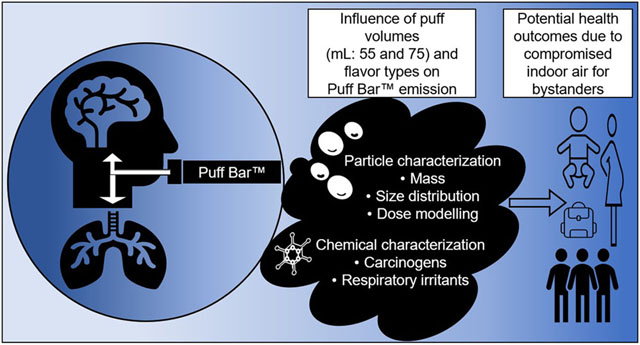
Abstract
Puff Bar™, one of the latest designs of e-cigarettes, heats a mixture of liquid using a battery-powered coil at certain temperatures to emit aerosol. This study presents a mass-based characterization of emissions from seven flavors of Puff Bar™ devices by aerosolizing with three puff topographies [(puff volume: 55 < 65 < 75-mL) within 4-seconds at 30-seconds interval]. We evaluated the effects of puff topographies on heating temperatures; characterized particles using a cascade impactor; and measured volatile carbonyl compounds (VCCs). Modeled dosimetry and calculated mass median aerodynamic diameters (MMADs) were used to estimate regional, total respiratory deposition of the inhaled aerosol and exhaled fractions that could pose secondhand exposure risk. Temperatures of Puff Bar™ e-liquids increased with increasing puff volumes: 55mL (116.6 °C), 65 mL (128.3 °C), and 75mL (168.9 °C). Flavor types significantly influenced MMADs, total mass of particles, and VCCs (μg/puff: 2.15–2.30) in Puff Bar™ emissions (p < 0.05). Increasing puff volume (mL:55 < 65 < 75) significantly increased total mass (mg/puff: 4.6 < 5.6 < 6.2) of particles without substantially changing MMADs (~1μm:1.02~0.99~0.98). Aerosol emissions were estimated to deposit in the pulmonary region of e-cigarette user (41–44%), which could have toxicological importance. More than 2/3 (67–77%) of inhaled particles were estimated to be exhaled by users, which could affect bystanders. The VCCs measured contained carcinogens—formaldehyde (29.6%) and acetaldehyde (16.4%)—as well as respiratory irritants: acetone (23.9%), isovaleraldehyde (14.5%), and acrolein (4.9%). As Puff Bar™ emissions contain respirable particles and harmful chemicals, efforts should be made to minimize exposures, especially in indoor settings where people (including vulnerable populations) spend most of their life-time.
Graphical Abstract
1. Introduction
Electronic cigarettes or vaping products (EVPs) operate by heating a liquid (“e-liquid”) to generate an aerosol that a user inhales (a portion of which is subsequently exhaled). This aerosol consists of liquid droplets (i.e., particles) and gases (i.e., chemicals) in air. Since their introduction in 2007, EVP design types have been under-going subsequent modifications (i.e., “generations”); current fourth generation EVPs are characterized by their pod-style design in which the e-liquid is housed in a disposable pod (e.g., JUULV® brand type) (Stefaniak et al. 2021). In February 2020, the U.S. Food and Drug Association (FDA) restricted the sale of flavored pods other than those with menthol and tobacco flavor (US FDA, 2020). Although this ban impacted pod-based EVPs such as JUULV® brand devices, it did not cover disposable EVPS that contain flavored e-liquids such as Puff Bars™ (Dai and Hao 2020). In June 2022, FDA denied authorization to market and ordered the removal of all JUULV® products from the U.S. market (US FDA, 2022). These enforcements might further increase usages of pod-alternative and non-regulated EVP devices like Puff Bars™ that offer an e-liquid variety of over 100 flavor types (https://puffbar.com/).
EVP emissions are not only harmful to device users, but can also compromise indoor air quality (IAQ) when users exhale, thereby posing a secondhand exposure risk (Li et al. 2020; Fernández et al. 2015; Avino et al. 2018; Tzortzi et al. 2020; McClelland et al. 2021; Johnson et al. 2019). According to Kleipes et al., people spend large proportions of time indoors at home or in cars (Klepeis et al. 2001). Indoor EVPs use potentially creates a secondhand exposure risk to occupants (Logue et al. 2017; Khachatoorian et al. 2022). Secondhand exposures to EVP emissions have been documented in retail shops, conventions, and other workplaces (Johnson et al. 2019; Khachatoorian et al. 2022; Li et al. 2021). Studies documenting EVP use at some indoor workplaces in the United States have demonstrated reduced productivity among non-users due to exposure to EVP emissions (Romberg et al. 2021; MMWR 2019). These emissions can contain considerable amounts of micrometer (μm)-sized particles and toxicants, including carcinogenic (e.g., formaldehyde, acetaldehyde) as well as irritant (e.g., acetone and acrolein) carbonyl compounds (Stefaniak et al. 2021; Li et al. 2021). Exposure to volatile carbonyl compounds (VCCs), which are associated with epithelial and mucosal damages in airways and immune response disruptions, have also been reported in primary EVP emissions (Stefaniak et al. 2021; Merecz-Sadowska et al. 2020; Hwang et al. 2016).
Characteristics of emissions in fourth generation EVP devices are dependent on many factors, including design type (e.g., pods or bars), e-liquid composition (e.g., density, proportion of polypropylene glycerin [PG] and vegetable glycerol [VG], diluents, flavor types, or additives in humectants) and users’ puffing behavior (i.e., puff topography) (Talih et al. 2019, 2020; Stefaniak et al. 2022; Ranpara et al. 2021b; Robinson et al. 2018). By heating either coil or wick, the temperature that aerosolizes flavored e-liquid has been reported as an influential operating parameter that affects physical and chemical characteristics of fourth generation EVP emissions (Margham et al. 2016; McAdam et al. 2019; Li et al. 2021; Manigrasso et al. 2021; Lechasseur et al. 2019). Physical characterization of EVP emissions, especially particle size distribution (PSD), is critical to evaluate respiratory deposition when an EVP user inhales emitted aerosols. Puffing volume (or puff flow rate) has been reported as an influential, modifiable factor that impacts PSD and the regional lung deposition on inhalation from pod-based EVP emissions with laboratory-based JUULV® alike e-liquids (Ranpara et al. 2021b). In that study, along with influence of different flavor type JUULV® e-liquids on PSD, approximately 50–70% of inhaled particles by JUULV® users were calculated to be exhaled (and potentially serve as a secondhand exposure risk), which was consistent with studies of earlier generation EVP (Ranpara et al. 2021b). However, environmental chamber studies conducted using human exhaled breath evaluation with other EVP device type, puff topography, and experimental set up differed from these mathematical predictions (Oldham et al. 2021a, 2021b). Son et al. documented harmful VCC emissions when various flavored e-liquids were aerosolized using previous (e.g., cig-a-like, top-coil, ‘mod’) and current (e.g., ‘pod’) generation EVP devices at different puff volumes and temperatures (Son et al. 2020).
This study focuses, how aerosol generation and characteristics from Puff Bar™ devices are impacted by changes in puff volume and subsequent flow rate (constant puff duration). Specifically, we investigated the influence of puff volumes on 1) temperature that aerosolizes e-liquids; 2) mass-based particle characterization (total mass/puff and PSD); and 3) mass-based VCC characterization (total mass/puff, and their relative proportion) in emissions.
2. Materials and methods
2.1. Puff bar™
We studied seven types of fruit flavored Puff Bar™ e-liquids (Wilmington, DE) The e-liquids contained 5% nicotine and were currently or previously available in the market out of more than 100 original flavors offered by the company (https://puffbar.com/). The seven flavors included Cucumber, Grape, Melon Ice, Orange-Mango-Guava (OMG), Pomegranate, Sour Apple, and Strawberry. The manufacturer reported the Puff Bar™ device contained 1.3 milliliter (mL) of e-liquid which allowed for ~300 puffs per device. We conducted three independent measurements to calculate the density in grams per mL (g/mL) of e-liquids by measuring the gravimetric mass at a volume of 200 microliter (μl) as previously described (Ranpara et al. 2021b). Puff Bar™ devices were purchased online from the company (https://puffbar.com/) in February 2021 with shelf life of 1–2 years. Puff Bar™ device were stored at laboratory conditions at 22.1 °C and 22.6% relative humidity with less than 5% relative standard deviation. Duration between acquisition and testing for herein studied experiments was less than 9 months. To avoid artificial elevation of coil temperature, first five puffs were excluded prior to considering measurements for evaluating temperatures, as well as particle and chemical characterization described in experimental set up below.
2.2. Experimental set up
An automated e-cigarette aerosol generator (ECAG+, e~Aerosols LLC, Central Valley, NY, USA) was programmed to aerosolize Puff Bar™ e-liquids. The ECAG+ works using positive pressure to aerosolize e-liquids; the EVP device is connected perpendicular to the generator to mimic an upright smoking position. Prior to conducting trials and recording measurements, each Puff Bar™ was puffed five times to ensure the coil was consistently heated at the calibrated puff topography. As a puff-flow-activated EVP, the modifiable Puff Bar™ factor was the puff flow rate (by varying puff volume or puff duration or both), which activates the coil to heat the e-liquid and create an aerosol that is inhaled by the user. In accordance with ISO method and modified CORESTA 81 method, three puff volumes were studied—55, 65, and 75mL—with fixed 4-second puff durations (rather than the CORESTA specified 3s duration). Each puff was 30 seconds apart (puff interval) (Ranpara et al. 2021b; ISO 20768, 2018; CORESTA 2015). To achieve these puff volumes with a fixed 4-second puff duration, the puff flow rates in liters per minute (LPM) were: 0.8 ± 0.002, 1.0 ± 0.002, and 1.1 ± 0.001, respectively and were calibrated using a soap-bubble flow meter (Borgwaldt KC GmbH, Hamburg, Germany) as a primary volumetric flow calibration device.
2.3. Effect of puff volumes on temperature heating of puff bar™ e-liquids
Previous studies have utilized a thermocouple to measure the temperature of the EVP coil that heats the wick soaked with e-liquid to form aerosol from various generations of EVP devices (Zhao, Shu, et al. 2016; Zhao, Pyrgiotakis, et al. 2016; Chen et al. 2018; Manigrasso et al. 2021; Lechasseur et al. 2019). Figure 1 shows a disassembled Puff Bar™. To maintain device integrity while measuring temperature during aerosolization of an e-liquid for each puff profile, we removed only the mouthpiece of the Puff Bar™ and placed the probe as close as possible to the coil (without contacting it) using a type-K thermocouple (Model# 4233, Fisher Scientific, Pittsburgh, PA, USA; error: ±2.2 °C). All tests were conducted under ambient environment in a laboratory at 22.1 °C and 22.6% relative humidity with relative standard deviation <5%. As illustrated in the schematic of the experimental setup (Figure 2a), three separate measurements (n = 3) for maximum temperature were recorded when all the flavored Puff Bar™ e-liquids (other than Grape) aerosolized at each puff flow rate.
Figure 1.
