Abstract
Introduction
Among chronically ill populations, affective disorders remain underdiagnosed and undertreated. A high degree of comorbidity exists between diabetes and affective disorders, particularly depression and anxiety. The mechanisms underlying stress-induced affective dysregulation are likely distinct from those induced by diabetes. A direct comparison between stress- and hyperglycemia-induced affective dysregulation could provide insight into distinct mechanistic targets for depression/anxiety associated with these different conditions.
Methods
To this end, the present study used male C57BL/6J mice to compare the independent and combined behavioral and neuroinflammatory effects of two models: (1) unpredictable chronic mild stress and (2) pharmacologically induced hyperglycemia.
Results
Streptozotocin-induced hyperglycemia was associated with a set of behavioral changes reflective of the neurovegetative symptoms of depression (i.e., reduced open field activity, reduced grooming, increased immobility in the forced swim task, and decreased marble burying), increased hippocampal Bdnf and Tnf expression, and elevations in frontal cortex Il1b expression. Our chronic stress protocol produced alterations in anxiety-like behavior and decreased frontal cortex Il1b expression.
Discussion
While the combination of chronic stress and hyperglycemia produced limited additive effects, their combination exacerbated total symptom burden. Overall, the data indicate that stress and hyperglycemia induce different symptom profiles via distinct mechanisms.
Keywords: Streptozotocin, Hyperglycemia, Stress, Depression, Anxiety, Neuroinflammation
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by chronic hyperglycemia resulting from either an inability to produce insulin or a dysregulated response to insulin [1]. Approximately 537 million adults aged 20–79 years are living with diabetes, and the number of diabetic cases worldwide continues to increase [2]. While this disease is associated with long-term complications including neuropathy, retinopathy, and increased risk of arteriosclerotic cardiovascular, peripheral arterial, and cerebrovascular disease, the high degree of comorbidity between diabetes and affective disorders, particularly depression and anxiety [3, 4], is less recognized and remains undertreated [5, 6].
Depression occurs twice as frequently among individuals with diabetes than among the general population [7], with an estimated prevalence rate of 17.6% among individuals with diabetes compared to 8.3% in the general population [8, 9]. Further, patients with comorbid major depression and diabetes appear to have higher rates of melancholic depression and suicide risk [10]. These differences may relate to increased inflammation, as measured by circulating C-reactive protein (CRP), observed among patients with DM [11, 12]. Inflammation-associated depression is often characterized by somatic symptoms (e.g., fatigue, sleep disruption) [13]; these symptoms may be more difficult for both patients and healthcare providers to recognize, leading to underdiagnosis and undertreatment [7, 14–17].
There are a number of factors that contribute to diabetic patients’ increased risk of affective dysregulation. The psychological burden of managing a chronic disease likely increases the risk of depression and anxiety in diabetic patients [17, 18]. Additionally, chronically ill patients with affective disorders are more likely to engage in unfavorable lifestyle behaviors [7, 15, 17, 19–21], further compounding existing issues. Hyperglycemia itself may also induce neurological changes that drive depression [22, 23].
This relationship has been experimentally confirmed using murine models. Pharmacologically inducing hyperglycemia in rodents using streptozotocin (STZ) has been shown to be associated with the development of depressive-like behavior [24–26]. STZ ablates insulin-producing pancreatic β cells inducing chronic, robust hyperglycemia [27]. The failure to produce insulin in this model is like that of type I DM. However, it deviates from the clinical condition in that STZ-treated mice remain in good health for months after induction without the need for insulin treatment. It may better be viewed as a model that allows us to isolate the impact of chronic hyperglycemia on the brain in the absence of insulin treatment and obesity. Previously, chronic hyperglycemia has been associated with impaired hippocampal neurogenesis, systemic peripheral inflammation, neuroinflammation, and both central and peripheral oxidative stress [7, 26, 28–30]. While the affective dysregulation associated with this model is likely linked to these mechanisms, further mechanistic research is needed.
Stress is, arguably, the most common factor associated with depression, and both clinical and preclinical research have demonstrated robust causal links [31–37]. While our understanding of stress-induced depressive symptoms is still developing, reduced synaptic plasticity and inhibited neurogenesis have been strongly implicated [33, 35, 38–40]. Understanding the shared and unique behavioral and neurobiological features of hyperglycemia- and stress-induced depression may allow us to develop more effective therapeutic interventions. If chronic stress and chronic hyperglycemia induce depression through different mechanisms this may allow them to act additively, further enhancing the risk for individuals with diabetes. Within this study, we used a murine model to compare the affective behavioral changes induced by (1) stress and (2) hyperglycemia. We used 2 weeks of unpredictable chronic mild stress (UCMS) [41] as our stress model and STZ to induce chronic hyperglycemia. We also evaluated commonly reported inflammatory targets (e.g., Il1b, Il6, Tnf), synaptic health-related targets (e.g., Bdnf, Snap25), and a critical glucose transporter (i.e., Slc2a1) within the hippocampus and frontal cortex, as these brain areas have been linked to depression [28, 38, 42, 43]. Both UCMS and STZ have been shown to independently induce depressive- and anxiety-like behavior in murine models [24, 28, 34, 44–50], however, there is limited research directly comparing these models.
Materials and Methods
Animals and Experimental Design
This study was conducted in male C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME, USA). Mice arrived at 6 weeks of age and were allowed to acclimate to the Baylor University vivarium for 2 weeks prior to the start of experiments. They were maintained at 22°C on a 12-h light/dark cycle with food and water ad libitum, were housed with 2–3 mice per cage, and were handled for 5 days prior to injections. All testing took place during the light cycle phase (0700–1900 h). All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Baylor University (IACUC protocol number: 1550457) and were in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
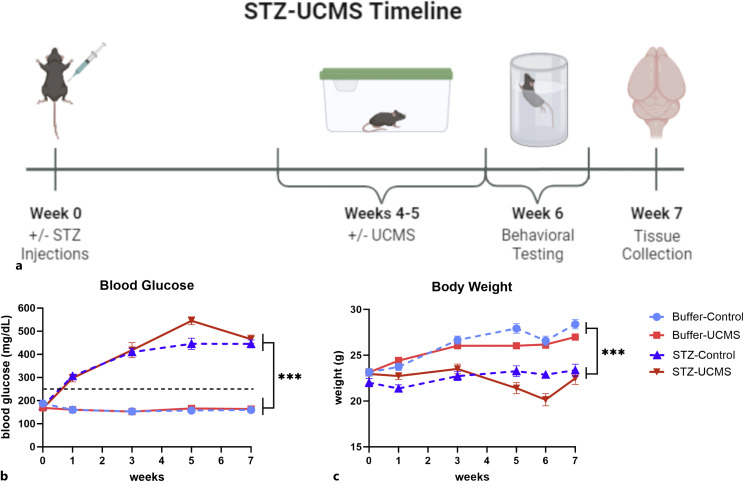
We used a 2 (STZ vs. buffer) × 2 (UCMS vs. control) factorial design with 12–13 mice per group (N = 49). The week of STZ or buffer administration was designated week 0. The UCMS protocol was conducted during weeks 4 and 5. Behavioral testing was conducted during week 6, and the mice were terminated at the start of week 7 (Fig. 1a). This study was run in two cohorts, with the same schedule except for the addition of the splash test (ST) in the second cohort. Tissue analyses were conducted on mice from the first cohort. Behavioral testing began the day after the final UCMS session with marble burying task on day 1, light/dark aversion (LDA) on day 2, open field task (OFT) and forced swim test (FST) on day 3, and ST (cohort 2 only) on day 4.
