Abstract
Background
Meningitis is a severe and fatal neurological disease and causes lots of disease burden. The purpose of this study was to assess the global, regional, and national burdens and trends of meningitis by age, sex, and etiology.
Methods
Data on the burden of meningitis were collected from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019. R and Joinpoint were used for statistical analysis and charting.
Results
In 2019, meningitis caused 236,222 deaths and 15,649,865 years of life lost (YLL) worldwide. The age-standardized death rate and age-standardized YLL rate of meningitis were 3.29 and 225, which decreased steadily. Burden change was mainly driven by epidemiological changes. Regionally, meningitis burden was the highest in Sub-Saharan Africa. Burden of disease increasingly concentrated in low sociodemographic index countries, and this was most pronounced in meningitis caused by N. meningitidis. Countries such as Mali, Nigeria, Sierra Leone, etc., especially need to enhance the rational allocation of public health resources to reduce the disease burden. Children and men were more likely to be affected by meningitis. PM2.5 was found to be an important risk factor.
Conclusions
This study provides the first comprehensive understanding of the global disease burden of meningitis caused by specific pathogens and highlights policy priorities to protect human health worldwide, with particular attention to vulnerable regions, susceptible populations, environmental factors, and specific pathogens.
Keywords: Global Burden of Disease, Meningitis, Socioeconomic status, Pathogen
Introduction
Meningitis is a severe, fatal disease that can cause permanent brain damage, death, and severe, long-term neurological sequelae [1–3]. Despite the availability of meningitis vaccines and antibiotics to prevent and treat meningitis, the current global burden of meningitis remains high, and meningitis is both a large component of the global neurological burden and a noteworthy cause of death among children worldwide [4–10]. The three common pathogens that cause meningitis are Haemophilus influenzae type B (Hib), Neisseria meningitidis (N. meningitidis), and Streptococcus pneumoniae (S. pneumoniae) [11].
Meningitis vaccination and other measures have led to a decline in the incidence of meningitis. However, there is still a considerable challenge for future meningitis prevention and treatment [12, 13]. However, in recent years there have been no epidemiological studies describing the global burden of meningitis caused by specific pathogens. We used data on the number of meningitis deaths, years of life lost (YLL), age-standardized death rates (ASDRs), age-standardized YLL rates (ASYRs), and annual rates of change (ARCs) from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019 (GBD 2019) to analyze the burden and trends of meningitis. Meningitis DALYs are mainly composed of YLL rather than years lived with disabilities [14]. We analyzed the burden and trends of meningitis with specific etiologies from 1990 to 2019 at the global, regional, and national levels by age and sex. Then, we analyzed cross-country health inequalities and major drivers of change in the burden of meningitis. Our research also revealed the potential improvement of meningitis burden on countries with different sociodemographic index (SDI) quantiles and relationship between national-level indicators and the burden of meningitis. We performed this study to raise awareness about meningitis among health care professionals and to improve policymakers’ understanding of the global burden of meningitis to provide a reference for strategies to reduce the burden of meningitis.
Methods
Data Sources
Data from GBD 2019 were obtained from 204 countries and territories from January 1, 1990, to December 31, 2019 [6]. These countries and territories were divided into 21 regions according to geographical location, grouped into five SDI quantiles. The SDI value is publicly available at the Institute for Health Metrics and Evaluation [15] and Human Development Reports [16], respectively. The GBD database uses the Institute for Health Metrics and Evaluation Bayesian regression tool DisMod-MR 2.1 to analyze, model and estimate the above indicators and standardize the world population [17]. The data of country-level indicators were collected from World Bank databases [18]. The number of meningitis deaths, YLLs, ASDR, ASYR, ARC and their 95% uncertainty interval were extracted for different countries, regions, and sexes. Relevant definitions of the work and specific country-level indicators are shown in online supplementary methods (for all online suppl. material, see https://doi.org/10.1159/000531508).
Statistical Methods
R (4.1.3) [19] was used for data organization, statistical analysis, and charting. Joinpoint (version 4.9.1.0; National Cancer Institute, Rockville, MD, USA) was used to create joinpoint regression models to assess trends in meningitis disease burden over time. A 95% confidence interval was calculated. p < 0.05 was considered statistically significant. We used fixed-effects mode panel data model analysis to explore the relationship between national level indicators and meningitis burden because it can effectively control unobserved time-invariant factors and location-invariant factors. To evaluate the relationship between burden of meningitis and sociodemographic development, we applied a frontier analysis as a quantitative methodology to identify the lowest potentially achievable ASDR/ASYR on the basis of development status as measured by the SDI. We used the slope index and concentration index to calculate the extent of health inequalities, specific methods are shown in online supplementary methods.
Results
Global Meningitis Burden
Deaths due to meningitis and age-standardized rates of YLL by region and sex worldwide in 1990–2019 are shown in Table 1 and online supplementary eTable 1. A total of 236,222 (95% uncertainty interval, 204,381–277,426) deaths and 15,649,865 (13,102,961–18,930,650) YLL occurred in 2019, which was almost one-half of the deaths (432,524 [376,419–494,074]) and YLL (32,699,213 [27,961,002–37,926,150]) in 1990. The ASDR and ASYR of meningitis decreased from 7.47 (6.58–8.45) and 531.85 (457.56–613.63) to 3.29 (2.82–3.89) and 225 (187.29–273.1), respectively, with ARCs of −0.56 (−0.62 to −0.48) and −0.58 (−0.65 to −0.49), respectively. After joinpoint regression analysis, there was a joinpoint for the meningitis ASDR and ASYR near 2008, after which the meningitis ASDR and ASYR decreased at an accelerated rate (shown in Fig. 1). In 2019, the burden of meningitis was concentrated in low SDI and low-middle SDI regions. In all SDI quintile regions, the disparity between the ASDRs and ASYRs in low SDI regions and other regions was enormous, with the ASDR 3.42 times the global average and 45 times that of high SDI regions. The downward trend in the ASDRs and ASYRs of meningitis was observed in all SDI regions by etiology and sex, but this trend was smaller in lower SDI regions. Compared with the ARCs in the ASDR of −0.69 (−0.7 to −0.67) and the ASYR of −0.73 (−0.75 to −0.72) in high SDI regions, the ARCs in the ASDR and ASYR in low SDI regions were only −0.55 (−0.62 to −0.46) and −0.58 (−0.65 to −0.48), respectively (shown in online suppl. eFig. 1–4).
Table 1.