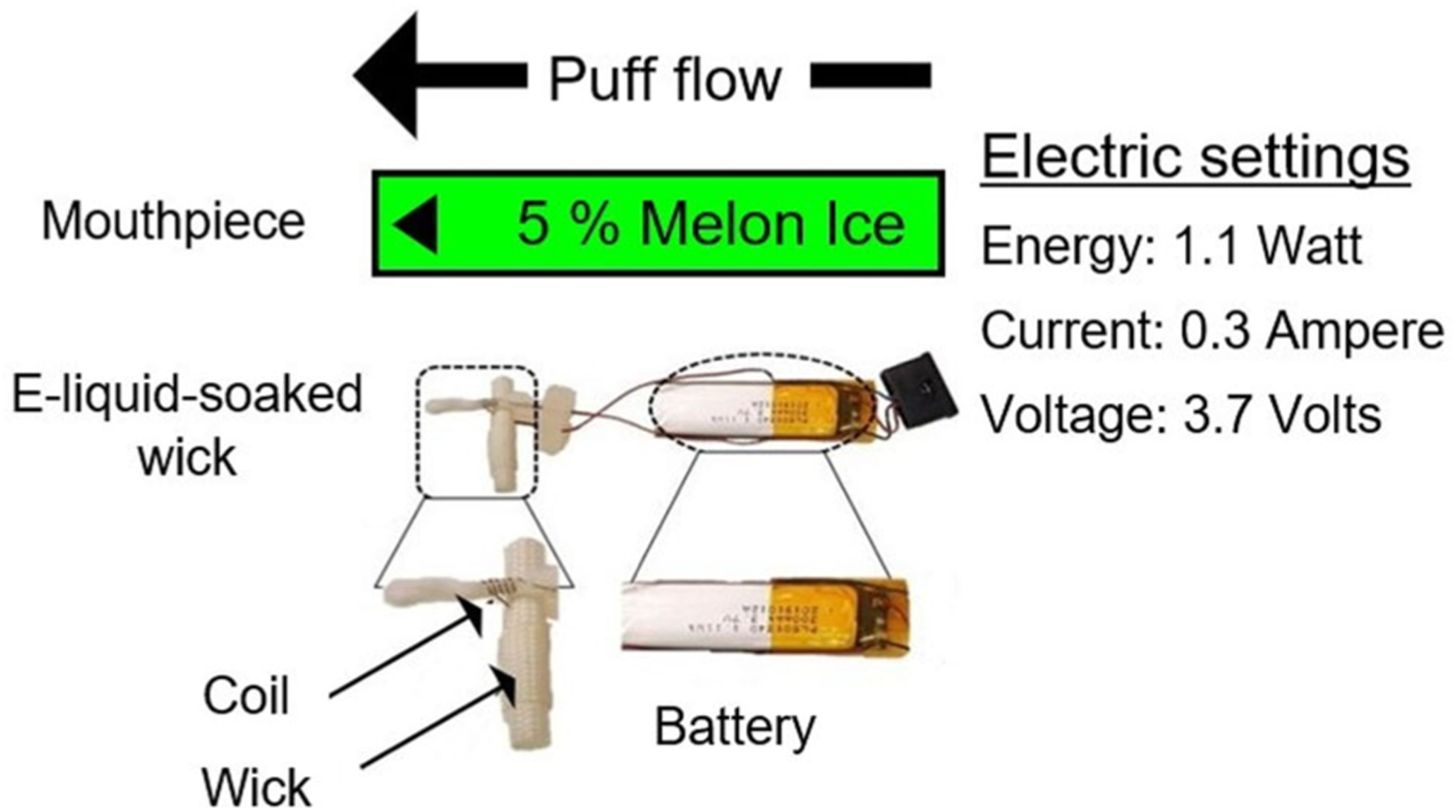
Structure of Puff Bar™ devices.
Figure 2.
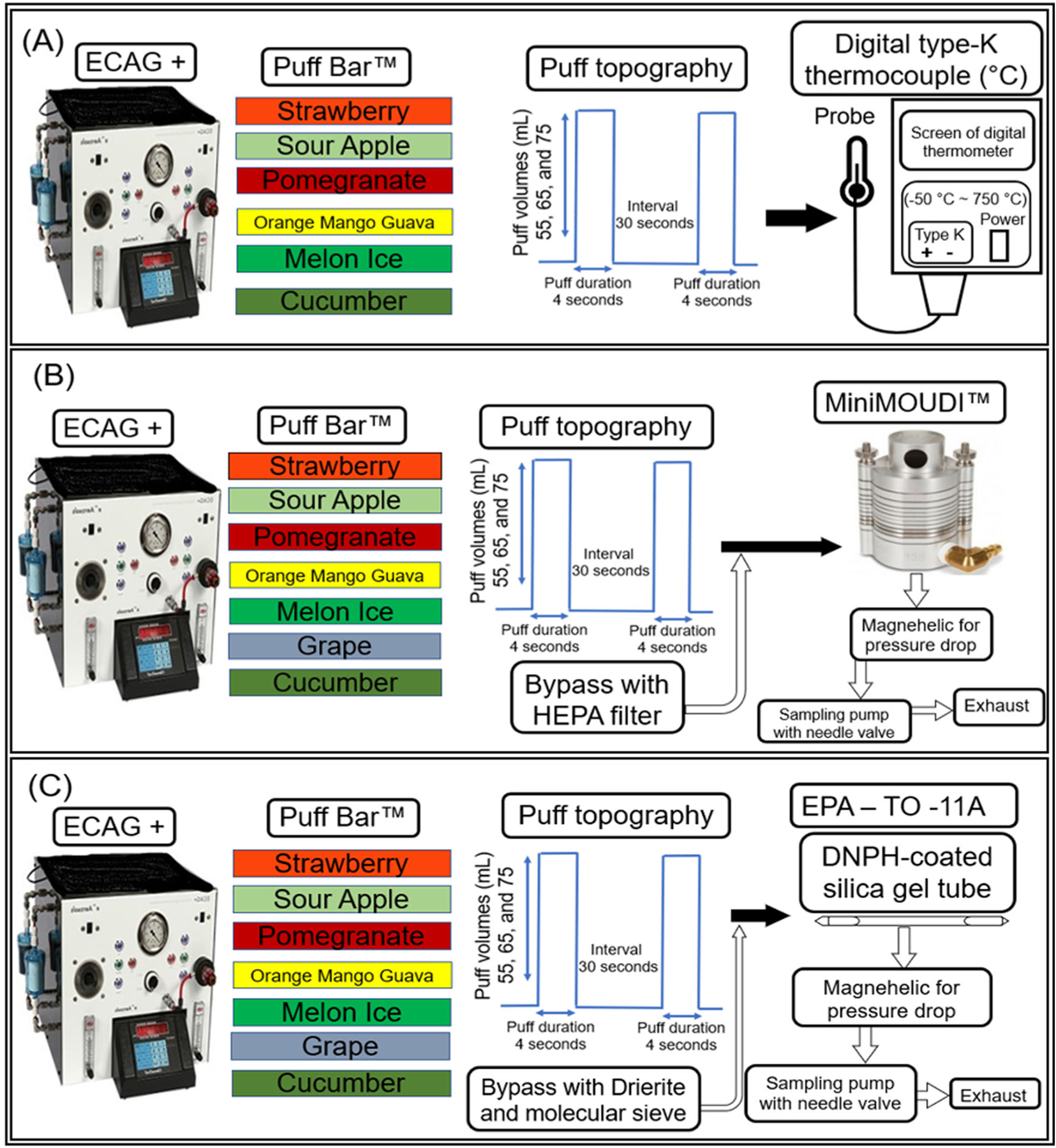
Schematic of experimental set up. Influence of puff volumes on (a) temperature to heating Puff Bar™ e-liquid (b) mass-based particle size distribution (c) chemical characterization using EPA method TO-11A.
2.4. Mass-based particle characterization and dosimetric estimates
Previous studies have emphasized mass-based PSD of EVP aerosols as an influential factor for estimating their regional respiratory deposition during inhalation and exhalation (Sosnowski and Kramek-Romanowska 2016; Rostami, Samuel, and Pithawalla 2018; Oldham et al. 2018; Zhao et al. 2018). Though critical, determining the PSD of EVP aerosols is complex because these liquid droplets contain, and are, suspended in volatile chemicals meaning their innate size can change during sampling and in the respiratory tract depending on various conditions, such as evaporation, hygroscopic growth, and thermodynamic behavior (Asgharian et al. 2018a, 2018b; Sosnowski and Kramek-Romanowska 2016; Pankow 2017; Pankow et al. 2018). Changes in PSD from sampling conditions (e.g., high flow rates that induce evaporation) or from aerosol behavior in the respiratory tract (e.g., bolus effects, hygrosocopic growth) can translate into uncertainty in predictions of regional particle deposition. Changes in PSD from sampling conditions can be controlled by the experimental setup. As in prior studies by our group and others, EVP aerosol were sampled using a cascade impactor to maintain the native properties of the aerosol intact (Zhao, Pyrgiotakis, et al. 2016; Ranpara et al. 2021a, b; Oldham et al. 2018; Kane and Li 2021; Pourchez et al. 2018). Figure 2b shows a schematic of our testing apparatus used to evaluate mass-based PSD with minimal dilution to sample total EVP aerosol constituting particles and chemicals such as VCCs. Using the experimental set up, no significant statistical deviations in mass-based aerodynamic diameters were observed by aerosolizing e-liquids at different puff volumes (mL: 55, 65, and 75) at 7.5W with pod-based EVP device (Ranpara et al. 2021b). A MiniMOUDI™ (MSP Corporation, Shoreview, MN, USA) cascade impactor was used to size-fractionate EVP aerosols (size range: 0.056–10 μm) at a sampling flow rate of 2.0 LPM. Three trials with two puffs per trial were conducted for each Puff Bar™ flavor type. The self-powered Puff Bar™ and ECAG+forced emitted EVP aerosols with the established puff topography directly into the MiniMOUDI™. While the ECAG+ was operating at the specified puff topography, the rest of the MiniMOUDI™ sampling flow rate of 2.0 LPM entered from a bypass air flow that passed through a high-efficiency particulate air filter (HEPA; Whatman® Schleicher & Schuell; Stockbridge, GA, USA).
Knowledge of mass-based PSD (MMAD: mass median aerodynamic diameter; GSD: geometric standard deviation), coupled with physiological information of respiratory tract characteristics (anatomy, ventilation parameters), can be used to model dosimetry for EVP users (Rostami, Samuel, and Pithawalla 2018; Oldham et al. 2018). Among existed dosimetry models (e.g., computational fluid dynamics approaches, the International Commission for Radiological Protection human respiratory tract model, and multiple path particle dosimetry model (MPPD)), the MPPD model is based on realistic lung geometry, physiology and deposition mechanisms, and it provides estimates of both the whole-lung and the regional particle deposition fractions that were validated with experimental data (Li et al. 2021). Hence, for our purposes, the freely available MPPD model (version 3.04, ARA, Albuquerque, NM, USA) was considered to conceptually illustrate regional and total particle deposition throughout the respiratory tract of e-cigarette users and derive an estimate of the exhaled fraction. It is important to note that MPPD can be been modified to account for the thermodynamic behavior of EVP aerosol in the respiratory tract (Asgharian et al. 2018a, 2018b); however, this version of the program is not yet publicly available. As such, estimated numerical values reported herein using the current free version of MPPD should be interpreted with caution.
The fraction of particles deposited in the respiratory tract was calculated based on a default adult human symmetric model as an oronasal mouth breather (the Yeh–Schum model) in an upright position. MPPD (version 3.04) uses empirically-derived equations to provide deposition estimates for aerosols inhaled via the nose and mouth, which is characteristic breathing pattern of traditional cigarette smokers or EVP users (i.e., oro-nasal breathing). Additionally, MPPD calculates specific details of regional deposition in the lungs (e.g., regional, total, lobar, airway generation levels) in different species such as human, rats, and mice. Therefore, researchers (e.g., toxicologist) can replicate the inputs and correlate the interpretations of our results with existing literature as well as across the future studies with human or animal participants for exposure assessments. Regional depositions were reported as mass deposited in the head, tracheobronchial (TB), and pulmonary regions, so estimated results correspond with the results from realistic lung structures in accordance to upright smoking positions depicted in experiment set up (section 2.2). Total inhaled respiratory deposition fractions were calculated by summing the regional (the head, TB and pulmonary) deposition fractions. The fraction of the aerosol exhaled by an EVP user was estimated as 1 minus the total inhaled deposited fraction.