Fig. 1.
a A timeline of 7 weeks was applied for this study, in which male C57BL/6J were injected with STZ (50 mg/kg/day) for a 5-day period. Starting 3 weeks after induction of diabetes, mice underwent 2 weeks of UCMS. This was followed by behavioral testing and tissue collection. Created with BioRender.com. b Fasting blood glucose was monitored with the hyperglycemic threshold set at 250 mg/dL; all STZ mice exceeded this threshold post-induction. c Body weight was measured consistently throughout the study, and mice with STZ-induced hyperglycemia had consistently lower body weights compared to buffer mice. Data are graphed as means ± SEM; ***p < 0.001 for the main effect of STZ. n = 12–13 mice per group. UCMS, unpredictable chronic mild stress; STZ, streptozotocin; SEM, standard error of the mean.
Induction of Hyperglycemia and Monitoring
Hyperglycemia was induced through administration of STZ [27]. Starting at 8 weeks of age, mice were injected intraperitoneally with 50 mg/kg/day of STZ in a volume of 10 μL/g body weight citrate buffer for 5 consecutive days. Control mice received an equal volume of buffer. Body weight and blood glucose (post 4 h of fasting) were monitored at least biweekly to ensure the health of the mice as well as confirm hyperglycemic status. Hyperglycemia was defined as a blood glucose level over 250 mg/dL. Blood glucose was measured using an AUVON handheld glucometer and test strips following a nick to the tail vein.
UCMS Procedure
Mice in the UCMS treatment groups were exposed to a quasi-randomized schedule of twice-daily stressors for 14 consecutive days [41]. The stressors used are described in Table 1. The predator odor stressor protocol was run as previously described [51].
Table 1.
Description of stressors applied during the 2 weeks of UCMS
| Stressor | Description |
|---|---|
| Food restriction | Food was removed at the end of the business day (∼5:00 p.m.) and returned the next morning (∼8:00 a.m.). Stressor did not occur 2 days in a row or within 24 h of water restriction |
| Water restriction | Water was removed at the end of the business day (∼5:00 p.m.) and returned the next morning (∼8:00 a.m.). Stressor did not occur 2 days in a row or within 24 h of food restriction |
| Wet bedding | Approximately 250–500 mL of clean water was poured onto bedding. The bedding was left damp for 4 h during the light cycle |
| No bedding | Mice were placed in an empty cage void of bedding for 3 h during the light cycle |
| Tilted cage | Cage was tilted at a 45° angle for 2 h during the light cycle |
| Light cycle disruption | Mice were moved into a dark room for 4 h during the light cycle |
| Stroboscope exposure | Mice were placed in a room with flashing lights from a stroboscope for 2 h during the light cycle |
| Restraint | Mice were placed in a 50-mL tube positioned in the home cage and equipped with holes for ventilation and breathing. Restraint occurred for 2 h during the light cycle |
| Predator odor | 2,4,5,-trimethylthiazole solution, a mimic of fox urine, was pipetted onto a small cotton swab, secured to the top of the cage, and left in place for 2 h during the light cycle |
Behavioral Tests
Marble Burying
The marble burying task has been used to assess anxiety-like and/or compulsive behavior, although the task responds to various pharmacological interventions including antidepressants [52–55]. Marble burying has previously been shown to be reduced in STZ-treated mice [56, 57], and while the precise dimension it is evaluating is unclear, it provides a robust behavioral endpoint for the STZ model. Each mouse was placed in an arena (30 cm × 19 cm × 13 cm) with fresh bedding and 20 marbles (arranged in 5 rows of 4 marbles) for 30 min. The number of marbles buried at least two-thirds of the way under the bedding was recorded by an experimenter blind to experimental conditions [58].
Light/Dark Aversion
The LDA task has been used to evaluate anxiety-like behavior [59–61]. This task uses a two-chamber arena (each chamber 28 cm × 14 cm × 25 cm) with one open chamber illuminated by white lights and a dark, enclosed chamber. Mice were placed into the dark chamber, and the door separating the two compartments was removed. The mice were allowed to move freely between the chambers for 10 min. Each trial was recorded, and the latency to first exit the dark chamber was recorded by an experimenter blind to experimental conditions, while the number of transitions was determined by Noldus EthoVision XT software (Leesburg, VA, USA).
Open Field
The OFT can be used to assess activity in a novel environment, while center time in the field is used to assess anxiety-like behavior [62]. Mice were placed in a novel plexiglass arena (30 cm × 30 cm × 40 cm) for 5 min and activity was recorded. Distance traveled and center duration were determined using Noldus EthoVision XT software (Leesburg, VA, USA).
Splash Test
The ST has been used to assess motivation toward grooming and has been demonstrated to be sensitive to stress and responsive to antidepressants [63, 64]. Mice were placed in an arena (30 cm × 30 cm × 40 cm), and the dorsal surface, from tail to nose of the mouse, was sprayed with a 10% sucrose solution. Grooming behavior was recorded for 5 min. An experimenter blind to experimental conditions analyzed the time to first groom and the total amount of time the mouse spent grooming.
