Abstract
Peptic ulcer disease is the greatest digestive disorder that has increased incidence and recurrence rates across all nations. Prangos pabularia (L.) has been well documented as a folkloric medicinal herb utilized for multiple disease conditions including gastric ulcers. Hence, the target study was investigation the gastro-protection effects of root extracts of Prangos pabularia (REPP) on ethanol-mediated stomach injury in rats. Sprague Dawley rats were clustered in 5 cages: A and B, normal and ulcer control rats pre-ingested with 1 % carboxymethyl cellulose (CMC)); C, reference rats had 20 mg/kg omeprazole; D and E, rats pre-supplemented with 250 and 500 mg/kg of REPP, respectively. After one hour, group A was given orally 1 % CMC, and groups B-E were given 100 % ethanol. The ulcer area, gastric acidity, and gastric wall mucus of all stomachs were determined. The gastric tissue homogenates were examined for antioxidant and MDA contents. Moreover, the gastric tissues were analyzed by histopathological and immunohistochemically assays. Acute toxicity results showed lack of any toxic effects or histological changes in rats exposed to 2 and 5 g/kg of REPP ingestion. The ulcer controls had extensive gastric mucosal damage with lower gastric juice and a reduced gastric pH. REPP treatment caused a significant reduction of the ethanol-induced gastric lacerations represented by an upsurge in gastric mucus and gastric wall glycoproteins (increased PAS), a decrease in the gastric acidity, leukocyte infiltration, positively modulated Bax and HSP 70 proteins, consequently lowered ulcer areas. REPP supplementation positively modulated oxidative stress (increased SOD, CAT, PGE2, and reduced MDA) and inflammatory cytokines (decreased serum TNF-α, IL-6, and increased IL-10) levels. The outcomes could be scientific evidence to back-up the folkloric use of A. Judaica as a medicinal remedy for oxidative stress-related disorders (gastric ulcer).
Keywords: Prangos pabularia, Stomach ulcer, Histology, Antioxidant enzymes, Acute toxicity
1. Introduction
Gastric ulcers are abundant gastrointestinal disorders that occur in nearly 10 % of the world population (Salari et al., 2022). Scientists have named PUD as the plague of the 19th and early 20th century due to its increased occurrence across the globe as a result of poor sanitation and overcrowding (Malfertheiner and Schulz, 2020). Stomach ulceration incidents are majorly due to disturbances in the mechanical balance between the constructive and destructive factors of gastric digestion. The defensive factors comprise gastric mucin and prostaglandin secretion, and blood flow, while destructive factors include gastric acid, pepsin secretion, and H. pylori. The gastric tissue damage in any layer can create serious physiological damage, resulting in a cascade of events including increased gastric acid secretion, releasing more reactive oxygen species, nitric oxide synthase, and generating lipid peroxidation. Gastric ulcers can also be an outcome of alcohol overuse, pathogenic infection, Non-steroidal anti-inflammatory drugs, refluxed bile salts, and stress (Woolf A and Rehman, 2023). Although the exact physiological mechanism of gastric ulcer is yet to be fully understood, the latest outcomes revealed many interacting factors associated with the severity and during of this disease, including genetics, the host immune system, and the environment (Gilani et al., 2022). Ethanol is the most commonly used alcohol to produce gastric ulcers in rats. Absolute ethanol oral delivery can create cell necrosis and vascular injury, consequently leading to ulceration and gastric lesions. The gastric damage of ethanol could be correlated with the increased production of hydroperoxy free radicals and superoxide anions, causing more lipid peroxidation and oxidative stress in the gastric tissues (Beiranvand, 2022).
The pharmaceutical industry has provided numerous chemical synthetics for treating gastric ulcers validated by the U.S. Food and Drug Administration (Das et al., 2018). Western medicinal protocol relies on triple treatment (proton pump inhibitor plus 2 antibiotics) to manage this disease. The therapeutic principle is to provide a defensive layer that covers the gastric mucosa and lowers gastric acid secretion. In the clinical management of gastric ulcers, lowering gastric acidity is one of the targets for designing stomach therapeutics (J. Huang et al., 2022). Omeprazole is the most well-known chemical synthetic that has been widely as a proton pump inhibitor in clinical practice regardless of its unsatisfactory and insufficient effects (Kangwan et al., 2014, Matuszczyk et al., 2021). Despite its therapeutic potential in ameliorating symptoms of gastric ulcers, which is reduction of acid secretion, omeprazole has been linked with numerous side effects Back, leg, or stomach pain (bleeding or crusting, blister, cloudy urine, burning and painful urination, and sores on the lips (Hamzeloo-Moghadam et al., 2021). Therefore, searching for better and safer alternatives as therapeutic agents for gastric ulcers become a major scientific mission in recent years (Al Batran et al., 2013, Jabbar, 2022, Jabbar et al., 2022a, Jabbar et al., 2022b, Jabbar et al., 2023a).
Traditional herbal medicine comprises major therapeutic solutions for numerous health issues including gastrointestinal diseases and gastric ulcers. Plants and their active ingredients have served as major natural resources for curing human illness in many countries for thousands of years (Ahmed, 2017, Jabbar et al., 2022c, Ullah et al., 2014). Scientists have found that the therapeutic efficacy of folkloric Chinese herbal medicine was higher compared to omeprazole in a 6 follow-up procedure for gastric ulcer patients without any recurrence records in cases ingested herbal medicines (Ling, 2018). Moreover, combination therapy of herbal medicine and omeprazole exhibited more therapeutic potential (effective rate and hemostasis time) than omeprazole alone for gastric ulcers (Xie et al., 2021). Natural products can have an inhibitory effect on the various inflammatory processes associated with gastric ulcer initiation; however, their utilization as pharmaceutically active agents has been continuously modified to reach optimum therapeutic action (Jabbar et al., 2022a, Jabbar et al., 2022b, Sidahmed et al., 2015). Chemical contents (terpenoids, alkaloids, and phenols) of herbal medicines are considered major contributors associated with their antioxidant potentials (increasing CAT, SOD, and PGE2 and decreasing lipid MDA levels) thereby avoiding oxidative stress, a crucial inflammatory mediator of the gastric ulcer (Jabbar, 2021, Jabbar, 2022, Salama et al., 2016).
Prangos species (Apiaceae family) is a well-documented plant utilized as spices and herbal medicine in Asia, especially in Iran, Turkey, and Iraq. Different plant parts and the isolated essential oil of Prango species are ingested as internal therapy and applied externally for various health purposes as well. The therapeutic potentials of these plants mainly include the amelioration of gastrointestinal disorders, but various other medicinal effects have been reported (Mottaghipisheh et al., 2020). Ethnobotanical records show that the extracts of P. pabularia roots have been utilized as curative agents for gastric problems and as stimulants by the people living in Hakary, Turkey (Kaval et al., 2014). Moreover, the Tajikistanian people have considered the fresh roots and fruits of P. pabularia (local names: Yugan) as medicinal agents for managing vitiligo because of its tonic action (Numonov et al., 2018). In Indian folkloric remedies, the roots and fruits of P. pabularia (local names: Komal, Kurangas) are ingested for their putative action as laxative, liver tonic, stimulant, diuretic, and carminative. Infusion from the roots is ingested to manage flatulence, and indigestion and treat menstrual cycle irregularities (Staff, 2014).
In recent years, researchers revealed numerous biological potentials of P. pabularia including, antimicrobial and cytokine inhibition (Tada et al., 2002), antioxidant (Kogure et al., 2004), antifungal (Banday, 2018), antibacterial (Banday et al., 2022), and antiproliferative actions (Zahri and Razavi, 2016). The essential oils of P. pabularia showed noticeable free radical quenching action and anti-diabetic, neuroprotective, and lipid-lowering (anti-obesity) actions (Bahadori et al., 2017). It is believed that the biological actions of P. pabularia are due to their phytochemical contents, phenolic, flavonoids, coumarins, terpenoids, and glycosides (Bahadori et al., 2017, Numonov et al., 2018, Tada et al., 2002). Roots of P. pabularia show significant enzymatic inhibition of PTP-1B (anti-diabetic action) based on molecular docking, which was linked with its phytochemical (coumarine) contents, namely 5-pentylcyclohexa-1,3-diene, menthone, 1-tridecyne, and osthole (Numonov et al., 2019). Moreover, root extracts of P. pabularia were reported as aphrodisiac agents because of their strong relaxation effect on mouse corpus cavernosum and they were correlated with its chemical potentials ((+)-Oxypeucedanin) in the modulation of the NO and H2S formation (Sevin et al., 2022).
Although several in vitro and in vivo studies highlighted the biological and therapeutic potentials of P. pabularia roots (Numonov et al., 2019, Staff, 2014, Zahri and Razavi, 2016), However, its toxicity and gastroprotective effects are yet to be explored. Thus, this work was undertaken to determine the tolerable safe dosage and the preclinical potentials of REPP as a gastroprotective agent to clarify its folkloric utilization in a scientific investigation.
2. Materials and methods
2.1. Plant collection
The aerial parts of Artemisia judaica L. were collected from Safeen Mountain, Shaqlawa, Erbil during spring 2022 and the authentication was done by the taxonomists in the Department of Biology, College of Education, Salahaddin University. The plant was dried in a shadow place at room temperature. An amount of 200 g plant parts was fine coarse for powder formation, backed to a 2 L conical flask with a stopper, and mixed with 250 mL of methanol and homogenized for fifteen minutes. The mixture was transferred into a dark glass bottle, the filtered supernatant was separated (0.2 mm) and ethanol was evaporated. The extract was kept in black containers for later investigation.
2.2. Animals
The experimental Albino male rats (180–200 g), aged between 7 and 8 weeks, were bought from the Animal House Unit, (Ethics form BIO/14/10/2022/M.A.A.), Cihan University-Erbil. Rats were kept in polypropylene cages (4 per cage) provided with sterilized paddy husk as bedding surface and were given pellet diet and water ad libitum. The animal handling was according international rules for animal laboratories set by Institutional Animal Care and Use Committee (IACUC) (Couto and Cates, 2019).
2.3. Acute toxicity test
The current toxicity procedure follows the guidelines set by national and international organizations for experimental and clinical studies (Ofori et al., 2021). Sprague Albino Dawley male rats (36) were bought from the Animal House Unit of Cihan University-Erbil and clustered (12 rats each) as follows: A, rats received 1 % CMC; B and C, rats ingested orally 2500 and 5000 mg/kg of REPP, respectively (A.A.J. Jabbar et al., 2023). A 24-hour fasting of rats was followed before the treatment. The animals were weighed regularly and the behavioral changes and response of the animals were continuously checked. At the end of the procedure, the animals were anesthetized with ketamine (87 mg/kg) and xylazine (12 mg/kg), whereby, blood was collected from intracardiac puncture for the biochemical evaluations. the orbital plexus for various hematological and biochemical analyses. The rats were sacrificed by cervical dislocation and their organs were obtained, analyzed for any gross pathology, and kept in 10 % formalin for later histological examination.
