mitchell DM. 2010. The economics of global light pollution. Ecol Econo 69(3):658–665. doi:10.1016/J.ECOLECON.2009.10.003.
- Garrett JK, Donald PF, Gaston KJ. 2019. Skyglow extends into the world’s key biodiversity areas. Anim Conserv 23(2):153–159. doi:10.1111/acv.12480.
- Hamann M, Jessop TS, Schäuble CS. 2007. Fuel use and corticosterone dynamics in hatchling green sea turtles (Chelonia mydas) during natal dispersal. J Exp Mar Biol Ecol 353(1):13–21. doi:10.1016/J.JEMBE.2007.08.017.
- Hirama S, Witherington B, Kneifl K, Sylvia A, Wideroff M, Carthy R. 2021. Environmental factors predicting the orientation of sea turtle hatchlings on a naturally lighted beach: a baseline for light-management goals. J Exp Mar Biol Ecol 541:515–568. doi:10.1016/J.JEMBE.2021.151568.
- Horch KW, Gocke JP, Salmon M, Forward RB. 2008. Visual spectral sensitivity of hatchling loggerhead (Caretta caretta L.) and leatherback (Dermochelys coriacea L.) sea turtles, as determined by single-flash electroretinography. Mar Fresh Behav Physiol 41(2):107–119. doi:10.1080/10236240802106556.
- Horrocks J. 2001. Sea turtles and beachfront lighting: an interactive workshop for industry professionals and policy-makers in Barbados. Mar Tur Newsl 93:18–19.
- Kamrowski RL, Limpus C, Moloney J, Hamann M. 2012. Coastal light pollution and marine turtles: assessing the magnitude of the problem. Endan Spec Res 19(1):85–98. doi:10.3354/ESR00462.
- Kamrowski RL, Limpus C, Jones R, Anderson S, Hamann M. 2014. Temporal changes in artificial light exposure of marine turtle nesting areas. Global Chang Biol 20(8):2437–2449. doi:10.1111/gcb.12503. [DOI] [PubMed]
- Karnad D, Isvaran K, Kar CS, Shanker K. 2009. Lighting the way: Towards reducing misorientation of olive ridley hatchlings due to artificial lighting at Rushikulya, India. Biol Conserv 142(10):2083–2088. doi:10.1016/J.BIOCON.2009.04.004.
- Ko J-Y. 2020. Influence of artificial light sources to the sea finding behavior of green turtle h on Lanyu Island, Taitung County, Taiwan. Master thesis, National Taiwan Ocean University.
- Kramer KM, Birney EC. 2001. Effect of light intensity on activity patterns of Patagonian leaf-eared mice, Phyllotis xanthopygus. J Mammal82(2):535–544. doi:10.1644/1545-1542(2001) 082<0535:EOLIOA>2.0.CO;2.
- Levenson D, Eckert S, Crognale M, Deegan I, Jacobs G. 2004. Photopic spectral sensitivity of green and loggerhead sea turtles. Copeia 2004(4):908–914. doi:10.1643/CP-03-217R1.
- Limpus CJ. 1971. Sea turtle ocean finding behaviour. Search 2(10):385–387.
- Limpus C, Kamrowski RL. 2013. Ocean-finding in marine turtles:the importance of low horizon elevation as an orientation cue. Behav 150(8):863–893. doi:10.1163/1568539X-00003083.
- Lohmann KJ, Witherington BE, Lohmann CM, Salmon M. 2017. Chap. 5. Orientation, navigation, and natal beach homing in sea turtles. In: PL Lutz, JA Musick eds. The Biology of Sea Turtles. CRC Press, New York.
- McFarlane RW. 1963. Disorientation of loggerhead hatchlings by artificial road lighting. Copeia 1963(1):153. doi:10.2307/1441283.
- Mrosovsky N. 1972. The water-finding ability of sea turtles. Behavioural studies and physiological speculations. Brain, Behav Evol 5(2–3):202–225. doi:10.1159/000123748. [DOI] [PubMed]
- Nicholls RJ. 1995. Coastal megacities and climate change. Geo J37(3):369–379. doi:10.1007/BF00814018.
- Pendoley KL. 2005. Sea turtles and the environmental management of industrial activities. MS thesis. Northwest Western Australia Murdoch University 284 pages.
- Pendoley K, Kamrowski RL. 2016. Sea-finding in marine turtle hatchlings: what is an appropriate exclusion zone to limit disruptive impacts of industrial light at night? J Nat Conserv 30:1–11. doi:10.1016/j.jnc.2015.12.005.