2.5. Mass-based chemical characterization of puff bar™ emissions
As shown in Figure 2c, we measured 14 chemicals emitted from Puff Bar™ on 2,4-dinitrophenylhydrazine (DNPH)-coated silica gel tubes (catalog # 226-119-7: SKC Inc., Eight Four, PA, USA) at a flow rate of 1.5 LPM using EPA method TO-11A. The self-powered Puff Bar™ and ECAG+ forced emitted chemicals with the established puff topography into the DNPH tube. While the ECAG+ was operating at determined puff topography, the rest of the sampling flow rate of 1.5 LPM entered from a bypass air flow that was dried and cleaned using a Drierite gas purifier (W.A. Hammond drierite company LTD, Xenia, OH, USA) and molecular sieve (Sigma-Aldrich CO, St. Louis, MO, USA).
We conducted a sensitivity analysis for assessing these chemicals from Puff Bar™ emissions at various conditions such as different puff numbers (2, 3, 4, and 5) within 3 min, and different puffing durations (minutes: 5, 6, 7, and 8) (online supplementary information [SI] Figures S1A and B). Based on this analysis, we chose 3 trials each with 3 puffs within 3 min per trial to assess emissions from each Puff Bar™ flavor type using 3 puff topographies. We focused on 8 of the 14 chemicals analyzed (see Table 1 for chemical information) because the other six VCCs (benzaldehyde, butyraldehyde, crotonaldehyde, hexaldehyde, pentanal (valeraldehyde), and 2,5-dimethylbenzaldehyde) were below detection limits or reported as consistently less than 1% of total mass of chemicals for more than 3 puffs within 3 min of sampling in our sensitivity analysis (Figures S1A and B). The total mass of VCCs was calculated by summing the masses of all eight individual VCCs quantified in the Puff Bar™ emissions. The proportion of individual VCCs out of total mass of chemicals evaluated in percentages (% = mass of chemical/total mass of chemicals) was also calculated. Total mass and proportion of VCCs in the emission of all trials with Puff Bar™ for puff volume 55, 65, and 75 mL were presented in the SI, Tables S4–6.
Table 1.
VCCs of focus emitted from Puff Bar™.
| # | Analyte | CAS # | Hazardous classification* | Irritant (Yes/no)* | Sensitizer (Yes/no)* | Carcinogen (Yes/no)** | Exposure limits (μg/m3) |
|---|---|---|---|---|---|---|---|
| 1 | 2-butanone+ (methyl ethyl ketone) | 1338-23-4 | Flammable, Corrosive | Yes | No | No | REL: 1500 PEL: 5000 |
| 2 | Acetone | 67-64-1 | Flammable | Yes | No | No | REL: 590000 PEL: 2400000 |
| 3 | Acrolein | 107-02-8 | Flammable, Corrosive Acute Toxic, Environmental hazard | Yes | No | Yes, Group 2 A: Probably carcinogenic to human | REL: 250 PEL: 250 |
| 4 | Acetaldehyde | 75-07-0 | Flammable, Health Hazard | Yes | Yes | Yes, Group 2B: Possibly carcinogenic to human | REL: NE PEL: 360000 |
| 5 | Formaldehyde | 50-00-0 | Corrosive, Acute Toxic, Health hazard | Yes | Yes | Yes, Group 1: Carcinogenic to human | REL: 2 PEL: 921 |
| 6 | Isovaleraldehyde | 590-86-3 | Flammable, Environmental hazard | Yes | Yes | No | REL: NE PEL: NE |
| 7 | o, m, p -Tolualdehyde | o-529-20-4 m-620-23-5 p-104-87-0 |
Corrosive, Toxic | Yes | No | No | REL: NE PEL: NE |
| 8 | Propionaldehyde (propanal) | 123-38-6 | Flammable | Yes | No | No | REL: NE PEL: NE |
Other than 2-butanone, rest of VCCs were analyzed in accordance with EPA method TO-11A, but standard stock solutions were prepared by Restek and Supelco. To prepare the 2-butanone standards, two aliquots of 2-butanone neat material were weighed separately and derivatized in an Acetonitrile/2,4-Dinitrophenylhydrazine (DNPH) solution. A dilution of both standards was then analyzed and agreed within 1.5%.
According to Globally Harmonized System (GHS) for classification and labeling of chemicals;
International Agency for Research on Cancer, NE – Not established
All the analytes (exception: o, m, p - Tolualdehyde) are toxic on inhalation, intraperitoneal, oral, and skin exposure. Results of o, m, p – Tolualdehyde are presented as total Tolualdehyde for simplicity.
REL – recommended exposure limit; PEL – permissible exposure limit
2.6. Statistical analysis
Data were log-transformed and managed using JMP 15.1.0 (SAS Institute, Inc., Cary, NC, USA). Deposited mass (in milligrams [mg]) of EVP aerosols for each impactor stage of the MiniMOUDI™ was calculated by measuring the aluminum substrate before (pre-) and after (post-) sampling. Trials were performed in triplicate (n = 3 per Puff Bar™ type per puff volume). Significant differences (p < 0.05) between MMADs (μm), total mass of aerosol (mg/puff), and total mass of VCCs (μg/puff) were determined using an ANOVA and Tukey’s HSD for multiple comparisons. These regression models investigated how puff volumes and flavor types affected the total mass of aerosol, MMAD, and total mass of chemicals. Average and standard deviations (SD) for triplicate measurements were considered for total mass of aerosol (mg/puff) and MMAD (μm) when flavored Puff Bar™ e-liquids were aerosolized with 55-, 65-, and 75- mL puff volumes. Total mass of aerosol, mass-based PSD, and lung deposition of all trials with Puff Bar™ for puff volume 55, 65, and 75 mL were presented in the SI, Tables S1–S3. For particle characterization, SD indicates variability in triplicate MMAD measurements and GSD indicates width of PSD. We studied the influence of puff volume on temperature (Section 2.3 and Figure 2a) separately from the characterization of emissions for particles (Section 2.4 and Figure 2a) and for chemicals (Section 2.5 and Figure 2c). Therefore, we did not evaluate the association between temperature and the characterized emission for particles (total mass of aerosols, MMAD) and chemicals (total mass of VCCs).
3. Results
3.1. Influence of puff volume on temperature of e-liquids
When flavored Puff Bar™ e-liquids were aerosolized at three puff volumes (mL: 55, 65, and 75), corresponding temperatures of the flavored e-liquids ranged from 85 °C to 250 °C, with an average ± SD of 140.9 ± 44.6 °C. We observed a significant influence of puff volume (p < 0.05), but not of flavor type, on the heated temperature of e-liquids. Specifically, a 75mL puff volume (168.9 °C) was significantly different than a 55mL (116.6 °C) or a 65mL (128.3 °C) puff volume. However, we did not observe a significant difference in temperature of e-liquids between 55mL and 65mL puff volumes.
3.2. Influence of puff volume and flavor type on particle emissions
Table 2 shows comparisons of e-liquid densities, total mass (mg/puff), MMAD (μm: average ± SD of three trials), and average GSD of flavored Puff Bar™ e-liquids when aerosolized using three puff volumes. Density of the flavored Puff Bar™ e-liquids ranged from 1.08 g/mL (Cucumber) to 1.23 g/mL (Strawberry) with an average ± SD (g/mL: 1.14 ± 0.1). We observed significant influences of puff volumes (p < 0.001) and flavor type (p < 0.0001) on total mass of emitted aerosols. Tukey’s HSD revealed significant differences (p < 0.05) in total mass of aerosols emitted between puff volumes of 55mL (4.6 mg/puff) and 75mL (6.2 mg/puff), as well as between 55mL (4.6 mg/puff) and 65mL (5.6 mg/puff) but not between 65mL and 75mL. Overall, we noticed greater total mass emissions with increasing puff volumes (55mL: 4.6 mg/puff, 65mL: 5.6 mg/puff, and 75mL: 6.2 mg/puff), except for the flavor OMG (65mL: 4.67 mg/puff, 75mL: 4.31 mg/puff).
Table 2.
Total mass, particle size distribution (PSD), regional respiratory deposition, and exhaled aerosol for Puff Bar™ flavor types** at three puff volumes (mL: 55* , 65, and 75*) with n = 3 per puff volume.
| Total mass of aerosol | Mass-based PSD | Inhaled deposition fraction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Puff Bar™ flavor types | Density of e-liquid (g/mL) | Puff volumes (mL) | Average (mg/puff) ± SD | Average MMAD (μm) | Average GSD | Head | TB | Pulmonary | Total | Exhaled fraction |
| Cucumber | 1.08 | 55 | 5.34 ± 0.28 | 1.01 | 1.50 | 0.11 | 0.06 | 0.12 | 0.29 | 0.71 |
| 65 | 6.60 ± 0.07 | 1.02 | 1.50 | 0.10 | 0.06 | 0.12 | 0.28 | 0.72 | ||
| 75 | 6.72 ± 0.08 | 0.99 | 1.40 | 0.09 | 0.06 | 0.12 | 0.27 | 0.73 | ||
| Grape | 1.09 | 55 | 4.89 ± 0.38 | 1.12 | 1.69 | 0.13 | 0.06 | 0.13 | 0.32 | 0.67 |
| 65 | 5.84 ± 0.09 | 1.01 | 1.50 | 0.10 | 0.06 | 0.12 | 0.28 | 0.72 | ||
| 75 | 6.41 ± 0.31 | 1.08 | 1.55 | 0.11 | 0.06 | 0.13 | 0.30 | 0.70 | ||
| Melon Ice | 1.12 | 55 | 4.44 ± 0.06 | 0.98 | 1.51 | 0.09 | 0.06 | 0.12 | 0.27 | 0.73 |
| 65 | 5.11 ± 0.26 | 0.97 | 1.40 | 0.09 | 0.06 | 0.11 | 0.26 | 0.74 | ||
| 75 | 7.73 ± 0.54 | 0.87 | 1.23 | 0.07 | 0.06 | 0.10 | 0.23 | 0.77 | ||
| OMG | 1.20 | 55 | 4.17 ± 0.22 | 1.04 | 1.54 | 0.11 | 0.06 | 0.13 | 0.30 | 0.70 |
| 65 | 4.67 ± 0.31 | 1.02 | 1.49 | 0.10 | 0.06 | 0.12 | 0.28 | 0.72 | ||
| 75 | 4.31 ± 0.73 | 1.03 | 1.48 | 0.10 | 0.06 | 0.12 | 0.28 | 0.72 | ||
| Pomegranate | 1.12 | 55 | 5.08 ± 0.06 | 1.03 | 1.48 | 0.10 | 0.06 | 0.12 | 0.28 | 0.72 |
| 65 | 6.06 ± 0.08 | 1.00 | 1.43 | 0.09 | 0.06 | 0.12 | 0.27 | 0.73 | ||
| 75 | 6.18 ± 0.17 | 0.98 | 1.42 | 0.09 | 0.06 | 0.12 | 0.27 | 0.73 | ||
| Sour Apple | 1.13 | 55 | 6.08 ± 0.13 | 0.97 | 1.38 | 0.09 | 0.06 | 0.11 | 0.26 | 0.74 |
| 65 | 7.42 ± 1.09 | 0.93 | 1.29 | 0.07 | 0.06 | 0.11 | 0.24 | 0.76 | ||
| 75 | 7.91 ± 0.24 | 0.95 | 1.35 | 0.08 | 0.06 | 0.11 | 0.25 | 0.75 | ||
| Strawberry | 1.23 | 55 | 2.02 ± 0.17 | 0.98 | 1.42 | 0.09 | 0.06 | 0.12 | 0.27 | 0.73 |
| 65 | 3.65 ± 0.13 | 0.96 | 1.36 | 0.09 | 0.06 | 0.11 | 0.26 | 0.74 | ||
| 75 | 4.09 ± 0.11 | 0.93 | 1.30 | 0.08 | 0.06 | 0.11 | 0.25 | 0.75 | ||
TB: Tracheobronchial; PSD = particle size distribution MMAD=mass median aerodynamic diameter; SD=standard deviation; GSD=geometric standard deviation; Over all, Puff Bar™ flavor type** (p < 0.0001) and puff volumes* (p < 0.001) influenced total mass of emitted aerosols; Significant differences evaluated between Puff volumes* on total mass of aerosol (55 mL [4.6 mg/puff] vs 75 mL [6.2 mg/puff] and 65 mL [5.6 mg/puff]) and MMADs (55 mL [1.02 μm ± 0.05] vs 75 mL [0.98 μm ± 0.07]) at p < 0.05.
