Abstract
Objective
To determine risk for incident basal cell carcinoma from cumulative low-dose ionising radiation in the US radiologic technologist cohort.
Methods
We analysed 65 719 Caucasian technologists who were cancer-free at baseline (1983–1989 or 1994–1998) and answered a follow-up questionnaire (2003–2005). Absorbed radiation dose to the skin in mGy for estimated cumulative occupational radiation exposure was reconstructed for each technologist based on badge dose measurements, questionnaire-derived work history and protection practices, and literature information. Radiation-associated risk was assessed using Poisson regression and included adjustment for several demographic, lifestyle, host and sun exposure factors.
Results
Cumulative mean absorbed skin dose (to head/neck/arms) was 55.8 mGy (range 0–1735 mGy). For lifetime cumulative dose, we did not observe an excess radiation-related risk (excess relative risk/Gy=−0.01 (95% CI −0.43 to 0.52). However, we observed that basal cell carcinoma risk was increased for radiation dose received before age 30 (excess relative risk/Gy=0.59, 95% CI −0.11 to 1.42) and before 1960 (excess relative risk/Gy=2.92, 95% CI 1.39 to 4.45).
Conclusions
Basal cell carcinoma risk was unrelated to low-dose radiation exposure among radiologic technologists. Because of uncertainties in dosimetry and sensitivity to model specifications, both our null results and our findings of excess risk for dose received before age 30 and exposure before 1960 should be interpreted with caution.
Keywords: ionising radiation, basal cell carcinoma, epidemiology, radiologic technologist
Introduction
Mortality for basal cell carcinoma (BCC) is low, but because over two million cases are diagnosed annually in the United States, this places a huge burden on health care resources[1]. Epidemiological research on BCC is difficult since most US cancer registries do not record non-melanoma skin cancers (NMSC) diagnoses including BCC[2]. Risk factors include increased solar ultraviolet radiation (UVR) exposure[2], serious sunburns at young ages and host characteristics including skin, eye and hair pigmentation[2]. A less common risk factor for BCC is exposure to moderate to high doses of ionising radiation (IR)[2–4]. An association between IR and increased BCC risk has been reported in the atomic bomb survivors[5–7], and in patients irradiated for medical reasons[3 8–10]. In these studies, dose was acute or high and fractionated. There are few studies of BCC risk and chronic low (under 100 mGy) IR dose[11–14]. One study of workers exposed to chronic low IR showed a dose-related increased risk for NMSC, although the analysis did not adjust for other BCC risk factors[14]. We do not know of any population based studies of BCC risk and low chronic radiation exposure with quantitative IR dose estimates that substantially account for UVR and other major BCC risk factors; these factors may confound the radiation-related risk of BCC.
In a previous report from the United States Radiologic Technologists (USRT) cohort, there was a suggestion that radiation exposure increased BCC risk. The finding was based on questionnaire-based work history that revealed elevated risks for technologists who first worked as radiologic technologists before 1960 when the occupational IR doses were higher compared to those who started work in 1960 or after[15]. The purpose of the present study was to update the previous USRT study with additional follow-up time and to evaluate radiation-related BCC risk using recently reconstructed individual quantitative estimates for cumulative dose[16] . We used recently improved individual solar UVR exposure measurements and other BCC risk covariates not previously available. We further evaluated possible IR effect modification by UVR and by age at exposure, time since exposure and calendar year periods of exposure.
Materials and Methods
Overview
The USRT cohort consists of 146,022 radiologic technologists (73% female) living in the United States and its territories who were certified by the American Registry of Radiologic Technologists (ARRT) for at least two years between 1926 and 1982. Methods of the study have been previously described [17] and detailed information can be found online at www.radtechstudy.nci.nih.gov. Periodic surveys were undertaken between 1983 and 2005. For the first survey, questionnaires were sent during 1983-89 to 132,298 surviving cohort members with a postal address; 90,305 (68%) technologists completed the questionnaire. A second questionnaire was sent to 126,628 living and located members during 1994-1998; 90,972 (72%) technologists responded. If a technologist answered both the first and second questionnaire, the earlier of the two was designated as the baseline questionnaire. A third questionnaire was sent in 2003-2005 to 105,694 participants who had answered at least one previous questionnaire; of these, 73,838 (73%) responded.
Eligibility for current study
Eligible for the current study were the 69,272 technologists who completed the third questionnaire and did not report any specific cancer (including BCC) or an unknown type of cancer diagnosis prior to their baseline questionnaire. Non-Caucasian (3,553 technologists: 5.1%) respondents were excluded due to the notably lower BCC incidence rates compared to Caucasians, and the relatively small number of these technologists. Of those who were followed to the third questionnaire, 55,823 technologists answered the first questionnaire and 9,896 were added at the time of the second questionnaire, yielding a total analytic cohort of 65,719 persons.
Data Collection and UVR Exposures
The first and second questionnaires collected demographic and BCC risk factor data, including gender; eye, skin, and hair color; and personal diagnostic x-ray history. Additional UVR-related risk factors for BCC, such as sunburn history, and residential location for five age periods (age<13, 13-19, 20-39, 40-64, 64+), were collected on the third questionnaire. (See Supplemental Table A for a full listing of potential risk factors.) Individual age-specific ambient UVR exposures were determined by linking the city-level residential locations with the Total Ozone Mapping Spectrometer (TOMS) database (http://toms.gsfc.nasa.gov), as previously described[18–20]. An average cumulative solar UVR exposure score up to the baseline questionnaire was computed by multiplying the personal questionnaire-derived number of hours of summer sun exposure (taking into account both weekday and weekend hours) by the ambient UVR exposure at each residential location for each age period weighted by the years of life at each location (“UVR Score”).