Forced Swim Test
The FST was developed as a screen for antidepressant activity of drugs in rodents [65]. It was run as previously described [66]. Each mouse was placed in a clear cylindrical swim chamber (diameter 19 cm) filled with ∼3.5 L of 24 ± 1°C water for 6 min. Each trial was recorded, and duration of immobility during the last 5 min of the trial was analyzed by an experimenter blind to experimental conditions.
Tissue Collection and Analysis
Following completion of behavioral testing, the mice were euthanized by CO2. Blood was collected via cardiac puncture, and mice were transcardially perfused. Tissue was collected, immediately snap frozen in liquid nitrogen, and stored at −80°C until processing.
Quantitative Polymerase Chain Reaction
RNA was extracted from the hippocampus and frontal cortex of the brain using the E.Z.N.A. RNA Isolation Kit II (Omega BioTek, Norcross, GA, USA). cDNA was transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Life Technologies, Grand Island, NY, USA), and quantitative real-time polymerase chain reaction (qPCR) was carried out in a QuantStudio 6 PCR machine using TaqMan gene expression assays (Applied Biosystems by Life Technologies, Grand Island, NY, USA). Reactions were performed in duplicate with the fold difference for each gene calculated using the 2−ΔΔCT method. Targets analyzed are as follows (Table 2).
Table 2.
Targets analyzed for qPCR
| Gene | Function | Catalog # |
|---|---|---|
| Il6 | Neuroinflammation | Mm00446190_m1 |
| Il1b | Neuroinflammation | Mm00434228_m1 |
| Tnf | Neuroinflammation | Mm00443258_m1 |
| Bdnf | Synaptic plasticity | Mm04230607_s1 |
| Gfap | Astrocytic reactivity | Mm01253033 |
| Snap25 | Synaptic plasticity | Mm.PT.58.11132039 |
| Slc2a1 | Glucose transporter 1 | Mm.PT.58.7590689 |
| Gapdh | Housekeeping gene | Mm99999915_g1 |
Statistical Analysis
Two-way ANOVAs were conducted for all behavioral tasks and qPCR analyses. Full ANOVA results are presented in supplemental Tables 1 and 2 (for all online suppl. material, see https://doi.org/10.1159/000534669) (online suppl. Table S1, S2). Effect size was calculated using partial eta squared for significant results. A mixed-effects model with two group factors (STZ and UCMS), time as fixed factor, and individual subjects as a random effect was conducted for body weight due to incomplete data in cohort 2 for the 6-week time point. Two-way repeated measures ANOVA was run for blood glucose. Tukey post hoc tests were performed to compare significant group differences as needed. Correlational analyses were conducted across the entire population to explore the relationships between outcome variables. These results are presented in online supplementary Tables S3 and S4. To gain perspective on overall affective dysregulation, we conducted an exploratory analysis on the computed composite symptom score. Such methodology has been utilized in a variety of similar contexts and has been shown to yield robust measures across complementary tasks [67–69]. Behavioral assessments were reverse-scored as needed, such that higher scores represent higher symptoms. The composite Z score was calculated and planned comparisons were conducted to compare the four groups. Statistical analyses were conducted using Prism 9 GraphPad and IBM SPSS Statistics 26. Effects were considered significant at p < 0.05. Data are displayed as mean ± standard error of the mean (SEM).
Results
Blood Glucose and Body Weight
Fasting blood glucose was measured throughout the experiment (Fig. 1b). There was a significant time × STZ interaction (F(4, 180) = 117.4, p < 0.0001, η2p = 0.7229) such that STZ-treated mice rapidly developed and maintained elevated blood glucose levels, as well as a significant time × UCMS interaction (F(4, 180) = 4.244, p = 0.0026, η2p = 0.0862). This appeared to be driven by stress-induced elevations in blood glucose at week 5. While the STZ × UCMS × time interaction failed to reach significance (p = 0.0608), this stress-induced elevation appears more pronounced in STZ mice, indicating that the STZ mice may be more vulnerable to stress-induced perturbations in blood glucose levels.
Body weight of mice was also monitored throughout to ensure the health of all animals (Fig. 1c). As anticipated, there was a significant time × STZ interaction (F(5, 201) = 57.38, p < 0.0001), such that STZ mice failed to gain weight during the study. A significant negative correlation was observed between body weight and blood glucose level (r2 = 0.49, p < 0.0001, see Table S3), such that mice with higher blood glucose had lower body weight. Further, there was a significant time × UCMS interaction (F(5, 201) = 25.46, p < 0.0001), such that we observed a dip in body weight corresponding to the stress exposure weeks.
Behavioral Results
Light/Dark Aversion
Latency to first exit the dark arena was analyzed for the LDA task (Fig. 2a). There was a main effect of UCMS on latency to exit, such that stress mice exited the dark arena more rapidly (F(1, 45) = 5.737, p = 0.0208, η2p = 0.1131). There was no main effect or interaction with STZ. We also looked at the total number of transitions between the light and dark arenas (Fig. 2b). As with the time to exit analysis, we observed a main effect of UCMS, such that UCMS significantly increased the number of transitions (F(1, 45) = 7.714, p = 0.0080, η2p = 0.1463). No main effect or interactions were observed for STZ.
Fig. 2.
Effect of STZ administration and UCMS protocol on behaviors measuring affective dysregulation. UCMS mice showed shorter latency to exit the dark arena (a) and more transitions between arenas in the LDA task compared to control mice (b). c STZ mice traveled less distance in the open field task compared to buffer mice. d UCMS mice spent less time in the center of the open field compared to control mice. Data are graphed as means ± SEM; *p < 0.05; **p < 0.01. n = 12–13 mice per group. UCMS, unpredictable chronic mild stress; STZ, streptozotocin; LDA, light/dark aversion; SEM, standard error of the mean.
Open Field
The distance traveled and total duration a mouse remained in the center of a novel empty open field were analyzed (Fig. 2c, d). Mice treated with STZ showed a reduction in total distance traveled (F(1, 45) = 9.584, p = 0.0034, η2p = 0.1756). UCMS did not significantly impact distance traveled. However, we did observe a main effect of UCMS on center duration, such that stressed mice spent significantly less time in the center (F(1, 45) = 10.92, p = 0.0019, η2p = 0.1952). There were no main effects or interactions for STZ on center duration.