2.4. Gastric ulcer experiment
The animals were kept in cages (widespread net bottom) for adaptation purposes. Thirty male Sprague rats were clustered into 5 clusters (6 rats each):
A and B, rats were treated with an oral dosage of 1 % CMC.
C, rats supplemented with 20 mg/kg omeprazole in CMC (reference group).
D and E, rats dieted with 250 and 500 mg/kg of REPP, respectively.
After 60 min, all rats (except group A) received orally absolute alcohol (5 mL/kg) as an inducer of gastric ulceration. After another hour, all animals received anesthesia (ketamine (87 mg/kg) & xylazine 12 mg/kg), blood was withdrawn by an intracardial perforation, and then they were sacrificed. The dissected stomach was transferred into formalin for gross and histopathological examinations (Jabbar et al., 2022a, Jabbar et al., 2022b, Omar et al., 2017).
2.4.1. Gastric fluid acidity
The obtained stomachs were carefully opened from the bigger curvature for the gastric juice collection. The gastric fluid was centrifuged at 1008 g for 10 min and the separated supernatant was analyzed for hydrogen ion intensity by a pH meter (mEq/L) (Weilheim, Germany) and NaoH 0.1 N (Mariod et al., 2023).
2.4.2. Estimation of gastric wall mucus
The stomach samples were weighed and buffer washed (phosphate-buffered saline (PBS)) and glandular tissues of the stomach were prepared on clean slides for the microscopic examination. The gastric mucus amount was determined using an electrical balance (Lokman et al., 2022).
2.4.3. Estimation of ulcer areas
The stomach samples were buffer washed and the length and width (mm) of each hemorrhagic lesion of the gastric tissues were found by (10 × 10 mm = ulcer area [UA]) under a dissecting microscope (magnification = 1.8×). The sum of the area of the gastric lesions for each gastric specimen was relied on to determine the UA and the ulcer inhibition % (I %) is calculated as follows:
I% = UA control– UA treated ⁄ UA control × 100 (Al-Wajeeh et al., 2017, Rudra et al., 2022).
2.4.4. Preparation of gastric tissue homogenates
The gastric tissues were homogenized according to the previously published procedure (S. Li et al., 2022). The preparation of a homogenized tiny portion of the gastric glandular tissues was possible by using 50 mM PBS (pH = 7.2) at 4 °C with a sigma–aldrich homogenizer (Merck, Germany), after centrifugation at 2580 g for 15 min, the supernatants examined for the antioxidant and MDA contents (A. A. Jabbar et al., 2023).
2.4.5. Antioxidants of tissue homogenates
The supernatants of gastric tissue homogenates were evaluated for the antioxidants (SOD (E-BC-K020-M), CAT (E-AB-11036), PGE2 (E-EL-0034) and MDA (E-EL-0060) contents. Laboratory protocols were following the producer’s instructions (Elab Science, Wuhan, China) (Vuolo et al., 2022).
2.4.6. Histological analysis
Gastric sections (1–2 cm) were immediately transferred into a formalin container (10 %) at room temperature for 24 h. Then gastric tissues were underwent a tissue-processing procedure on a semi-automated tissue processing instrument (Leica, Solms, Germany). Finally, the gastric sections produced at 5 μm fixed on slides and deepened in Hematoxylin and Eosin stains. After washing, the slides were observed under light microscopic (Leica Rotation Microtome) (Mousa et al., 2019).
2.4.7. Gastric mucosal glycoprotein evaluation
The amount of gastric epithelial mucus secretion and modulation of either acidic or basic glycoprotein was found by staining gastric glandular tissues with PAS stain according to the previously mentioned protocols via Sigma (PAS) Kit (Merck, Germany). The slides were imaged by Image J software (Lokman et al., 2022).
2.4.8. Estimation of serum inflammatory cytokines
The obtained serum samples were analyzed for the amount of inflammatory cytokines by using a specialized ELISA kit (Merck, Germany). These procedure protocols were by the manufacturer’s instructions for the ELISA rat kits, TNF-α (RAB0479), IL-6 (RAB0311), and IL-0 (RAB0246-1KT). The strength of the inflammatory cytokine strength measurement was determined by using normal sanitized recombinant cytokines (Shareef et al., 2022).
2.5. Statistical analysis
The present data analysis was possible by using the IBM SPSS program, one-way analysis of variance (ANOVA), and Turkey’s post hoc estimation. The figures were designed by Graph-pad Prism version 9.0. Data were presented as Values of P < 0.05 and were labeled as significant.
3. Results
3.1. The toxicity trial
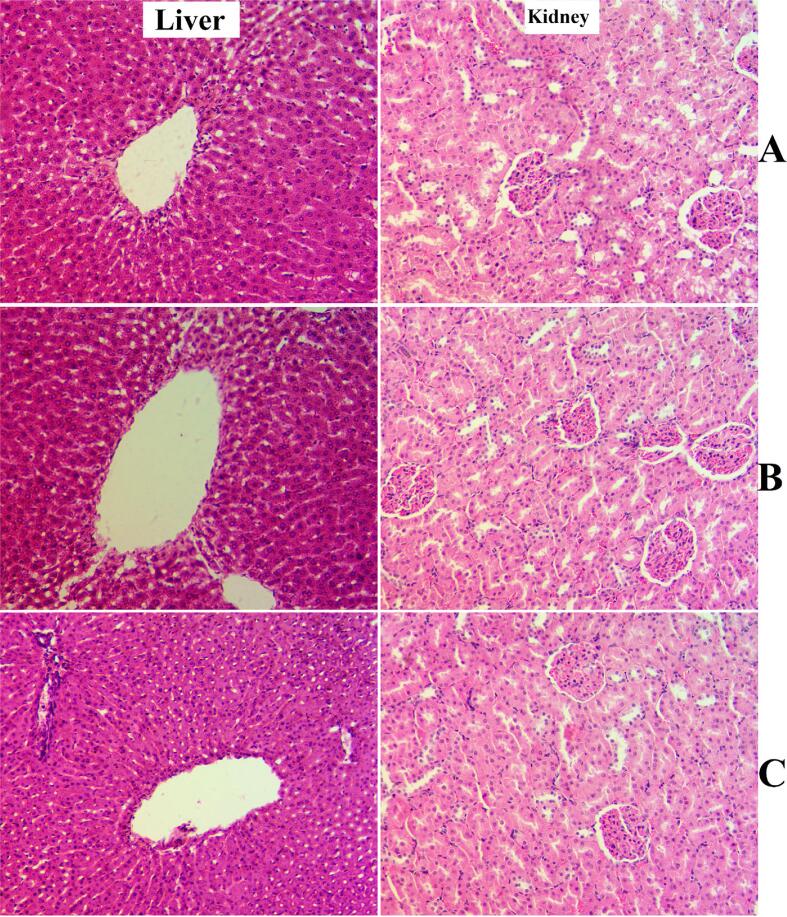
The current procedure showed that ingestion of 2 and 5 g/kg had no toxic effect or any noticeable behavioral changes in rats after 14 days’ trial. The consumed food and water by REPP-treated rats were very similar to that of normal controls. The two-week observational study did not detect any toxic signs and symptoms (dizziness, respiratory distress, tingling, burning sensation in tongue, throat, or skin; restlessness, muscular incoordination, vomiting, diarrhea, convulsions) without any mortality among rats supplemented orally with 2 and 5 g/kg of REPP. The histological analysis of obtained liver and kidney tissues showed similar tissue structure arrangement between normal control and REPP-ingested rats (Fig. 1). The biochemical investigation of serum samples from normal control rats and REPP-treated rats revealed non-significant modulation in different liver and kidney function tests (Data not shown can be provided on request).
Fig. 1.
Histology of liver and kidney in toxicity trial. A, rats ingested orally 1 % CMC; B, rats received 2 g/kg of REPP; C, rats received orally 5 g/kg of REPP. The three group rats showed comparable structure format of thei liver and kidney tissues (hematoxylin and eosin, 20x).
3.2. Gastric ulcer experiment
3.2.1. Results of gross morphology
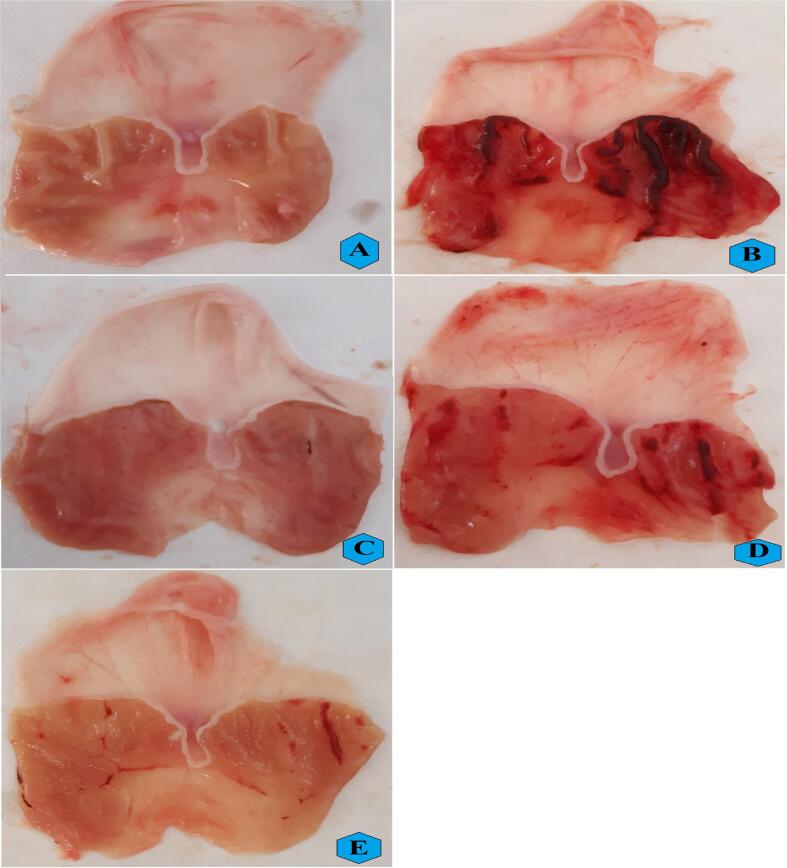
The present gross examination showed that normal controls (Fig. 2A) had normal tissue arrangement and usual stomach mucosa. Rats treated only with ethanol (Fig. 2B) experienced sever mucosal injury, hyperemia, hemorrhagic streaks, and numerous lager and deeper hemorrhagic lesion areas across mucosal layers (ulceration). Rats ingested with omeprazole (Fig. 2C) had smaller and shallower ulceration areas in their gastric mucosal layers compared to group B. The REPP-treated rats (2 and 5 g/kg) had reduced ulceration area and fewer ulcer patches on their stomach mucosal surface compared to vehicle rats (Fig. 2D and E).