Occupational IR dose
Annual and cumulative radiation absorbed doses to specific organs from occupational exposure for each radiologic technologist were estimated through a historical dose reconstruction[16]. Data for the dose reconstruction for the entire study from which the analytic cohort was drawn, included: 921,134 annual badge dose measurements available for 79,959 cohort members between 1960 and 1997 from the largest U.S. commercial dosimetry provider, three U.S. military dose registries, civilian employers, and a major hospital; detailed information on protection practices and work procedures obtained from the three questionnaire surveys; and a comprehensive review of the literature on published badge doses (especially for the early years) and changes in x-ray imaging technology, radiation protection standards, individual protection practices, typical radiation energies and filtration, and other factors that contributed to exposure over calendar time. Annual reported badge doses were used for each technologist when available; otherwise, the annual badge dose was estimated from annual probability density distributions based on badge doses of the technologists who did have exposure data for that particular year. Organ doses were estimated from measured or estimated badge doses, estimates of the percentage of time protective lead aprons were worn (derived from individual questionnaires and the literature), badge dose-to-organ dose conversion factors based on kV and filtration (which varied by time period), and apron attenuation factors (specific to each kV and time period)[21] Absorbed dose to the head, neck and arms, which represents dose to skin unprotected by a lead apron and the most common BCC anatomic locations[2], was used for the main analysis[16].
BCC case ascertainment and medical validation
Eligible cases were self-reported incident first primary BCCs (with year of diagnosis) occurring after subjects’ completion of their baseline questionnaire. Validation of self-reported BCC cases in the USRT cohort had been conducted previously[15]. Of the 1,355 self-reported BCCs from the second questionnaire, medical records were obtained on approximately 50% [15]. Among those, 97% (668 BCCs) were confirmed. For the 4,862 BCCs self-reported on the third questionnaire, medical records were obtained on 2,058 (42.3%) and of these 1,762 (85.6%) were confirmed as a BCC. Because of the high medical record confirmation, we defined both confirmed as well as self-reported BCCs for which medical validation was not obtained, as cases.
Statistical analysis
We fitted a Poisson relative risk model via maximum likelihood to analyze risk in relation to a linear function of cumulative occupational IR dose. The cumulative doses were time-dependent and lagged by five years, as recommended by most scientific committees[22 23]. For sensitivity analysis, we also tested 10- and 15-year lagged dose.
The model assumes that the expected number of BCC cases in stratum, with cumulative lagged radiation dose (in Gy), and person years of follow-up is given by:
| (1) |
for parameters (determined by the maximum likelihood fit) . is the ERR per unit dose in units of Gy−1. The baseline risk was described using a logarithmic function of variables Xi which include gender, calendar year period (1983-1984, 1985-89 …2000-05) and attained age.
Due to the strong relationship between dose and attained age[16], we tried several methods to adjust for attained age in the baseline risk including categorical age effects using 12, 7, 5 and 4 attained age groups and polynomials in age (age + age2). The Akaike Information Criteria (AIC)[24] was used to choose the best age adjustment method (Supplemental Table C). We found that a model with 12 attained age groups [<30, 30-35….80+ (5 year intervals)] yielded the lowest AIC values and was therefore considered the main model.
The choice of variables in the final multivariate models was determined by comparison of nested models with additional risk factors (from Appendix A) and based on evidence of significant improvement in deviance for each nested model (Supplemental Table B in Appendix B). The variables for Xi in final multivariate model included gender, calendar year period (1983-1984, 1985-89 …2000-05), attained age, sunburn history, complexion, BMI, skin reaction to strong sunlight, UVR Score, hours of exercise per week, income, dental x-rays, eye color, cigarette smoking, alcohol consumption and education. In Table 2 we present the minimally adjusted model (stratification by gender and adjustment for birth cohort) additionally adjusted for age using 12 attained age groups as well as the final multivariate model.
Table 2.
Excess relative risk (ERR/Gy) of incident basal cell carcinoma for ionising radiation in a cohort of U.S. radiologic technologists a, 1983-2005.
| Model | Method of Attained Age Adjustment | Model 1 b | Model 2 c | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| ERR/Gy | 95% CI | P value | ERR/Gy | 95% CI | P value | ||||
| Maind | 12 age groups: <30,30-35….80+ (5 year intervals) | 0.03 | −0.39 | 0.56 | >0.5 | −0.01 | −0.43 | 0.52 | > 0.5 |
Absorbed ionising radiation dose to the skin of the head, neck and arms. Caucasians only
Model 1: Poisson regression with ionising radiation on the linear scale. Time begins at the return of the first questionnaire (1983) and ends at the end of the last follow-up (2005). Adjustments are made by five calendar periods: 1983-84, 1985-89, 1990-94, 1995-99, 2000-05. Confidence intervals are based on profile likelihoods. Additional adjustments by sex (stratified).
Model 2: Additional adjustments by education, income, cigarette smoking, alcohol consumption, body mass index, hours of exercise per week, eye color, skin complexion, ever sunburnt so as to cause blisters, number of blistering sunburns before age 15, skin reaction to 30 minutes of strong sunlight, number of dental x-rays and cumulative solar ultra violet exposure score five years prior to baseline questionnaire.
Additional sensitivity analysis using different methods for attained age adjustment can be found in Supplemental Table C.
To analyze possible interaction between solar UVR and occupational IR on the risk of BCC, we used the following model:
| (2) |
β11 is the ERR per unit dose in units of Gy−1 and is the ERR per unit UVR exposure. is the solar UVR score created based on residential location and outdoor activity as previously described. The coefficient expresses the effect of the possible interaction between solar UVR and IR dose.