Splash Test
Within the ST, we quantified the latency to first groom and total grooming time (Fig. 3a, b). STZ increased the latency to start grooming (F(1, 20) = 5.562, p = 0.0286, η2p = 0.2176) and decreased the total time grooming (F(1, 20) = 4.928, p = 0.0382, η2p = 0.1977). Further, we observed an STZ × UCMS interaction for total groom time (F(1, 20) = 8.738, p = 0.0078, η2p = 0.3041). Post hoc analyses indicate that this effect was driven by a reduction in the non-stressed mice, without an effect in the UCMS mice (p = 0.0078).
Fig. 3.
Effect of STZ administration and UCMS protocol on behaviors measuring affective dysregulation. STZ mice showed longer latency to first groom (a) and shorter duration spent grooming in the splash test compared to buffer mice (b). c STZ mice spent longer immobile during FST compared to buffer mice. d STZ mice buried less marbles compared to buffer mice, while UCMS mice buried less marbles compared to control mice. Data are graphed as means ± SEM; *p < 0.05; **p < 0.01, ****p < 0.0001; # indicates significantly different from all other groups. n = 6–7 mice per group for the splash test and n = 12–13 mice per group for FST and marble burying. UCMS, unpredictable chronic mild stress; STZ, streptozotocin; FST, forced swim test; SEM, standard error of the mean.
Forced Swim Test
Immobility duration was recorded during the last 5 min of the FST (Fig. 3c). STZ significantly increased immobility time (F(1, 45) = 31.39, p < 0.0001, η2p = 0.4109), and the effect of UCMS on immobility time approached significance (F(1, 45) = 3.930, p = 0.0535, η2p = 0.0803).
Marble Burying
The number of marbles buried in the marble-burying task was recorded (Fig. 3d). It was demonstrated that both STZ (F(1, 45) = 24.51, p < 0.0001, η2p = 0.3526) and UCMS (F(1, 45) = 12.06, p = 0.0012, η2p = 0.2113) significantly decreased the number of marbles buried.
Composite Behavioral Score
The composite behavioral score was calculated by averaging Z scores across the affective assessments (Table 3). Planned comparisons demonstrated that STZ-control mice showed a worse symptom profile than buffer-control mice and that STZ-UCMS mice had a higher composite symptom score than all other groups (ps < 0.001).
Table 3.
Composite behavioral score
| N | Mean | Std. error | 95% confidence interval for mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|---|
| lower bound | upper bound | ||||||
| Buffer-control | 12 | −0.504 | 0.142 | −0.816 | −0.192 | −1.42 | 0.36 |
| Buffer-UCMS | 12 | −0.167 | 0.117 | −0.424 | 0.090 | −0.71 | 0.52 |
| STZ-control | 12 | 0.016 | 0.113 | −0.232 | 0.265 | −0.51 | 0.52 |
| STZ-UCMS | 13 | 0.629 | 0.077 | 0.462 | 0.796 | 0.26 | 1.13 |
UCMS, unpredictable chronic mild stress; STZ, streptozotocin.
Evaluation of Neuroinflammation
To determine if STZ and UCMS differentially impact neuroinflammation, we ran qPCR on tissue collected from the hippocampus and frontal cortex (Fig. 4). Within the hippocampus, we observed a significant STZ-induced increase in Tnf (F(1, 19) = 6.009, p = 0.0241, η2p = 0.2403) and Bdnf (F(1, 19) = 5.784, p = 0.0265, η2p = 0.2334). Further, these two markers were correlated (r2 = 0.286, p = 0.008, see Table S2). No effects were observed for any of the other markers, and UCMS did not significantly impact the markers we evaluated in the hippocampus.
Fig. 4.
Effect of STZ administration and UCMS protocol on mRNA expression in hippocampus and frontal cortex. a STZ increased hippocampal expression of Tnf and Bdnf compared to buffer mice. b STZ increased frontal cortex expression of Il1b compared to buffer mice while UCMS decreased frontal cortex expression of Il1b compared to control mice. Data are graphed as means ± SEM; *p < 0.05. #p < 0.05 stress effect. n = 4–7 mice per group. UCMS, unpredictable chronic mild stress; STZ, streptozotocin; SEM, standard error of the mean.
Within the frontal cortex, we observed an effect of both STZ and UCMS on Il1b expression. STZ increased Il1b expression (F(1, 21) = 5.647, p = 0.0271, η2p = 0.2119), while UCMS significantly decreased its expression (F(1, 21) = 4.971, p = 0.0368, η2p = 0.1914). The increase in Il1b expression in the cortex was not significantly correlated to changes in hippocampal Tnf or Bdnf (Table S4).
Discussion
Within this study, we aimed to compare the affective and neuroinflammatory profiles of UCMS and chronic hyperglycemia. STZ-treated mice displayed the anticipated increase in fasting glucose levels and reduced body weight. We also observed increased neurovegetative depressive-like behavioral symptoms and elevations in markers of neuroinflammation. In response to UCMS, mice displayed transient reductions in body weight as well as alterations in anxiety-like behavior, a trend toward increased immobility in the FST, and decreased Il1b expression in the frontal cortex. Evidence of increased overall affective dysregulation from the addition of UCMS to STZ was also shown through exploratory analyses. These data supports our hypothesis that stress and hyperglycemia have distinct behavioral profiles and indicates that these profiles are likely mediated by distinct neurobiological profiles.
STZ induced robust changes in immobility time in the FST and groom time in the ST. Both are classic tests of antidepressant sensitivity. They have also been associated with lack of motivation or increased apathy [63]. These particular depressive-like behaviors have been reported and replicated following STZ administration by a variety of research groups [24, 25, 28, 43, 46, 48, 70, 71]. STZ also significantly reduced distance traveled in the OFT. This effect has also been reported previously [24, 50, 72]. There are a variety of reasons that a mouse may have less activity in a novel environment; it could be indicative of a depressive-like lack of exploratory interest [73, 74] or it could also point to increased fatigue [75]. Both depression and fatigue are reported at higher rates in diabetic individuals than in the general population [76–79]. Further research is needed to parse out this effect, as fatigue can represent a neurovegetative symptom of depression as well as exist independently of mood symptoms. In the context of the STZ model, it is possible that reduced energy availability due to poor glucose uptake may contribute to behavioral changes. While there are a number of studies that report reduced open field center time [46, 50, 70, 72], we were not able to detect any significant anxiety-like behaviors in our STZ mice. We also observed decreases in marble burying in response to STZ.