Fig. 2.
Gross appearance of stomach mucosal surface of different treated rats A, normal controls; B, vehicle (ulcer) rats; C, ulcer + omeprazole-pre-treated rats; D and E, ulcer + 250 and 500 mg/kg of REPP-pretreated rats.
3.2.2. Effect of REPP on gastric juice volume, pH, ulcer area
The normal controls showed the highest amount of gastric juice (2.45 g) and gastric pH (6.47) as expected. The present study revealed a significantly (P > 0.05) lower gastric juice content (0.73 g) and gastric pH (3.82) in ulcer controls compared to treated rats. Moreover, the ulcer area was clearly and expectedly higher in group B rats (621.34 mm2) compared to that (121.45, 226.70, 151.28 mm2) of omeprazole or REPP -treated (250 and 500 mg/kg) rats, respectively. Rats ingested orally 20 mg/kg omeprazole had comparable gastric juice amount and gastric pH to that of normal controls. The REPP treatment (250 and 500 mg/kg) caused noticeable up-regulation of gastric juice and reduced gastric pH, which was significantly (P > 0.05) different from that of ulcer controls. Gastric pH was gradually up-regulated depending on the ingested dosage of REPP. Which, rats received 250 mg/kg REPP had elevated gastric pH compared to ulcer controls, but not as significant as the rats ingested 500 mg/kg REPP. Moreover, the inhibition percentage of ulceration was significantly (P > 0.05) reduced (80.45, 63.51, 75.64 %) in omeprazole or REPP (250 and 500 mg/kg)-treated rats, respectively, compared to ulcer controls (Table 1).
Table 1.
Effects of REPP on gastric parameters of rats.
| Animal groups | Mucus weight (g) | pH | Ulcer area (mm)2 | Inhibition (%) |
|---|---|---|---|---|
|
A |
2.45 ± 0.38a | 6.47 ± 0.30a | - | |
| B | 0.73 ± 0.21e | 3.82 ± 0.71e | 621.34 ± 9.20d | – |
| C | 1.92 ± 0.48b | 6.0 ± 0.14b | 121.45 ± 4.87a | 80.45 % |
| D | 1.87 ± 0.39d | 4.78 ± 0.39d | 226.70 ± 7.54c | 63.51 % |
| E | 1.78 ± 0.11c | 5.03 ± 0.29c | 151.28 ± 8.32b | 75.64 % |
Data revealed as Mean ± SEM (n = 6). Values were found non-significant at p < 0.05 and labeled with same within same column. A, normal controls received only 1 % CMC; B, Ulcer control had only ethanol; C, ulcer + 20 mg/kg omeprazole; D and E, ulcer + 250 and 500 mg/kg REPP, respectively.
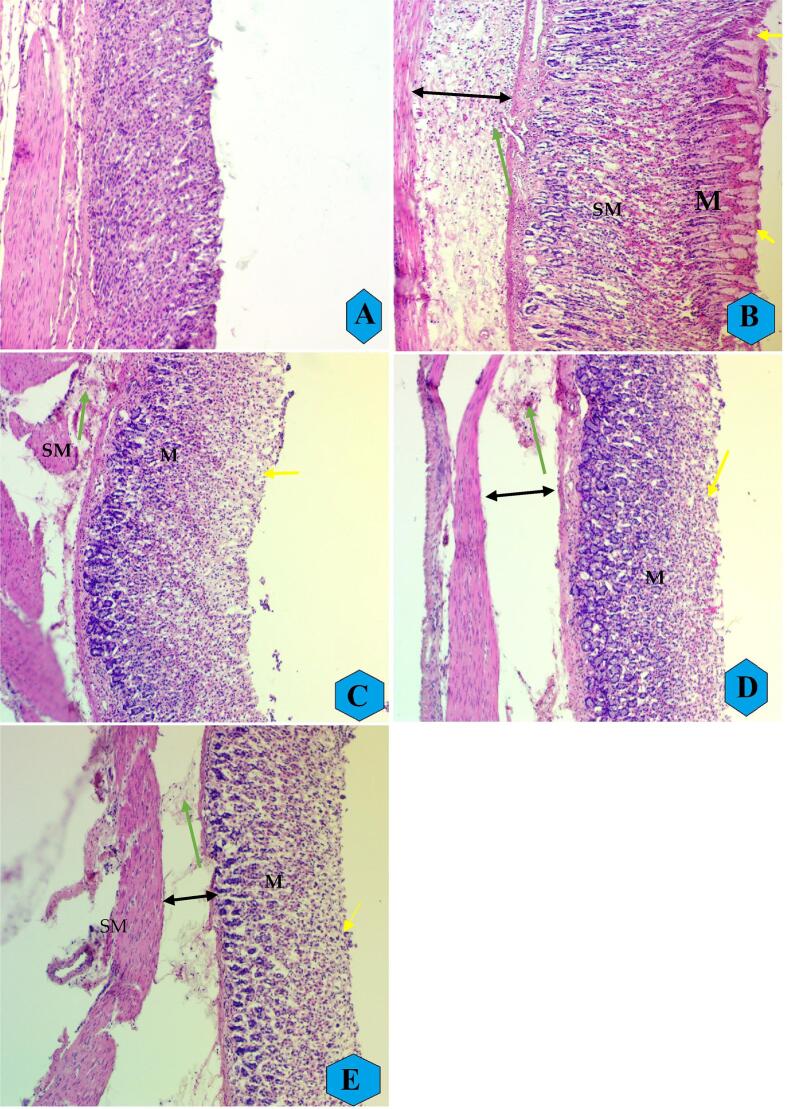
3.2.3. H & E stain
The histological results of gastric tissues showed normal tissue arrangement of gastric mucosal layers in normal controls (Fig. 3A) as usual. Ethanol ingestion caused significant gastric tissue penetration, ulceration, and a extensive edema in the mucosal and submucosal layers with leukocyte infiltration (Fig. 3B). The reference drug-treated rats had reduced gastric tissue damage represented by fewer mucosal injuries, lower submucosal edema, and reduced leukocyte infiltrations compared to ulcer controls (Fig. 3C). REPP treatment (250 and 500 mg/kg) formed a protective lower over the mucosal surface evidenced by lower lesion areas and gastric tissue penetrations with fewer leukocyte infiltration and reduced edema incidence compared to that of ulcer controls (Fig. 3D and E).
Fig. 3.
The microscopic appearance of stomach tissues in different experimental rats. A, normal controls showed usual tissue structure of their gastric mucosal layer; B, ulcer controls experienced numerous gastric tissue penetrations, mucosal edema, and leukocyte infiltration; C, reference rats (C), had a mild gastric tissue damage and fewer mucosal penetration compared to ulcer controls; rats ingested orally REPP (250 and 500 mg/kg) had moderately gastric tissue penetrations and significantly lower edema and reduced leukocyte infiltration rate compared to ulcer controls (H & E stain, 10x). Yellow arrows show of ulcer areas and focal erosion, double-headed black arrows reveals submucosal edema, and green arrows show leucocytes infiltration. M: mucosa; SM: submucosa.
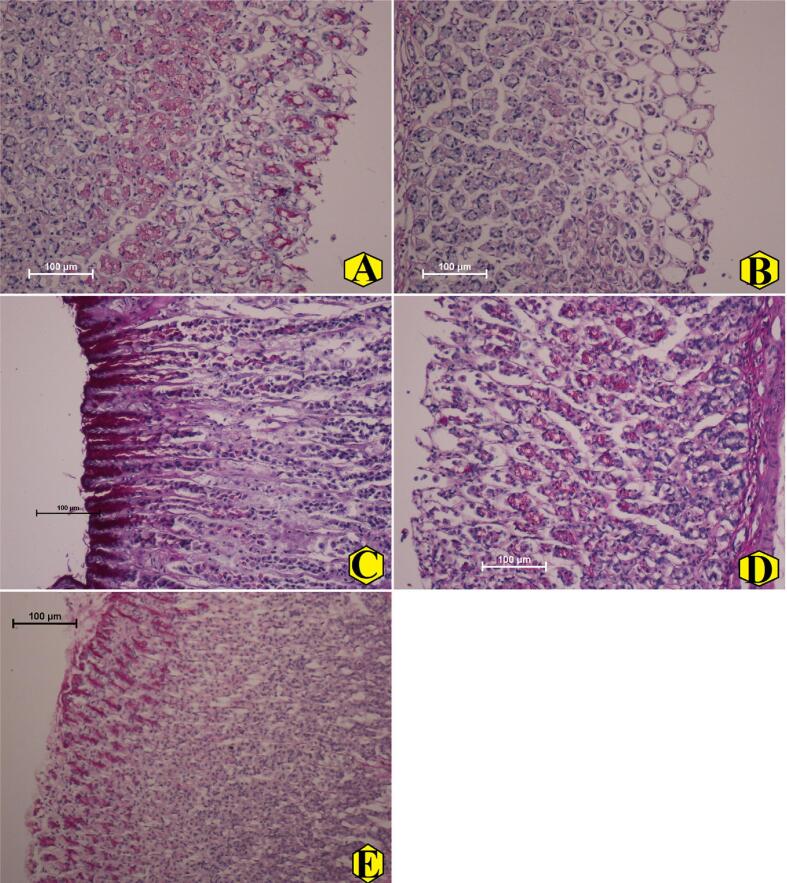
3.2.4. AME effects on PAS stain intensity
The present data revealed that normal controls had increased expression of PAS stain as expected (Fig. 4A). The ethanol ingestion caused negative modulation of the glycoproteins, glycolipids and mucins in gastric tissues represented by significantly lower expression of PAS stain in the gastric epithelial layers of ulcer controls compared to other treated rats (Fig. 4B). The reference drug or REPP-pre-supplemented rats showed higher PAS intensity in their epithelial tissues, which indicates more goblet cell hyperplasia and mucus content comparable to that of ulcer controls (Fig. 4C-E).
Fig. 4.
The microscopic views of gastric tissues expressing different levels of PAS stain in the gastric layers in rats. A, normal control showed efficient amount of PAS stain in their gastric tissues; B, ulcer controls revealed reduced expression of PAS, indicating lower glycoprotein and mucin content (magenta appearance); C, omeprazole-treated rats showed increased PAS stain in their gastric tissues; (D), rats treated orally with 250 mg/kg REPP had mild to moderate gastric mucosal damage with mild PAS intensity; (E), rats supplementation with 500 mg/kg REPP caused increased the mucin and glycoprotein content in gastric tissues evidenced by higher PAS expression in their stomach mucosal tissues compared to that of ulcer controls (PAS stain magnification 20×).