Global and regional ASDR, ASYR, and cases of meningitis in 1990 and 2019, and the annualized rate of change in ASDR/ASYR
| Location | Sex | Death (95% UI) | YLL (95% UI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 | 2019 | 1990 | 2019 | ||||||||
| count | ASR (per 100,000) | count | ASR (per 100,000) | annual rate of change in ASR | count | ASR (per 100,000) | count | ASR (per 100,000) | annual rate of change in ASR | ||
| Global | Both | 432,524 (376,419–494,074) | 7.47 (6.58–8.45) | 236,222 (204,381–277,426) | 3.29 (2.82–3.89) | −0.56 (−0.62 to −0.48) | 32,699,213 (27,961,002–37,926,150) | 531.85 (457.56–613.63) | 15,649,865 (13,102,961–18,930,650) | 225 (187.29–273.1) | −0.58 (−0.65 to −0.49) |
| Male | 228,903 (198,514–264,712) | 7.87 (6.9–8.99) | 128,802 (110,632–153,328) | 3.56 (3.05–4.27) | −0.55 (−0.62 to −0.45) | 17,366,184 (14,881,696–20,442,486) | 552.25 (475.82–645.49) | 8,641,816 (7,185,721–10,667,842) | 242.59 (200.71–301.07) | −0.56 (−0.64 to −0.46) | |
| Female | 203,621 (174,589–236,785) | 7.1 (6.14–8.22) | 107,419 (91,361–126,253) | 3.01 (2.53–3.57) | −0.58 (−0.64 to −0.49) | 15,333,029 (12,839,421–18,132,993) | 510.96 (429.81–602.38) | 7,008,049 (5,746,567–8,539,772) | 206.64 (168.04–253.78) | −0.6 (−0.67 to −0.5) | |
| High SDI | Both | 6,592 (6,374–6,806) | 0.82 (0.79–0.85) | 3,303 (3,088–3,477) | 0.25 (0.24–0.27) | −0.69 (−0.7 to −0.67) | 323,915 (313,181–337,110) | 46.39 (44.71–48.51) | 111,606 (105,485–117,629) | 12.35 (11.5–13.18) | −0.73 (−0.75 to −0.72) |
| Male | 3,541 (3,408–3,678) | 0.94 (0.9–0.98) | 1,793 (1,693–1,896) | 0.29 (0.28–0.31) | −0.69 (−0.71 to −0.67) | 182,491 (175,244–191,170) | 52.01 (49.84–54.74) | 63,578 (59,470–67,684) | 13.83 (12.78–14.85) | −0.73 (−0.76 to −0.71) | |
| Female | 3,050 (2,908–3,198) | 0.71 (0.68–0.75) | 1,510 (1,373–1,614) | 0.22 (0.21–0.23) | −0.69 (−0.71 to −0.67) | 141,424 (135,437–149,809) | 40.79 (38.78–43.68) | 48,028 (45,396–50,705) | 10.84 (10.08–11.59) | −0.73 (−0.76 to −0.72) | |
| High-middle SDI | Both | 27,387 (24,978–30,047) | 2.59 (2.35–2.84) | 8,517 (7,841–9,170) | 0.6 (0.55–0.66) | −0.77 (−0.8 to −0.73) | 1,924,908 (1,721,767–2,148,686) | 181.84 (162.05–203.2) | 389,349 (354,312–426,620) | 34.21 (30.63–38.22) | −0.81 (−0.84 to −0.78) |
| Male | 15,882 (14,338–17,542) | 3.02 (2.73–3.33) | 5,020 (4,567–5,538) | 0.73 (0.66–0.81) | −0.76 (−0.79 to −0.72) | 1,116,031 (994,141–1,255,930) | 205.94 (183.27–232.2) | 237,205 (214,464–262,971) | 40.16 (35.8–45.19) | −0.8 (−0.84 to −0.77) | |
| Female | 11,504 (10,296–12,881) | 2.18 (1.94–2.46) | 3,497 (3,218–3,789) | 0.49 (0.44–0.53) | −0.78 (−0.81 to −0.74) | 808,877 (705,648–923,964) | 157.11 (136.38–180.29) | 152,144 (138,073–167,993) | 28.05 (24.95–31.8) | −0.82 (−0.85 to −0.78) | |
| Middle SDI | Both | 86,197 (76,774–96,739) | 4.82 (4.34–5.35) | 29,389 (26,336–33,120) | 1.37 (1.22–1.55) | −0.72 (−0.75 to −0.67) | 6,587,367 (5,790,514–7,487,080) | 334.17 (294.63–378.61) | 1,632,738 (1,421,966–1,877,659) | 79.03 (68.24–91.68) | −0.76 (−0.8 to −0.72) |
| Male | 49,014 (43,135–55,845) | 5.36 (4.76–6.01) | 16,822 (14,778–19,549) | 1.57 (1.37–1.82) | −0.71 (−0.76 to −0.64) | 3,771,620 (3,281,360–4,403,070) | 372.02 (325.08–429.83) | 954,692 (822,715–1,122,443) | 89.87 (76.95–106.06) | −0.76 (−0.8 to −0.7) | |
| Female | 37,183 (32,132–42,319) | 4.26 (3.75–4.79) | 12,568 (11,174–14,060) | 1.17 (1.04–1.32) | −0.72 (−0.76 to −0.68) | 2,815,748 (2,383,821–3,245,431) | 294.02 (250.63–337.51) | 678,046 (584,344–784,418) | 67.75 (57.83–79.91) | −0.77 (−0.81 to −0.72) | |
| Low-middle SDI | Both | 147,955 (128,749–170,146) | 11.74 (10.49–13.16) | 65,802 (57,769–75,428) | 4.07 (3.58–4.64) | −0.65 (−0.7 to −0.6) | 11,113,082 (9,499,409–12,998,789) | 738.8 (641.12–851.53) | 4,090,122 (3,501,435–4,828,168) | 235.99 (202.12–279.02) | −0.68 (−0.74 to −0.61) |
| Male | 73,825 (63,762–85,294) | 11.43 (10.16–12.85) | 33,990 (28,876–40,494) | 4.18 (3.58–4.94) | −0.63 (−0.7 to −0.56) | 5,560,704 (4,721,110–6,546,194) | 719.24 (620.03–832.53) | 2,151,707 (1,789,933–2,641,668) | 243.8 (203.39–298.64) | −0.66 (−0.73 to −0.58) | |
| Female | 74,129 (62,428–87,797) | 12.05 (10.43–13.92) | 31,812 (27,235–37,163) | 3.95 (3.39–4.61) | −0.67 (−0.72 to −0.6) | 5,552,378 (4,566,452–6,695,809) | 759.19 (636.72–902.88) | 1,938,415 (1,620,416–2,341,803) | 227.89 (190.39–275.79) | −0.7 (−0.76 to −0.61) | |
| Low SDI | Both | 164,117 (136,691–195,630) | 24.83 (21.52–28.54) | 129,072 (106,069–157,253) | 11.25 (9.6–13.22) | −0.55 (−0.62 to −0.46) | 12,728,291 (10,400,161–15,344,414) | 1,532.63 (1,287.64–1,814.78) | 9,416,356 (7,536,502–11,724,516) | 647.06 (530.96–789.17) | −0.58 (−0.65 to −0.48) |
| Male | 86,490 (71,675–105,832) | 25.58 (21.99–30.08) | 71,100 (58,060–88,468) | 12.17 (10.34–14.44) | −0.52 (−0.6 to −0.42) | 6,723,601 (5,439,402–8,334,360) | 1,587.32 (1,324.05–1,919.77) | 5,229,192 (4,188,145–6,714,762) | 707.15 (577.51–879.67) | −0.55 (−0.64 to −0.44) | |
| Female | 77,627 (63,945–93,560) | 24.03 (20.5–28.17) | 57,972 (47,017–71,238) | 10.31 (8.7–12.17) | −0.57 (−0.64 to −0.49) | 6,004,690 (4,847,411–7,388,889) | 1,475.18 (1,219.77–1,769.48) | 4,187,164 (3,282,536–5,289,542) | 585.61 (472.51–720.29) | −0.6 (−0.68 to −0.5) | |
| Andean Latin America | Both | 1,315 (1,146–1,530) | 2.93 (2.59–3.36) | 395 (314–494) | 0.65 (0.52–0.81) | −0.78 (−0.83 to −0.71) | 101,355 (87,337–119,446) | 200.3 (173.91–233.78) | 22,227 (17,418–28,422) | 35.08 (27.48–44.87) | −0.82 (−0.87 to −0.77) |
| Male | 719 (614–857) | 3.22 (2.8–3.75) | 226 (177–286) | 0.74 (0.58–0.94) | −0.77 (−0.83 to −0.69) | 55,149 (46,168–67,000) | 217.02 (185.02–259.96) | 12,747 (9,797–16,637) | 39.9 (30.76–52.09) | −0.82 (−0.87 to −0.74) | |
| Female | 597 (488–726) | 2.64 (2.23–3.12) | 169 (133–218) | 0.55 (0.43–0.71) | −0.79 (−0.84 to −0.72) | 46,205 (37,173–57,075) | 183.46 (149.64–223.47) | 9,481 (7,209–12,489) | 30.27 (23–39.83) | −0.84 (−0.88 to −0.76) | |
| Australasia | Both | 125 (117–134) | 0.67 (0.63–0.72) | 59 (54–64) | 0.19 (0.17–0.21) | −0.72 (−0.75 to −0.69) | 7,457 (6,859–8,163) | 43.46 (39.66–47.83) | 2,553 (2,269–2,849) | 10.73 (9.26–12.3) | −0.75 (−0.79 to −0.71) |