To analyze the modifying effect on BCC risk of radiation accumulated in various time periods of exposure, we constructed time windows based on age, time since exposure and calendar time. For example, in relation to age at exposure, by the following equation:
| (3) |
where, ERRD is the ERR per unit dose, AgeD<30, AgeD30-39 and AgeD≥40 represent cumulative doses at ages accrued up to age 30, cumulative doses accrued within ages 30-39 and cumulative doses accrued after age 39. α1, α2, α3 are the ERR per Gy for these exposure ages.
Time since exposure is a time-varying measure. It is the cumulative exposure that was accrued during a time period before a particular point of time at risk for an individual. The following equation was constructed in relation to time since exposure:
| (4) |
where TimeSince D0-4, TimeSince D5-9, TimeSince D10-14, and TimeSince D≥15 represent cumulative doses for 0-4, 5-9, 10-14 and 15 years and longer before time at risk. γ1, γ2, γ3 and γ4 are the ERR per Gy for these time since exposure periods. Likewise in relation to calendar time period of exposure:
| (5) |
whereYearDPre1950 , YearD1950-9, YearD1960-9 and YearD≥1970 represent cumulative doses during the calendar periods before 1950, 1950-9, 1960-9, and 1970 and beyond . ε1, ε2, ε3 and ε4 are the ERR per Gy for these calendar periods.
Sensitivity analyses were conducted including multivariate models which excluded self-reported sunburn history, skin sensitivity to sun exposure and self-reported income since these questions were asked on the 3rd questionnaire and could potentially introduce recall bias. We also adjusted for the number of years worked as a technologist in order to address potential bias introduced by a potential healthy worker selection effect (HWSE). Cumulative years worked is a commonly used measure to control for HWSE [25]. In order to determine if there was any difference in radiation related BCC risk for cases diagnosed earlier compared to later, we stratified our analysis by time period before the administration of the second questionnaire and time period after the initial administration of the second questionnaire. We tested for curvilinearity in the radiation dose response by using a linear-exponential model.
All tests of statistical significance were based on the likelihood ratio test. Confidence intervals are also likelihood based; confidence intervals were derived using the profile likelihood when the profile likelihood converged, otherwise they were Wald-based. Estimated parameters from the partial-likelihood maximization of the Cox proportional hazards models were derived using SAS (SAS Institute, Cary, NC). The construction of the person year/event table and Poisson regression modeling was conducted using EPICURE (Hirosoft, Seattle, WA).
Ethical approvals
The research protocol for the USRT cohort study of cancer risks has been approved annually by the National Cancer Institute Special Studies Institution Review Board (SSIRB Protocol OH97-C-N053) and the University of Minnesota Human Research Protection Program Institution Review Board (Federal Wide Assurance (FWA) number 8005M02489). Completion of any questionnaire was considered implied consent for study participation.
Results
On average, subjects were followed for 17 years (range 0-23), with a total of 1,113,298 person-years. Table 1 shows the characteristics of the study cohort and crude rates. Most technologists were female (75%), the mean age at entry (baseline) was 39 years (range 22-90) and the mean year of birth was 1948 (range 1905-1966). There were 3,615 technologists who reported an incident BCC (5.5% of the analytic cohort). The crude BCC rate was 3.25 per 1,000 person years (Table 1). Among the 3553 non-Caucasians not included in the analytic cohort, the crude rate was 0.24 per 1,000 person years (Supplemental Table D). Supplemental Table A shows the age-adjusted hazard ratios. Age-adjusted risk for a BCC was lower for those born in earlier decades compared to those born in later decades. The average age of diagnosis for the BCC cases was 52.3 years (standard deviation (SD): 10.2). Eighty-eight percent of technologists began working at age 24 or less, and 52% of them worked for over 20 years; 51% of the analytic cohort first worked as a radiologic technologist in the 1970s (or later), and 16% began work as a technologist before 1960.
Table 1:
Demographic and other characteristics and crude incidence rates of basal cell carcinoma in a cohort of U.S. radiologic technologists a, 1983-2005
| Variable | Cases | Person-years | BCC Rate/1000 Person-Years | |
|---|---|---|---|---|
| Total | 3,615 | 1,113,298 | 3.25 | |
| Gender | Men | 796 | 219,504 | 3.63 |
| Women | 2,819 | 893,794 | 3.15 | |