3.2.5. Effect of REPP on the expression of immunohistochemical proteins (Bax and HSP 70)
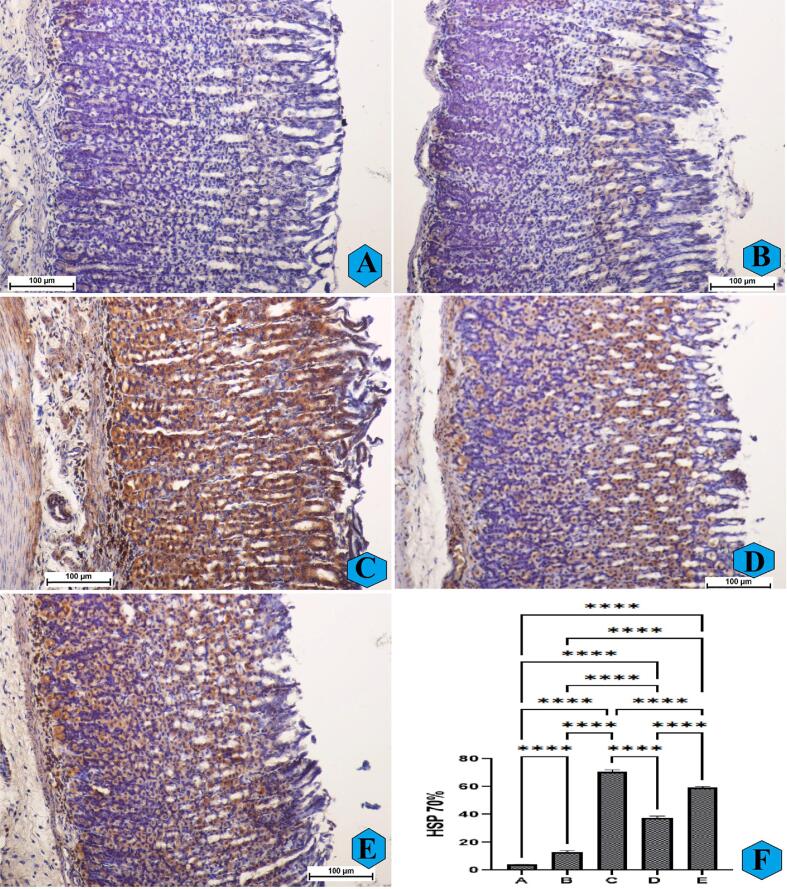
HSP 70 has been well-known low-molecular weight protein involved in the posttranslational recognition of polypeptides acting as protective protein under stress conditions (Fig. 5A). The histological analysis revealed that very reduced expression of HSP 70 in normal controls. The ulcer controls exposed to absolute ethanol had reduced HSP 70 intensity in their gastric tissues (Fig. 5B). The intensity of HSP 70 protein was up-regulated (intense brown color-stained antigen) in rats pretreated with omeprazole or REPP (250 and 500 mg/kg) compared to ulcer controls, denoting a cytoprotective effect of these treatments against ethanol-induced stress conditions (Fig. 5C-E).
Fig. 5.
The microscopical observation of gastric tissues expressing different intensity of HSP 70 protein expression (A–E) and quantitative analysis (F) of HSP 70 protein in rats. A and F, normal controls had normal structure of their gastric mucosa and very reduced expression of HSP 70 protein in their stomach tissues; B and F, ulcer controls experienced sever gastric tissue damage and a decreased expression of HSP 70 intensity in their stomach tissues; C and F, omeprazole-treated rats showed mild gastric tissue damage and an elevated HSP 70 protein expression in their gastric mucosa. D and E, rats ingested with REPP (250 and 500 mg/kg, respectively) had mild to moderate gastric tissue injury and an increase in the HSP 70 protein concentration in their stomach mucosa compared to ulcer controls (HSP 70 stain, magnification 20×). ****, p < 0.0001.
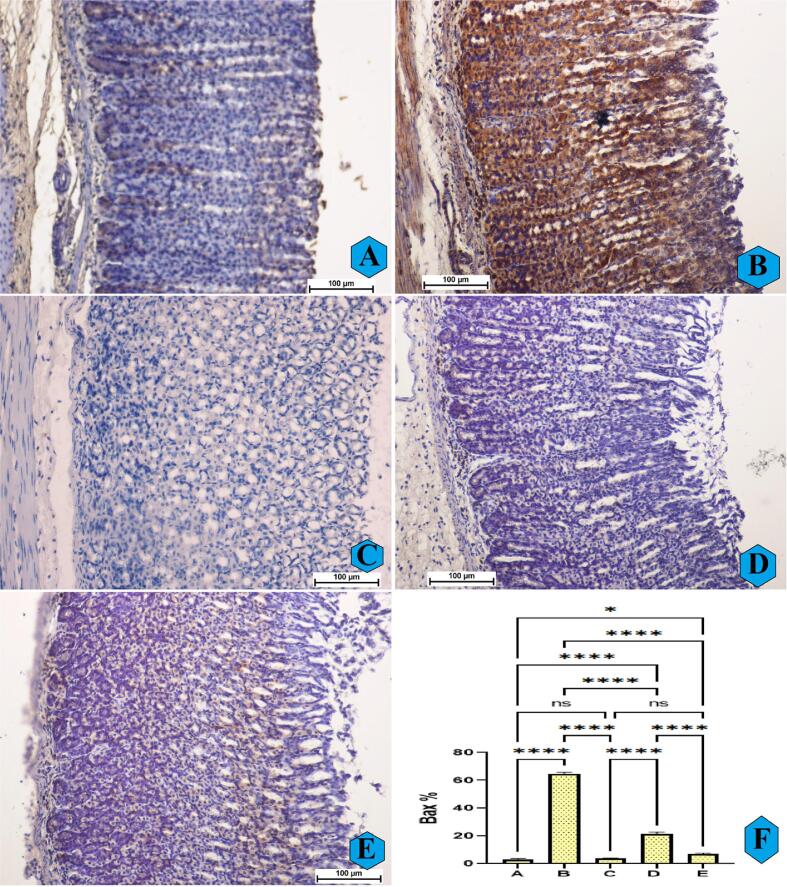
The immunohistochemical results showed reduced expression of pro-apoptotic Bax proteins in normal control rats (Figur 6A). The Bax protein expression was noticeably higher in ulcer controls compared to normal controls, indicating clear apoptotic process in the gastric tissues. Rats ingested with omeprazole or REPP (250 and 500 mg/kg) had signifcantly (p < 0.0001) down-regulated Bax protein accumulations, denoting their protective and anti-apoptotic actions (Fig. 6C-E).
Fig. 6.
The microscopic observation of Bax protein appearance (A–E) and quantitative analysis (F) of Bax protein expression in rats. A and F, normal controls had normal stomach tissue layers and very reduced Bax protein intensity; B and F, ulcer controls experienced severe stomach tissue injury and an increase in the Bax protein expressions; C and F, omeprazole-treated rats had mild gastric mucosal injury and reduced Bax protein compared to ulcer controls; D and E, rats pre-supplemented with REPP (250 and 500 mg/kg) rats had moderate gastric tissue damage and a decrease in the Bax protein expression (Bax stain, magnification 20×). REPP treatment caused a down-regulation of a pro-apoptotic Bax protein that prevented further gastric tissue damage. ns, non-significant; *, p < 0.05; ****, p < 0.0001.
3.2.6. Effects of REPP on gastric antioxidants and MDA content
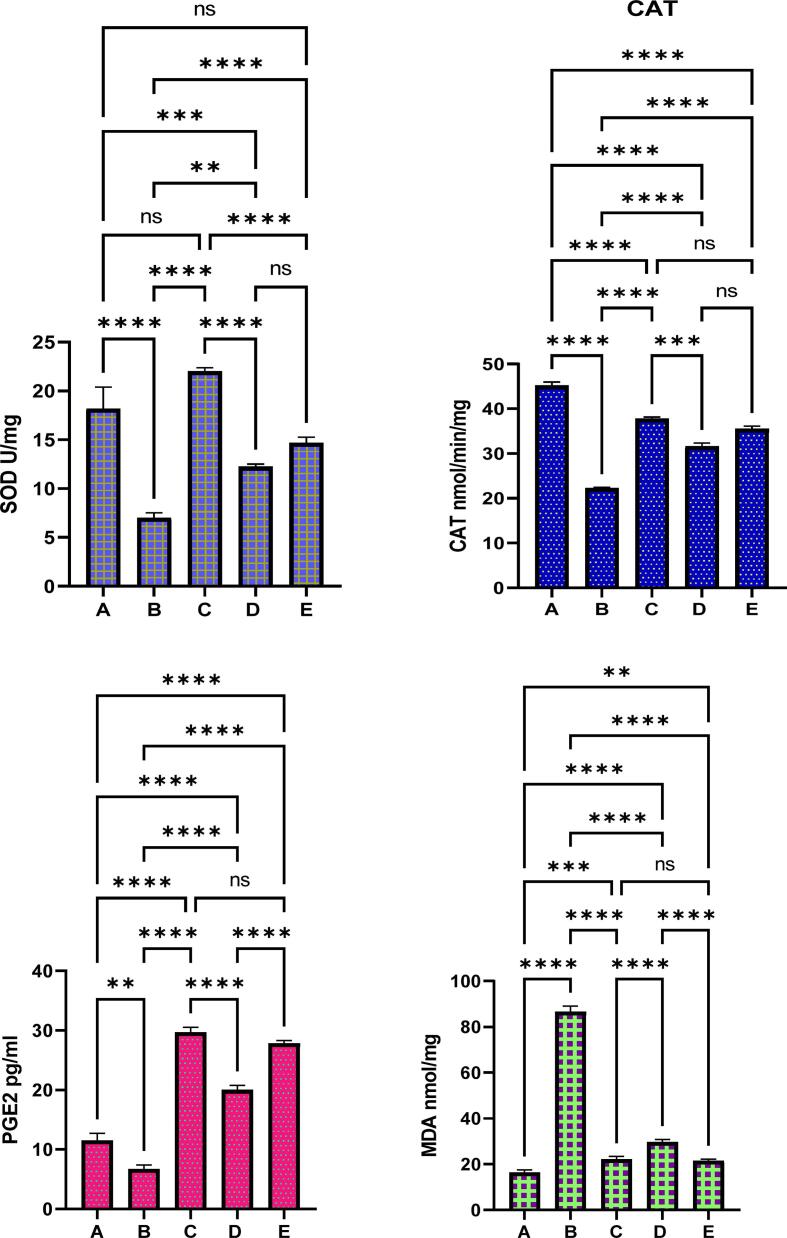
The biochemical results showed normal values of antioxidant and MDA contents in normal controls. The ulcer controls showed significantly (p < 0.05) lowest endogenous antioxidants (SOD, CAT, and PGE2) compared to reference (C) or REPP-treated rats (D and E) (Fig. 7B). Rats ingested orally omeprazole drug showed reasonable amount of antioxidant enzymes, which were very comparable to that of normal controls. REPP treatment caused positive modulation of gastric endogenous antioxidants compared to ulcer controls. Rats received 250 and 500 mg/kg REPP showed higher SOD (12.24 and 14.67 U/mg, respectively), CAT (31.57 and 35.48 nmol/min/mg, respectively), and PGE2 (20 and 27.81 pg/ml, respectively) levels compared to that that (SOD, 7 U/mg; CAT, 22.27 nmol/min/mg; PEG3, 6.65 pg/ml) of ulcer controls. The present results showed increased lipid peroxidation levels in ulcer controls represented by elevated MDA content compared to all other treated rats. Rats ingested omeprazole or REPP (250 and 500 mg/kg) had significantly (p < 0.01) lower MDA values (22.13, 29.60, 21.53 nmol/mg, respectively) compared to that (86.64 nmol/mg) of ulcer controls (Fig. 7B-E).