| Male | 69 (64–75) | 0.77 (0.71–0.83) | 29 (26–32) | 0.19 (0.17–0.22) | −0.75 (−0.78 to −0.71) | 4,292 (3,912–4,683) | 49.63 (45.03–54.47) | 1,315 (1,154–1,494) | 11.07 (9.43–12.83) | −0.78 (−0.82 to −0.73) | |
| Female | 56 (52–61) | 0.58 (0.53–0.64) | 30 (27–32) | 0.18 (0.16–0.2) | −0.69 (−0.73 to −0.65) | 3,165 (2,850–3,549) | 37.09 (33.13–42.13) | 1,238 (1,106–1,378) | 10.38 (9.01–11.85) | −0.72 (−0.77 to −0.67) | |
| Caribbean | Both | 3,475 (2,934–4,076) | 8.93 (7.63–10.38) | 1,424 (1,075–1,864) | 3.39 (2.54–4.49) | −0.62 (−0.73 to −0.48) | 271,634 (225,102–324,182) | 662.13 (550.62–786.77) | 103,981 (74,822–140,656) | 255.74 (182.95–348.43) | −0.61 (−0.74 to −0.44) |
| Male | 1,860 (1,525–2,253) | 9.53 (7.96–11.39) | 810 (571–1,124) | 3.85 (2.68–5.4) | −0.6 (−0.73 to −0.41) | 145,582 (116,399–179,397) | 702.44 (566.27–861.26) | 59,751 (39,806–86,333) | 290.33 (191.95–422.19) | −0.59 (−0.75 to −0.37) | |
| Female | 1,615 (1,268–2,011) | 8.33 (6.64–10.24) | 615 (445–854) | 2.92 (2.06–4.12) | −0.65 (−0.77 to −0.49) | 126,052 (96,321–159,963) | 621.09 (476.79–784.79) | 44,230 (30,365–64,011) | 220.19 (150.17–322.23) | −0.65 (−0.78 to −0.46) | |
| Central Asia | Both | 3,031 (2,787–3,287) | 3.73 (3.47–4) | 638 (552–752) | 0.7 (0.61–0.82) | −0.81 (−0.84 to −0.78) | 232,495 (211,349–254,647) | 267.96 (245.27–291.59) | 39,510 (33,434–48,282) | 41.87 (35.39–51.2) | −0.84 (−0.87 to −0.8) |
| Male | 1,694 (1,537–1,847) | 4.22 (3.89–4.56) | 380 (324–444) | 0.85 (0.73–1) | −0.8 (−0.83 to −0.76) | 130,874 (117,715–144,360) | 299.07 (270.67–326.81) | 23,355 (19,671–27,830) | 49.08 (41.35–58.33) | −0.84 (−0.86 to −0.8) | |
| Female | 1,337 (1,223–1,465) | 3.27 (3.02–3.55) | 258 (219–329) | 0.56 (0.48–0.72) | −0.83 (−0.85 to −0.77) | 101,621 (91,801–112,563) | 236.76 (215.52–260.39) | 16,155 (13,343–22,073) | 34.78 (28.76–47.64) | −0.85 (−0.88 to −0.79) | |
| Central Europe | Both | 1,738 (1,672–1,828) | 1.65 (1.57–1.75) | 421 (357–484) | 0.3 (0.25–0.35) | −0.82 (−0.85 to −0.79) | 108,417 (102,649–116,097) | 112.79 (105.97–121.62) | 15,263 (12,798–17,711) | 15.14 (12.55–17.95) | −0.87 (−0.89 to −0.84) |
| Male | 1,044 (1,003–1,103) | 2 (1.91–2.13) | 256 (209–302) | 0.39 (0.32–0.45) | −0.81 (−0.84 to −0.77) | 65,219 (61,590–70,186) | 133.39 (125.17–144.84) | 9,623 (7,844–11,293) | 18.56 (15.14–22.06) | −0.86 (−0.89 to −0.83) | |
| Female | 694 (661–741) | 1.31 (1.23–1.42) | 165 (143–190) | 0.22 (0.19–0.26) | −0.83 (−0.86 to −0.8) | 43,197 (40,352–47,263) | 92.1 (85.21–101.92) | 5,639 (4,793–6,582) | 11.77 (9.82–14.04) | −0.87 (−0.9 to −0.85) | |
| Central Latin America | Both | 4,720 (4,401–5,068) | 2.59 (2.44–2.74) | 1,670 (1,365–2,028) | 0.7 (0.57–0.85) | −0.73 (−0.78 to −0.67) | 357,812 (330,003–388,760) | 170.51 (158.68–183.53) | 86,226 (68,902–107,037) | 36.33 (28.98–45.29) | −0.79 (−0.84 to −0.73) |
| Male | 2,688 (2,488–2,907) | 2.98 (2.81–3.18) | 992 (787–1,229) | 0.86 (0.69–1.07) | −0.71 (−0.78 to −0.64) | 203,009 (185,298–221,896) | 193.69 (178.91–209.76) | 51,187 (40,202–64,231) | 43.48 (34.11–54.72) | −0.78 (−0.83 to −0.71) | |
| Female | 2,032 (1,887–2,190) | 2.2 (2.07–2.35) | 678 (555–820) | 0.55 (0.45–0.67) | −0.75 (−0.8 to −0.69) | 154,803 (142,274–168,527) | 147.4 (136.47–159.48) | 35,039 (28,166–43,411) | 29.51 (23.53–36.78) | −0.8 (−0.84 to −0.74) | |
| Central Sub-Saharan Africa | Both | 14,516 (11,406–18,258) | 19.6 (15.86–23.71) | 9,995 (7,725–12,885) | 8.9 (6.76–11.21) | −0.55 (−0.65 to −0.43) | 1,141,880 (881,126–1,454,326) | 1,249.09 (1,004.1–1,545.45) | 658,348 (506,885–854,911) | 440.18 (340.06–566.28) | −0.65 (−0.74 to −0.55) |
| Male | 6,759 (5,010–8,915) | 19.26 (14.65–24.39) | 5,004 (3,692–6,724) | 9.43 (6.75–12.63) | −0.51 (−0.64 to −0.37) | 526,030 (384,843–713,872) | 1,177.9 (887.26–1,508.22) | 325,512 (239,716–438,963) | 451.33 (330.26–599.55) | −0.62 (−0.73 to −0.48) | |
| Female | 7,757 (5,888–10,395) | 20.07 (15.76–25.42) | 4,991 (3,716–6,563) | 8.45 (6.35–10.93) | −0.58 (−0.69 to −0.44) | 615,850 (458,375–845,413) | 1,324.94 (1,015.68–1,738.48) | 332,836 (244,892–453,126) | 430.6 (319.8–564.4) | −0.68 (−0.77 to −0.55) | |
| East Asia | Both | 38,629 (33,771–43,698) | 3.41 (3–3.82) | 6,793 (5,940–7,683) | 0.51 (0.45–0.57) | −0.85 (−0.88 to −0.82) | 2,930,231 (2,521,760–3,357,984) | 243.66 (209.63–278.82) | 310,529 (271,340–350,673) | 28.99 (25.01–33.27) | −0.88 (−0.9 to −0.85) |
| Male | 22,578 (19,383–25,741) | 3.87 (3.35–4.37) | 4,071 (3,414–4,812) | 0.61 (0.52–0.71) | –−0.84 (−0.87 to −0.8) | 1,721,707 (1,459,414–2,000,572) | 272.95 (231.83–316.74) | 189,472 (160,854–221,684) | 33.17 (28.12–38.94) | −0.88 (−0.91 to −0.84) | |
| Female | 16,052 (13,651–18,484) | 2.94 (2.53–3.37) | 2,723 (2,313–3,154) | 0.42 (0.35–0.48) | −0.86 (−0.88 to −0.83) | 1,208,524 (995,740–1,407,287) | 211.65 (174.22–246.09) | 121,057 (100,906–138,851) | 24.55 (19.59–28.5) | −0.88 (−0.91 to −0.85) | |
| Eastern Europe | Both | 3,420 (3,276–3,565) | 1.71 (1.63–1.8) | 1,640 (1,476–1,803) | 0.71 (0.64–0.78) | −0.58 (−0.63 to −0.54) | 211,829 (201,794–224,642) | 116.73 (110.72–124.82) | 72,023 (64,802–79,326) | 38.53 (34.43–42.89) | −0.67 (−0.71 to −0.63) |
| Male | 2,063 (1,959–2,195) | 2.16 (2.06–2.29) | 1,000 (871–1,136) | 0.94 (0.83–1.05) | −0.57 (−0.63 to −0.51) | 129,058 (121,975–136,959) | 141.94 (134.17–151.72) | 45,738 (40,543–51,467) | 48.54 (43–54.5) | −0.66 (−0.7 to −0.61) | |
| Female | 1,357 (1,296–1,436) | 1.31 (1.24–1.4) | 640 (558–730) | 0.51 (0.45–0.58) | −0.61 (−0.66 to −0.55) | 82,771 (78,180–89,147) | 92.27 (86.77–99.89) | 26,286 (22,987–29,706) | 29.22 (25.5–33.27) | −0.68 (−0.73 to −0.64) | |
| Eastern Sub-Saharan Africa | Both | 68,952 (57,841–81,908) | 32.66 (28.1–37.39) | 42,412 (35,839–50,651) | 13.22 (11.51–15.13) | −0.6 (−0.65 to −0.53) | 5,169,271 (4,241,186–6,257,079) | 1,820.32 (1,542.09–2,137.35) | 2,736,211 (2,238,193–3,400,288) | 597.51 (506.77–710.6) | −0.67 (−0.73 to −0.6) |