| Year of birth | < 1930 | 282 | 37,159 | 7.59 |
| 1930-1934 | 266 | 46,059 | 5.78 | |
| 1935-1939 | 433 | 85,193 | 5.08 | |
| 1940-1944 | 539 | 145,278 | 3.71 | |
| 1945-1949 | 712 | 234,663 | 3.03 | |
| 1950-1954 | 804 | 313,808 | 2.56 | |
| 1955-1959 | 563 | 240,664 | 2.34 | |
| 1960+ | 16 | 10,474 | 1.53 | |
| Age at Baseline | <30 | 458 | 220,330 | 2.08 |
| 30-39 | 1,429 | 523,524 | 2.73 | |
| 40-49 | 1,020 | 259,806 | 3.93 | |
| 40+ | 708 | 109,638 | 6.46 | |
| Education b | 2-year hospital radiologic technologist program | 1,464 | 470,860 | 3.11 |
| College or graduate school | 1,555 | 439,744 | 3.54 | |
| Other/unknown | 596 | 202,694 | 2.94 | |
| UVR Score c | 1 (lowest) | 662 | 224,495 | 2.95 |
| (quintiles) | 2 | 707 | 219,793 | 3.22 |
| 3 | 689 | 219,915 | 3.13 | |
| 4 | 747 | 220,546 | 3.39 | |
| 5 (highest) | 760 | 211,287 | 3.60 | |
| Unknown/missing | 50 | 17,262 | 2.90 | |
| Age First Worked | <20 years old | 1,546 | 495,535 | 3.12 |
| 20-24 years old | 1,623 | 500,182 | 3.24 | |
| 25-29 years old | 261 | 67,796 | 3.85 | |
| 30+ years old | 140 | 39,496 | 3.54 | |
| Unknown/missing | 45 | 10,288 | 4.37 | |
| Total Years Worked | <10 years | 761 | 215,589 | 3.53 |
| 10-19 years | 765 | 234,118 | 3.27 | |
| 20-29 years | 1,026 | 357,260 | 2.87 | |
| 30+ years | 794 | 232,552 | 3.41 | |
| Unknown/missing | 269 | 73,778 | 3.65 | |
| First Year Worked | <1950 | 234 | 25,674 | 9.11 |
| 1950-1959 | 717 | 135,886 | 5.28 | |
| 1960-1969 | 1,110 | 350,096 | 3.17 | |
| 1970+ | 1,509 | 591,354 | 2.55 | |
| Unknown/missing | 45 | 10,288 | 4.37 | |
| Absorbed dose to the skin of | <10 | 396 | 174,850 | 2.26 |
| the head, neck and arms | 10-25 | 705 | 256,795 | 2.75 |
| (mGy) | 25-50 | 885 | 294,775 | 3.00 |
| 50-75 | 549 | 158,301 | 3.47 | |
| 75-100 | 327 | 85,168 | 3.84 | |
| 100-200 | 505 | 108,608 | 4.65 | |
| 200-300 | 155 | 22,506 | 6.89 | |
| 300-500 | 72 | 9,460 | 7.61 | |
| 500-750 | 16 | 2,142 | 7.47 | |
| 750+ | 5 | 694 | 7.20 |
Caucasian only
Assessed on the third questionnaire
Based on Total Ozone Mapping Spectrometer satellite instrument data. The UVR Score was created by ultraviolet radiation levels in mWatt/m2 (based on linking residential location to NASA satellite data) for each of 5 age periods, weighting each ambient exposure for each age period by summer hours outdoors (weekend and weekdays) and creating a weighted cumulative average over the lifetime
Adjusting for age and birth cohort, we found several factors associated with BCC risk (see Appendix A). Higher education and income were associated with increased risk for BCC, but not gender or marital status. Higher alcohol consumption, lower BMI, and more frequent exercise were associated with increased BCC risk. Current cigarette smokers had lower BCC risk compared to never smokers; former smokers had a higher risk compared to never smokers. Elevated risk for BCC was associated with lighter eye, skin and hair color, history of ever having a sunburn that caused blisters, higher number of blistering sunburns under age 15 and number of blistering sunburns over age 15, sunburn after 30 minutes of strong sunlight without sunscreen, and higher lifetime cumulative personal UVR exposure score. Higher numbers of self-reported personal medical dental x-rays were associated with increased risk of BCC, as well as a history of ever having a tomogram or CT scan.
The mean cumulative occupational badge dose, cumulative absorbed dose to the skin of the head, neck and arms, and cumulative absorbed dose to the skin of the trunk (at the time of the baseline questionnaire) was 47.9 mGy (SD: 57.4), 55.8 mGy (SD: 69.9), and 31.1 mGy (SD: 57.0), respectively. Using cumulative dose to the skin of the head, neck, and arms, 14% of the technologists had cumulative doses above 100 mGy and less than 1% (200 technologists) had doses above 0.5 Gy (see Appendix A). Cumulative doses were higher for those born in earlier decades compared to those born in later decades.
Fits of Poisson models explicitly accounting for radiation dose
Table 2 shows the minimally adjusted as well as multivariate adjusted excess relative risk per Gy (ERR/Gy) for IR absorbed dose to the skin of the head, neck and arms using a Poisson regression model with dose on the linear scale using attained age adjustment of 12 age groups. Results of other models for attained age adjustment are shown in Supplemental Table C. Cumulative lifetime occupational radiation exposure (main model) showed no radiation associated excess relative risk (ERR/Gy=−0.01; 95% CI: −0.43, 0.52). This result is also shown graphically in Figure 1. Increasing the number of age groups used to adjust the underlying rates attenuated the dose and BCC relationship (see Supplemental Table C).
Figure 1.