Fig. 7.
The gastric antioxidants and MDA contents in different experimental rats. A, normal controls received only 1 % CMC; B, Ulcer control had only ethanol; C, ulcer + 20 mg/kg omeprazole; D and E, ulcer + 250 and 500 mg/kg REPP, respectively. ns, non-significant; *, p < 0.05; **, p < 0.001; ***, p < 0.001; ****, p < 0.0001.
3.2.7. Effect of REPP on inflammatory cytokines
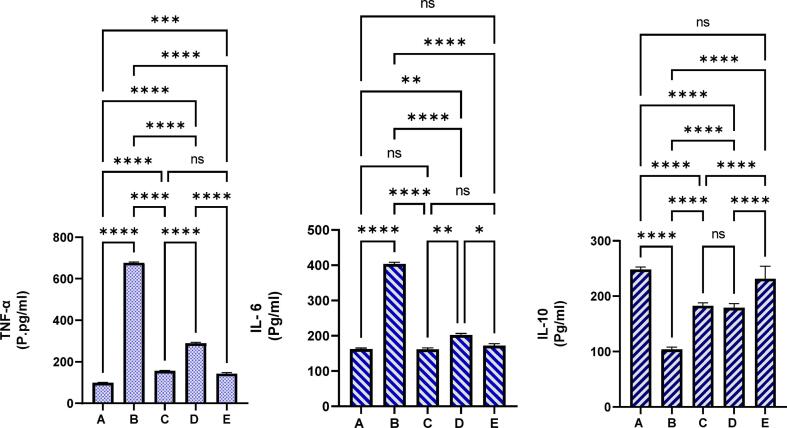
The present results showed immunomodulatory actions of REPP supplementation in ethanol-mediated ulcer in rats. The normal controls had lower TNF-α, IL-6 levels and higher IL-10 cytokines in their serum samples. Rats received orally 20 mg omeprazole had significant (p < 0.001) down-regulation of pro-inflammatory cytokines (TNF-α, IL-6) and an elevated anti-inflammatory cytokines (IL-10) than in the ulcer controls. The present supplementation of REPP (250 and 500 mg/kg) led to noticeable reduction in the TNF-α (289.0, 142.80P.pg/ml, respectively), IL-6 (202.10, 172.18 pg/ml, respectively) levels and an increase in the IL-10 levels (179.21, 231.73 pg/ml, respectively) compared to that (676.1P. pg/ml, 403.0, 102.3 pg/ml, respectievely) of ulcer controls (Fig. 8).
Fig. 8.
The influence of REPP on the inflammatory cytokines in experimental rats. A, rats received only 1 % CMC and distal water; B, rats received 1 % CMC and absolute ethanol; C, rats pre-ingested with omeprazole (20 mg/kg) and given absolute ethanol; D and E, rats pre-ingested with 250 and 500 mg/kg of REPP and were given absolute ethanol. REPP treatment caused positive immunomodulatory effect represented by lower TNF-α and IL-6 and significantly higher IL-10 cytokines compared to that ulcer controls. REPP supplementation caused positive modulation of inflammatory parameters in the serum of ethanol-mediated stomach ulceration. ns, non-significant; *, p < 0.05; **, p < 0.001; ***, p < 0.001; ****, p < 0.0001.
4. Discussion
Scientist have evaluated the toxic effects of herbal plants on animal physiology and survival by implementing regulatory guidelines for acute toxicity, providing recommendations for their tolerable dosage, and avoiding undesirable modifications (A.A.J. Jabbar et al., 2023). The present toxicity evaluation showed the absence of toxic signs or death in rats ingested single oral dosages of 2 and 5 mg/kg REPP, denoting a toxic dosage of this herb would be more than 5 mg/kg. Similarly, previous researchers have shown the non-toxic effect and strong relaxation effects of P. pabularia extract (10−7-10−4 g/mL) on mouse corpus cavernosum (Sevin et al., 2022). Accordingly, literature data reported the non-toxic effects of P. pabularia extracts based on various experimental studies (Mottaghipisheh et al., 2020).
Stomach ulceration is a well-documented peptic ulcer that commonly occurs in clinical practice but the etiology of the majority of gastric ulcer cases is shrouded in mystery. It has, however, been found that stomach ulcers are an outcome of an imbalance between destructive and maintenance factors of gastric mucosal layers through different endogenous pathways (Shady et al., 2022). The defense lines of the stomach can be weakened and penetrated by chemicals (alcohol) by creating lesions in the stomach epithelium and manipulating the permeability of vascular layers (edema). The gastric lesions can be initiated by alcohol (ethanol) through different mechanisms, lowering gastric motility, decreasing gastric pH, and reducing mucus and bicarbonate secretions (Jabbar et al., 2022a, Jabbar et al., 2022b). The current procedure showed that oral delivery of absolute ethanol caused a significant reduction of the gastric mucosal layers, lowered gastric juice release, and decreased endogenous antioxidants of gastric tissues. As a consequence, ethanol ingestion created severe gastric lesions, increased acid secretion, and interrupted the stomach epithelial layers as well as numerous hemorrhagic and ulcer areas. Similar effects of ethanol ingestion on the gastric mucosal and submucosal layers in different rat trials (Jabbar et al., 2022a, Jabbar et al., 2022b, Nazarbahjat et al., 2016). The current supplementation of rats by REPP caused a noticeable reduction of the gastric tissue damage mediated by ethanol. Rats Pre-treated with REPP had higher mucosal surface area and flattened linings of the mucosa, as a result, less gastric injury formed compared to that of ulcer controls.
Accordingly, previous investigations revealed the gastroprotective roles of medicinal plants and their natural products and they have been linked with the plant's phytochemical potentials in the positive modulation of various physiological processes (Hama Amin and Aziz, 2022, Ibrahim et al., 2016). Moreover, the current study revealed lower ulcer area (index) and total ulcer score in REPP-treated groups were very comparable to reference groups (omeprazole). There was also a comparable rate of hemorrhagic lesion incidence between REPP (500 mg/kg) and omeprazole-treated rats. The current histological evaluation showed less ulcerative area, ulcer index, and total ulcer scores in REPP-treated rats, which were very comparable to reference rats (omeprazole). There was also a comparable amount of gastric ulceration and gastric lesion between REPP and omeprazole-treated rats. This outcome could be linked with the REPP phytochemicals, mainly coumarins (furocoumarin) and γ-pyrone, which was repeatedly reported as gastroprotective agent (isolated from other herbal species) because of its biological potentials in modulating various defensive factors of gastric tissues (Cruz et al., 2020, Pavlović et al., 2022, Razuvaeva et al., 2023).
The present gastroprotection effect of REPP against ethanol-mediated ulcers could be also linked with the potential in lowering cellular permeability of the stomach submucosal layers, up-regulating gastric juice secretion, and reduced leukocyte infiltration, thereby lowering inflammation rates. Similarly, researchers have linked the anti-ulcer action of coumarin (the main REPP chemical) with its positive regulation of the cellular signaling and biological pathways involved in the formation and reduction of stomach ulcers, including inflammation, oxidative stress, apoptosis, tumors, and angiogenesis (Cruz et al., 2020, Fahmi et al., 2019).
In the PAS staining expression, an estimator of mucopolysaccharide secretion which is one of the main components of the gastric mucus. The current data revealed that REPP supplementation amplified the glycoprotein content, a defensive barrier of gastric tissues against various epithelial damaging factors (toxins, microbes, gastric acids). According to protocols of the PAS technique, the higher PAS stains expressed in the stomach tissues indicate the presence of higher gastric mucus secretions. In the current procedure, ulcer controls had significantly reduced PAS stain appearance in their gastric tissues, while omeprazole or REPP-treated rats had up-regulated PAS stain expression as an indication of glycoprotein content that creates a protective barrier and lowers gastric damage. These modulations of gastric defense factors could be linked with REPP phytochemical (coumarins), which were following the previous outcomes regarding the gastroprotective role and positive regulations of mucopolysaccharides in different rat trials (Abd-Alla et al., 2022, Albaayit et al., 2016, Sidahmed et al., 2019).
Apoptosis is a major contributor to lowering gastric tissue damage induced by different intrinsic and extrinsic factors. Hsp 70, a heat shock protein family, is ubiquitously found in mammalian cells and this protein level increased in response to stress (oxidative stress) because it mainly preserves the functional structure of tissue proteins while rebuilding or eliminating denatured proteins. Ethanol ingestion caused hemorrhagic mucosal damage, generating ROS formation and lowering Hsp 70 proteins, thereby making gastric mucosa layers more vulnerable to ulcerative injuries (Fahmy et al., 2020, Saremi et al., 2020). The present immunohistochemically evaluation of anti-apoptotic protein (Hsp 70) in the gastric tissues showed decreased and increased expressions in ulcer control and REPP (500 mg/kg)-treated, respectively. The Bax protein is a pro-apoptotic protein and a member of the Bcl-2 family that is associated with the regulation of apoptosis in mitochondrial damages. Alcohol overuse (absolute ethanol) can have a stimulatory effect on the apoptosis process and stomach injury by increasing the expression of pro-apoptotic (Bax) proteins, which later down-regulates the expression of anti-apoptotic proteins (Bcl-2). Our results were inconsistent with the previous outcomes that revealed significant potentials of the coumarin (the main chemical component of REPP) in the positive modulation of immunohistochemical (increasing Hsp 70 and decreasing Bax) proteins, thereby strengthening the defense systems of the gastric mucosa against oxidative stress-mediated tissue injury (Aas et al., 2015, Fouman-Ajirlou et al., 2020, Song et al., 2022, Zheng et al., 2022).