| Male | 36,600 (29,820–44,055) | 33.88 (28.41–39.58) | 23,001 (19,405–27,982) | 14.34 (12.43–16.58) | −0.58 (−0.65 to −0.48) | 2,768,588 (2,212,446–3,406,753) | 1,917.95 (1,573.55–2,291.71) | 1,500,986 (1,228,378–1,893,637) | 657.21 (556.55–795.71) | −0.66 (−0.73 to −0.57) | |
| Female | 32,352 (26,564–39,520) | 31.36 (26.72–36.65) | 19,411 (15,874–23,799) | 12.12 (10.27–14.33) | −0.61 (−0.68 to −0.54) | 2,400,683 (1,925,113–3,002,280) | 1,719.48 (1,425.24–2,075.49) | 1,235,225 (971,712–1,568,789) | 538.83 (441.55–661.37) | −0.69 (−0.75 to −0.6) | |
| High-income Asia Pacific | Both | 1,225 (1,168–1,275) | 0.77 (0.73–0.82) | 491 (427–531) | 0.13 (0.12–0.14) | −0.83 (−0.84 to −0.81) | 56,277 (52,334–60,106) | 40.66 (37.08–44.29) | 10,559 (9,786–11,150) | 5.17 (4.81–5.53) | −0.87 (−0.89 to −0.86) |
| Male | 664 (632–699) | 0.88 (0.83–0.94) | 277 (252–295) | 0.17 (0.16–0.18) | −0.81 (−0.82 to −0.79) | 31,596 (29,359–34,285) | 45 (41.14–49.99) | 6,388 (6,010–6,757) | 6.13 (5.67–6.57) | −0.86 (−0.88 to −0.85) | |
| Female | 561 (524–590) | 0.67 (0.6–0.72) | 214 (174–240) | 0.1 (0.09–0.11) | −0.85 (−0.86 to −0.82) | 24,681 (21,870–26,733) | 36.45 (30.61–40.34) | 4,171 (3,675–4,518) | 4.26 (3.88–4.66) | −0.88 (−0.9 to −0.85) | |
| High-income North America | Both | 1,961 (1,865–2,080) | 0.7 (0.67–0.75) | 1,236 (1,175–1,282) | 0.29 (0.28–0.3) | −0.59 (−0.61 to −0.57) | 103,562 (98,311–110,878) | 41.39 (39.2–44.67) | 47,052 (45,065–48,938) | 14.47 (13.69–15.24) | −0.65 (−0.68 to −0.62) |
| Male | 1,064 (994–1,134) | 0.8 (0.75–0.86) | 656 (613–685) | 0.32 (0.3–0.34) | −0.6 (−0.62 to −0.57) | 58,691 (54,628–63,863) | 46.74 (43.49–51.34) | 25,905 (24,134–27,162) | 15.93 (14.81–16.86) | −0.66 (−0.69 to −0.63) | |
| Female | 898 (848–983) | 0.61 (0.57–0.67) | 579 (543–609) | 0.26 (0.24–0.27) | −0.58 (−0.62 to −0.55) | 44,872 (41,973–49,771) | 36.06 (33.49–40.27) | 21,147 (20,222–22,048) | 13.03 (12.37–13.74) | −0.64 (−0.68 to −0.61) | |
| North Africa and Middle East | Both | 17,494 (14,394–21,533) | 4.24 (3.6–5.07) | 6,275 (5,332–7,405) | 1.2 (1.03–1.4) | −0.72 (−0.77 to −0.65) | 1,369,801 (1,111,963–1,714,493) | 280.73 (230.99–345.86) | 376,540 (310,241–457,477) | 64.07 (53–77.56) | −0.77 (−0.82 to −0.71) |
| Male | 9,318 (7,392–11,870) | 4.41 (3.54–5.32) | 3,428 (2,829–4,145) | 1.28 (1.07–1.52) | −0.71 (−0.77 to −0.61) | 731,620 (569,570–956,341) | 292.34 (231.92–372.44) | 205,311 (163,584–257,643) | 67.76 (54.04–84.88) | −0.77 (−0.83 to −0.69) | |
| Female | 8,176 (6,651–10,279) | 4.06 (3.41–4.98) | 2,848 (2,329–3,501) | 1.12 (0.93–1.37) | −0.72 (−0.78 to −0.64) | 638,181 (508,371–815,013) | 268.52 (218.34–336.82) | 171,228 (136,683–221,085) | 60.13 (48.04–77.32) | −0.78 (−0.83 to −0.69) | |
| Oceania | Both | 783 (599–1,001) | 9.34 (7.4–11.54) | 693 (497–937) | 4.42 (3.28–5.72) | −0.53 (−0.66 to −0.34) | 63,316 (47,733–82,126) | 680.9 (521.88–869.3) | 54,291 (38,290–74,849) | 311.73 (223.39–423.46) | −0.54 (−0.68 to −0.34) |
| Male | 430 (314–577) | 10.05 (7.67–13.01) | 377 (268–526) | 4.74 (3.51–6.36) | −0.53 (−0.67 to −0.31) | 34,531 (24,578–47,331) | 723.63 (530.53–969.22) | 29,043 (20,126–41,462) | 326.73 (231.29–457.62) | −0.55 (−0.7 to −0.31) | |
| Female | 353 (260–477) | 8.62 (6.59–11.19) | 316 (208–472) | 4.09 (2.83–5.94) | −0.53 (−0.69 to −0.29) | 28,785 (20,705–39,522) | 635.58 (466.26–860.06) | 25,248 (15,712–39,198) | 296.04 (192.59–446.5) | −0.53 (−0.72 to −0.26) | |
| South Asia | Both | 130,619 (113,278–149,667) | 11.24 (10.01–12.54) | 55,538 (48,412–64,415) | 3.49 (3.04–4.03) | −0.69 (−0.74 to −0.63) | 9,652,414 (8,215,080–11,222,877) | 678.55 (586.78–778.61) | 3,457,640 (2,935,306–4,129,991) | 203.97 (172.54–244.57) | −0.7 (−0.76 to −0.63) |
| Male | 65,169 (55,886–75,185) | 10.83 (9.5–12.19) | 28,031 (23,407–33,754) | 3.45 (2.9–4.15) | −0.68 (−0.74 to −0.61) | 4,804,956 (4,051,888–5,666,690) | 651.93 (557.67–753.63) | 1,781,010 (1,440,531–2,198,607) | 203.87 (165.38–252.61) | −0.69 (−0.76 to −0.6) | |
| Female | 65,450 (55,635–76,959) | 11.69 (10.13–13.43) | 27,507 (23,547–32,452) | 3.52 (3.01–4.16) | −0.7 (−0.75 to −0.63) | 4,847,458 (4,064,330–5,757,627) | 707.39 (600.96–832.61) | 1,676,630 (1,388,570–2,054,559) | 204.01 (167.61–252.16) | −0.71 (−0.77 to −0.63) | |
| Southeast Asia | Both | 37,360 (30,311–46,805) | 6.86 (5.69–8.44) | 10,733 (9,320–12,432) | 1.85 (1.59–2.15) | −0.73 (−0.79 to −0.66) | 3,029,202 (2,414,843–3,839,274) | 522.39 (420.63–657.96) | 690,178 (582,614–812,930) | 118.24 (98.99–140.65) | −0.77 (−0.83 to −0.71) |
| Male | 20,952 (16,833–26,832) | 7.64 (6.24–9.56) | 6,559 (5,583–7,724) | 2.25 (1.91–2.66) | −0.71 (−0.77 to −0.62) | 1,699,650 (1,354,692–2,212,321) | 577.33 (462.39–745.34) | 429,660 (357,445–521,368) | 143.89 (118.9–176.82) | −0.75 (−0.82 to −0.67) | |
| Female | 16,408 (11,565–21,789) | 6.09 (4.46–7.94) | 4,175 (3,528–4,987) | 1.45 (1.21–1.74) | −0.76 (−0.83 to −0.67) | 1,329,552 (906,989–1,795,931) | 465.98 (321.97–625.75) | 260,517 (210,875–322,441) | 91.7 (73.17–115.16) | −0.8 (−0.86 to −0.71) | |
| Southern Latin America | Both | 1,193 (1,136–1,252) | 2.46 (2.34–2.58) | 580 (524–631) | 0.82 (0.74–0.9) | −0.67 (−0.7 to −0.63) | 72,617 (67,886–77,488) | 144.84 (135.5–154.51) | 24,001 (21,323–26,934) | 38.6 (33.68–44.08) | −0.73 (−0.77 to −0.69) |
| Male | 685 (646–722) | 2.96 (2.8–3.11) | 311 (269–351) | 0.95 (0.82–1.07) | −0.68 (−0.73 to −0.64) | 42,025 (38,894–45,337) | 168.46 (156.21–181.36) | 13,768 (11,840–15,743) | 45.15 (38.33–52.18) | −0.73 (−0.78 to −0.68) | |
| Female | 508 (481–541) | 2.03 (1.92–2.16) | 269 (243–297) | 0.7 (0.63–0.77) | −0.66 (−0.69–−0.61) | 30,592 (28,233–33,128) | 121.81 (112.37–132.05) | 10,233 (9,118–11,544) | 32.17 (28.02–37.2) | −0.74 (−0.77 to −0.69) | |
| Southern Sub-Saharan Africa | Both | 4,072 (3,501–4,608) | 8.02 (6.98–8.94) | 4,130 (3,523–4,774) | 6.09 (5.27–6.94) | −0.24 (−0.36 to −0.11) | 278,049 (233,870–321,295) | 457.01 (391.43–519.46) | 218,303 (183,917–260,223) | 279.8 (236.69–331.45) | −0.39 (−0.5 to −0.24) |
| Male | 2,295 (1,935–2,701) | 9.44 (8.21–10.83) | 2,404 (2,023–2,801) | 7.46 (6.36–8.5) | −0.21 (−0.36 to −0.05) | 159,367 (130,815–191,034) | 533.77 (448.03–628.99) | 132,208 (109,679–158,501) | 346.17 (289.53–410.76) | −0.35 (−0.49 to −0.19) | |