An MMAD particle size of approximately 1 μm was observed regardless of puff volume (55 mL: 1.02 μm, 65 mL: 0.99 μm, and 75 mL: 0.98 μm). Additionally, we observed comparable GSD measurements among puff volumes (55 mL: 1.50, 65 mL: 1.42, and 75 mL: 1.39). We observed significant influences of puff volume and flavor type (p < 0.0001) on MMADs of emitted particles. We only observed significant differences in MMADs between 55mL (1.02 μm ± 0.05) and 75 mL (0.98 μm ± 0.07) puff volumes, but this small difference of 0.04 μm did not affect regional dosimetry estimates for aerosol deposition in the respiratory tract (see Section 3.3). The greatest average MMAD ± SD (1.12 μm ± 0.02) and GSD (1.69) resulted with 55 mL puff volume aerosolizing Grape flavor. The smallest average MMAD ± SD (0.87 μm ± 0.02) and GSD (1.23) were measured when aerosolizing Melon Ice flavor at 75mL puff volume.
3.3. Dosimetry estimates for puff bar™ emissions
Based on MMAD and GSD, dosimery estimates resulted in similar total and regional respiratory tract depositions, which translated to similar exhaled fractions (Table 2). Comparing the three puff volumes, a wider range in the estimated total respiratory deposition was observed at 55mL (26–33%) and 75mL (23– 30%) compared to 65mL (24–28%). Hence, a greater puff volume coupled with smaller observed MMADs will, upon inhalation, result in greater deposition in the pulmonary region, lower total respiratory deposition, and therefore greater percentage of exhaled aerosol. For example, Melon Ice at 75-mL puff volume resulted in the smallest MMAD (0.87 μm ± 0.02), greatest pulmonary region deposition (44%), lowest total respiratory deposition (23%), and greatest exhaled aerosol estimates (77%). Generally, regional deposition estimates were comparable between the 55mL (head: 36%, pulmonary: 43%) and 75mL (head: 35%, pulmonary: 43%) puff volumes. Generally, increasing puff volumes resulted in subsequently smaller GSDs but were estimated to deposit a similar percentage of aerosol in the head region. For example, increasing puff volumes from 55mL (GSD: 1.50) to 65mL (GSD: 1.42) and 75mL (GSD: 1.39) resulted in smaller GSDs but estimated percentage of aerosol deposit in the head region were similar (55 mL: 36%, 65 mL:35%, 75 mL: 34%). The Grape flavor at 55mL puff volume had the greatest GSD (1.69) and greatest MMAD (1.12 μm ± 0.02) and resulted in the greatest predicted deposition in the head (39%). Overall, most (average 73% ± 2) total inhaled aerosols emitted from Puff Bar™ were estimated to be exhaled by users, which could negatively impact IAQ and pose a risk of secondhand exposure to bystanders.
3.4. Influence of puff volume and flavor type on chemical emissions
Figure 3 shows total mass (μg/puff) of eight out of fourteen VCCs emitted when flavored Puff Bar™ e-liquids were aerosolized at three puff volumes. For brevity, the results of the other six VCCs that contributed less to the total mass (as less than detection limits or reported as consistently less than 1% of total mass of chemicals for more than 3 puffs within 3 min of sampling) are presented in the SI Figures S1A and B. Overall, flavor type but not puff volume, resulted in significant differences in total mass of emitted VCCs. The smallest and the largest total mass of VCCs were reported with the OMG flavor at 55mL (0.76 mg/puff ± 0.09) and Strawberry at 75 mL (5.48 μg/puff ± 0.48), respectively. Generally, the total mass of VCCs emitted from different flavor types demonstrated non-linear patterns on increasing puff volumes other than Strawberry and Cucumber. For Strawberry (55 mL: 3.38 μg/puff, 65 mL: 4.84 μg/puff, and 75 mL: 5.48 μg/puff) and Cucumber (55 mL: 1.27 μg/puff, 65 mL: 1.55 μg/puff, and 75 mL: 1.72 μg/puff), the total VCCs mass consistently increased with increasing puff volumes.
Figure 3.
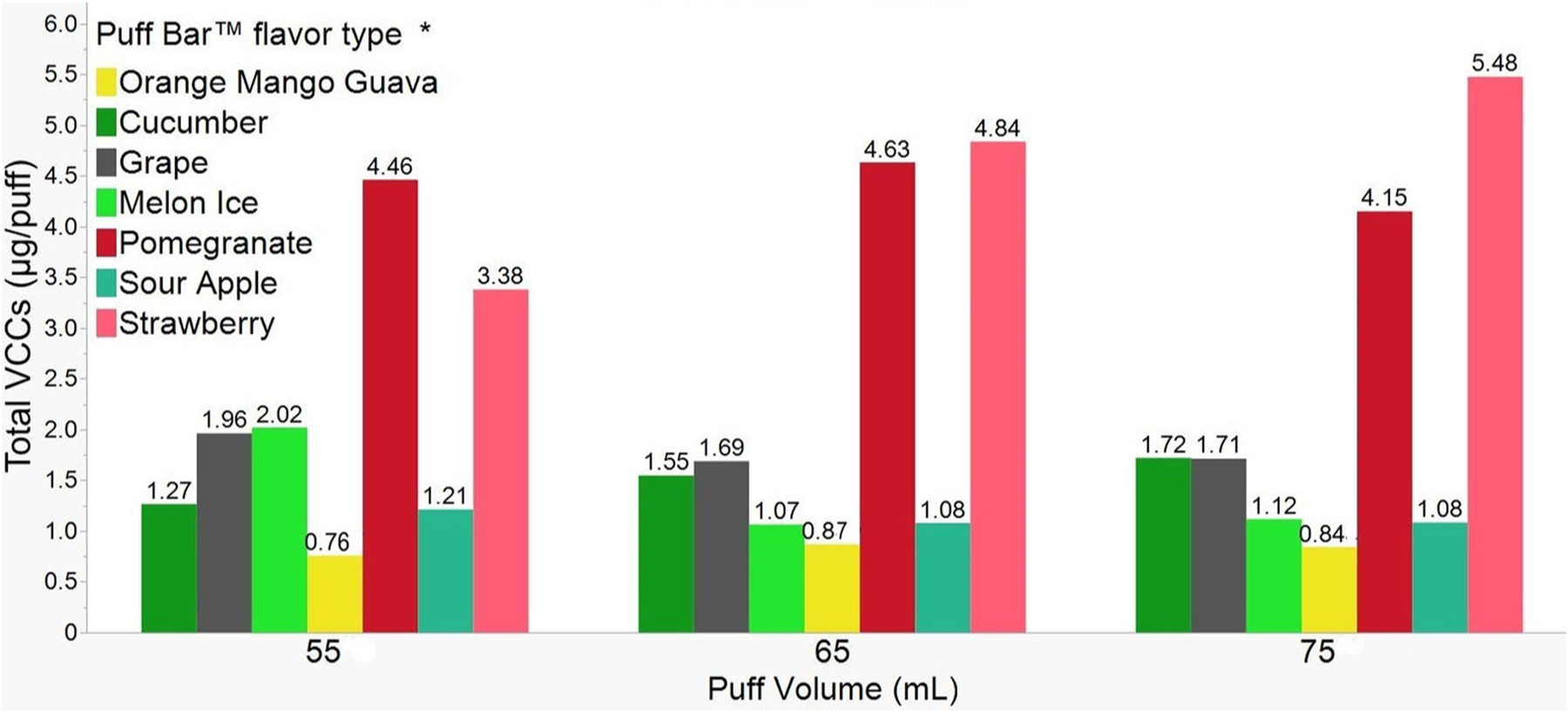
Total mass (average (μg/puff) ± SD) of VCCs emitted by different flavor types of Puff Bar™ on aerosolizing at puff volumes (mL: 55, 65, and 75) for n = 3 per puff volume. *Puff Bar™ flavor types but not puff volumes significantly influence (at p < 0.05) total mass of VCCs.
To further address the influence of different flavors, the SI Figure S2 presents the total mass of VCCs normalized by puff volume and number for comparability across different flavor type. Generally, total VCCs (μg/ml/puff) for a given flavor e-liquid was reduced or remained constant on increasing puff flow rate from 0.8 LPM to 1.1 LPM. The only exception was Strawberry, with which normalized total VCC increased when aerosolized from puff flow rate 0.8 LPM (0.061 μg/mL/puff) to 1.0 LPM (0.074 μg/mL/puff).
The top five VCCs emitted by mass from Puff Bar™ e-liquids (μg/puff) are presented in Figure 4. These five VCCs are carcinogens (formaldehyde [0.61 ± 0.35] and acetaldehyde [0.46 ± 0.47]) and respiratory irritants (acetone [0.42 ± 0.14], isovaleraldehyde [0.22 ± 0.05], and acrolein [0.18 ± 0.25]). Figure 5 shows the proportion of specific VCCs out of the total mass of VCCs. The largest to smallest proportion of VCCs for all flavors and puff volumes were formaldehyde (29.6% ± 0.12), acetone (23.9% ± 0.09), acetaldehyde (16.4% ± 0.09), isovaleraldehyde (14.5% ± 0.08), and acrolein (5.0% ± 0.05). We also noticed trace amounts (less than 5.0% of the total) of other respiratory irritants such as propionaldehyde (4.7% ± 0.04), 2-butanone (3.1% ± 0.04), and total tolualde-hyde (2.3% ± 0.02) (SI Figures S1A and B).
Figure 4.