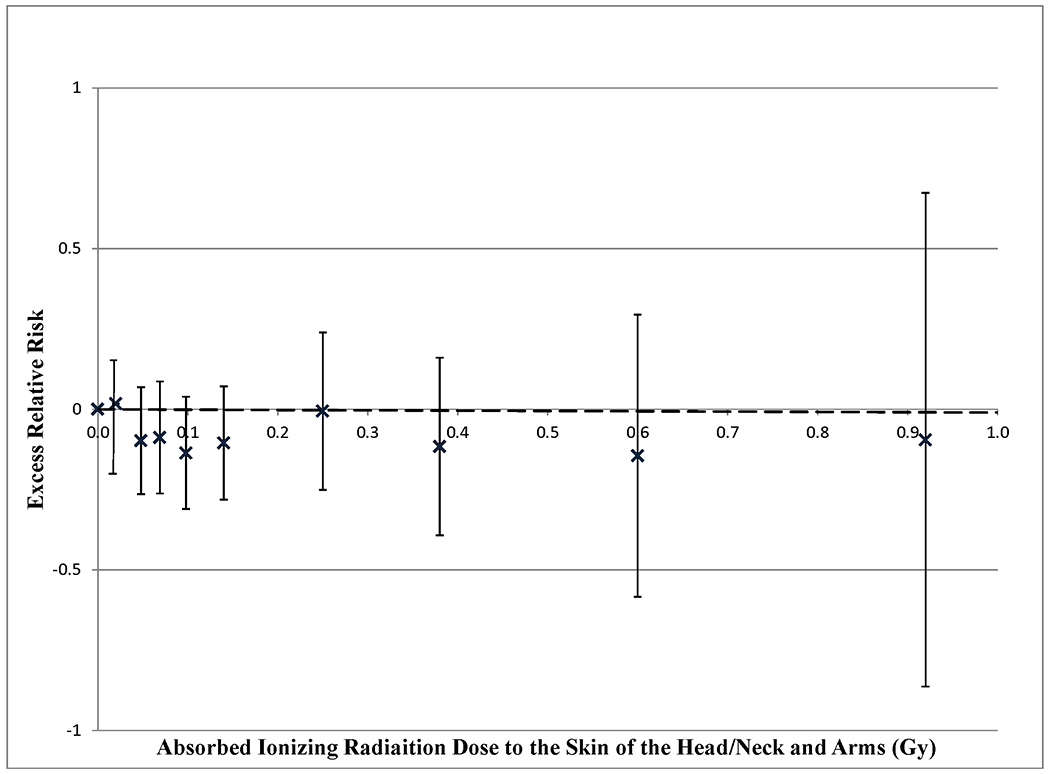
Excess relative risk of incident basal cell carcinoma by absorbed ionising radiation dose to the skin of the head, neck and arms in a cohort of U.S. radiologic technologists, 1983-2005, calculated using Poisson regression. Follow-up begins at the return of the first questionnaire (1983-1989) or second questionnaire (1994-1998) and ends at 31 December 2005. Calendar period adjustments are made by five calendar periods: 1983-84, 1985-89, 1990-94, 1995-99, 2000-05. Additional adjustments by sex (stratified), education, income, cigarette smoking, alcohol consumption, body mass index, exercise, eye color, complexion, sun burn history, tanning sensitivity, dental x-rays and solar ultra violet exposure score. The dashed ( ) line represents a linear Poisson model using 12 age groups for attained age adjustment. Points with their respective confidence intervals use the same attained age adjustment ( X ). The ERRs are for radiation dose categories of : <10, 10-24, 25-49, 50-74, 75-99, 100-199, 200-299, 300-499, 500- mGy.
Possible curvilinearity in the dose response was explored but, linear-exponential models did not improve the fit compared to linear models for any method of age adjustment (p>0.5) (data not shown). Other multivariate models using a different background model with birth cohort and calendar period interactions, models without questionnaire responses for sunburn history, skin sensitivity to sun exposure and income and models using different lag periods for IR dose did not materially change the results (data not shown). Sensitivity analyses controlling for the number of years worked did not materially change our findings (data not shown). Separate analysis of the time period before the second questionnaire compared to the time period after the second questionnaire were not significantly different (Supplemental Table E).
We tested for interaction between UVR and IR on the risk of BCC by adding a solar UVR/IR interaction term to our model. The addition of the interaction term did not improve the model fit (p>0.5), and while solar UVR was associated with BCC, the solar UVR/IR interaction term was not associated with BCC risk (p>0.05). We did not observe any obvious patterns suggesting interaction when we analyzed IR risk by UVR score quartiles (Supplemental Table F).
Table 3 shows the multivariate adjusted time window analysis of age at IR exposure, time since IR exposure and calendar year period of IR exposure using attained age adjustment by 12 age groups. Using age-at-exposure time windows improved the model fit (p=0.038) and suggested that occupational radiation exposure at younger ages had a higher BCC risk. There was a borderline suggestive effect (p=0.081) of time since exposure, with risk tending to decrease with increasing time after exposure. Calendar year time windows improved the fit of the model (p-value <0.001); occupational radiation exposure during periods before 1960 had higher radiation- associated excess BCC risk compared to the periods after 1960. The sensitivity analysis results of multivariate models (using other methods of attained age adjustment (7, 5, 4) age groups and age polynomials) and adjustment by birth cohort and gender are shown in Supplemental Table G.
Table 3.
Time windows of excess relative risks (ERR/Gy) for occupational ionising radiation dose for risk of basal cell carcinoma in a cohort of U.S. radiologic technologists a, 1983-2005. All analyses have multivariate adjustment for various risk factors b, and are adjusted for attained age in 12 groups c.
| Age During Exposure d | ||||
|---|---|---|---|---|
|
| ||||
| ERR/Gy | 95% CI | |||
|
|
||||
| Age <30 e | 0.59 | −0.11 | 1.42 | |
| Age 30-39 | −0.53 | −2.09 | 1.03 | |
| Age ≥40 | −1.33 | −3.21 | 0.72 | |
| Comparison to null model using the likelihood ratio test f | P value | 0.038 | ||
|
| ||||
| Years Since Exposure g | ||||
|
| ||||
| ERR/Gy | 95% CI | |||
|
|
||||
| 5-9 | −0.43 | −7.48 | 6.62 | |
| 10-14 | 1.85 | −3.32 | 7.02 | |
| ≥15 | 0.14 | −0.63 | 0.36 | |
| Comparison to null model using the likelihood ratio test f | P value | 0.081 | ||
|
| ||||
| Calendar Year of Exposure h | ||||
|
| ||||
| ERR/Gy | 95% CI | |||
|
|
||||
| Pre 1950 | 0.63 | −0.45 | 1.70 | |
| 1950-59 | 2.92 | 1.39 | 4.45 | |
| 1960-69 | −1.80 | −2.90 | −0.69 | |
| ≥1970 | −0.88 | −1.88 | 0.12 | |
| Comparison to null model using the likelihood ratio test f | P value | <0.001 | ||
Absorbed ionising radiation dose to the skin of the head, neck and arms Caucasians only.