| Female | 1,777 (1,393–2,093) | 6.74 (5.45–7.8) | 1,726 (1,426–2,082) | 4.86 (4.07–5.78) | −0.28 (−0.42 to −0.12) | 118,682 (88,806–144,174) | 383.02 (292.16–456.53) | 86,095 (68,842–107,712) | 216.83 (173.92–268.39) | −0.43 (−0.56 to −0.27) | |
| Tropical Latin America | Both | 8,545 (7,701–9,664) | 5.28 (4.79–5.91) | 2,072 (1,869–2,288) | 1.01 (0.9–1.14) | −0.81 (−0.84 to −0.78) | 673,434 (599,112–770,428) | 395.05 (352.37–451.05) | 119,799 (104,515–135,454) | 63.29 (53.93–72.69) | −0.84 (−0.87 to −0.81) |
| Male | 4,899 (4,385–5,605) | 6.06 (5.48–6.85) | 1,224 (1,077–1,372) | 1.22 (1.06–1.38) | −0.8 (−0.83 to −0.76) | 384,734 (339,181–444,916) | 448.76 (396.97–516.99) | 71,602 (61,450–82,265) | 75.05 (63.52–87.13) | −0.83 (−0.86 to −0.79) | |
| Female | 3,646 (3,215–4,234) | 4.5 (4–5.18) | 848 (772–930) | 0.82 (0.73–0.91) | −0.82 (−0.85 to −0.79) | 288,701 (251,599–340,303) | 340.76 (297.61–400.32) | 48,197 (42,193–54,351) | 51.58 (44.26–59.32) | −0.85 (−0.88 to −0.82) | |
| Western Europe | Both | 3,184 (3,080–3,295) | 0.85 (0.82–0.89) | 1,454 (1,346–1,546) | 0.24 (0.22–0.26) | −0.71 (−0.74 to −0.69) | 149,854 (144,083–157,396) | 50.71 (48.4–53.86) | 45,224 (41,972–48,137) | 11.93 (10.85–13.01) | −0.76 (−0.79 to −0.74) |
| Male | 1,673 (1,607–1,739) | 0.97 (0.93–1.02) | 765 (690–823) | 0.28 (0.25–0.3) | −0.71 (−0.74 to −0.69) | 84,073 (80,050–88,768) | 57.04 (53.87–60.85) | 25,380 (23,205–27,437) | 13.43 (12.09–14.82) | −0.76 (−0.79 to −0.74) | |
| Female | 1,510 (1,443–1,579) | 0.74 (0.71–0.79) | 690 (620–752) | 0.21 (0.19–0.23) | −0.72 (−0.74 to −0.69) | 65,781 (62,837–70,498) | 44.38 (42.01–48.4) | 19,843 (18,388–21,413) | 10.44 (9.46–11.49) | −0.76 (−0.79 to −0.74) | |
| Western Sub-Saharan Africa | Both | 86,166 (69,261–107,387) | 34.67 (29.06–41.25) | 87,572 (68,315–110,041) | 18.37 (15.02–22.1) | −0.47 (−0.56 to −0.35) | 6,718,306 (5,316,545–8,544,248) | 2,152.83 (1,735.86–2,658.12) | 6,559,408 (5,033,585–8,422,674) | 1,058 (825.44–1,323.28) | −0.51 (−0.61 to −0.37) |
| Male | 45,681 (36,555–58,368) | 35.94 (29.79–43.76) | 49,004 (38,104–62,906) | 20.35 (16.43–25.06) | −0.43 (−0.55 to −0.28) | 3,585,432 (2,822,949–4,681,309) | 2,274.06 (1,830.2–2,880.92) | 3,701,853 (2,833,862–4,838,617) | 1,188.87 (927.53–1,508.21) | −0.48 (−0.6 to −0.32) | |
| Female | 40,485 (31,203–50,857) | 33.22 (27.27–40.17) | 38,568 (29,640–49,091) | 16.45 (13.11–20.15) | −0.5 (−0.6 to −0.38) | 3,132,873 (2,359,605–3,985,781) | 2,027.28 (1,573.27–2,541.78) | 2,857,555 (2,122,602–3,752,067) | 928.59 (712.41–1,180.42) | −0.54 (−0.64 to −0.4) | |
ASDR, age-standardized death rate; ASYR, age-standardized years of life lost rate; UI, uncertainty interval.
Fig. 1.
Trends in ASDR and ASYR for meningitis overall and for meningitis due to three etiologies, Hib, N. meningitidis, and S. pneumoniae, 1990–2019. a Meningitis; b meningitis due to Hib; c meningitis due to N. meningitidis; d meningitis due to S. pneumoniae. ASDR, age-standardized death rate; ASYR, age-standardized years of life lost rate; Hib, Haemophilus influenzae type B; N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae; YLL, years of life lost.
Of the three common meningitis pathogens Hib, N. meningitidis, and S. pneumoniae, S. pneumoniae caused the highest meningitis burden, with 82,842 (31,610–140,151) deaths, 5,008,568 (1,790,724–8,683,386) YLL, an ASDR of 1.13 (0.42–1.92), and an ASYR of 70.64 (24.82–123.91). Among the three pathogens, the burden of meningitis caused by Hib showed the greatest decrease over the past 30 years, with ARCs in the ASDR and ASYR of −0.69 (−0.77 to −0.62) and −0.68 (−0.76 to −0.6), respectively (shown in Fig. 1).
Regional Meningitis Burden
Substantial disparities in the meningitis burden were observed in different regions (shown in online suppl. eFig. 5–12). In 2019, western sub-Saharan Africa had the highest ASDR (18.37 [15.02–22.1]) and ASYR (1,058 [825.44–1,323.28]), followed by eastern sub-Saharan Africa, central sub-Saharan Africa, southern sub-Saharan Africa, and Oceania. The high-income Asia-Pacific region had the lowest ASDR (0.13 [0.12–0.14]) and ASYR (5.17 [4.81–5.53]) in the world. The gap between the ASDR and ASYR of this region and those of western sub-Saharan Africa was greater than 100-fold, suggesting large regional gaps in the meningitis burden (shown in Fig. 2; online suppl. eFig. 13). The burden of meningitis caused by different pathogens was also concentrated in Africa and Oceania. In addition, the Caribbean had a high burden of meningitis caused by Hib.
Fig. 2.
Regional ASDRs of meningitis in 2019 and their ARCs from 1990 to 2019 for different sexes. a ASDR and ARC in ASDR of meningitis; b ASDR and ARC in ASDR of meningitis due to Hib; c ASDR and ARC in ASDR of meningitis due to N. meningitidis; d ASDR and ARC in ASDR of meningitis due to S. pneumoniae. ARC, annual rate of change; ASDR, age-standardized death rate; Hib, Haemophilus influenzae type B; N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae.
The Meningitis Burden at the National Level
The ASDRs and ASYRs by country and territory are shown in Figure 3 and online supplementary eTable 2. The top 10 countries in terms of ASDRs were Somalia, Mali, Niger, Chad, Guinea, Burkina Faso, Sierra Leone, South Sudan, Nigeria, and Guinea-Bissau, all of these countries were invariably located in sub-Saharan Africa. The etiologies of meningitis ASDRs and ASYRs varied by country and territory (shown in online suppl. eFig. 14–17; online suppl. eTable 3). Four countries showed an upward trend in burden (Lesotho: ASDR and ASYR for meningitis due to N. meningitidis, ASDR for meningitis due to S. pneumoniae; Northern Mariana Islands: ASDR and ASYR for meningitis due to Hib; Chad: ASDR for meningitis due to S. pneumoniae; Zimbabwe: ASDR and ASYR for meningitis due to N. meningitidis), while all other countries showed a decrease in burden. In most countries, S. pneumoniae caused the highest ASDRs and ASYRs, and Hib caused the lowest ASDRs and ASYRs among Hib, N. meningitidis, and S. pneumoniae.
Fig. 3.