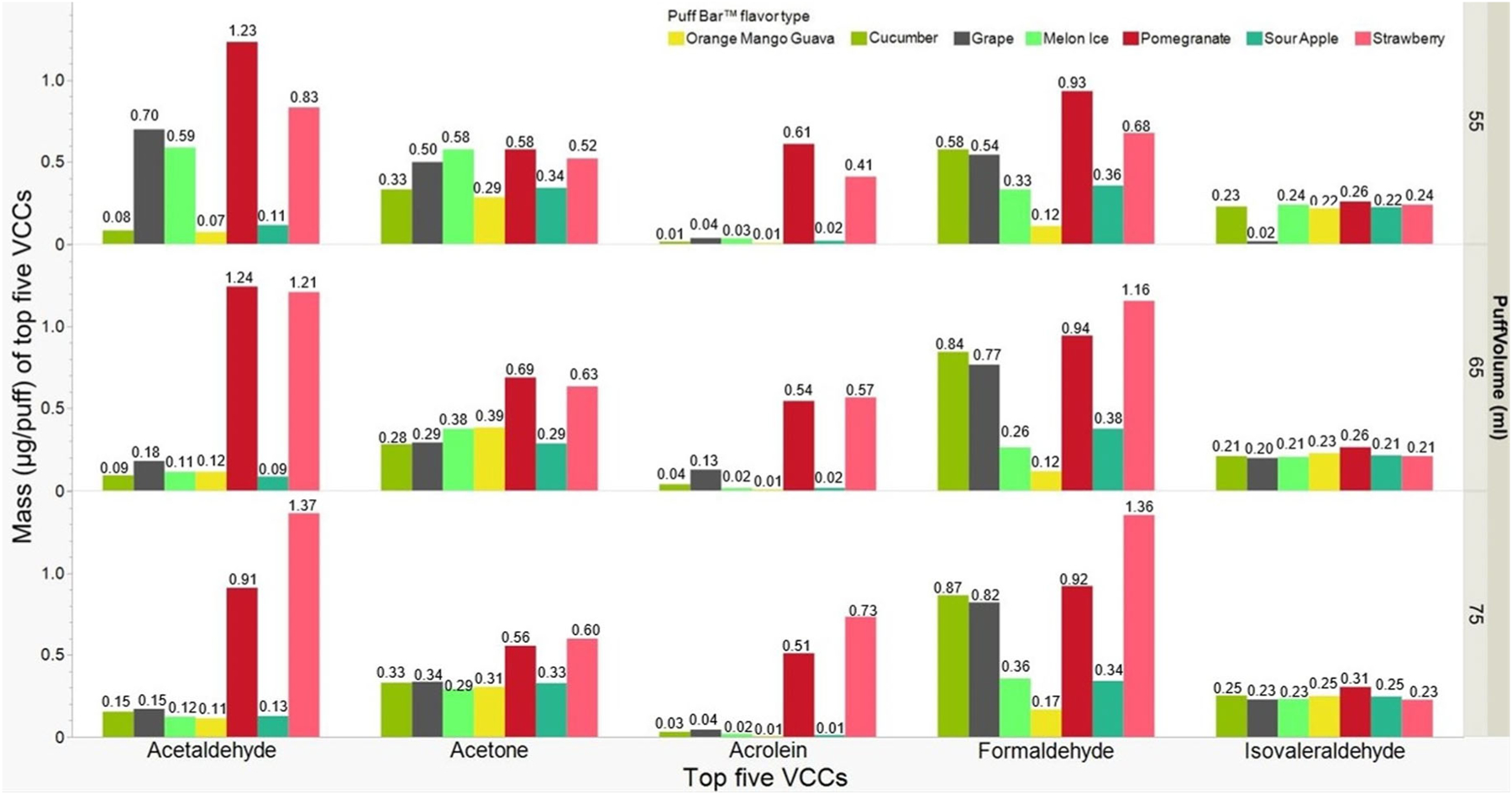
Mass (μg/puff) of top five VCCs emitted in different flavor types of Puff Bar™ on aerosolizing at puff volumes (mL: 55, 65, and 75) for n = 3 per puff volume (average ± SD): Formaldehyde (0.61 ± 0.35), Acetaldehydes (0.46 ± 0.47), Acetone (0.42 ± 0.14), Isovaleraldehyde (0.22 ± 0.05), and Acrolein (0.18 ± 0.25).
Figure 5.
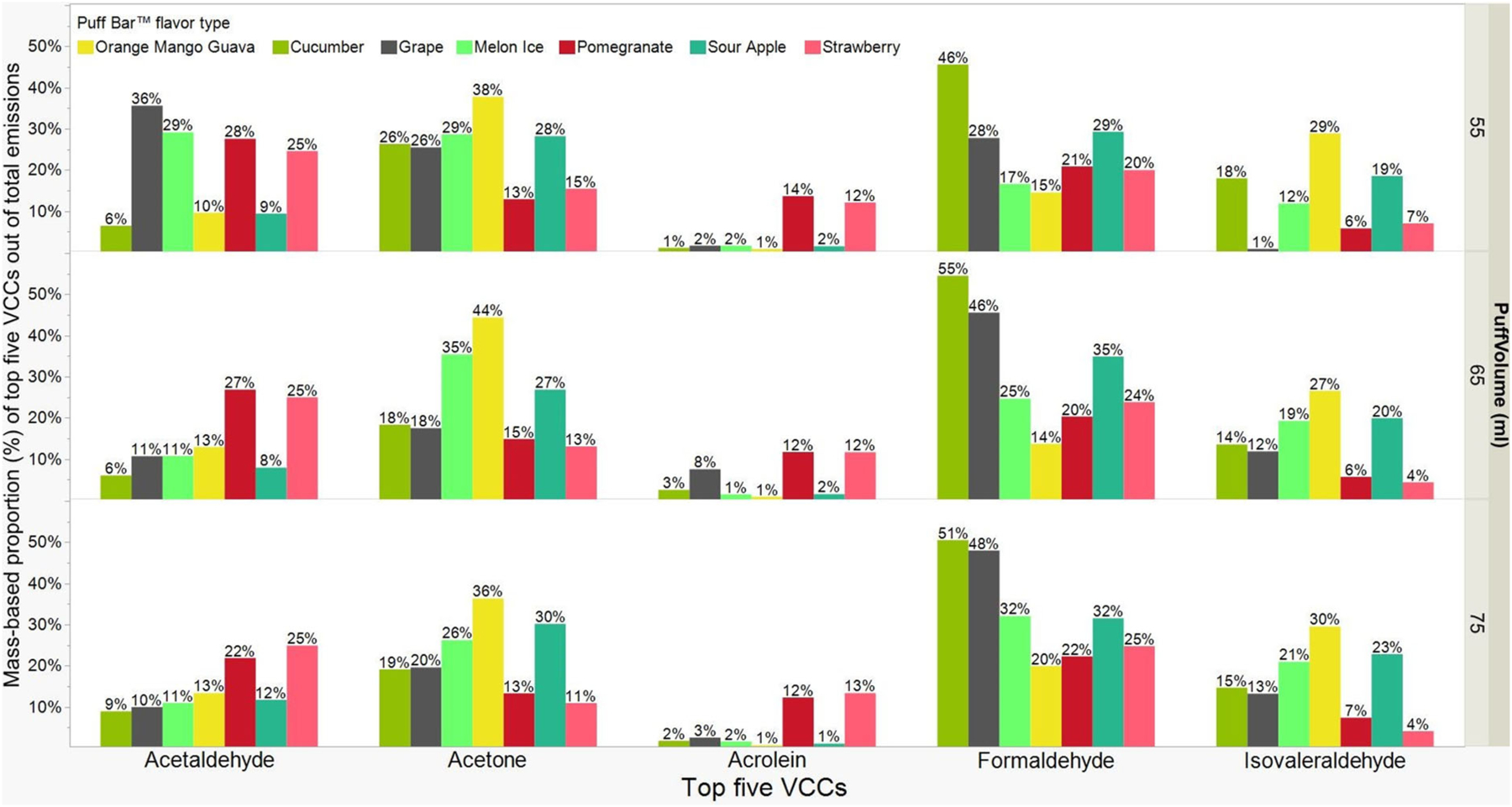
Mass-based proportion (%) of top five VCCs out of total emissions in different flavor types of Puff Bar™ on aerosolizing at puff volumes (mL: 55, 65, and 75) for n = 3 per puff volume (average ± SD): Formaldehyde (29.6% ± 0.12), Acetone (23.9% ± 0.09), Acetaldehyde (16.4% ± 0.09), Isovaleraldehyde (14.5% ± 0.08), Acrolein (5.0% ± 0.05).
The mass and proportions of VCCs present in emissions from flavored Puff Bar™ e-liquids varied nonlinearly by puff volumes. For example, the largest masses of acetaldehyde (1.37 μg/puff) and formalde-hyde (1.36 μg/puff) were observed with 75mL puff volume for Strawberry flavor but accounted for 25% of proportions for both VCCs. The largest proportion of acetaldehyde (36%) and formaldehyde (55%) were observed with 55mL puff volume for Grape and 65mL puff volume for Cucumber, respectively. The largest proportion of acetone (44%) and isovaleralde-hyde (30%) were observed with OMG for 65mL and 75mL puff volumes. However, OMG had the smallest proportion of formaldehyde for all the puff volumes (55, 65, and 75 mL). Similarly, with Grape flavor, acetaldehyde was greater at 55mL (36%) compared to 65mL (11%) and 75mL (10%) puff volumes. However, for the same Grape flavor, formaldehyde was smaller at 55mL (28%) puff volume compared to 65mL (46%) and 75mL (48%) puff volumes. The only observed statistical significance of puff volumes on mass (μg/puff) of VCCs was for isovaleraldehyde. For this VCC, the mass emitted at 55mL (0.21 μg/puff) was significantly smaller than the mass emitted at 75mL (0.25 μg/puff), but not with the mass at 65mL (0.22 μg/puff).
4. Discussion
Even though the sale of flavored e-liquid containing pods (other than menthol and Tobacco) have been restricted or completely banned, other fourth generation EVP devices, especially disposable Puff Bar™ prefilled with a variety of flavored e-liquids, continue to be available and popular in the market in the United States (Dai and Hao 2020). To our knowledge, this is the first controlled study assessing the effect of puff volume on Puff Bar™ e-liquid temperature, aerosol and chemical emissions, and inhalation dosimetry evaluations of different Puff Bar™ e-liquid flavors.
4.1. Puff volumes and flavor types influence mass-based aerosol emissions
For all Puff Bar™ flavors, the total mass of aerosol emitted increased with increasing puff volume, i.e., (55mL (4.6 mg/puff), 65mL (5.6 mg/puff), 75mL (6.2 mg/puff)). We observed higher puff volume (75mL compared with 55 mL, p < 0.05) emitted increased total aerosol mass, ranging by flavor from 2.02 mg/puff to 7.73 mg/puff. Sampling 15 puffs with 4-second of puff durations with JUUL® e-liquids, Talih et al. (2019) reported comparable total mass of aerosols between JUUL® (2.59 ± 0.1 mg/puff) and combustible cigarette (2.64 ± 0.1 mg/puff). Even with dissimilar puff topographies from Talih’s studies, greater (1.8–2.4-fold) total aerosol mass per puff was observed in Puff Bar™ emissions (4.6–6.2 mg/puff) compared with both JUUL® and combustible cigarette emissions (~2.6 mg/puff). Puffing behavior (e.g., puff volumes, duration, frequency) crucially influence e-liquid heating mechanics that eventually impact aerosol emissions (McAdam et al. 2019). Using a different generation EVP (i.e., “cig-a-like” disposables), McAdam et al. (2019) concluded that airflow (i.e., puff volume or rate) could not “heavily” impact aerosol generation at higher puff volumes inside the relatively smaller volume of a cig-a-like device. In the current study, though Puff Bar™ have a relatively smaller internal volume compared with the volume of a puff, the mass of aerosol per puff increased only slightly (1.6 mg/puff) from 55mL to 75mL. At rapid puff flow rates, factors such as heating mechanics (cooling of the wick, aerosol residence time, etc.) might explain our observation (Margham et al. (2016). Puff Bar™ emissions resulted in similar MMADs of approximately 1 μm at each puff volume. The data here are generally consistent with MMADs reported in other studies that use a low-flow cascade impactor; for example, Oldham et al. (0.9–1.2 μm), Kane and Li (0.5–0.9 μm) for various e-cigarette devices, and Pourchez et al. (0.7–1.2 μm) for various e-liquids that were aerosolized at different power settings. Though consistent, inter-comparisons among studies are not straightforward because, along with different EVP generations, other associated factors were not standardized across the studies such as sampling setup, e-liquid composition (including PG:VG proportions and impurities in commercial products), and e-liquid heating parameters (Stefaniak et al. 2021). A more direct comparison of the influence of puff volume and flavor types on PSD and dosimetry estimates from Puff Bar™ emissions can be made with our previous publication on refillable pods and JUUL® brand EVPs (same generation type), which used the same puff topographies (i.e., puff volumes using 55, 65, 75mL within a 4-second puff duration, and 30 second puff interval) and mass-based sampling parameters for (Ranpara et al. 2021b). For Puff Bar™, we observed smaller sized particles at greater puff volumes with significant differences between 55mL and 75mL. Using JUUL® brand EVPs, we observed a similar trend in MMADs when aerosolized using the same puff topography (Ranpara et al. 2021b).