The UCMS mice showed an anxiety-like phenotype with significantly decreased center duration in the OFT. This result has been observed in other studies of UCMS [80–83]. In the LDA task, the UCMS groups displayed a significantly shorter latency to exit the dark arena as well as a significantly higher number of transitions between arenas. A longer latency to exit the dark area is typically associated with anxiety-like behavior [61], and therefore this brings into question why the UCMS groups showed robust anxiety-like behavior in OFT but not in LDA. The protocol we used initially places the mouse in the dark arena; another study using this protocol showed an STZ-induced increased latency to exit the dark arena [50]. However, a number of groups place mice in the illuminated arena first and measure the latency to enter the dark arena [25, 46, 84]. One such study found that chronic stress significantly decreased latency to enter the dark area [47]. A potential explanation for our unexpected result may be a consequence of the stress procedure. Mice may have developed a learned aversion to novel environments due to repeated exposure to novel stressors. If the UCMS mice were expecting a stressor when placed in the dark box, they may have exited quickly as an avoidance response. The lack of correlation between the LDA task and other affective measures also suggests that we were not measuring the affective domain we were targeting (see Table S3). Further research is needed to elucidate the impact of UCMS on the LDA task as well as our failure to detect anxiety in the STZ mice. We anticipate that the behavioral profiles of the STZ and UCMS groups would be similar if they shared neurobiological mechanisms; however, their distinct profiles suggest that chronic hyperglycemia and stress modulate affective dysregulation through distinct mechanisms.
While most assessments did not show additive effects between STZ and UCMS, additivity was observed for marble burying. In this task, the STZ-UCMS group buried significantly less marbles compared to all other groups. While there is lack of clarity in the literature concerning the domain this task evaluates, greater number of marbles buried is generally interpreted as a compulsive or anxiety-like phenotype. Our laboratory has repeatedly found that STZ induces a robust decrease in marbles buried. This reduced marble burying behavior in response to STZ has been reported previously [56, 57]. In our model, this task may be indicative of reduced motivation or fatigue-like behavior, as evidenced by its correlation with FST performance. While a stepwise pattern was observed in FST, such that STZ-UCMS mice showed the highest immobility times, the effect of UCMS on immobility was only trending toward significance. The already high immobility time in this task may produce a bit of a ceiling effect, making it difficult to observe a mild stress-induced exacerbation in the depressive phenotype. When considering all tasks analyzed, the STZ-UCMS group had the greatest number of significant dysregulated behavioral responses, and our exploratory analysis indicated more severe affective dysregulation.
With the hippocampus and frontal cortex, we evaluated a variety of neurobiological markers, examining neuroinflammation, neuroplasticity, and glucose transport. The only markers we saw dysregulated in our hippocampal samples were Tnf and Bdnf. The hippocampal mRNA expression for both markers was significantly elevated in STZ-treated mice. Current literature confirms that STZ induces a significant upregulation of hippocampal expression of Tnf at times of affective dysregulation [28, 48, 85]. While STZ has previously been reported to decrease hippocampal expression of Bdnf [28, 48, 71], indicative of a decrease in synaptic plasticity, our study found elevated Bdnf expression. It is possibly a consequence of timing, potentially as a late compensatory response caused by early-on downregulation of Bdnf. Further research is necessary to elucidate the mechanism of this upregulation. In the frontal cortex, STZ induced an elevated expression of Il1b, which is confirmed by other STZ studies [28, 86].
While UCMS did not significantly dysregulate neuroinflammatory response in the hippocampus, a downregulation in Il1b expression was observed in the frontal cortex. This was not the direction we had anticipated, as various studies have demonstrated elevations in Il1b mRNA expression following stress protocols [33, 49, 83, 87, 88]. However, other studies that have shown that UCMS can produce a depressive-like phenotype without a neuroinflammatory profile [32, 89], implicating HPA-axis dysregulation and neurotrophic changes as potential neurobiological mediators instead [32, 90]. The immunomodulatory effects of stress are complex and often variable; for a review of this topic, see [91]. Although we used a 2-week stressor protocol, there is significant variability in protocol length – ranging from 2 to 10 weeks [32, 40, 81, 92, 93]. Studies with longer stressor protocol lengths have more consistently reported increases in neuroinflammatory markers (e.g., Il1b, Il6, Tnf). The lack of neuroinflammatory consequences observed in our protocol may relate to our shorter stress protocol duration. Alternatively, the length of time between the end of the stress protocol and tissue collection may have impacted our results. While we expected the stress protocol to induce persistent neuroinflammatory effects, it is possible that having just over a week between the cessation of stress and tissue collection resulted in us missing an inflammatory signal and instead picking up a compensatory, recovery response. Further research is necessary to parse out the timeline of the pro-inflammatory profile in response to chronic stress and how it pairs to the recovery from behavioral effects.
Overall, the behavioral and neuroinflammatory results suggest that UCMS and STZ act via distinct mechanisms and, thereby, promoting their ability to have additive effects. While our data did not show increased sensitivity to stress-induced depressive symptoms in hyperglycemic mice, the known struggles associated with disease management in diabetic individuals may contribute to their high incidence of depression.
Our study did have two important limitations that should be noted. First, we only utilized male mice. Male mice have historically been used to study effects of STZ, in large part due to the difficulty of inducing hyperglycemia in female mice [94–99]. Given the higher prevalence of depressive-like behavior in female clinical populations, it is critically important to study female models of hyperglycemia-associated depression. Current research using a higher STZ dose to induce hyperglycemia in female mice is underway within our laboratory.
Another limitation, mentioned earlier, concerns the UCMS protocol. While we used a 2-week stressor, we recognize that longer protocols are often used. We utilized a shorter stress protocol as we wanted to determine if there would be potential synergy in symptom severity between STZ and UCMS. However, it is possible that a longer protocol known to induce more robust depressive-like and neuroinflammatory responses [37, 49, 82, 83] may have had a different effect. Additionally, there is significant variability in the set of mild stressors utilized between research groups. We also chose to end the stress protocol prior to starting behavioral data collection and approximately 1 week prior to tissue collection. This selection was to avoid the highly variable acute effects of the stressor on behavioral and inflammatory outcomes. As such, we do not know what the behavioral effects and neuroinflammatory signature of the brain were during and immediately following the stress protocol.