Disease burden and ARC of meningitis in 204 countries and territories. a ASDR of meningitis in 2019; b ASYR of meningitis in 2019; c ARC in ASDR of meningitis between 1990 and 2019; d ARC in ASYR of meningitis between 1990 and 2019. ARC, annual rate of change; ASDR, age-standardized death rate; ASYR, age-standardized years of life lost rate; YLL, years of life lost. Note: The raw data are from GBD2019, and possible zoning issues are not our focus.
Cross-Country SDI-Related Health Inequalities
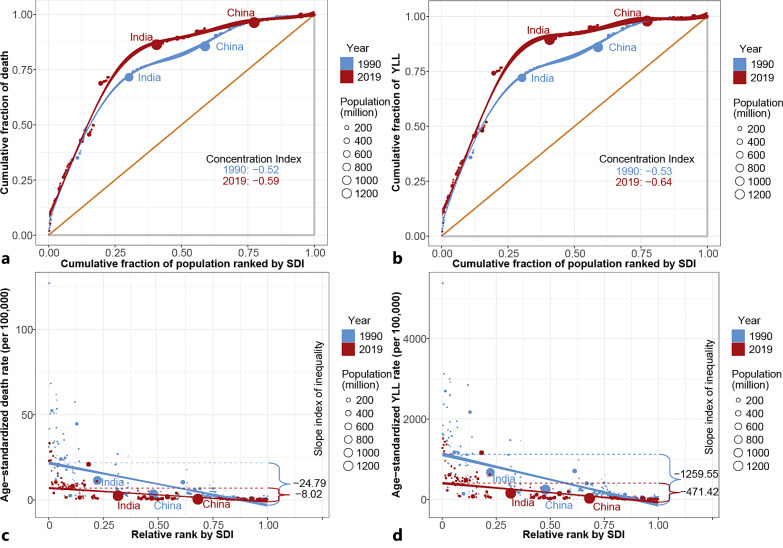
We found substantial absolute and relative SDI-related inequalities in the 204 countries and territories analyzed, and the burden of meningitis was disproportionately concentrated among poorer countries (shown in Fig. 4; online suppl. eFig. 18–20). As the slope index of inequality showed, the predicted excess ASDR/ASYR of 24.79/1,259.55 existed between countries with the lowest and highest SDIs in 1990, which decreased to an ASDR/ASYR of 8.02/471.42 in 2019. The results of the slope index of inequality indicated decreased but huge absolute SDI-related cross-country inequality. The concentration index of death/YLL was −0.52/−0.53 in 1990 and −0.59/−0.64 in 2019, representing a significantly high level of relative inequality. In contrast to the slope index, the concentration index showed that the relative SDI-related cross-country inequality increased from 1990 to 2019. The burden of disease was increasingly disproportionately concentrated in areas with lower SDIs.
Fig. 4.
SDI-related health inequality concentration curves and regression lines for the burden of meningitis across the world, 1990 and 2019. a Health inequality concentration curve of death; b health inequality concentration curve of YLL; c health inequality regression line of ASDR; d health inequality regression line of ASYR. ASDR, age-standardized death rate; ASYR, age-standardized years of life lost rate; SDI, sociodemographic index; YLL, years of life lost.
At the etiological level, the meningitis burden caused by S. pneumoniae had the highest slope index of inequality among the three common pathogens in 1990 and 2019. The slope index of S. pneumoniae suggested an important part of the excess meningitis ASDRs and ASYRs. In 2019, the burden of meningitis caused by N. meningitidis had the highest concentration index, indicating the highest relative SDI-related cross-country health inequality among the three common pathogens.
Drivers of Meningitis Epidemiology: Population Growth, Aging, and Epidemiological Changes
We conducted a decomposition analysis for the changes in deaths and YLL caused by population growth, aging, and epidemiological changes between 1990 and each year from 1991 to 2019 (shown in online suppl. eFig. 21–28). Overall, there was a significant decrease in meningitis deaths and YLL worldwide and in each SDI quintile. There was an approximately continuous downward trend in every SDI quintile except the low SDI quintile. Deaths/YLL in the low SDI quintile increased starting in 1990 and decreased below the level in 1990 until 2010/2011. Globally, epidemiological changes followed by aging contributed 141.15%/121.14% and 25.06%/33.93%, respectively, to the decreased burden of meningitis deaths/YLL between 1990 and 2019. In contrast to the effect of the two factors above, population growth contributed −66.21%/−55.06% to the decreased burden of meningitis deaths/YLL, but this did not offset the impact of population growth and aging. The contributions of epidemiological changes to deaths/YLL were most pronounced in the low SDI quintile (404.53%/335.48%).
Potential Improvement in Meningitis ASDR/ASYR
A frontier analysis was conducted to gain a better understanding of the potential improvement in meningitis ASDR and ASYR that are potentially achievable given a country’s development status. This analysis was based on ASDR/ASYR and SDI using data from 1990 to 2019. The frontier line indicates countries and territories with the lowest burden of disease for a given SDI. The effective difference is the distance from the frontier line and represents the gap between a country’s real burden and the lowest possible burden that could be achieved based on its level of development.
The frontier line and the effective difference are shown in Figure 5 and online supplementary eFigures 29–31. Countries with a lower SDI tend to have great effective variation, particularly low SDI countries in Africa. The top 10 countries with highest effective difference from the frontier of ASDR of meningitis included Burkina Faso, Chad, Guinea, Guinea-Bissau, Malawi, Mali, Nigeria, Sierra Leone, South Sudan, and Zambia. And the top 10 countries with highest effective difference from the frontier of ASYR of meningitis included Benin, Burkina Faso, Central African Republic, Chad, Guinea, Guinea-Bissau, Mali, Nigeria, Sierra Leone, and South Sudan. These countries have disproportionally higher meningitis ASDR/ASYR than other countries with comparable sociodemographic resources. With relatively low SDI, Cambodia, Somalia, and Yemen have relatively low effective difference from the frontier of ASDR, Nepal, Somalia, and Yemen have relatively low effective difference from the frontier of ASYR. These countries have disproportionally low meningitis ASDR/ASYR than other countries with comparable sociodemographic resources. With relatively high SDI, Kuwait, Netherlands, and United Arab Emirates have relatively high effective difference from the frontier of ASDR/ASYR. Etiologically, ASDR/ASYR of meningitis caused by Hib has lower effective difference from the frontier than ASDR/ASYR of meningitis caused by S. pneumoniae/N. meningitidis.
Fig. 5.
Frontier analysis based on SDI and age-standardized meningitis rate. Frontier analysis based on SDI and age-standardized meningitis a death rate and b YLL rate from 1990 to 2019. The frontier is delineated in solid black color. Frontier analysis based on SDI and age-standardized meningitis c death rate d YLL rate in 2019. The frontier is delineated in solid black color. Countries and territories are represented as dots. The top 10 countries with the largest effective difference (largest burden gap from the frontier) are labeled in black; examples of frontier countries with low SDI (<0.5) and low effective difference are labeled in blue, and examples of countries and territories with high SDI (>0.85) and relatively high effective difference for their level of development are labeled in red. SDI, sociodemographic index; YLL, years of life lost.
Sex and Age Distribution in the Meningitis Burden
The number of deaths, ASDRs, and ASYRs of meningitis worldwide in 2019 differed slightly between males and females (Table 1), with the number of male deaths at 128,802 (110,632–153,328) being approximately 1.2 times higher than the number of female deaths at 107,419 (91,361–126,253). The death rate and YLL rate were higher among males of almost all ages (shown in Fig. 6). The sex differences in the ASDRs and ASYRs were not obvious in pediatric populations but mainly in older age groups.
Fig. 6.
Death rate and YLL rate due to meningitis and three etiologies: Hib, N. meningitidis, and S. pneumoniae for different ages from 1990 to 2019 and for different ages and sexes in 2019. a Trends of death rate and YLL rate of different age groups due to meningitis and three etiologies, 1990–2019; b death rate and YLL rate due to meningitis and three etiologies by age and sex in 2019. ASDR, age-standardized death rate; ASYR, age-standardized years of life lost rate; Hib, Haemophilus influenzae type B; N. meningitidis, Neisseria meningitidis; S. pneumoniae, Streptococcus pneumoniae; YLL, years of life lost. Note: Upper columns stand for male, and lower columns stand for female.
The death rate peaked in the 0–4 age-group, after the age of 30, the mortality rate of meningitis increased yearly with increasing age. During the last 30 years, the death rate and YLL rate caused by meningitis showed a downward trend in all age-groups, and the 0–4 age-group showed the greatest decrease. Among the three common pathogens, N. meningitidis predominantly infected children, and S. pneumoniae had the highest death and YLL rates in all age-groups.