4.2. Modeled regional respiratory tract deposition and exhaled fractions
Along with e-liquid compositions, EVP user’s puffing behavior (e.g., puff volume, puff duration, etc.), sampling conditions, physiological parameters such as mouth hold, inhalation depth, temperature of oral cavity, anatomical variabilities of the lungs, and thermodynamic behavior of EVP aerosol in the respiratory tract can affect modeled dosimetry estimates of emitted aerosol (Asgharian et al. 2018a; Asgharian et al. 2018b; Ooi et al. 2019; Pichelstorfer et al. 2021); however, the current freely available version 3.04 of MPPD does not account for all of these relevant considerations. Recognizing these limitations, similar with the computed deposition fractions demonstrated in Pichelstorfer et al. (2021), our study showed that total particle mass fraction is preferentially deposited in the deeper lung region (i.e., pulmonary and TB) of the lung during inhalation. In our previous study, modeling predicted total respiratory tract deposition of JUUL® emissions of 28–47% (Ranpara et al. 2021b), which is greater compared with results from the current study for Puff Bar™ emissions (22–33%). However, within the respiratory tract, modeling predicted that a greater proportion of the total inhaled aerosol will deposit in the pulmonary region for Puff Bar™ emissions (41–44%) compared with JUUL® emissions (30–37%) though deposition in the TB region was similar (JUUL®: 18–23%, Puff Bar™: 20–24%). Overall, more than 60% of the total inhaled particles from Puff Bar™ are predicted to deposit in the pulmonary and TB regions, which might help to better understand respiratory health effect endpoints reported in the literature (Cho and Paik 2016; Wang et al. 2016). Other than herein considered dosimetric estimates for the Pulmonary region, a dynamic model that accounts for changes in EVP aerosol properties predicted 65% particle deposition, whereas an insoluble model that does not account for changes in EVPA aerosol properties predicted 25% particle deposition (Asgharian et al. 2018a). Pichelstorfer et al. (2021) also compared dynamic and insoluble particle models and reported that dynamic models yielded median number-based and mass-based particle sizes that were 242 and 466% larger than situations where dynamics were ignored, which again means that the actual amount deposited would be higher than predicted using an insoluble model such as MPPD version 3.04. The primary reason for this difference in deposition predictions between dynamic and insoluble models is the volatility of e-cigarette aerosol constituents and the corresponding contribution to deposition from vapor uptake, which were not accounted for in the insoluble particle models (Asgharian et al.2018a, 2018b). Assuming that all the non-deposited particles are exhaled, the dynamic model indicated ~15% of particles would be available for secondhand exposure, whereas the insoluble model indicated 65% of particles could be a source of secondhand exposure (similar to our estimates: 67–77%). More than 2/3 of inhaled particles (67–77%) are estimated to be exhaled by Puff Bar™ users. Similarly, Sundahl, Berg, and Svensson (2017) reported that after inhalation from user, 75–90% of e-cigarette droplets containing nicotine would be exhaled into the surrounding atmosphere.
These exhaled fractions estimated with Puff Bar™ emissions were greater compared with that of JUUL® (52–64%) (Ranpara et al. 2021b). Exhaled fraction estimates from previous JUUL® (Ranpara et al. 2021b) and NJOY top tanks (NJOY, Inc., Scottsdale, AZ) (Stefaniak et al. 2022) based humectant research are consistent with Puff Bar™ (>70%) studied here at puff volumes of 65 and 75 mL. Though our exhaled EVP aerosol estimation were limited to particles only, other researchers have conducted exhaled breath studies to evaluate chemicals including VCCs (Long 2014; St Helen et al. 2016; Samburova et al. 2018; Papaefstathiou et al. 2020; Edmiston et al. 2021) as well as at real-world exposure scenario such as in vape shop (Zwack, Stefaniak, and LeBouf 2017). Confirming significant amounts of VCCs produced during e-cigarette emissions observed in laboratory studies (Gillman et al. 2016; Jensen et al. 2015; Sleiman et al. 2016; Salamanca et al. 2018), Samburova et al. (2018) concluded that concentrations of formaldehyde and acetaldehyde were higher (2–125 times) in exhaled e-cigarette aerosols than in background breath of e-cigarette users among 12 human participants. Other studies reported no incremental change or below detection limit exposure levels of VCCs in exhaled e-cigarette aerosols, indicative of full deposition in the respiratory tract of the user (Long 2014; Edmiston et al. 2021; Zwack, Stefaniak, and LeBouf 2017).
4.3. Flavor types, not puff volume, influenced mass-based VCCs emitted from puff bar™
Increasing puff volume (or flow rates) from 55mL (0.8 LPM) to 75mL (1.1 LPM) increased (non-significantly) total mass of VCCs emitted from 2.15 to 2.30 μg/puff and this slight increase (~0.15 μg/puff) was not significant at p < 0.05. Previously, Son et al. (2020) reported that greater puff flow rates did not change carbonyl emissions for a cig-a-like and JUUL® EVPs (p > 0.052). Talih et al. (2020) reported the total mass of VCCs with JUUL® e-liquids as 1.63–1.71 μg/puff (Talih et al. 2020). Other than Strawberry and Pomegranate (≥3.3 μg/puff), the remaining flavor types of Puff Bar™ (Cucumber, OMG, Grape, Melon Ice, and Sour Apple) emitted less than 2.02 μg/puff total VCCs for all puff volumes. Son et al. (2020) also reported that cig-a-like EVP with a Grape-flavored e-liquid had lower VCC emissions than other flavors. Similar to our findings characterizing Puff Bar™ chemical emissions, other studies have demonstrated the influence of different flavor types on total mass of emitted VCCs for different devices: JUUL® (Talih et al. 2019, 2020) and cig-a-like EVP (Son et al. 2020). Studying a variety of Puff Bar™ flavors, Yogeswaran, Muthumalage, and Rahman (2021) presented ROS concentrations varied within the same flavors (e.g., OMG) across different EVP brand types and with various flavored e-liquids in Puff Bar™ emissions. Influence of flavor types of e-liquids on emitted harmful chemicals is consistent with Puff Bar™ emission characterization presented in this study.
After normalizing total VCC mass to puff volume and number (SI Figure S2), values were reduced or remained constant (i.e., upon increasing puff flow rate from 0.8 LPM to 1.1 LPM) within same flavor type, except for Strawberry. Alteration in the trend without normalization (Figure 3) for emitted total mass of VCCs for Strawberry and other studied flavor types accounted for a significant influence of flavor type. In general, regardless of puff volumes, total mass of VCC in Puff Bar™ emissions remained fairly constant within a flavor type and was influenced significantly by flavor type. For example, after normalization to puff volume and number, total mass of VCCs emitted with Cucumber were similar (μg/mL/puff: 0.023~0.024~0.023) but considerably different than other flavor types such as Strawberry (μg/mL/puff: 0.062~0.074~0.073). Margham et al. (2016) reported that emissions of formaldehyde, acetaldehyde, and acrolein decreased with increasing puff volume and suggested that increased flow rate at higher puff volumes reduced the temperature of the wick or changes in aerosol residence time in previous generation EVP (i.e., Vype ePen). In our study, total mass of VCCs (~0.15 μg/puff or ~0.01 μg/mL/puff) in Puff Bar™ emissions slightly increased with higher puff volumes; this change was not statistically significant (p < 0.05) and whether it is practically relevant (e.g., health relevant levels of VCC deposition in the lung) is unknown at this time. Like evaluations of emissions with previous generation EVP conducted in Margham et al. (2016), we observed that VCC mass in Puff Bar™ emissions was not influenced by puff volume and puff number (SI Figure S2).
Other studies have reported the formation of formaldehyde, acetaldehyde, propanol, acrolein, acetone, and benzene by reactions (e.g., degradation) upon heating of PG:VG e-liquids at different coil temperatures, with the proportion of carbonyls varying among EVP devices, puff topographies, and e-liquid types (Margham et al. 2016; Ogunwale et al. 2017; Qu et al. 2019; Sleiman et al. 2016). Evaluating VCC emissions from JUUL® e-liquids, Talih et al. 2019, 2020) found acetone (highest measured), acetaldehyde, formaldehyde, methacrolein, and valeraldehyde to account for most of the mass proportion of total VCCs, which is similar to our study results. Son et al. (2020 reported that fruit-flavored JUUL® e-liquid generated more formaldehyde (μg/puff: 0.14 ± 0.04) than other flavor JUUL® e-liquids (μg/puff: 0.07–0.10). In our study, formaldehyde (1.36 μg/puff, 55%), acetone (0.69 μg/puff, 44%) and acetaldehyde (1.37 μg/puff, 36%) had the greatest masses and proportions of total VCCs in Puff Bar™ emissions at herein evaluated puff topographies. The discrepancy between studies for the mass results of formaldehyde could be due to difference in EVP device type, sampling methods, puff topographies, or experimental setups. Even at different puff topography, fruit-flavored JUUL® generated the greatest mass of formaldehyde compared with other flavor types (Son et al. 2020). Studying emission profiles of flavored e-liquids in different EVP types, Klager et al. (2017) reported that the main VCC exposures to EVP users are from formaldehyde and acetaldehyde, like we evaluated in our study with Puff Bar™ emissions. The greater proportions of formal-dehyde observed in our results are similar to those quantified by other research groups (Flora et al. 2016; Gillman et al. 2016; Goniewicz et al. 2014; Jensen et al. 2015). Ooi et al. (2019) reported that levels of acetaldehyde and acrolein were increased for e-liquids as VG content increased from 0 of 80%, and our results for Puff Bar™ (~70% VG) are consistent with their findings. Acrolein (Son et al. 2020) and methacrolein (Talih et al. 2019, 2020) were previously reported in JUUL® emissions and were quantified in the current study in Puff Bar™ emissions. Isovaleraldehyde (as one of the top five VCCs) and other carbonyls measured in Puff Bar™ emissions have also been found in emissions from tobacco combustible cigarettes and flavored e-liquids of different EVP brands (Talih et al. 2019; Klager et al. 2017).
4.4. Contribution to the field of public health
Our findings demonstrate the potential impact of puff volume, and fruit flavor types on Puff Bar™ emissions to EVP users and bystanders in indoor environments at occupational or residential settings. Aerosolized fruit flavored e-liquid emissions, which contain more carbonyls than unflavored e-liquids, have been shown to generate reactive oxygen species that promote oxidative-stress induced damages in lung cells (Yogeswaran, Muthumalage, and Rahman 2021). In addition to nicotine, the literature has demonstrated that numerous chemicals (i.e., flavorings) and particles from EVP emissions impact body systems besides the respiratory system such as brain function (Heldt et al. 2020; Ruszkiewicz et al. 2020), even following short-term exposures (Kuntic et al. 2020), though much remains to be understood on the systemic distribution of inhaled aerosols and chemicals. Additionally, secondhand exposures to EVP emissions in indoor settings could pose considerable health risks to nearby vulnerable populations, such as children with higher breathing rates (Manigrasso et al. 2021) and pregnant women with hyperventilated breathing patterns (LoMauro and Aliverti 2015). Like combustible tobacco cigarettes and other EVP generations, exhaled Puff Bar™ emissions could compromise air quality in homes and automobiles where people spend more than 80% of their lifetime. Our results with Puff Bar™ emission characterization will serve as a foundation for future research with exposure assessments and epidemiological studies required to further understand and thereby, elucidate adverse health outcomes among EVP users and bystanders.
4.5. Study limitations, assumptions, and challenges
In this study, we evaluated emissions from only seven types of Puff Bar™, and limited the analysis to fruit flavors. The results of this study should not be extended to other Puff Bar™ flavor types not tested because they might emit different amounts of aerosols or chemicals even with the same experimental parameters. Another point to consider is that e-liquid temperature and Puff Bar™ emissions were characterized in this experimental study with three puff volumes (mL: 55, 65, and 75) at constant puff duration (4 seconds) and puff interval (30 seconds), but other topographies could yield different results. The limited number of puffs from each Puff Bar™ device were analyzed, with an assumption regarding aerosol constituent levels to be reasonably constant throughout the emission from the product. Translating results of laboratory studies performed using fixed puff parameters to EVP user exposures becomes problematic because puff topography among EVP users varies widely and influences emission profiles. The lack of a standardized protocol, other than CORESTA Method 81 which likely does not reflect human consumption patterns, would limit the generalizability in real-world scenarios and the comparability of our findings under experimental parameters considered in this laboratory study with wide-scale EVP-related research (Stefaniak et al. 2021; Farsalinos et al. 2013).
