Abstract
Site-specific modification of amino acid residues in protein binding pockets using sulfonyl exchange chemistry expands the druggable proteome by enabling the development of covalent modulators that target residues beyond cysteine. Sulfonyl fluoride and triazole electrophiles were incorporated previously into the cereblon (CRBN) molecular glue degrader EM12, to covalently engage His353 within the CRBN sensor loop, but these probes had poor human plasma stability. Attenuation of intrinsic reactivity through the development of sulfonyl pyrazoles, imidazoles, and nucleobases enhanced plasma stability, and several compounds retained efficient labeling of His353. For example, sulfonyl imidazole EM12-SO2Im covalently blocked the CRBN binding site and possessed excellent metabolic stability in human plasma, liver microsomes, and hepatocytes. These results highlight the potential suitability of sulfonyl imidazole and related sulfur(VI)-diazole exchange (SuDEx) warheads for covalent drug development and further exemplify the therapeutic promise of site-specific histidine targeting.
Keywords: SuFEx, SuTEx, SuDEx, Sulfonyl imidazole, Cereblon, Metabolic stability, Histidine targeting, Covalent drug discovery
Targeted covalent inhibitors (TCIs) possess potential advantages over reversible binding ligands, including enhanced potency, selectivity, and pharmacodynamic duration.1,2 Usually, TCIs are designed to target the nucleophilic cysteine thiol/thiolate side chain,3 but the amino acid is rarely available for engagement in protein binding pockets due to its low frequency, and its high nucleophilicity may hinder the development of selective inhibitors.4 Sulfur(VI) fluoride exchange (SuFEx) electrophiles enable covalent modification of tyrosine, lysine, serine, and threonine in a site-specific manner.5−7 As a result, chemical probes and target occupancy tools have been developed to engage these residues across a diverse range of protein binding sites.
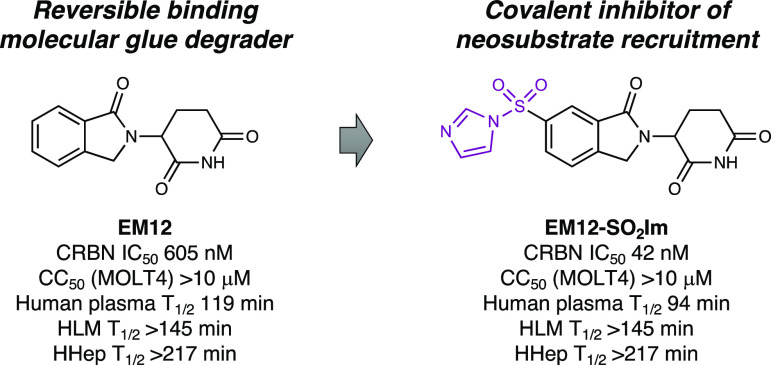
Immunomodulatory imide drugs (IMiDs) thalidomide, lenalidomide, and pomalidomide (Figure 1a) bind cereblon (CRBN), a component of the E3 ubiquitin ligase CRL4CRBN.8 The IMiDs are termed molecular glue degraders because they remodel the surface of CRBN, mediating interactions with neosubstrates, resulting in polyubiquitination and proteasomal degradation of the target. We recently reported the first examples of rational histidine targeting using SuFEx warheads incorporated into the 6-position of the CRBN modulator EM12 (Figure 1a), which binds in the thalidomide binding domain (TBD) that lacks a targetable cysteine residue.9 EM12-SO2F potently engaged His353 within the β-hairpin arrangement of the sensor loop, thus acting as a covalent intramolecular glue by stapling CRBN in the closed conformation, which is ordinarily a prerequisite for neosubstrate binding (Figure 1b,c).10 Covalent docking suggested that His353 is held in a different conformation to that seen in ternary complex structures with reversible binding CRBN molecular glues.11 Moreover, the sulfonyl oxygen atoms of the sulfonylated adduct appeared to clash with the neosubstrate structural G-loop degron, and thus unsurprisingly, EM12-SO2F did not induce any significant protein degradation, as determined using mass spectrometry (MS) proteomics.9
Figure 1.

(a) Structures of reversible binding of CRBN molecular glues. (b) Lenalidomide-CRBN-DDB1-CK1α crystal structure (PDB 5FQD)12 showing proximity of the 6-position of the IMiD template to His353 in the CRBN sensor loop.10 (c) EM12 derivatives incorporating a variety of sulfonyl exchange covalent warheads.