In summary, we used a murine model to investigate the effects of chronic stress and hyperglycemia on neuroinflammatory and affective behavioral profiles. While chronic stress and hyperglycemia individually produced neurovegetative and affective symptoms, their combination exacerbated the overall symptom burden. Further, they were associated with distinct neuroinflammatory profiles. This is indicative of distinct neurobiological mechanisms of depression. Since chronic illness is often accompanied by chronic stress, it is vital to investigate how chronic stress interacts with mechanisms such as hyperglycemia. Elucidating these mechanisms will provide insight into more efficient and targeted treatment for diabetic patients suffering from depression/anxiety.
Statement of Ethics
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Baylor University (IACUC protocol number: 1550457) and were in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was funded by Baylor University.
Author Contributions
Riley G. McCready and Kayla R. Gilley contributed to designing and performing the experiment, analyzing the data, and writing the manuscript. Grace M. Hall and Laura E. Kusumo contributed to performing the experiment and analyzing the data. Elisabeth G. Vichaya guided the planning of this project and the writing of this article. All authors have read and approved the final manuscript.
Funding Statement
This work was funded by Baylor University.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author. Raw data will be made available via the Baylor Electronically Accessible Research Data (BEARdata) repository.
Supplementary Material
References
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004 Jan;27Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- 2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lloyd CE, Dyer PH, Barnett AH. Prevalence of symptoms of depression and anxiety in a diabetes clinic population. Diabet Med. 2000 Mar;17(3):198–202. [DOI] [PubMed] [Google Scholar]
- 4. Smith KJ, Béland M, Clyde M, Gariépy G, Pagé V, Badawi G, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013 Feb;74(2):89–99. [DOI] [PubMed] [Google Scholar]
- 5. Lustman PJ, Harper GW. Nonpsychiatric physicians’ identification and treatment of depression in patients with diabetes. Compr Psychiatry. 1987 Jan–Feb;28(1):22–7. [DOI] [PubMed] [Google Scholar]
- 6. Menear M, Doré I, Cloutier AM, Perrier L, Roberge P, Duhoux A, et al. Chronic physical comorbidity burden and the quality of depression treatment in primary care: a systematic review. J Psychosom Res. 2015 Apr;78(4):314–23. [DOI] [PubMed] [Google Scholar]
- 7. Holt RI, de Groot M, Golden SH. Diabetes and depression. Curr Diab Rep. 2014 Jun;14(6):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Substance Abuse and Mental Health Services Administration . Key substance use and mental health indicators in the United States: results from the 2021 national survey on drug use and health. 2022. [Google Scholar]
- 9. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006 Nov;23(11):1165–73. [DOI] [PubMed] [Google Scholar]
- 10. Fugger G, Dold M, Bartova L, Kautzky A, Souery D, Mendlewicz J, et al. Major depression and comorbid diabetes: findings from the European group for the study of resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2019 Aug 30;94:109638. [DOI] [PubMed] [Google Scholar]
- 11. Tabassum R, Mia AR, Reza-Ul-Haq KM, Yesmin M, Faruqui JM. C-Reactive protein level in type-2 diabetic patients attending mymensingh medical college hospital, mymensingh. Mymensingh Med J. 2017 Jan;26(1):56–60. [PubMed] [Google Scholar]
- 12. Thorand B, Löwel H, Schneider A, Kolb H, Meisinger C, Fröhlich M, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003 Jan 13;163(1):93–9. [DOI] [PubMed] [Google Scholar]
- 13. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. 2021 Dec;26(12):7393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khuwaja AK, Lalani S, Dhanani R, Azam IS, Rafique G, White F. Anxiety and depression among outpatients with type 2 diabetes: a multi-centre study of prevalence and associated factors. Diabetol Metab Syndr. 2010 Dec 20;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon GE. Treating depression in patients with chronic disease: recognition and treatment are crucial; depression worsens the course of a chronic illness. West J Med. 2001 Nov;175(5):292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bădescu SV, Tătaru C, Kobylinska L, Georgescu EL, Zahiu DM, Zăgrean AM, et al. The association between Diabetes mellitus and Depression. J Med Life. 2016 Apr–Jun;9(2):120–5. [PMC free article] [PubMed] [Google Scholar]
- 18. Berge LI, Riise T. Comorbidity between type 2 diabetes and depression in the adult population: directions of the association and its possible pathophysiological mechanisms. Int J Endocrinol. 2015;2015:164760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008 Dec;31(12):2398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics. 2011 Jan–Feb;52(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Dooren FE, Nefs G, Schram MT, Verhey FR, Denollet J, Pouwer F. Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2013;8(3):e57058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolo NR, Jacobson AM, Musen G, Keshavan MS, Simonson DC. Acute hyperglycemia increases brain pregenual anterior cingulate cortex glutamate concentrations in type 1 diabetes. Diabetes. 2020 Jul;69(7):1528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilsanz P, Karter AJ, Beeri MS, Quesenberry CP Jr., Whitmer RA. The bidirectional association between depression and severe hypoglycemic and hyperglycemic events in type 1 diabetes. Diabetes Care. 2018 Mar;41(3):446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen C, Wang Y, Zhang J, Ma L, Gu J, Ho G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis Model Mech. 2014 Jun;7(6):723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta D, Radhakrishnan M, Kurhe Y. Ondansetron, a 5HT3 receptor antagonist reverses depression and anxiety-like behavior in streptozotocin-induced diabetic mice: possible implication of serotonergic system. Eur J Pharmacol. 2014 Dec 5;744:59–66. [DOI] [PubMed] [Google Scholar]
- 26. Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013 Sep;37(8):1346–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011 Aug;61(4):356–60. [PMC free article] [PubMed] [Google Scholar]
- 28. Bampi SR, Casaril AM, Fronza MG, Domingues M, Vieira B, Begnini KR, et al. The selenocompound 1-methyl-3-(phenylselanyl)-1H-indole attenuates depression-like behavior, oxidative stress, and neuroinflammation in streptozotocin-treated mice. Brain Res Bull. 2020 Aug;161:158–65. [DOI] [PubMed] [Google Scholar]