Puff Bar™ design type does not allow the user to change the e-liquid heating temperature by modifying the electric settings such as voltage and power applied to the coil. To maintain the integrity of the Puff Bar™ devices, we conducted separate sets of experiments to measure the temperature of the e-liquid during puffing and to characterize emissions while aerosolizing e-liquids. Therefore, association between e-liquid temperature and emission characteristics (i.e., total aerosol mass, PSD, total VCCs mass and their relative proportions) was not analyzed in this study. The placement of the thermocouple could affect the measured e-liquid temperature depending on its proximity to the coil.
We did not analyze the bulk composition of e-liquids (e.g., chemical constituents and proportions) in Puff Bars™. Variations in sampling set ups as well as e-liquid formulation could affect the characterization of particles and VCCs emitted when aerosolized. Clearance of aerosols and chemicals during inhalation and exhalation process were not considered in the dosimetry model and would likely affect the fraction of EVP emissions that are exhaled by users. Possible deviations in particle mass measurements of MMAD due to hygroscopic growth and evaporative loss were not evaluated in this study. Therefore, applicability of MPPD which was designed for solid particles might present different results for the dynamic VCCs from e-cigarette emission (Ingebrethsen, Cole, and Alderman 2012; David et al. 2020; Kane and Li 2021). Considering our previous experience with JUUL® and looking at the similarities in operating parameters (e.g., e-liquid density, electric settings, experimental set up and sampling methods) between Puff Bar™ and JUUL®, these deviations in mass might not substantially impact PSD and thereby dosimetry estimations. In the future, laboratory-based reference e-liquids with flavorings and nicotine in a “blank” Puff Bar™ device type should be considered to evaluate the effect of constituents on PSD to explore any deviations in measurements. Further evaluation should be considered to extend chemical characterization of aerosol constituents (that are missed in the study such as metals and tobacco specific nitrosamines) for advanced toxicity profile on Puff Bar™ emissions.
5. Summary
With Puff Bar™ device type, the only factor an EVP user can modify is puff volume and flow rate. Hence, we studied the impact of different puff volumes at constant puff duration and interval on heating e-liquids and subsequent particle and gas-phase emissions. We observed that changing puff volume translated into changes in e-liquid temperature ranging from 85 °C to 250 °C. The average temperatures increased more than 50 °C when the puff volume increased from 55mL to 75mL. Depending on puff volume and flavor types, total aerosol mass of the smaller-sized particles (~1 μm) emitted from Puff Bar™ EVPs ranged from 2.02 mg/puff to 7.7 mg/puff and the total chemical mass of VCCs ranged from 0.76 μg/puff to 5.5 μg/puff. Further, the majority of VCCs emitted from Puff Bar™ included carcinogens such as formaldehyde (29.6%), and acetaldehyde (16.4%) as well as respiratory irritants such as acetone (23.9%), isovaleraldehyde (14.5%), acrolein (4.9%), propionaldehyde (4.7%), 2-butanone (3.1%), and total tolualdehyde (2.3%). Only trace amount (i.e., below detection limits or consistently less than 1% of total mass of VCCs) of other VCCs such as benzaldehyde, butyraldehyde, crotonaldehyde, hexaldehyde, pentanal (valeraldehyde), and 2,5-dimethylbenzaldehyde were reported with a particular testing condition in Puff Bar™ emissions. Inhalation of total mass of aerosols and chemicals from Puff Bar™ emissions could contribute to a range of adverse health outcomes (respiratory, cardiovascular, cerebrovascular, etc.) among EVP users, and exhaled particles (>67%) could compromise air quality in occupational or non-occupational indoor settings (Wold et al. 2022). Exhaled aerosols can potentially compromise IAQ and negatively impact overall health for bystanders, especially vulnerable populations (Aboaziza et al. 2023; Aslaner et al. 2022; Burrage et al. 2021).
Supplementary Material
Acknowledgment
The authors would like to thank Clifford Watson and Alyson Fortner for technical review of this manuscript prior to submission to the journal.
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law.
Disclosure statement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Mention of any company or product does not constitute endorsement by the U.S. Government, National Institute for Occupational Safety and Health, or Centers for Disease Control and Prevention. This work was supported by NIOSH intramural research funds. All web addresses referenced in this document were accessible as of the publication date.
Funding
This study was supported by CDC/NIOSH intramural research funds.
Footnotes
Supplemental data for this article can be accessed online at https://doi.org/10.1080/02786826.2023.2190786.
Data availability statement
Anonymized data and original contributions presented in the research study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
References
- Aboaziza E, Feaster K, Hare L, Chantler PD, and Olfert IM. 2023. Maternal electronic cigarette use during pregnancy affects long-term arterial function in offspring. J Appl Physiol (1985) 134 (1):59–71. Access date 2022 Nov 29. doi: 10.1152/japplphysiol.00582.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgharian B, Rostami AA, Price OT, and Pithawalla YB. 2018a. Regional deposition of inhaled aerosol constituents from Electronic Nicotine Delivery Systems (ENDS) in the respiratory tract. J. Aerosol Sci 126:7–20. doi: 10.1016/j.jaerosci.2018.08.006. [DOI] [Google Scholar]
- Asgharian B, Price OT, Rostami AA, and Pithawalla YB. 2018b. Deposition of inhaled electronic cigarette aerosol in the human oral cavity. J. Aerosol Sci 116: 34–47. doi: 10.1016/j.jaerosci.2017.11.014. [DOI] [Google Scholar]
- Aslaner DM, Alghothani O, Saldana TA, Ezell KG, Yallourakis MD, MacKenzie DM, Miller RA, Wold LE, and Gorr MW. 2022. E-cigarette vapor exposure in utero causes long-term pulmonary effects in offspring. Am. J. Physiol. Lung Cell. Mol. Physiol 323 (6):L676–L682. doi: 10.1152/ajplung.00233.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino P, Scungio M, Stabile L, Cortellessa G, Buonanno G, and Manigrasso M. 2018. Second-hand aerosol from tobacco and electronic cigarettes: Evaluation of the smoker emission rates and doses and lung cancer risk of passive smokers and vapers. Sci. Total Environ 642: 137–47. doi: 10.1016/j.scitotenv.2018.06.059. [DOI] [PubMed] [Google Scholar]
- Burrage EN, Aboaziza E, Hare L, Reppert S, Moore J, Goldsmith WT, Kelley EE, Mills A, Dakhlallah D, Chantler PD, et al. 2021. Long-term cerebrovascular dysfunction in the offspring from maternal electronic cigarette use during pregnancy. Am. J. Physiol. Heart Circ. Physiol 321 (2):H339–H352. doi: 10.1152/ajpheart.00206.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang P, Ito K, Fowles J, Shusterman D, Jaques PA, and Kumagai K. 2018. Measurement of heating coil temperature for e-cigarettes with a top-coil clearomizer. PLoS One 13 (4):e0195925. doi: 10.1371/journal.pone.0195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, and Paik SY. 2016. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One 11 (3):e0151022. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, and Hao J. 2020. Online popularity of JUUL and Puff Bars in the USA. Tobacco Control 31 (1):2019–20. doi: 10.1136/tobaccocontrol-2020-055727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Parmentier EA, Taurino I, and Signorell R. 2020. Tracing the composition of single e-cigarette aerosol droplets in situ by laser-trapping and Raman scattering, Sci. Rep 10:7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston JS, Rostami A, Liang Q, Miller S, and Sarkar M. 2021. Computation modelling method to estimate secondhand exposure potential from exhalations during e-vapor use under various real-world scenarios. PREPRINT (Version 1) available at Research Square doi: 10.21203/rs.3.rs-341609/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, and Voudris V. 2013. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public Health 10 (6):2500–14. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández E, Ballbè M, Sureda X, Fu M, Saltó E, and Martínez-Sánchez JM. 2015. Particulate matter from electronic cigarettes and conventional cigarettes: A systematic review and observational study. Curr. Environ. Health Rep 2 (4):423–9. doi: 10.1007/s40572-015-0072-x. [DOI] [PubMed] [Google Scholar]
- Flora JW, Meruva N, Huang CB, Wilkinson CT, Ballentine R, Smith DC, Werley MS, and McKinney WJ. 2016. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol 74:1–11. doi: 10.1016/j.yrtph.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Gillman IG, Kistler KA, Stewart EW, and Paolantonio AR. 2016. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol 75:58–65. doi: 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, et al. 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23 (2):133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt NA, Seliga A, Winfield M, Gajghate S, Reichenbach N, Yu X, Rom S, Tenneti A, May D, Gregory BD, et al. 2020. Electronic cigarette exposure disrupts blood-brain barrier integrity and promotes neuroinflammation. Brain. Behav. Immun 88:363–80. doi: 10.1016/j.bbi.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, et al. 2016. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. (Berl.) 94 (6):667–79. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- Ingebrethsen BJ, Cole SK, and Alderman SI. 2012. Electronic cigarette aerosol particle concentration and size distribution in the mainstream of e-cigarette arettes. Inhal. Toxicol 24 (14):976–84. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- ISO 20768 2018. Vapour products-routine analytical vaping machine-definitions and standard conditions Geneva, Switzerland: ISO. [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, and Peyton DH. 2015. Hidden formaldehyde in e-cigarette aerosols. N Engl. J. Med 372 (4):392–4. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Naeher LP, Yu X, Sosnoff C, Wang L, Rathbun SL, De Jesus VR, Xia B, Holder C, Muilenburg JL, et al. 2019. A biomonitoring assessment of secondhand exposures to electronic cigarette emissions. Int. J. Hyg. Environ. Health 222 (5):816–23. doi: 10.1016/j.ijheh.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DB, and Li W. 2021. Particle size measurement of electronic cigarette aerosol with a cascade impactor. Aerosol Sci. Technol 55 (2):205–14. doi: 10.1080/02786826.2020.1849536. [DOI] [Google Scholar]
- Khachatoorian C, McWhirter KJ, Luo W, Pankow JF, and Talbot P. 2022. Tracing the movement of electronic cigarette flavor chemicals and nicotine from refill fluids to aerosol, lungs, exhale, and the environment. Chemosphere 286 (Pt 3):131494. doi: 10.1016/j.chemo-sphere.2021.131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klager S, Vallarino J, MacNaughton P, Christiani DC, Lu Q, and Allen JG. 2017. Flavoring chemicals and aldehydes in e-cigarette emissions. Environ. Sci. Technol 51 (18):10806–13. doi: 10.1021/acs.est.7b02205. [DOI] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, and Engelmann WH. 2001. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol 11 (3):231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Kuntic M, Oelze M, Steven S, Kröller-Schön S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Bayo Jimenez MT, Kvandova M, et al. 2020. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur. Heart J 41 (26):2472–83. doi: 10.1093/eurheartj/ehz772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechasseur A, Altmejd S, Turgeon N, Buonanno G, Morawska L, Brunet D, Duchaine C, and Morissette MC. 2019. Variations in coil temperature/power and e-liquid constituents change size and lung deposition of particles emitted by an electronic cigarette. Physiol. Rep 7 (10):e14093. doi: 10.14814/phy2.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lin Y, Xia T, and Zhu Y. 2020. Effects of electronic cigarettes on indoor air quality and health. Annu. Rev. Public Health 41:363–80. doi: 10.1146/annurev-publ-health-040119-094043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Burns AE, Tran LN, Abellar KA, Poindexter M, Li X, Madl AK, Pinkerton KE, and Nguyen TB. 2021. Impact of e-liquid composition, coil temperature, and puff topography on the aerosol chemistry of electronic cigarettes. Chem. Res. Toxicol 34 (6):1640–54. doi: 10.1021/acs.chemrestox.1c00070. [DOI] [PubMed] [Google Scholar]