Modified Effects of National-Level Indicators on the Meningitis Burden
We used panel data model analysis to explore the relationship between national-level indicators and the burden of meningitis (shown in online suppl. eFig. 32–39). Regarding air pollution, high levels of PM2.5 exposure increased the meningitis ASDR (β = 0.09, p < 0.01) and ASYR (β = 6.15, p < 0.01). This effect is more obvious in the ASDR and ASYR of meningitis caused by pneumococcus. Countries with longer life expectancies and higher proportions of urban populations had lower meningitis ASDRs and ASYRs, and regarding socioeconomic indicators, we found that countries with higher levels of educated populations had lower meningitis ASDRs.
Discussion
Principal Findings
This research comprehensively explored the global burden of meningitis, and it generated multiple new discoveries. From 1990 to 2019, the ASDR and ASYR of meningitis generally declined globally, but there were still some countries with rising burdens that could not be ignored, such as Lesotho, Chad, and the Northern Mariana Islands. The decrease in burden was mainly driven by aging and epidemiological changes, while population growth tended to increase the burden. At the regional level, Africa and Oceania have a higher burden. We found significant relative and absolute cross-country SDI-related health inequalities, with the burden of meningitis increasingly disproportionately concentrated in countries with low SDI. Frontier analysis showed that many countries with low SDI had a much higher observed burden than might be achieved at a given SDI, suggesting that they have a significant opportunity to reduce the burden of disease, particularly in African countries with low SDI. The burden of meningitis was more concentrated in young children and in the male population. However, meningitis caused by S. pneumoniae also posed a huge burden in elderly individuals. This study also examined the factors associated with meningitis burden. Countries with lower life expectancy at birth, primary completion rate, urban population, and higher level of PM2.5 air pollution have a higher meningitis burden. Among meningitis caused by specific pathogens, PM2.5 pollution was more associated with the burden of meningitis caused by S. pneumoniae.
Comparison with Other Studies
We discovered that the global ASDR and ASYR of meningitis declined by more than half from 1990 to 2019, which can be partly attributed to the large-scale use of antibiotics and the availability of vaccines. While the overall burden is declining globally, the burden caused by specific pathogens is on the rise in four countries (Lesotho: N. meningitidis and S. pneumoniae; Chad: S. pneumoniae; Northern Mariana Islands: Hib; Zimbabwe: N. meningitidis). However, there is very little research in these countries on meningitis caused by specific pathogens.
The regional differences in the meningitis burden remain high, and the burden in sub-Saharan Africa is still of particular concern. This area is also known as the “meningitis belt” in Africa [20]. There are signs that the gap between this region and other regions is growing. This gap may be due to multiple reasons, such as sub-Saharan Africa having a less developed health care system and lower density of human resources for health, with a shortage of approximately 1.9 million physicians [21]. In addition to Africa, we found that Oceania, South Asia, and the Caribbean also have a high burden of meningitis deaths, and these regions need additional attention.
We found large relative and absolute SDI-related cross-country inequalities. Previous research showed that people in countries with lower SDI have poor hygiene habits, such as handwashing [22], which may lead to more transmission of meningitis-associated pathogens. Countries with lower SDIs may also have low vaccination rates [23], advocacy issues, etc., which also leads to higher prevalence and death rates. In addition, in countries with lower SDIs, health care infrastructure is poorer, and people have less access to antibiotics, which makes death more likely to occur after meningitis infection. This could explain why the burden of meningitis was higher in countries with lower SDI. In addition, we found that the burden caused by N. meningitidis exhibited the highest relative inequality. In the future, there is also a need to strengthen meningitis vaccination and antibiotic supply, especially against meningitis due to N. meningitidis, in countries with low SDI. Interestingly, however, Somalia or Ethiopia, despite lower SDI, has a better prognosis for meningitis than wealthier countries in the meningitis belt (e.g., Nigeria). The reasons for this phenomenon can be complex. For Somalia, it has been weakened by more than two decades of conflict and natural disasters, so it has yet to implement a health information system that allows for electronic collection and sharing of information nationwide [24, 25]. As a result, the data availability of Somalia is severely limited, and the burden of disease may be significantly underestimated. In Ethiopia, the public health sector bears a higher proportion of health care expenditure (Ethiopia: 22.6%, Nigeria: 15.7%), which ensures that more low-income people have access to health services and is important in improving meningitis outcomes [26]. Although a conjugate meningococcal vaccine against serotype A was promoted in Africa, the prevalence of novel strains of other serotypes has been observed in countries such as Nigeria, Chad, and Niger in recent years, posing a serious challenge to the local elimination of meningococcal meningitis epidemics [27, 28].
This study showed that the decrease in meningitis burden is mainly driven by aging and epidemiological changes, probably because the burden of meningitis is mainly concentrated in pediatric populations, and aging reduced the proportion of pediatric populations in the population, which validated our finding that meningitis burden is more concentrated in pediatric populations [29]. The driving force of population growth is intended to increase the burden of meningitis but is far from offset by the impact of aging and epidemiological changes.
The frontier analysis shows that a number of countries are far from the frontier, suggesting unrealized opportunities to close the disease burden gap. Some of the low SDI countries show leading performance, such as Yemen, despite limited resources. These countries can serve as models for optimizing health outcomes in less developed conditions. Conversely, there are countries with high SDI that show lagging performance, such as the Netherlands. Burkina Faso, Mali, Chad, Guinea, and Nigeria are far from the frontier in the burden of meningitis caused by multiple pathogens, and these countries have significant potential for improvement in public health policy.
The burden of meningitis is more concentrated in the male group and pediatric populations. Differences in the meningitis burden by sex may be due to immune differences. In mammals, innate immunity differs between males and females in many ways [30]. Males have a greater susceptibility to bacterial or viral infections and weaker immune responses to related pathogens. In addition, androgen may affect males’ aggression and dispersal behaviors, making males more susceptible to exposure to pathogens [31, 32]. The burden of meningitis in children must also be noted. In Africa, meningitis has become the leading bacterial cause of morbidity and mortality in children [33]. Furthermore, the burden of meningitis caused by S. pneumoniae is also large in elderly individuals, which needs special attention.
This study found that air pollution had a positive association with the burden of meningitis, which may be related to multiple mechanisms. Air pollution particles may make pathogen colonization possible by reducing mucosal ciliary clearance, reducing the barrier effect of the airways, and dysregulating the airway microbiota, while air pollution may enhance the inflammatory response of the body and enhance the transmission of pathogens such as S. pneumoniae to tissues and blood, causing carriers to be more likely to develop meningitis and resulting in higher ASDRs and ASYRs [34, 35]. Before our study, a large body of current research focused on the association between meningitis incidence and dust/pm2.5, and most of the studies were limited to a single country [36, 37]. Compared to prior studies, our research used data from 1990 to 2019 and revealed the relationship between PM2.5 and meningitis burden on a global scale, finding that PM2.5 has a positive association with the ASDR and ASYR of meningitis. Moreover, it was most associated with the burden of meningitis caused by S. pneumoniae, indicating that countries with a high burden of meningitis caused by S. pneumoniae are more required to take measures to reduce air pollution. In addition, planned urbanization and increasing the primary education completion rate may also be useful to reduce the adverse health impacts of the meningitis burden.
Strengths and Limitations
Our study had the following strengths. First, to our knowledge, this is the most up-to-date and comprehensive analysis of the global burden of meningitis available. We analyzed the disease burden of meningitis caused by specific pathogens at the global, regional, and national levels by age and sex. Compared to other studies with similar themes to ours, our study used more recent data, and we optimized the extraction metrics to better assess the burden of meningitis because deaths were more responsible for the burden of meningitis than disabling outcomes. Second, this was the first study to analyze trends in the meningitis burden by age over time, and we also analyzed sex differences in ASDRs and ASYRs by region and by meningitis type. Third, we used the concentration index and slope index to quantify cross-country inequalities. To the best of our knowledge, ours is one of the first studies to examine cross-country inequalities in the burden of meningitis.
This study also had some limitations. Our data source is from the GBD study. The reliability of the original data determines the accuracy of our analysis. In some countries suffering from conflicts or terrorism for a long time, the original data may be insufficient or of poor accuracy. Vital registration systems are an important source of GBD research data, but there are many countries with limited or missing vital registration systems, which may increase data bias.
Future Directions
More research is still needed on meningitis, especially in countries with lower SDI because the burden of disease has been increasingly concentrated in low SDI countries. Countries in volatile situations have limited access to data, their burden is often underestimated, and more research is needed to obtain true data on the burden. Although the burden of meningitis has been generally declining, it is still on the rise in individual countries, where research on the response is urgently needed to curb its growth. The burden of meningitis caused by S. pneumoniae is high in elderly individuals, but there are relatively few studies on the subject. In the context of increasing aging, it needs more attention. More research is also needed for countries where the burden is much higher than the achievable burden at a given SDI, as these countries have significant opportunities for improvement, and upgraded responses may significantly reduce the burden of meningitis in these areas. Because reliance on antibiotics and serotype replacement of pathogens has occurred in many countries, research and development of novel vaccines and antibiotics against meningitis-associated pathogens should be pursued, and more effective diagnostic methods of meningitis pathogens should be continually developed.