Oxidative stress is considered as an imbalance between the rate of ROS formation and elimination in tissues. Oxidative stress can accelerate gastric mucosal damage and enhance further tissue injury because under stress conditions (ethanol ingestion) gastric tissues generate increased production of detrimental free radicals and insufficient or imbalance in the gastric endogenous antioxidants can be part of the development and pathogenesis of gastric ulcers (Moawad et al., 2019). MDA is a well-documented secondary molecule of polyunsaturated fatty acids to peroxidation and is the main indicator for the evaluation of lipid peroxidation in tissues (Ibrahim et al., 2016). In the current work, oral ingestion of ethanol caused significant oxidative stress in ulcerative rats (positive controls) represented by decreased endogenous antioxidants (SOD, CAT, and PEG2) and increased MDA contents in gastric tissue homogenates. While rats pre-treated with omeprazole or REPP had significantly higher antioxidant enzymes and lower MDA values in gastric tissues. The current outcomes validate increased antioxidant potentials of REPP that can actively scavenge free radicles to lower ratś gastric mucosa against ethanol-mediated injury. Accordingly, previous data showed significant antioxidant potentials of coumarin (a major REPP chemical) found in different plant species, which was considered as one of the molecular mechanisms behind its biological (anti-ulcer) potentials (Pavlović et al., 2022, Razuvaeva et al., 2023, Serrano-Román et al., 2023).
Lipid peroxidation byproducts can be lipid peroxyl radicals and hydroperoxides that cause further oxidative stress and the initiation of tissue inflammation leading to attenuation of the gastric defense lines (antiradical enzymes) (A.A. Jabbar, Abdullah, Hassan, et al., 2022). Scavengers including phase 2 antioxidant and cellular protection genes [NQO1 (NAD(P)H quinone oxidoreductase 1), and HO-1 (heme oxygenase-1)], were stimulated by Nrf2 (nuclear factor erythroid-2-), has been labeled as strong reductants of pro-inflammatory and oxidants (Liu et al., 2022). The dimerization of Nrf2 with Maf proteins normally occurs in the cell nuclei, thereby forming radical scavengers to start a new round of transcription, which could be inhibited through different genetic pathways (NQO1 and HO-1) as previously declared (Liu et al., 2022). The dimerization of Nrf2 with Maf proteins normally occurs in the cell nuclei, thereby forming radical scavengers to start a new round of transcription, which could be inhibited through different genetic pathways (NQO1 and HO-1) as previously declared. While, the NF-Κb (nuclear Factor-kappa-light-chain-inducer of B cells) signaling mechanism has been recognized as a strong inhibitor of the antioxidant enzymes due to the suppressed effect on the Nrf2-Keap1 pathway by stimulating various genes (Keap1 and the p65) (Motohashi and Yamamoto, 2004). Therefore, scientists continuously searching for natural products that could modulate the Ndf2 because of its biological roles in the expression of cellular defensive genes (HO-1, NQO1, and GCLC) and enhancement of the immune system (Hasanvand et al., 2018). The present free radical quenching potentials of REPP could be through the induction of anti-radical genes mediated by the Nrf2 signaling pathway. Similarly, previous studies found significant antioxidant potentials of Prangos pabularia in different in vivo and in vitro trials, which were linked with its phytochemical contents (furanocoumarin) (Banday et al., 2022, Kogure et al., 2004, Sevin et al., 2022). A very recent study revealed that coumarin can be utilized as an active ingredient in the production and development of intestinal anti-inflammatory drugs due to its significant potential in the positive modulation of Nrf2 pathways, thereby lowering apoptosis and inflammation rates (Di Stasi, 2023).
NF-κB is a transcription factor that modulates various biological processes, the induction of NF- κB requires phosphorylation of the suppressors of the κB (IκB) kinases (IKK) complex. The later complex phosphorylated the IκB molecules and created proteasomal denaturation of IκB and nuclear translocation of NF-κB (Kumar et al., 2020), consequently increasing the secretion of pro-inflammatory cytokines (TNF-α and IL6). During stomach ulceration, macrophages generate an increased amount of TNF-α that halts the curing process by many mechanisms, inhibition of gastric microcirculation, induction of neutrophil infiltration, and stimulates inflammatory mechanisms, including the formation of other inflammatory cytokines and NF-κB activation, up-regulating its production. IL-6 is a well-known pro-inflammatory cytokine associated with acute inflammation by triggering neutrophils, macrophages, and lymphocytes at the inflammatory site, stimulating further inflammatory mediators, and amplifying gastric mucosal damages (Mariod et al., 2023). On the other hand, IL-10 is an anti-inflammatory cytokine that decreases the Th1 cytokine secretion, reduces expression of MHC class II antigens, and co-activator molecules on macrophages. IL-10 facilitates B cell survival, cell proliferation, antibody generation, and suppression of NF-κB pathway, thereby lowering the incidence of oxidative stress-related gastric ulcers (W.-S. Li et al., 2021). The current results showed ulcer controls had increased levels of TNF-α and IL-6 cytokines, while reduced IL-10 levels in blood plasma. REPP supplementation caused positive modulation of inflammatory cytokines shown by lower TNF-α, IL-6, and higher IL-10 cytokine levels compared to ulcer controls. Consistently, numerous researchers have shown the anti-inflammatory potentials of furocoumarin (a major constituent of REPP), which were detected in other plant species (Agour et al., 2022, Balkrishna et al., 2022, Huang et al., 2022a).
5. Conclusion
The gastro-protective effects of REPP in ethanol-induced ulcers in rats were investigated by different histopathological methods for the first time. Acute toxicity trials revealed non-toxic signs and symptoms in rats exposed to REPP even after two trials. REPP-treated rats had fewer gastric lesions and ulcer index anti-ulcer compared to ulcer controls based on the estimation of gastric mucus content, PAS expression, and gastric tissue anti-radical enzymes. REPP supplementation increased the SOD, CAT, and PGE2, and decreased MDA levels in the gastric tissues. Moreover, rats pre-ingested with REPP had lower pro-inflammatory and higher anti-inflammatory cytokines. This biological actions of REPP could be due to its phytochemicals (coumarins) that have already been validated as active compounds that can lower free radicals and ROS formation by activating antioxidant response–based genes facilitated by the Nrf2 mechanism, thereby lowering tissue damage (gastric ulcers) oxidative stress. The present data might serve as a viable ground source for the generation of new alternative medicine for gastric ulcers.
CRediT authorship contribution statement
Ahmed A.J. Jabbar: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Ramzi A. Mothana: Data curation, Formal analysis, Funding acquisition, Writing – review & editing. Mahmood Ameen Abdulla: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. Fuad Othman Abdullah: Resources. Khaled Abdul-Aziz Ahmed: Data curation, Formal analysis, Project administration. Rawaz Rizgar Hussen: Project administration. Mohammed F.Hawwal: Resources. Omer I.Fantoukh: Project administration. SidgiHasson: Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to Researchers Supporting Project number (RSP2023R119), King Saud University, Riyadh, Saudi Arabia for funding this work.
Footnotes
Peer review under responsibility of King Saud University
Contributor Information
Ahmed A.J. Jabbar, Email: ahmed.abuljabbar@epu.edu.iq.
Ramzi A. Mothana, Email: rmothana@ksu.edu.sa.
Mahmood Ameen Abdulla, Email: mahmood.ameen@cihanuniversity.edu.iq.
Fuad Othman Abdullah, Email: fuad.abdullah@su.edu.krd.
Khaled Abdul-Aziz Ahmed, Email: k.ahmed@ammanu.edu.jo.
Rawaz Rizgar Hussen, Email: rawaz.hassan@knu.edu.iq.
Mohammed F. Hawwal, Email: mhawwal@ksu.edu.sa.
Omer I. Fantoukh, Email: ofantoukh@ksu.edu.sa.
Sidgi Hasson, Email: s.s.hasson@ljmu.ac.uk.
References
- Aas Z., Babaei E., Feizi M.A.H., Dehghan G. Anti-proliferative and apoptotic effects of dendrosomal farnesiferol C on gastric cancer cells. Asian Pacific J. Cancer Prev. 2015;16(13):5325–5329. doi: 10.7314/apjcp.2015.16.13.5325. [DOI] [PubMed] [Google Scholar]
- Abd-Alla H.I., Ibrahim Fouad G., Ahmed K.A., Shaker K. Alloimperatorin from Ammi majus fruits mitigates Piroxicam-provoked gastric ulcer and hepatorenal toxicity in rats via suppressing oxidative stress and apoptosis. Biomarkers. 2022;27(8):727–742. doi: 10.1080/1354750X.2022.2102213. [DOI] [PubMed] [Google Scholar]
- Agour A., Mssillou I., Es-Safi I., Conte R., Mechchate H., Slighoua M., Amrati F.-E.-Z., Parvez M.K., Numan O., Bari A. The antioxidant, analgesic, anti-inflammatory, and wound healing activities of Haplophyllum tuberculatum (Forsskal) A. Juss Aqueous and Ethanolic Extract. Life. 2022;12(10):1553. doi: 10.3390/life12101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H.M. Traditional uses of Kurdish medicinal plant Pistacia atlantica subsp. kurdica Zohary in Ranya, Southern Kurdistan. Genet. Resour. Crop Evol. 2017;64(6):1473–1484. doi: 10.1007/s10722-017-0522-4. [DOI] [Google Scholar]
- Al Batran R., Al-Bayaty F., Abdulla M.A., Al-Obaidi M.M.J., Hajrezaei M., Hassandarvish P., Fouad M., Golbabapour S., Talaee S. Gastroprotective effects of Corchorus olitorius leaf extract against ethanol-induced gastric mucosal hemorrhagic lesions in rats. J. Gastroenterol. Hepatol. 2013;28(8):1321–1329. doi: 10.1111/jgh.12229. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Albaayit S.F.A., Abba Y., Abdullah R., Abdullah N. Prophylactic effects of Clausena excavata Burum. f. leaf extract in ethanol-induced gastric ulcers. Drug Des. Devel. Ther. 2016;10:1973–1986. doi: 10.2147/DDDT.S103993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Wajeeh N.S., Hajrezaie M., Al-Henhena N., Kamran S., Bagheri E., Zahedifard M., Saremi K., Noor S.M., Mohd Ali H., Abdulla M.A. The antiulcer effect of Cibotium barometz leaves in rats with experimentally induced acute gastric ulcer. Drug Des. Devel. Ther. 2017;11:995–1009. doi: 10.2147/DDDT.S107018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bahadori M.B., Zengin G., Bahadori S., Maggi F., Dinparast L. Chemical composition of essential oil, antioxidant, antidiabetic, anti-obesity, and neuroprotective properties of Prangos gaubae. Nat. Prod. Commun. 2017;12(12) 1934578X1701201233. [Google Scholar]
- Balkrishna A., Arya V., Sharma I.P. Anti-Cancer and Anti-Inflammatory Potential of Furanocoumarins from Ammi majus L. Anti-Cancer Agents Med. Chem. (formerly Curr Med Chem Agents) 2022;22(6):1030–1036. doi: 10.2174/1871520621666210824113128. [DOI] [PubMed] [Google Scholar]
- Banday J.A., Yatoo G.N., Hajam M.A., Bhat S.A., Santhanakrishnan V.P., Farozi A., Rather M.A., Rasool S. Gas chromatographic-mass spectrometric analysis, antioxidant, antiproliferative and antibacterial activities of the essential oil of prangos pabularia. Microb. Pathog. 2022;166 doi: 10.1016/j.micpath.2022.105540. [DOI] [PubMed] [Google Scholar]
- Banday, J.A., 2018. Preparation, Characterization and Antimicrobial and Antifungal activities of 7-Methoxy-8-{(3, 3-dimethyloxiran-2-yl) methyl}-2H-chromen-2-one, an Analogue of Osthol. Extraction.