- Logue JM, Sleiman M, Montesinos VN, Russell ML, Litter MI, Benowitz NL, Gundel LA, and Destaillats H. 2017. Emissions from electronic cigarettes: assessing vapers’ intake of toxic compounds, secondhand exposures, and the associated health impacts. Environ. Sci. Technol 51 (16):9271–9. doi: 10.1021/acs.est.7b00710. [DOI] [PubMed] [Google Scholar]
- LoMauro A, and Aliverti A. 2015. Respiratory physiology of pregnancy. Breathe (Sheff) 11 (4):297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GA 2014. Comparison of select analytes in exhaled aerosol from e-cigarettes with exhaled smoke from a conventional cigarette and exhaled breaths. Int. J. Environ. Res. Public Health 11 (11):11177–91. doi: 10.3390/ijerph111111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso M, Protano C, Vitali M, and Avino P. 2021. Passive vaping from sub-ohm electronic cigarette devices. Int. J. Environ. Res. Public Health 18 (21):11606. doi: 10.3390/ijerph182111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, and Proctor C. 2016. Chemical composition of aerosol from an a-cigarette: a quantitative comparison with cigarette smoke. Chem. Res. Toxicol 29 (10):1662–78. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- McAdam K, Davis P, Ashmore L, Eaton D, Jakaj B, Eldridge A, and Liu C. 2019. Influence of machine-based puffing parameters on aerosol and smoke emissions from next generation nicotine inhalation products. Regul. Toxicol. Pharmacol 101:156–65. doi: 10.1016/j.yrtph.2018.11.006. [DOI] [PubMed] [Google Scholar]
- McClelland ML, Sesoko CS, MacDonald DA, Davis LM, and McClelland SC. 2021. The immediate physiological effects of e-cigarette use and exposure to secondhand e-cigarette vapor. Respir. Care 66 (6):943–50. doi: 10.4187/respcare.08596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, Malinowska K, Zajdel K, Zakonnik L, and Zajdel R. 2020. A summary of in vitro and in vivo studies evaluating the impact of e-cigarette exposure on living organisms and the environment. Int. J. Mol. Sci 21 (2):652. doi: 10.3390/ijms21020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR, 2019: Su C, Syamlal G, Tamers S, Li J and Luckhaupt SE Workplace secondhand tobacco smoke exposure among U.S. nonsmoking workers, 2015. MMWR Morb Mortal Wkly Rep 68:604–607. doi: 10.15585/mmwr.mm6827a2.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunwale MA, Li M, Raju MVR, Chen Y, Nantz MH, Conklin DJ, and Fu XA. 2017. Aldehyde detection in electronic cigarette aerosols. ACS Omega 2 (3):1207–14. doi: 10.1021/acsomega.6b0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham M, Zhang J, Rusyniak MJ, Kane DB, and Gardner WP. 2018. Particle size distribution of selected electronic nicotine delivery system products. Food Chem. Toxicol 113:236–40. doi: 10.1016/j.fct.2018.01.045. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Bailey PC, Castro N, Lang Q, Salehi A, and Rostami AA. 2021a. Prediction of potential passive exposure from commercial electronic nicotine delivery systems using exhaled breath analysis and computational fluid dynamic techniques. J. Breath Res 15 046006. doi: 10.1088/1752-7163/ac2884. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Sehgal A, Cohen G, Chen J, Evans B, and Heraldez D. 2021b. Room air constituent concentrations from use of electronic nicotine delivery systems and cigarettes using different ventilation conditions. Sci. Rep 11 (1):1736.doi: 10.1038/s41598-021-80963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi BG, Dutta D, Kazipeta K, and Chong NS. 2019. Influence of the e-cigarette emission profile by the ratio of glycerol to propylene glycol in e-liquid composition. ACS Omega 4 (8):13338–48. doi: 10.1021/acsomega.9b01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaefstathiou E, Bezantakos S, Stylianou M, Biskos G, and Agapiou A. 2020. Comparison of particle size distributions and volatile organic compounds exhaled by e-cigarette and cigarette users. J. Aerosol Sci 141:105487. [Google Scholar]
- Pankow JF 2017. Calculating compound dependent gasdroplet distributions in aerosols of propylene glycol and glycerol from electronic cigarettes. J. Aerosol Sci 107:9–13. doi: 10.1016/j.jaerosci.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JF, Kim K, Luo W, and McWhirter KJ. 2018. Gas/particle partitioning constants of nicotine, selected toxicants, and flavor chemicals in solutions of 50/50 propylene glycol/glycerol as used in electronic cigarettes. Chem. Res. Toxicol 31:985–90. doi: 10.1021/acs.chemrestox.8b0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichelstorfer L, Winkler-Heil R, Boy M, and Hofmann W. 2021. Aerosol dynamics simulations of the anatomical variability of e-cigarette particle and vapor deposition in a stochastic lung. J. Aerosol Sci 158:105706. doi: 10.1016/j.jaerosci.2020.105706. [DOI] [Google Scholar]
- Pourchez J, Parisse S, Sarry G, Perinel-Ragey S, Vergnon JM, Clotagatide A, and Prévôt N. 2018. Impact of power level and refill liquid composition on the aerosol output and particle size distribution generated by a new-generation e-cigarette device. Aerosol Sci. Technol 52 (4): 359–69. doi: 10.1080/02786826.2017.1422857. [DOI] [Google Scholar]
- Qu Y, Szulejko JE Kim KH, Jo SH 2019. The effect of varying battery voltage output on the emission rate of carbonyls released from e-cigarette smoke. Microchem. J 145:47–54. doi: 10.1016/j.microc.2018.10.019. [DOI] [Google Scholar]
- Ranpara A, Stefaniak AB, Williams K, Fernandez E, and LeBouf RF. 2021a. Modeled respiratory tract deposition of aerosolized oil diluents used in 19-THCbased electronic cigarette liquid products. Front. Public Health 9: 744166. doi: 10.3389/fpubh.2021.744166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranpara A, Stefaniak AB, Fernandez E, and LeBouf RF. 2021b. Effect of puffing behavior on particle size distributions and respiratory depositions from pod-style electronic cigarette, or vaping, products. Front. Public Health 9:750402. doi: 10.3389/fpubh.2021.750402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Eddingsaas NC DiFrancesco AG Jayasekera S, and Hensel EC Jr 2018. A framework to investigate the impact of topography and product characteristics on electronic cigarette emissions. PLoS One 13 (11):e0206341. doi: 10.1371/journal.pone.0206341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg AR, Diaz MC, Briggs J, Stephens DK, Rahman B, Graham AL, and Schillo BA. 2021. Vaping in the workplace prevalence and attitudes among employed US adults. J. Occup. Environ. Med 63 (1):10–7. doi: 10.1097/JOM.0000000000002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami AA, Samuel A, and Pithawalla YB. 2018. A distributed computational model for estimating room air level of constituents due to aerosol emission from evapor product use. Food Chem. Toxicol 116 (Part B): 114–28. ISSN 0278–6915, doi: 10.1016/j.fct.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Routine analytical machine for e-cigarette aerosol generation and collection – definitions and standard conditions 2015. CORESTA recommended method N◦ 81 Cooperation Centre for Scientific Research Relative to Tobacco, Paris, France. Accessed October 12, 2021. https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf [Google Scholar]
- Ruszkiewicz JA, Zhang Z, Gonçalves FM, Tizabi Y, Zelikoff JT, and Aschner M. 2020. Neurotoxicity of e-cigarettes. Food Chem. Toxicol 138:111245. doi: 10.1016/j.fct.2020.111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca JC, Meehan-Atrash J, Vreeke S, Escobedo JO, Peyton DH, and Stronging RM. 2018. E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci. Rep 8:7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samburova V, Bhattarai C, Strickland M, Darrow L, Angermann J, Son Y, and Khlystov A. 2018. Aldehydes in exhaled breath during e-cigarette vaping: Pilot study results. Toxics 6 (3):46. doi: 10.3390/toxics6030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, and Destaillats H. 2016. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ. Sci. Technol 50 (17):9644–51. doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- Son Y, Bhattarai C, Samburova V, and Khlystov A. 2020. Carbonyls and carbon monoxide emissions from electronic cigarettes affected by device type and use patterns. Int. J. Environ. Res. Public Health 17 (8):2767. doi: 10.3390/ijerph17082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowski TR, and Kramek-Romanowska K. 2016. Predicted deposition of e-cigarette aerosol in the human lungs. J. Aerosol Med. Pulm. Drug Deliv 29 (3):299–309. doi: 10.1089/jamp.2015.1268. [DOI] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P, and Benowitz NL. 2016. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111 (3):535–44. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak AB, LeBouf RF, Ranpara A, and Leonard SS. 2021. Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems. Pharmacol. Ther 224:107838. doi: 10.1016/j.pharmthera.2021.107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak AB, Ranpara AC, Virji MA, and LeBouf RF. 2022. Influence of e-liquid humectants, nicotine, and flavorings on aerosol particle size distribution and implications for modeling respiratory deposition. Front. Public Health 10:782068. doi: 10.3389/fpubh.2022.782068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundahl M, Berg E, and Svensson M. 2017. Aerodynamic particle size distribution and dynamic properties in aerosols from electronic cigarettes. J. Aerosol. Sci 103:141–50. doi: 10.1016/j.jaerosci.2016.10.009. [DOI] [Google Scholar]
- Talih S, Salman R, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, and Shihadeh A. 2019. Characteristics and toxicant emissions of JUUL electronic cigarettes. Tob. Control 28 (6):678–80. doi: 10.1136/tobaccocontrol-2018-054616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Salman R, El-Hage R, Karam E, Salam S, Karaoghlanian N, El-Hellani A, Saliba N, and Shihadeh A. 2020. A comparison of the electrical characteristics, liquid composition, and toxicant emissions of JUUL USA and JUUL UK e-cigarettes. Sci. Rep 10 (1):7322. doi: 10.1038/s41598-020-64414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzortzi S, Teloniatis G, Matiampa G, Bakelas C, Tzavara VK, Vyzikidou C, Vardavas P, Behrakis E, and Fernandez A 2020. Passive exposure of non-smokers to E-Cigarette aerosols: sensory irritation, timing and association with volatile organic compounds. Environ. Res 182: 108963. doi: 10.1016/j.envres.2019.108963. [DOI] [PubMed] [Google Scholar]
- US FDA. 2020. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint US Food and Drug Administration news release. FDA, Silver Spring, MD. Accessed October 1, 2022. https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children [Google Scholar]
- Wang MP, Ho SY, Leung LT, and Lam YH. 2016. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA Pediatr 170 (1):89–91. doi: 10.1001/jamapediatrics.2015.3024. [DOI] [PubMed] [Google Scholar]
- Wold LE, Tarran R, Crotty ALE, Hamburg NM, Kheradmand F, St Helen G, and Wu JC. 2022. Cardiopulmonary consequences of vaping in adolescents: A scientific statement from the American Heart Association. Circ. Res 131 (3):e70–e82. doi: 10.1161/RES.0000000000000544. [DOI] [PubMed] [Google Scholar]
- Yogeswaran S, Muthumalage T, and Rahman I. 2021. Comparative reactive oxygen species (ROS) content among various flavored disposable vape bars, including cool (iced) flavored bars. Toxics 9 (10):235. doi: 10.3390/toxics9100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Shu SS, Guo Q, and Zhu Y. 2016. Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos. Environ 134:61–9. doi: 10.1016/j.atmosenv.2016.03.027. [DOI] [Google Scholar]
- Zhao J, Pyrgiotakis G, and Demokritou P. 2016. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal. Toxicol 28 (14):658–69. doi: 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Bello D, and Demokritou P. 2018. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater 344:549–57. doi: 10.1016/j.jhazmat.2017.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwack LM, Stefaniak AB, and LeBouf RF. 2017. Evaluation of chemical exposures at a vape shop U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. 2015–0107–3279 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data and original contributions presented in the research study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.