- 29. Montilla PL, Vargas JF, Túnez IF, Muñoz de Agueda MC, Valdelvira ME, Cabrera ES. Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J Pineal Res. 1998 Sep;25(2):94–100, [DOI] [PubMed] [Google Scholar]
- 30. Niu S, Bian Z, Tremblay A, Luo Y, Kidder K, Mansour A, et al. Broad infiltration of macrophages leads to a proinflammatory state in streptozotocin-induced hyperglycemic mice. J Immunol. 2016 Oct 15;197(8):3293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PLoS One. 2009;4(1):e4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cardinal P, Monchaux de Oliveira C, Sauvant J, Foury A, Darnaudéry M, Vancassel S, et al. A new experimental design to study inflammation-related versus non-inflammation-related depression in mice. J Neuroinflammation. 2021 Dec 11;18(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan C, Song Q, Wang P, Li Y, Yang M, Liu B, et al. Curcumin protects against chronic stress-induced dysregulation of neuroplasticity and depression-like behaviors via suppressing IL-1β pathway in rats. Neuroscience. 2018 Nov 10;392:92–106. [DOI] [PubMed] [Google Scholar]
- 34. Nollet M. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc. 2021 Aug;1(8):e208. [DOI] [PubMed] [Google Scholar]
- 35. Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008 Jan;33(1):88–109. [DOI] [PubMed] [Google Scholar]
- 36. Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28(2):77–88. [DOI] [PubMed] [Google Scholar]
- 37. Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G, et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav Brain Res. 2019 May 17;364:494–502. [DOI] [PubMed] [Google Scholar]
- 38. Filho CB, Jesse CR, Donato F, Giacomeli R, Del Fabbro L, da Silva Antunes M, et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na(+),K(+)-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015 Mar 19;289:367–80. [DOI] [PubMed] [Google Scholar]
- 39. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006 Jun 15;59(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 40. Mao QQ, Huang Z, Zhong XM, Xian YF, Ip SP. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res. 2014 Mar 15;261:140–5. [DOI] [PubMed] [Google Scholar]
- 41. Wohleb ES, Terwilliger R, Duman CH, Duman RS. Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol Psychiatry. 2018 Jan 1;83(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li W, Ali T, Zheng C, Liu Z, He K, Shah FA, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021 Feb 1;18(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, et al. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res. 2014 Jan 1;258:52–64. [DOI] [PubMed] [Google Scholar]
- 44. Hilakivi-Clarke LA, Wozniak KM, Durcan MJ, Linnoila M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol Behav. 1990 Sep;48(3):429–33. [DOI] [PubMed] [Google Scholar]
- 45. Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry. 2015 Aug 15;78(4):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mbiantcha M, Khalid R, Dawe A, Mehreen A, Atsamo DA, Ateufack G, et al. Antihypernociceptive and neuroprotective effects of Combretin A and Combretin B on streptozotocin-induced diabetic neuropathy in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019 Jun;392(6):697–713. [DOI] [PubMed] [Google Scholar]
- 47. Naqvi F, Saleem S, Naqvi F, Batool Z, Sadir S, Tabassum S, et al. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak J Pharm Sci. 2019 Jul;32(4 Suppl):1893–900. [PubMed] [Google Scholar]
- 48. Patel SS, Ray RS, Sharma A, Mehta V, Katyal A, Udayabanu M. Antidepressant and anxiolytic like effects of Urtica dioica leaves in streptozotocin induced diabetic mice. Metab Brain Dis. 2018 Aug;33(4):1281–92. [DOI] [PubMed] [Google Scholar]
- 49. Xue J, Li H, Deng X, Ma Z, Fu Q, Ma S. L-Menthone confers antidepressant-like effects in an unpredictable chronic mild stress mouse model via NLRP3 inflammasome-mediated inflammatory cytokines and central neurotransmitters. Pharmacol Biochem Behav. 2015 Jul;134:42–8. [DOI] [PubMed] [Google Scholar]
- 50. Yuan P, Zhang J, Li L, Song Z. Fluoxetine attenuated anxiety-like behaviors in streptozotocin-induced diabetic mice by mitigating the inflammation. Mediators Inflamm. 2019;2019:4315038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Genné-Bacon EA, Trinko JR, DiLeone RJ. Innate fear-induced weight regulation in the C57bl/6J mouse. Front Behav Neurosci. 2016;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013 Dec 24(82):50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Brouwer G, Fick A, Harvey BH, Wolmarans W. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: mapping the way forward. Cogn Affect Behav Neurosci. 2019 Feb;19(1):1–39. [DOI] [PubMed] [Google Scholar]
- 54. Gyertyán I. Analysis of the marble burying response: marbles serve to measure digging rather than evoke burying. Behav Pharmacol. 1995 Jan;6(1):24–31. [PubMed] [Google Scholar]
- 55. Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009 Jun;204(2):361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gazzo G, Salgado Ferrer M, Poisbeau P. The non-benzodiazepine anxiolytic etifoxine limits mechanical allodynia and anxiety-like symptoms in a mouse model of streptozotocin-induced diabetic neuropathy. PLoS One. 2021;16(8):e0248092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zappa Villar MF, López Hanotte J, Pardo J, Morel GR, Mazzolini G, García MG, et al. Mesenchymal stem cells therapy improved the streptozotocin-induced behavioral and hippocampal impairment in rats. Mol Neurobiol. 2020 Feb;57(2):600–15. [DOI] [PubMed] [Google Scholar]
- 58. Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1(1):122–4. [DOI] [PubMed] [Google Scholar]
- 59. Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980 Aug;13(2):167–70. [DOI] [PubMed] [Google Scholar]
- 60. Hascoët M, Bourin M, Nic Dhonnchadha BA. The mouse light-dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2001 Jan;25(1):141–66. [DOI] [PubMed] [Google Scholar]
- 61. Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006 Nov13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015 Feb 6(96):e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One. 2010 Apr 28;5(4):e10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yalcin I, Belzung C, Surget A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res. 2008 Nov 3;193(1):140–3. [DOI] [PubMed] [Google Scholar]
- 65. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978 Feb 15;47(4):379–91. [DOI] [PubMed] [Google Scholar]
- 66. Vichaya EG, Laumet G, Christian DL, Grossberg AJ, Estrada DJ, Heijnen CJ, et al. Motivational changes that develop in a mouse model of inflammation-induced depression are independent of indoleamine 2,3 dioxygenase. Neuropsychopharmacology. 2019 Jan;44(2):364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011 Apr 15;197(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Harrison DJ, Creeth HDJ, Tyson HR, Boque-Sastre R, Isles AR, Palme R, et al. Unified behavioral scoring for preclinical models. Front Neurosci. 2020;14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kraeuter AK. The use of integrated behavioural z-scoring in behavioural neuroscience: a perspective article. J Neurosci Methods. 2023 Jan 15;384:109751. [DOI] [PubMed] [Google Scholar]