Poisson regression with ionising radiation dose on the linear scale. Time begins at the return of the first questionnaire (1983) and ends at the end of the last follow-up (2005). Adjustments are made by five calendar periods: 1983-84, 1985-89, 1990-94, 1995-99, 2000-05. Additional adjustments by sex (stratified), education, income, cigarette smoking, alcohol consumption, body mass index, exercise, eye color, complexion, sun burn history, tanning sensitivity, dental x-rays and solar ultra violet exposure score. Confidence intervals are Wald-based (unless otherwise stated).
12 groups: <30, 30-35….80+ (5 year intervals)
Based on equation 2 in the main text.
Confidence intervals are profile-likelihood based.
Comparison of the fit of the time windows model with the null model (without time windows) by the likelihood ratio test
Based on equation 3 in the main text.
Based on equation 4 in the main text.
Discussion
We found no association between cumulative lifetime occupational radiation exposure and risk of BCC. We found evidence of higher risk for occupational radiation exposure received under age 30 years, and increased risk for exposure that occcured during earlier time periods. The USRT cohort members are geographically dispersed, but the addition of BCC risk factors, including solar UVR exposure and sunburn history did not materially change our findings (Supplemental Table C).
This present report, an update of a previous analysis of occupational IR and BCC incidence in the USRT cohort[15], had seven years of additional follow-up and a notably increased number of cases from 1,355 to 3,615. Additionally, we used detailed questionnaire responses including more refined measures of solar UVR exposure to determine the background risk for BCC and recently updated quantitative dose estimates for cumulative occupational IR exposure, which had not been available previously.
Although we did not see an association of lifetime occupational radiation exposure and risk of BCC using our main model for adjustment of attained age, our findings of an increased radiation-related risk in sensitivity analyses and our findings of a higher excess risk for exposure at younger ages suggests that a radiation-related excess risk may exist.
The studies of Japanese atomic bomb survivors[3],[6],[7] [26] and data on radiotherapy patients[3],[27],[9],[10],[28] have established an association between IR and BCC for doses of about 1 Gy, the former providing information on acute exposures and the latter on fractionated exposures.. However, these studies are uninformative as to the relation between low dose chronic IR exposure and BCC risk[22]. The Japanese atomic bomb survivors reported an ERR/Sv=0.7 (95% CI: 0.3, 1.6), and the data suggest a possible threshold at 0.6 Gy.[7] The Israeli tinea capitis cohort, exposed in childhood, had an ERR/Gy=0.7 (95% CI: 0.3, 1.4) [3] and the New York tinea capitis cohort, also exposed in childhood, had an ERR/Gy=1.6 (95% CI: 1.3, 2.1) [9].
Our study is one of few with information on the relationship between low chronic radiation doses and BCC risk with a rich covariate set (Supplemental Table H). Some incidence studies of occupationally IR exposed populations have reported an increased skin cancer risk, however the histological type of skin cancer in these studies was not recorded[13],[29]. A study of the UK National Registry for Radiation Workers (NRRW) reported a statistically significant increasing trend with dose for incident NMSC[14]. In the NRRW, exposed at a comparable low dose-rate as the present study, the authors found an ERR/Sv=1.5 (95% CI: 0.1, 3.9) [14] For the NRRW cohort of mostly Caucasian radiation workers, it was likely that about 80% of NMSC were BCC. Adjustment for other BCC risk factors, such as solar UVR exposure, was not done, although based on our findings (Table 2) there would be little expected effect of adjusting for such factors. Increased risk of BCC among uranium workers has been reported, but details on other exposures and risk factors and IR dose estimates were not analyzed[11]. As has been previously concluded, the alpha-particle radiation that uranium miners would be exposed to is unlikely to cause BCC, since this type of radiation would not penetrate to the basal layer, where the relevant stem cells are thought to reside[30].
Some studies of airline crew, who have increased exposure to cosmic radiation, show elevated incidence rates of BCC compared to the general public, but there is no convincing association with occupational radiation exposure in these workers[12],[31] [32]. It is unclear whether the increased risk among aircrews was related to occupationally associated IR or to recreational solar UVR exposure; studies have reported that aircrew take more vacations in sunny locations compared to the general public[33],[31]. Overall, these studies suggest that elevated BCC incidence for flight crew is not likely to be due to occupational radiation exposure.
Our observations of a greater BCC risk at younger exposure ages are supported by other studies, in particular in the Japanese atomic bomb survivors[5] and in the two groups of patients treated with radiation for tinea capitis[3 9]. More generally, there is abundant evidence of increased relative risk at younger exposure age for most types of radiation-related cancers[26],[34],[35] . We did not see any marked variations in risk with time since exposure, consistent with results from other studies on radiation and BCC risk[3 5 9] .
In our analysis by calendar time period, the ERR/Gy for exposure during the 1950s and during the years before 1950 were strongly and significantly associated with increased BCC risk while risks for radiation exposure in subsequent time periods decreased. We were unable to locate other publications with similar analyses to compare our findings. One possible contribution to the observed higher excess risk for earlier years could be the increased x-ray energies over time due to improvements in filtering and higher energy of the beam, combined with the known greater biological effectiveness of lower energy radiation[36 37]. However, the modifications in radiation energy spectrum are modest and unlikely to fully explain our observations. For the earlier years, there is greater uncertainty for the assessment of doses and it is possible that doses were underestimated during the earlier years, when they were largely derived from literature surveys, so that radiation-associated excess risk in those earlier years may have been overestimated.