- Beiranvand M. A review of the most common in vivo models of stomach ulcers and natural and synthetic anti-ulcer compounds: A comparative systematic study. Phytomedicine Plus. 2022;2(2) doi: 10.1016/j.phyplu.2022.100264. [DOI] [Google Scholar]
- Couto, M., Cates. C., 2019. Laboratory guidelines for animal care. Vertebr Embryog Embryol Cell Genet Methods, 407–430. [DOI] [PubMed]
- Cruz L.F., de Figueiredo G.F., Pedro L.P., Amorin Y.M., Andrade J.T., Passos T.F., Rodrigues F.F., Souza I.L.A., Gonçalves T.P.R., dos Santos Lima L.A.R. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110432. [DOI] [PubMed] [Google Scholar]
- Das P., Delost M.D., Qureshi M.H., Smith D.T., Njardarson J.T. A survey of the structures of US FDA approved combination drugs. J. Med. Chem. 2018;62(9):4265–4311. doi: 10.1021/acs.jmedchem.8b01610. [DOI] [PubMed] [Google Scholar]
- Di Stasi L.C. Natural coumarin derivatives activating Nrf2 signaling pathway as lead compounds for the design and synthesis of intestinal anti-inflammatory drugs. Pharmaceuticals. 2023;16(4):511. doi: 10.3390/ph16040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmi A.A., Abdur-Rahman M., Aboul Naser A.F., Hamed M.A., Abd-Alla H.I., Nasr M.I. Pulicaria crispa mitigates gastric ulcer induced by ethanol in rats: role of treatment and auto healing. Biomarkers. 2019;24(3):286–294. doi: 10.1080/1354750X.2018.1556340. [DOI] [PubMed] [Google Scholar]
- Fahmy N.M., Al-Sayed E., Michel H.E., El-Shazly M., Singab A.N.B. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2020;248 doi: 10.1016/j.jep.2019.112297. [DOI] [PubMed] [Google Scholar]
- Fouman-Ajirlou P., Ahmadizadeh C., Zaeifizadeh M. The effect of combination of Lactobacillus reuteri and Coumarin on the inhibition of gastric cancer cells, AGS Cell Line. KAUMS J. 2020;24(2):122–132. [Google Scholar]
- Gilani S.J., Bin-Jumah M.N., Al-Abbasi F.A., Nadeem M.S., Imam S.S., Alshehri S., Ahmed M.M., Ghoneim M.M., Afzal M., Alzarea S.I. Protective effect of fustin against ethanol-activated gastric ulcer via downregulation of biochemical parameters in rats. ACS Omega. 2022;7(27):23245–23254. doi: 10.1021/acsomega.2c01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama Amin R.R., Aziz T.A. Gastroprotective Effect of Azilsartan through ameliorating oxidative stress, inflammation, and restoring hydroxyproline, and gastrin levels in ethanol-induced gastric ulcer. J. Inflamm. Res. 2022;15:2911–2923. doi: 10.2147/JIR.S365090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeloo-Moghadam M., Tavirani M.R., Jahani-Sherafat S., Tavirani S.R., Esmaeili S., Ansari M., Ahmadzadeh A. Side effects of omeprazole: a system biology study. Gastroenterol Hepatol from Bed Bench. 2021;14(4):334. [PMC free article] [PubMed] [Google Scholar]
- Hasanvand D., Amiri I., Soleimani Asl S., Saidijam M., Shabab N., Artimani T. Effects of CeO(2) nanoparticles on the HO-1, NQO1, and GCLC expression in the testes of diabetic rats. Can. J. Physiol. Pharmacol. 2018;96(9):963–969. doi: 10.1139/cjpp-2017-0784. [DOI] [PubMed] [Google Scholar]
- Huang L., Li H., Huang S., Wang S., Liu Q., Luo L., Gan S., Fu G., Zou P., Chen G. Notopterol attenuates monocrotaline-induced pulmonary arterial hypertension in rat. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.859422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhao Y., Cao Y., Zhang Q., Ran D., Li J., Luo L., Qiu F., Meng L. Anaplastic lymphoma kinase tyrosine kinase inhibitors associated gastrointestinal obstruction, perforation, and ulceration: an analysis of the FDA adverse event reporting system database (FAERS) Int. J. Clin. Pharm. 2022;44(4):993–1003. doi: 10.1007/s11096-022-01425-4. [DOI] [PubMed] [Google Scholar]
- Ibrahim I.A.A., Abdulla M.A., Hajrezaie M., Bader A., Shahzad N., Al Ghamdi S.S., Gushash A.S., Hasanpourghadi M. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug Des. Devel. Ther. 2016;10 doi: 10.2147/DDDT.S91247. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jabbar A.A. Onosma mutabilis: Phytochemical composition, antioxidant, cytotoxicity, and acute oral toxicity. Food Sci. Nutr. 2021;9(10):5755–5764. doi: 10.1002/fsn3.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar A.A. Gastroprotective and Immuno-supportive Role of Alcea kurdica against Stress Induced Lesion in Japanese Quails. Baghdad Sci. J. 2022;19(4):716–724. doi: 10.21123/bsj.2022.19.4.0716. [DOI] [Google Scholar]
- Jabbar A.A., Abdullah F.O., Abdoulrahman K., Galali Y., Ibrahim I.A., Alzahrani A.R., Hassan R.R. Gastroprotective, Biochemical, and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats. Processes. 2022;10(10):1985. doi: 10.3390/pr10101985. [DOI] [Google Scholar]
- Jabbar A.A., Abdullah F.O., Abdulrahman K.K., Galali Y., Sardar A.S. GC-MS Analysis of Bioactive Compounds in Methanolic Extracts of Papaver decaisnei and Determination of Its Antioxidants and Anticancer Activities. J. Food Qual. 2022;2022:1405157. doi: 10.1155/2022/1405157. [DOI] [Google Scholar]
- Jabbar A.A., Abdullah F.O., Hassan A.O., Galali Y., Hassan R.R., Rashid E.Q., Salih M.I., Aziz K.F. Ethnobotanical, Phytochemistry, and Pharmacological Activity of Onosma (Boraginaceae): An Updated Review. Molecules. 2022;27:8687. doi: 10.3390/molecules27248687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar A.A.J., Alamri Z.Z., Abdulla M.A., Salehen N.A., Salim Amur Al Sinawi Z., Alfaifi S.M. Hepatoprotective effects of Gynura procumbens against thioacetamide-induced cirrhosis in rats: Targeting inflammatory and oxidative stress signalling pathways. Heliyon. 2023;9(9) doi: 10.1016/j.heliyon.2023.e19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbar A.A., Ibrahim I.A.A., Abdullah F.O., Aziz K.F., Alzahrani A.R., Abdulla M.A. Chemopreventive Effects of Onosma mutabilis against Azoxymethane-Induced Colon Cancer in Rats via Amendment of Bax/Bcl-2 and NF-κB Signaling Pathways. Curr. Issues Mol. Biol. 2023;45(2):885–902. doi: 10.3390/cimb45020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangwan N., Park J.-M., Kim E.-H., Hahm K.B. Quality of healing of gastric ulcers: natural products beyond acid suppression. World J. Gastrointest. Pathophysiol. 2014;5(1):40. doi: 10.4291/wjgp.v5.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaval I., Behçet L., Cakilcioglu U. Ethnobotanical study on medicinal plants in Geçitli and its surrounding (Hakkari-Turkey) J. Ethnopharmacol. 2014;155(1):171–184. doi: 10.1016/j.jep.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Kogure K., Yamauchi I., Tokumura A., Kondou K., Tanaka N., Takaishi Y., Fukuzawa K. Novel antioxidants isolated from plants of the genera Ferula, Inula, Prangos and Rheum collected in Uzbekistan. Phytomedicine. 2004;11(7–8):645–651. doi: 10.1016/j.phymed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kumar A., Kaur V., Pandit K., Tuli H.S., Sak K., Jain S.K., Kaur S. Antioxidant phytoconstituents from onosma bracteata wall. (boraginaceae) ameliorate the CCl4 induced hepatic damage. In Vivo study in male wistar rats. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01301. https://www.frontiersin.org/article/10.3389/fphar.2020.01301 pp. 7(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-S., Lin S.-C., Chu C.-H., Chang Y.-K., Zhang X., Lin C.-C., Tung Y.-T. The gastroprotective effect of naringenin against ethanol-induced gastric ulcers in mice through inhibiting oxidative and inflammatory responses. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222111985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ye A., Pan Z., Cui J., Dave A., Singh H. Dynamic in vitro gastric digestion behavior of goat milk: Effects of homogenization and heat treatments. J. Dairy Sci. 2022;105(2):965–980. doi: 10.3168/jds.2021-20980. [DOI] [PubMed] [Google Scholar]
- Ling Y. Clinical observation on treating gastric ulcer with TCM medicine and omeprazole. Clin. Study Tradit. Chin. Med. 2018;10:135–136. [Google Scholar]
- Liu L., Yuan Y., Zuo J., Tao J. Composition and antioxidant activity of Paeonia lactiflora petal flavonoid extract and underlying mechanisms of the protective effect on H2O2-induced oxidative damage in BRL3A cells. Hortic. Plant J. 2022;9(2):335–344. [Google Scholar]
- Lokman M.S., Zaafar D., Althagafi H.A., Abdel Daim M.M., Theyab A., Hasan Mufti A., Algahtani M., Habotta O.A., Alghamdi A.A.A., Alsharif K.F., Albrakati A., Oyouni A.A.A., Bauomy A.A., Baty R.S., Zhery A.S., Hassan K.E., Abdel Moneim A.E., Kassab R.B. Antiulcer activity of proanthocyanidins is mediated via suppression of oxidative, inflammatory, and apoptotic machineries. J. Food Biochem. 2022;46(2):e14070. doi: 10.1111/jfbc.14070. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Schulz C. Peptic ulcer: chapter closed? Dig. Dis. 2020;38(2):112–116. doi: 10.1159/000505367. [DOI] [PubMed] [Google Scholar]