- Perry G, Fisher RN. 2006. Night lights and reptiles: observed and potential effects. Ecological consequences of artificial night lighting, pp. 169–191.
- Pilcher N, Enderby S, Stringell T, Bateman L. 2000. Nearshore turtle hatchling distribution and predation. Sea turtles of the IndoPacific. ASEAN Academic Press, London, pp. 151–166.
- Price JT, Drye B, Domangue RJ, Paladino FV. 2018. Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol Conser Biol 13(2):415–422.
- Rivas ML, Tomillo PS, Uribeondo JD, Marco A. 2015. Leatherback hatchling sea-finding in response to artificial lighting: Interaction between wavelength and moonlight. J Exp Mar Biol Ecol 463:143–149. doi:10.1016/J.JEMBE.2014.12.001.
- Robertson K, Booth DT, Limpus CJ. 2016. An assessment of ‘turtlefriendly’ lights on the sea-finding behaviour of loggerhead turtle hatchlings (Caretta caretta). Wild Res Limpus 43(1):27–37. doi:10.1071/WR15138.
- Salmon M. 2003. Artificial night lighting and sea turtles. Biologist 50(4):163–168.
- Salmon M. 2006. Protecting sea turtles from artificial night lighting at Florida’s oceanic beaches. Ecological consequences of artificial night lighting, pp. 141–168.
- Salmon M, Witherington BE. 1995. Artificial lighting and seafinding by loggerhead hatchlings: evidence for lunar modulation. Copeia 4:931–938. doi:10.2307/1447042.
- Salmon M, Witherington BE, Elvidge CD. 2000. Artificial lighting and the recovery of sea turtles. Sea turtles of the Indo-Pacific: research, management and conservation. ASEAN Academic Press, London, pp. 25–34.
- Silva E, Marco A, da Graça J, Pérez H, Abella J, Patin-Martinez, Martins S, Almeida C. 2017. Light pollution affects nesting behavior of loggerhead turtles and predation risk of nests and hatchlings. J Photochem Photobiol B: Biol 173:240–249. doi:10.1016/j.jphotobiol.2017.06.006. [DOI] [PubMed]
- Taitung County Report. 2020. Tourism Statistics. Available at: https://tour.taitung.gov.tw/zh-tw/visitorstatics/detail/247.
- Tsai I-L. 2016. Disturbance of coastal light pollution to the green turtle hatchlings on Lanyu Island. BS thesis, Institute of Marine Biology, National Taiwan Ocean University.
- Tuxbury SM, Salmon M. 2005. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biol Conserv 121(2):311–316. doi:10.1016/J.BIOCON.2004.04.022.
- Verheijen F. 1985. Photopollution:artificial light optic spatial control systems fail to cope with. Incidents, causation, remedies. Exp Biol 44(1):1–18. . [PubMed]
- Verutes GM, Huang C, Estrella RR, Loyd K. 2014. Exploring scenarios of light pollution from coastal development reaching sea turtle nesting beaches near Cabo Pulmo, Mexico. Global Ecol Conserv 2:170–180. doi:10.1016/J.GECCO.2014.09.001.
- Witherington BE. 1986. Human and natural causes of marine turtle clutch and hatchling mortality and their relationship to hatchling production on an important Florida nesting beach. Ms thesis University of Central Florida, 141 pages.
- Witherington BE. 1991. Orientation of hatchling loggerhead turtles at sea off artificially lighted and dark beaches. J Exp Mar Biol Ecol 149(1):1–11. doi:10.1016/0022-0981(91)90113-B.
- Witherington BE, Bjorndal KA. 1991. Influences of wavelength and intensity on hatchling sea turtle phototaxis: implications for seafinding behavior. Copeia 4:1060–1069. doi:10.2307/1446101.
- Witherington BE, Martin RE. 2000. Understanding, assessing, and resolving light-pollution problems on sea turtle nesting beaches. In: Florida Fish and Wildlife Conservation Commission, Marine Research Institute, St. Petersburg, FL, 84 pp.
- Wyneken J, Salmon M, Fisher L, Weege S. 2000. Managing relocated sea turtle nests in open-beach hatcheries. Lessons in hatchery design and implementation in Hillsboro Beach, Broward County, Florida. In Proceedings of Nineteenth Annual Symposium on Sea Turtle Biology and Conservation. US Dept. Commence NOAA Tech. Memo. NMFS-SEFSC-443.
- Zar JH. 1999. Biostatistical analysis. Pearson Education India.