Limitations of this study include uncertainties in dose estimation and recollection of past personal events and activities related to assessing confounders. Our UVR exposure assessment may be incomplete as we assessed UVR exposure by residential locations for different age periods of the technologist’s life but, did not account for other sources of UVR, including for example exposure during vacations. In selection of our analytic cohort, we excluded those who had BCCs diagnosed prior to baseline. If these BCCs were associated with higher doses in early calendar periods[38] then the excess risks we found could have been underestimated. We assessed this potential bias by stratifying our analysis into two time periods: follow-up until the second questionnaire and follow-up after the second questionnaire. There was little difference between the results for these time periods (Supplemental Table E). There is a possibility that those who worked longer have a different risk for BCC which could introduce bias. We addressed this by adjusting in our analysis the number of years worked and our findings were not materially changed. Other limitations of this study include uncertainty in the best method of adjustment for attained age. Age is a known correlate of dose and related to BCC risk. As shown in Supplemental Table C, increasing the number of age groups attenuated the dose and BCC relationship. It is possible that our main model using 12 attained age categories, may have over-adjusted for age . It is also possible there was insufficient variation in dose, after accounting for age, to detect an association. Although the survey response was relatively high (68-73%), selection bias due to non-response may have influenced our results. However, we found previously that adjustment for missing data in cohort-based analyses did not substantially change results [39].
Our study had several strengths. It is a large prospective cohort with low to moderate chronic IR exposure and one of the few cohorts that identified BCC cases and comprehensive risk factor information, including individual technologist’s values for lifetime UVR exposure. The results of the present analysis assessing these postulated risk factors are in general agreement with previous analyses in the USRT [15 20 40]. The number of BCC cases was large (n= 3,615) and the follow-up time was substantial (mean, 17 years). The questionnaire response rate was relatively high, 73%, and of those subjects with BCCs for whom medical records were obtained, over 85% were confirmed.
BCC is the most common cancer in Caucasian populations and incidence is rising [1], thus determinations of the risk factors and elucidation of its etiology is important. There is limited evidence from epidemiological studies for excess BCC risk at low radiation doses. Overall, we did not observe an excess radiation-related risk for lifetime cumulative occupational dose. Because of uncertainties in dosimetry and sensitivity to model specifications, both our null results and our findings of excess risk for exposure received before 1960 and dose received before age 30 should be interpreted with caution.
Supplementary Material
What this paper adds.
This analysis of the nationwide cohort of U.S. radiologic technologists, the largest population of medical radiation workers with long-term follow-up, examines the relation of low protracted exposure to occupational radiation with risk of basal cell carcinoma. Individual cumulative occupational radiation doses were estimated using recently improved dose reconstruction. The risk assessment accounted for many potentially confounding demographic, lifestyle, host and sun exposure factors.
Basal cell carcinoma risk was generally unrelated to occupational radiation exposure of the technologists, however there were indications of excess risk for dose received before age 30 and exposure before 1960.
To our knowledge, this is the only cohort of medical radiation workers with a large number of radiation-exposed female workers, over 900,000 badge dose measurements, questionnaire-derived work history and extensive information on potential confounders. Our findings contribute to the knowledge of basal cell carcinoma risk associated with low-dose, protracted radiation exposure.
Acknowledgements
The authors thank Drs. Elizabeth Platz and Paul Strickland for their input in the design of the study and review of early results of the study, and for the detailed and helpful comments of the three referees. We also thank the radiologic technologists who participated in the USRT Study; Jerry Reid of the ARRT for continued support of this study and Diane Kampa, Allison Iwan, and Richard Hoffbeck of the University of Minnesota for study management and data collection and management. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
References
- 1.Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg 2011;30(1):3–5 doi: S1085-5629(11)00013-7 [pii] 10.1016/j.sder.2011.01.002[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Karagas MR, Weinstock MA, Nelson HH Karatinocyte Carcinomas (Basal and Squamous Cell Carcinomas of the Skin). In: Schottenfeld DF, J. F, ed. Cancer Epidemiology and Prevention, Third Edition. New York: Oxford, 2006. [Google Scholar]
- 3.Ron E, Modan B, Preston D, et al. Radiation-induced skin carcinomas of the head and neck. Radiat Res 1991;125(3):318–25 [PubMed] [Google Scholar]
- 4.Shore RE. Radiation-induced skin cancer in humans. Med Pediatr Oncol 2001;36(5):549–54 doi: 10.1002/mpo.1128[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Ron E, Preston DL, Kishikawa M, et al. Skin tumor risk among atomic-bomb survivors in Japan. Cancer Causes Control 1998;9(4):393–401 [DOI] [PubMed] [Google Scholar]
- 6.Kishikawa M, Koyama K, Iseki M, et al. Histologic characteristics of skin cancer in Hiroshima and Nagasaki: background incidence and radiation effects. Int J Cancer 2005;117(3):363–9 doi: 10.1002/ijc.21156[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama H, Misumi M, Kishikawa M, et al. Skin Cancer Incidence among Atomic Bomb Survivors from 1958 and 1996. Radiat Res 2014. doi: 10.1667/RR13494.1[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Shore RE. Radiation induced cancer: risk assessment and prevention. Cancer Detect Prev 1984;7(3):181–90 [PubMed] [Google Scholar]
- 9.Shore RE, Moseson M, Xue X, et al. Skin cancer after X-ray treatment for scalp ringworm. Radiat Res 2002;157(4):410–8 [DOI] [PubMed] [Google Scholar]