- Mariod A.A., Jabbar A.A.J., Alamri Z.Z., Al Rashdi A.S., Abdulla M.A. Gastroprotective effects of Polygonatum odoratum in rodents by regulation of apoptotic proteins and inflammatory cytokines. Saudi J. Biol. Sci. 2023;30(6) doi: 10.1016/j.sjbs.2023.103678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszczyk M., Mika-Stępkowska P., Szmurło A., Szary M., Perlinski M., Kierkuś J. Dietary management of infants and young children with feeding difficulties and unsatisfactory weight gain using a nutritionally complete hypercaloric infant formula. Practical considerations from clinical cases. Postgrad. Med. 2021;133(6):707–715. doi: 10.1080/00325481.2021.1941142. [DOI] [PubMed] [Google Scholar]
- Moawad H., El Awdan S.A., Sallam N.A., El-Eraky W.I., Alkhawlani M.A. Gastroprotective effect of cilostazol against ethanol- and pylorus ligation–induced gastric lesions in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392(12):1605–1616. doi: 10.1007/s00210-019-01699-y. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Yamamoto M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mottaghipisheh J., Kiss T., Tóth B., Csupor D. The Prangos genus: a comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020;19(6):1449–1470. doi: 10.1007/s11101-020-09688-3. [DOI] [Google Scholar]
- Mousa A.A., El-Gansh H.A.I., Eldaim M.A.A., Mohamed M.A.E.G., Morsi A.H., El Sabagh H.S. Protective effect of Moringa oleifera leaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers. Environ. Sci. Pollut. Res. 2019;26(31) doi: 10.1007/s11356-019-06368-4. [DOI] [PubMed] [Google Scholar]
- Nazarbahjat N., Kadir F.A., Ariffin A., Abdulla M.A., Abdullah Z., Yehye W.A. Antioxidant Properties and Gastroprotective Effects of 2-(Ethylthio)Benzohydrazones on Ethanol-Induced Acute Gastric Mucosal Lesions in Rats. PLoS One. 2016;11(6):e0156022. doi: 10.1371/journal.pone.0156022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Numonov S., Bobakulov K., Numonova M., Sharopov F., Setzer W.N., Khalilov Q., Begmatov N., Habasi M., Aisa H.A. New coumarin from the roots of Prangos pabularia. Nat. Prod. Res. 2018;32(19):2325–2332. doi: 10.1080/14786419.2017.1413558. [DOI] [PubMed] [Google Scholar]
- Numonov S., Sharopov F.S., Atolikhshoeva S., Safomuddin A., Bakri M., Setzer W.N., Musoev A., Sharofova M., Habasi M., Aisa H.A. Volatile Secondary Metabolites with Potent Antidiabetic Activity from the Roots of Prangos pabularia Lindl.—Computational and Experimental Investigations. Appl. Sci. 2019;9(11) doi: 10.3390/app9112362. [DOI] [Google Scholar]
- Ofori M., Danquah C.A., Ossei P.P.S., Rahamani G., Asamoah W.A., Ativui S., Doe P. Acute and sub-acute toxicity studies of the chloroform extract of Crinum asiaticum bulbs in mice. South African J. Bot. 2021;143:133–140. doi: 10.1016/j.sajb.2021.07.047. [DOI] [Google Scholar]
- Omar H., Nordin N., Hassandarvish P., Hajrezaie M., Azizan Syahadah A.H., Fadaeinasab M., Majid N.A., Abdulla M.A., Hashim N.M., Ali H.M. Methanol leaf extract of actinodaphne sesquipedalis (Lauraceae) enhances gastric defense against ethanol-induced ulcer in rats. Drug Des. Devel. Ther. 2017;11 doi: 10.2147/DDDT.S120564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlović I., Radenković M., Branković S., Milenković M.T., Niketić M., Ušjak L., Petrović S. Spasmolytic, gastroprotective and antioxidant activities of dry methanol extract of Ferula heuffelii underground parts. Chem. Biodivers. 2022;19(5):e202200047. doi: 10.1002/cbdv.202200047. [DOI] [PubMed] [Google Scholar]
- Razuvaeva Y.G., Toropova A.A., Salchak S.M., Olennikov D.N. Coumarins of Ferulopsis hystrix: LC–MS Profiling and Gastroprotective and Antioxidant Activities of Skimmin and Peucenidin. Appl. Sci. 2023;13(17) doi: 10.3390/app13179653. [DOI] [Google Scholar]
- Rudra D.S., Pal U., Chowdhury N., Maiti N.C., Bagchi A., Swarnakar S. Omeprazole prevents stress induced gastric ulcer by direct inhibition of MMP-2/TIMP-3 interactions. Free Radic. Biol. Med. 2022;181:221–234. doi: 10.1016/j.freeradbiomed.2022.02.007. [DOI] [PubMed] [Google Scholar]
- Salama S.M., Gwaram N.S., AlRashdi A.S., Khalifa S.A.M., Abdulla M.A., Ali H.M., El-Seedi H.R. A Zinc Morpholine Complex Prevents HCl/Ethanol-Induced Gastric Ulcers in a Rat Model. Sci. Rep. 2016;6 doi: 10.1038/srep29646. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salari N., Darvishi N., Shohaimi S., Bartina Y., Ahmadipanah M., Salari H.R., Mohammadi M. The global prevalence of peptic ulcer in the world: A systematic review and meta-analysis. Indian J. Surg. 2022;84(5):913–921. [Google Scholar]
- Saremi K., Rad S.K., Khalilzadeh M., Hussaini J., Majid N.A. In vivo acute toxicity and anti-gastric evaluation of a novel dichloro Schiff base: Bax and HSP70 alteration. Acta Biochim. Biophys. Sin. (Shanghai) 2020;52(1) doi: 10.1093/abbs/gmz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Román J., Herrera-Ruiz M., González-Cortazar M., Nicasio-Torres P. Anti-ulcerogenic Properties of Sphaeralcea angustifolia on Gastric Ulcer in Mice. Rev. Bras. Farmacogn. 2023:1–7. [Google Scholar]
- Sevin G., Alan E., Demir S., Albayrak G., Demiroz T., Yetik-Anacak G., Baykan S. Comparative evaluation of relaxant effects of three prangos species on mouse corpus cavernosum: Chemical characterization and the relaxant mechanisms of action of P. pabularia and (+)-oxypeucedanin. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114823. [DOI] [PubMed] [Google Scholar]
- Shady N.H., Abdullah H.S., Maher S.A., Albohy A., Elrehany M.A., Mokhtar F.A., Oraby H.F., Shawky A.M., Abdelmohsen U.R. Antiulcer potential of psidium guajava seed extract supported by metabolic profiling and molecular docking. Antioxidants. 2022;11(7) doi: 10.3390/antiox11071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareef S.H., Al-Medhtiy M.H., Ibrahim I.A., Alzahrani A.R., Jabbar A.A., Galali Y., Agha N.F., Aziz P.Y., Thabit M.A., Agha D.N.F., Salehen N.A., Ameen Z.M., Abdulla M.A. Gastroprophylactic Effects of p-Cymene in Ethanol-Induced Gastric Ulcer in Rats. Processes. 2022;10(7) doi: 10.3390/pr10071314. [DOI] [Google Scholar]
- Sidahmed H.M.A., Hashim N.M., Abdulla M.A., Ali H.M., Mohan S., Abdelwahab S.I., Taha M.M.E., Fai L.M., Vadivelu J. Antisecretory, gastroprotective, antioxidant and anti-helicobcter pylori activity of zerumbone from zingiber zerumbet (L.) smith. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sidahmed H.M.A., Vadivelu J., Loke M.F., Arbab I.A., Abdul B., Sukari M.A., Abdelwahab S.I. Anti-ulcerogenic activity of dentatin from clausena excavata Burm. f. against ethanol-induced gastric ulcer in rats: Possible role of mucus and anti-oxidant effect. Phytomedicine. 2019;55:31–39. doi: 10.1016/j.phymed.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Song J., Guan Y.-F., Liu W.-B., Song C.-H., Tian X.-Y., Zhu T., Fu X.-J., Qi Y.-Q., Zhang S.-Y. Discovery of novel coumarin-indole derivatives as tubulin polymerization inhibitors with potent anti-gastric cancer activities. Eur. J. Med. Chem. 2022;238 doi: 10.1016/j.ejmech.2022.114467. [DOI] [PubMed] [Google Scholar]
- Staff P.O.N.E. Correction: Isolation, Cytotoxicity Evaluation and HPLC-Quantification of the Chemical Constituents from Prangos pabularia. PLoS One. 2014;9(12):e115110. doi: 10.1371/journal.pone.0108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y., Shikishima Y., Takaishi Y., Shibata H., Higuti T., Honda G., Ito M., Takeda Y., Kodzhimatov O.K., Ashurmetov O., Ohmoto Y. Coumarins and γ-pyrone derivatives from Prangos pabularia: antibacterial activity and inhibition of cytokine release. Phytochemistry. 2002;59(6):649–654. doi: 10.1016/S0031-9422(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Ullah S., Rashid Khan M., Ali Shah N., Afzal Shah S., Majid M., Asad Farooq M. Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. J. Ethnopharmacol. 2014;158 Pt A:412–422. doi: 10.1016/j.jep.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Vuolo M.M., da Silva-Maia J.K., Batista Â.G. Basic Protocols in Foods and Nutrition. Springer; 2022. The GSH Colorimetric Method as Measurement of Antioxidant Status in Serum and Rodent Tissues; pp. 187–194. [Google Scholar]
- Woolf A, R.R., Rehman, R.B., 2023. Gastric ulcer. Treasure Isl StatPearls Publ. https://www.ncbi.nlm.nih.gov/books/NBK537128/.
- Xie C., Liu L., Zhu S., Wei M. Effectiveness and safety of Chinese medicine combined with omeprazole in the treatment of gastric ulcer: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100(17) doi: 10.1097/MD.0000000000025744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahri S., Razavi S.M. Cytotoxic effect of Prangos Pabularia extract on HELA cell line a medicinal plant. Int J Med Res Heal Sci. 2016;5(11):547–552. [Google Scholar]
- Zheng Z., Zhang L., Hou X. Potential roles and molecular mechanisms of phytochemicals against cancer. Food Funct. 2022;13(18):9208–9225. doi: 10.1039/d2fo01663j. [DOI] [PubMed] [Google Scholar]