Conclusions
The global burden of meningitis has generally decreased, but the burden in individual countries is on the rise. The burden varies substantially by location. Sub-Saharan Africa and Oceania have high meningitis burdens. There are substantial absolute and relative SDI-related inequalities in the meningitis burden, and relative SDI-related inequalities are increasing. The change in the meningitis burden is mainly driven by epidemiological changes. Many countries with lower SDIs have burdens well above those achievable at a given SDI and have significant opportunities for improvement. Pneumococci cause the greatest disease burden among the three common pathogens that cause meningitis. Males have a greater burden of disease than females. Children are an important group affected by meningitis, so it is also crucial to reduce the burden of meningitis in pediatric populations. However, the burden of meningitis caused by S. pneumoniae in the elderly is also significant and requires attention. Reducing the burden of meningitis requires the concerted efforts of governments, scientific research institutions, and hospitals. Countries can reduce the burden of meningitis by increasing planned urbanization, reducing air pollution and increasing primary education completion rates. Government and medical institutions should improve advocacy and vaccination strategies to help increase vaccination rates. It is also essential to continue developing new vaccines and antibiotics.
Acknowledgments
In this report, we used data from the GBD 2019 study. We appreciate the works of the GBD 2019 study collaborators, without whom this report would not be possible.
Statement of Ethics
All data used in this study were aggregated and did not contain identifiable personal or medical information. The original data for our study came from GBD 2019. For GBD studies, a waiver of informed consent was reviewed and approved by the Institutional Review Board of the University of Washington. All the information about ethical standards is available through the official website (http://www.healthdata.org/gbd/2019). Therefore, no additional ethical approval was required for this study. This study does not need consent to participate.
Conflict of Interest Statement
The authors declare they have no conflicts of interest regarding the content of this paper.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
C.Q. and Y.W. performed the study design, analyzed the data, interpreted the results and data collection, and wrote the manuscript. X.W., R.H., H.C., B.L., H.Z., and N.Z. revised the manuscript. Z.L. helped with revisions and beautification of tables and figures and manuscript editing. Z.D. and Q.C. reviewed and finalized the manuscript and conceptualized the study. All authors read and approved the final manuscript.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Publicly available datasets were used in this study. These can be found in GBD 2019 at https://ghdx.healthdata.org/gbd-2019.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Stevens JP, Eames M, Kent A, Halket S, Holt D, Harvey D. Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F179–84. 10.1136/fn.88.3.f179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merkler AE, Reynolds AS, Gialdini G, Morris NA, Murthy SB, Thakur K, et al. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci. 2017 Apr 15;375:460–3. 10.1016/j.jns.2017.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bor M, Çokuğraş H. Factors associated with early complications in inpatients who were treated in our clinic between 1992 and 2011 with a diagnosis of acute bacterial meningitis. Turk Pediatri Ars. 2020;55(2):149–56. 10.14744/TurkPediatriArs.2019.34445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erdem H, Inan A, Guven E, Hargreaves S, Larsen L, Shehata G, et al. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. Eur J Clin Microbiol Infect Dis. 2017 Sep;36(9):1595–611. 10.1007/s10096-017-2973-0. [DOI] [PubMed] [Google Scholar]
- 5. Oordt-Speets AM, Bolijn R, van Hoorn RC, Bhavsar A, Kyaw MH. Global etiology of bacterial meningitis: a systematic review and meta-analysis. PLoS One. 2018;13(6):e0198772. 10.1371/journal.pone.0198772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1204–22. 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020 Oct;81(4):483–98. 10.1016/j.jinf.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 8. Tenforde MW, Gertz AM, Lawrence DS, Wills NK, Guthrie BL, Farquhar C, et al. Mortality from HIV-associated meningitis in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc. 2020 Jan;23(1):e25416. 10.1002/jia2.25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peltola H, Roine I, Kallio M, Pelkonen T. Outcome of childhood bacterial meningitis on three continents. Sci Rep. 2021 Nov 3;11(1):21593. 10.1038/s41598-021-01085-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsai ST, Lin FY, Chen PS, Chiang HY, Kuo CC. Three-year mortality in cryptococcal meningitis: hyperglycemia predict unfavorable outcome. PLoS One. 2021;16(5):e0251749. 10.1371/journal.pone.0251749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers. 2016 Nov 3;2(1):16074. 10.1038/nrdp.2016.74. [DOI] [PubMed] [Google Scholar]
- 12. Mustapha MM, Harrison LH. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccin Immunother. 2018 May 4;14(5):1107–15. 10.1080/21645515.2017.1412020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koelman D, Brouwer MC, van de Beek D. Resurgence of pneumococcal meningitis in europe and northern America. Clin Microbiol Infect. 2020;26(2):199–204. 10.1016/j.cmi.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 14. GBD 2016 Meningitis Collaborators . Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018 Dec;17(12):1061–82. 10.1016/S1474-4422(18)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute for Health Metrics and Evaluation .
- 16. The United Nations Development Programme . Human development reports.
- 17. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1223–49. 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The World Bank Group . World Bank databases.
- 19. R Project . R: The R project for statistical computing.
- 20. Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, et al. Meningococcal meningitis surveillance in the african meningitis belt, 2004–2013. Clin Infect Dis. 2015 Nov 15;61(Suppl 5):S410–5. 10.1093/cid/civ597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. GBD 2019 Human Resources for Health Collaborators . Measuring the availability of human resources for health and its relationship to universal health coverage for 204 countries and territories from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022 Jun 4;399(10341):2129–54. 10.1016/S0140-6736(22)00532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman MC, Stocks ME, Cumming O, Jeandron A, Higgins JP, Wolf J, et al. Hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Trop Med Int Health. 2014 Aug;19(8):906–16. 10.1111/tmi.12339. [DOI] [PubMed] [Google Scholar]
- 23. Gilsdorf JR. Hib vaccines: their impact on Haemophilus influenzae type b disease. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S321–30. 10.1093/infdis/jiaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bank W. Completeness of birth registration (%): Somalia.
- 25. Bile K, Warsame M, Ahmed AD. Fragile states need essential national health research: the case of Somalia. Lancet Glob Health. 2022 May;10(5):e617–8. 10.1016/S2214-109X(22)00122-X. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization . WHO global health expenditure database.
- 27. Meningococcal disease in countries of the African meningitis belt, 2012: emerging needs and future perspectives. Wkly Epidemiol Rec. 2013 Mar 22;88(12):129–36. [PubMed] [Google Scholar]
- 28. Preparedness for outbreaks of meningococcal meningitis due to Neisseria meningitidis serogroup C in Africa: recommendations from a WHO expert consultation. Wkly Epidemiol Rec. 2015 Nov 20;90(47):633–6. [PubMed] [Google Scholar]
- 29. Bongaarts J. Human population growth and the demographic transition. Phil Trans R Soc B. 2009 Oct 27;364(1532):2985–90. 10.1098/rstb.2009.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016 Oct;16(10):626–38. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 31. Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000 Aug;24(6):627–38. 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 32. Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008 Sep;8(9):737–44. 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peltola H. Burden of meningitis and other severe bacterial infections of children in africa: implications for prevention. Clin Infect Dis. 2001 Jan;32(1):64–75. 10.1086/317534. [DOI] [PubMed] [Google Scholar]
- 34. Bahamonde C, Stuardo V, Hott-Harvey B, Manríquez J, Mardones P. Meningococcal disease in the Metropolitan Region of Chile and its correlation with environmental factors. Rev Chilena Infectol. 2014 Dec;31(6):645–50. 10.4067/S0716-10182014000600001. [DOI] [PubMed] [Google Scholar]
- 35. Beentjes D, Shears RK, French N, Neill DR, Kadioglu A. Mechanistic insights into the impact of air pollution on pneumococcal pathogenesis and transmission. Am J Respir Crit Care Med. 2022 Nov 1;206(9):1070–80. 10.1164/rccm.202112-2668TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tobías A, Caylà JA, Pey J, Alastuey A, Querol X. Are Saharan dust intrusions increasing the risk of meningococcal meningitis? Int J Infect Dis. 2011;15(7):e503. 10.1016/j.ijid.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 37. Dukić V, Hayden M, Forgor AA, Hopson T, Akweongo P, Hodgson A, et al. The role of weather in meningitis outbreaks in navrongo, Ghana: a generalized additive modeling approach. J Agric Biol Environ Stat. 2012;17(3):442–60. 10.1007/s13253-012-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were used in this study. These can be found in GBD 2019 at https://ghdx.healthdata.org/gbd-2019.