- 10.Watt TC, Inskip PD, Stratton K, et al. Radiation-Related Risk of Basal Cell Carcinoma: A Report From the Childhood Cancer Survivor Study. J Natl Cancer Inst 2012. doi: djs298 [pii] 10.1093/jnci/djs298[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sevcova M, Sevc J, Thomas J. Alpha irradiation of the skin and the possibility of late effects. Health Phys 1978;35(6):803–6 [DOI] [PubMed] [Google Scholar]
- 12.Pukkala E, Helminen M, Haldorsen T, et al. Cancer incidence among Nordic airline cabin crew. Int J Cancer 2012. doi: 10.1002/ijc.27551[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Wang JX, Zhang LA, Li BX, et al. Cancer incidence and risk estimation among medical x-ray workers in China, 1950-1995. Health Phys 2002;82(4):455–66 [DOI] [PubMed] [Google Scholar]
- 14.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100(1):206–12 doi: 6604825 [pii] 10.1038/sj.bjc.6604825[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshinaga S, Hauptmann M, Sigurdson AJ, et al. Nonmelanoma skin cancer in relation to ionizing radiation exposure among U.S. radiologic technologists. International journal of cancer Journal international du cancer 2005;115(5):828–34 doi: 10.1002/ijc.20939[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 16.Simon SL, Preston DL, Linet MS, et al. Radiation organ doses received in a nationwide cohort of u.s. Radiologic technologists: methods and findings. Radiat Res 2014;182(5):507–28 doi: 10.1667/RR13542.1[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurdson AJ, Doody MM, Rao RS, et al. Cancer incidence in the US radiologic technologists health study, 1983-1998. Cancer 2003;97(12):3080–9 doi: 10.1002/cncr.11444[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DM, Kimlin MG, Hoffbeck RW, et al. Multiple indicators of ambient and personal ultraviolet radiation exposure and risk of non-Hodgkin lymphoma (United States). Journal of photochemistry and photobiology B, Biology 2010;101(3):321–5 doi: 10.1016/j.jphotobiol.2010.08.001[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahoon E, Rajaraman P, Alexander B, et al. Use of non-steroidal anti-inflammatory drugs and risk of basal cell carcinoma in the united states radiologic technologists study. Int J Cancer 2011. doi: 10.1002/ijc.26286[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstenblith M, Rajaraman P, Khaykin E, et al. Basal cell carcinoma and anthropometric factors in the U.S. radiologic technologists cohort study. Int J Cancer 2011. doi: 10.1002/ijc.26480[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon SL. Organ-specific external dose coefficients and protective apron transmission factors for historical dose reconstruction for medical personnel. Health Phys 2011;101(1):13–27 doi: 10.1097/HP.0b013e318204a60a[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. . New York: United Nations, 2008:13–322. [Google Scholar]
- 23.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation NRC. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII - Phase 2. Washington, DC, USA: National Academy Press, 2006. [PubMed] [Google Scholar]
- 24.Akaike H Likelihood of a model and information criteria. J Econometrics 1981;16(1):3–14 [Google Scholar]
- 25.Gilbert ES. Some confounding factors in the study of mortality and occupational exposures. AmJEpidemiol 1982;116(1):177–88 [DOI] [PubMed] [Google Scholar]
- 26.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res 2007;168(1):1–64 doi: RR0763 [pii] 10.1667/RR0763.1[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Shore RE, Albert RE, Reed M, et al. Skin cancer incidence among children irradiated for ringworm of the scalp. Radiat Res 1984;100(1):192–204 [PubMed] [Google Scholar]
- 28.Karagas MR, McDonald JA, Greenberg ER, et al. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. For The Skin Cancer Prevention Study Group. J Natl Cancer Inst 1996;88(24):1848–53 [DOI] [PubMed] [Google Scholar]
- 29.Andersson M, Engholm G, Ennow K, et al. Cancer risk among staff at two radiotherapy departments in Denmark. Br J Radiol 1991;64(761):455–60 [DOI] [PubMed] [Google Scholar]
- 30.Charles MW. Radon exposure of the skin: II. Estimation of the attributable risk for skin cancer incidence. J Radiol Prot 2007;27(3):253–74 doi: S0952-4746(07)50290-9 [pii] 10.1088/0952-4746/27/3/R02[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 31.Kojo K, Helminen M, Pukkala E, et al. Risk Factors for Skin Cancer among Finnish Airline Cabin Crew. Ann Occup Hyg 2013;57(6):695–704 doi: 10.1093/annhyg/mes106[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 32.dos Santos Silva I, De Stavola B, Pizzi C, et al. Cancer incidence in professional flight crew and air traffic control officers: disentangling the effect of occupational versus lifestyle exposures. Int J Cancer 2013;132(2):374–84 doi: 10.1002/ijc.27612[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 33.Rafnsson V, Hrafnkelsson J, Tulinius H, et al. Risk factors for cutaneous malignant melanoma among aircrews and a random sample of the population. Occup Environ Med 2003;60(11):815–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little MP. Heterogeneity of variation of relative risk by age at exposure in the Japanese atomic bomb survivors. Radiat Environ Biophys 2009;48(3):253–62 doi: 10.1007/s00411-009-0228-x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 35.Little MP, De Vathaire F, Charles MW, et al. Variations with time and age in the risks of solid cancer incidence after radiation exposure in childhood. Statistics in medicine 1998;17(12):1341–55 [DOI] [PubMed] [Google Scholar]
- 36.Little MP, Kwon D, Doi K, et al. Association of chromosome translocation rate with low dose occupational radiation exposures in US radiologic technologists. Radiat Res 2014;182(1):1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NCRP. National Council on Radiation Protection and Measurements (NCRP) Report No. 104. The relative biological effectiveness of radiations of different quality. In: (NCRP) NCoRPaM, ed.: NCRP, 1990. [Google Scholar]
- 38.Linet MS, Kim KP, Miller DL, et al. Historical Review of Occupational Exposures and Cancer Risks in Medical Radiation Workers. Radiat Res 2010. doi: 10.1667/RR2014.1 [pii][published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao RS, Sigurdson AJ, Doody MM, et al. An application of a weighting method to adjust for nonresponse in standardized incidence ratio analysis of cohort studies. Annals of epidemiology 2005;15(2):129–36 doi: 10.1016/j.annepidem.2004.05.007[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DM, Sigurdson A, Doody MM, et al. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2003;12(12):1540–3 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