- 70. Amiri S, Haj-Mirzaian A, Momeny M, Amini-Khoei H, Rahimi-Balaei M, Poursaman S, et al. Streptozotocin induced oxidative stress, innate immune system responses and behavioral abnormalities in male mice. Neuroscience. 2017 Jan 6;340:373–83. [DOI] [PubMed] [Google Scholar]
- 71. Wang J, Duan P, Cui Y, Li Q, Shi Y. Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampus of streptozotocin-evoked mice. Metab Brain Dis. 2016 Oct;31(5):1113–22. [DOI] [PubMed] [Google Scholar]
- 72. Huang CW, Hong TW, Wang YJ, Chen KC, Pei JC, Chuang TY, et al. Ophiocordyceps formosana improves hyperglycemia and depression-like behavior in an STZ-induced diabetic mouse model. BMC Complement Altern Med. 2016 Aug 24;16(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li ZR, Han YS, Liu Z, Zhao HQ, Liu J, Yang H, et al. GR/NF-κB signaling pathway regulates hippocampal inflammatory responses in diabetic rats with chronic unpredictable mild stress. Eur J Pharmacol. 2021 Mar 15;895:173861. [DOI] [PubMed] [Google Scholar]
- 74. Wang YH, Yin LT, Yang H, Li XL, Wu KG. Hypoglycemic and anti-depressant effects of Zuogui Jiangtang Jieyu formulation in a model of unpredictable chronic mild stress in rats with diabetes mellitus. Exp Ther Med. 2014 Jul;8(1):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang-Fischer Y, Garyantes T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. J Diabetes Res. 2018;2018:8054073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010 Jul;69(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalra S, Sahay R. Diabetes Fatigue Syndrome. Diabetes therapy : research, treatment and education of diabetes and related disorders. Diabetes Ther. 2018 Aug;9(4):1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lasselin J, Layé S, Dexpert S, Aubert A, Gonzalez C, Gin H, et al. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav Immun. 2012 Nov;26(8):1211–9. [DOI] [PubMed] [Google Scholar]
- 79. Lasselin J, Layé S, Barreau JB, Rivet A, Dulucq MJ, Gin H, et al. Fatigue and cognitive symptoms in patients with diabetes: relationship with disease phenotype and insulin treatment. Psychoneuroendocrinology. 2012 Sep;37(9):1468–78. [DOI] [PubMed] [Google Scholar]
- 80. Ieraci A, Mallei A, Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lippi SLP. Chronic mild unpredictable stress and high-fat diet given during adolescence impact both cognitive and noncognitive behaviors in young adult mice. Brain Sci. 2021 Feb 19;11(2):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ping G, Qian W, Song G, Zhaochun S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol Biochem Behav. 2014 Sep;124:5–12. [DOI] [PubMed] [Google Scholar]
- 83. Zhao Z, Zhang L, Guo XD, Cao LL, Xue TF, Zhao XJ, et al. Rosiglitazone exerts an anti-depressive effect in unpredictable chronic mild-stress-induced depressive mice by maintaining essential neuron autophagy and inhibiting excessive astrocytic apoptosis. Front Mol Neurosci. 2017;10:293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84. Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006 Nov 25;175(1):43–50. [DOI] [PubMed] [Google Scholar]
- 85. Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. 2019;10:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kotagale N, Rahangdale S, Borkar A, Singh K, Ikhar A, Takale N, et al. Possible involvement of agmatine in neuropharmacological actions of metformin in diabetic mice. Eur J Pharmacol. 2021 Sep 15;907:174255. [DOI] [PubMed] [Google Scholar]
- 87. Avolio E, Fazzari G, Mele M, Alò R, Zizza M, Jiao W, et al. Unpredictable chronic mild stress paradigm established effects of pro- and anti-inflammatory cytokine on neurodegeneration-linked depressive states in hamsters with brain endothelial damages. Mol Neurobiol. 2017 Oct;54(8):6446–58. [DOI] [PubMed] [Google Scholar]
- 88. Fernandes J, Gupta GL. N-acetylcysteine attenuates neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Behav Brain Res. 2019 May 17;364:356–65. [DOI] [PubMed] [Google Scholar]
- 89. Yu H, Yu B, Qin X, Shan W. A unique inflammation-related mechanism by which high-fat diets induce depression-like behaviors in mice. J Affect Disord. 2023;339:180–93. [DOI] [PubMed] [Google Scholar]
- 90. Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017 Feb;6:78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang EV, Glaser R. Stress-induced immunomodulation and the implications for health. Int Immunopharmacol. 2002;2(2–3):315–24. [DOI] [PubMed] [Google Scholar]
- 92. Yang H, Li W, Meng P, Liu Z, Liu J, Wang Y. Chronic Unpredictable Mild Stress Aggravates Mood Disorder, Cognitive Impairment, and Brain Insulin Resistance in Diabetic Rat. Evid Based Complement Alternat Med. 2018;2018:2901863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. López-López AL, Jaime HB, Escobar Villanueva MDC, Padilla MB, Palacios GV, Aguilar FJA. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol Behav. 2016 Jul 1;161:15–23. [DOI] [PubMed] [Google Scholar]
- 94. Kim B, Kim YY, Nguyen PT, Nam H, Suh JG. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl Biol Chem. 2020;63(1):59. [Google Scholar]
- 95. Chandramouli C, Reichelt ME, Curl CL, Varma U, Bienvenu LA, Koutsifeli P, et al. Diastolic dysfunction is more apparent in STZ-induced diabetic female mice, despite less pronounced hyperglycemia. Sci Rep. 2018 Feb 5;8(1):2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim B, Park ES, Lee JS, Suh JG. Outbred mice with streptozotocin-induced diabetes show sex differences in glucose metabolism. Int J Mol Sci. 2023 Mar 8;24(6). 5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Saadane A, Lessieur EM, Du Y, Liu H, Kern TS. Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS One. 2020;15(9):e0238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tian L, Nikolic-Paterson DJ, Tesch GH. Establishing equivalent diabetes in male and female Nos3-deficient mice results in a comparable onset of diabetic kidney injury. Physiol Rep. 2019 Sep;7(18):e14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. O’Neill CC, Locke EJ, Sipf DA, Thompson JH, Drebushenko EK, Berger NS, et al. The effects of exercise training on glucose homeostasis and muscle metabolism in type 1 diabetic female mice. Metabolites. 2022 Oct 5;12(10):948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author. Raw data will be made available via the Baylor Electronically Accessible Research Data (BEARdata) repository.