EM12-SO2F inhibited the degradation of neosubstrates by other reversible binding CRBN-mediated degraders, and the compound is therefore a useful target validation tool to confirm CRBN dependency of IMiD-derived hits in phenotypic screens, complementing genetic methods of CRBN perturbation.13 The addition of an oxygen atom provided the intrinsically less reactive fluorosulfate EM12-FS (Figure 1c)14 that also labeled His353. Intriguingly, EM12-FS was found to recruit and degrade a neosubstrate (protein-N-terminal glutamine amidase 1, NTAQ1) that had not been reported previously for reversible binding thalidomide derivatives. These results demonstrate synthetic re-engineering of E3 ligases through covalent modification of the protein surface enables exploration of new structure–activity relationships (SARs).
Sulfur-triazole exchange (SuTEx) was recently reported as an alternative strategy to SuFEx, enabling further fine-tuning of the electrophile through modification of the leaving group heterocycle.15,16 We incorporated sulfonyl triazole warheads into the EM12 scaffold to yield probes that covalently engaged CRBN His353 (Figure 1c) and similarly lacked degradation capacity as expected based on the EM12-SO2F result.9 Unlike the fluorosulfate EM12-FS, aryl sulfonyl fluoride and triazole derivatives were found to be unstable in human plasma (Table 1), highlighting a key challenge regarding their incorporation into covalent drug design strategies.
Table 1. CRBN Binding Potency, Human Plasma Stability, and CRBN Labeling Efficiency for a Series of EM12 Derivatives.
| compd | CRBN IC50 (nM) | plasma T1/2 (min) | CRBN labeling |
|---|---|---|---|
| EM12 | 605 ± 106 | 119 | |
| EM12-SO2F | 1.1 ± 0.2 | 7 | >95% |
| EM12-FS | 255 ± 87 | 196 | 75% |
| EM12-SO2Tr1 | 21.1 ± 3.1 | 2 | 90% |
| EM12-SO2Tr2 | 77.9 ± 7.3 | <1 | 90% |
| EM12-SO2Im | 41.9 ± 3.7 | 94 | >95% |
| EM12-SO2Im(4-Me) | 454 ± 30 | 287 | <5% |
| EM12-SO2Im(4-F) | 324 ± 36 | 19 | 90% |
| EM12-SO2Im(4-CF3) | 54.9 ± 3.5 | 6.5 | 75% |
| EM12-SO2Pyr(4-Me) | 3752 ± 264 | >289 | <5% |
| EM12-SO2Pyr(3-F) | 1629 ± 221 | 115 | 37% |
| EM12-SO2Pyr(4-F) | 2341 ± 167 | 92 | 67% |
| EM12-SO2Pyr(3-CF3) | 50.4 ± 2.4 | 14 | 90% |
| EM12-SO2Pyr(4-CF3) | 43.6 ± 4.4 | 10 | 90% |
| EM12-SO2-T | 1377 ± 54 | 239 | 90% |
| EM12-SO2-U | 4298 ± 219 | 163 | 90% |
| EM12-SO2-C | 28630 ± 2877 | >289 | 55% |
We reasoned that reducing the number of heterocycle nitrogen atoms to two, yielding sulfur-diazole exchange (SuDEx) warheads, would attenuate intrinsic reactivity and potentially improve plasma stability in a manner that is complementary to that of EM12-FS. Substitution of the pyrazole and imidazole ring systems with electron donating and withdrawing motifs would further probe the electronic features of the heterocyclic leaving groups and expand exploration of CRBN SARs (Figure 1c). Similarly, sulfonucleobases utilizing thymine, cytosine, and uracil as leaving groups were prepared and profiled (Figure 1c). All derivatives were furnished in a single synthetic step from the sulfonyl chloride precursor treated with the respective heterocycle and Hünig’s base as a proton scavenger (Supporting Information (SI)).
To determine CRBN binding potency in cells, the compounds were screened in a NanoBRET assay that we reported recently.9 Displacement of a BODIPY-lenalidomide tracer from a NanoLuc-tagged CRBN by the EM12 derivatives dose-dependently reduced the intensity of the BRET signal. The library was also screened in a human plasma stability assay to understand the suitability of the sulfonyl exchange warheads for future drug development (Table 1, Figure 2, SI).17 Additionally, intact MS of the treated CRBN/DDB1 complex was used to determine the labeling efficiency and stoichiometry of covalent adduct formation (Table 1, SI).
Figure 2.
Plot of CRBN in-cell binding potency versus human plasma stability for a series of EM12 derivatives bearing sulfonyl exchange warheads (data from Table 1).
As expected, the new compounds possess a range of potencies and plasma stabilities, reflecting differences in equilibrium binding affinity, intrinsic electrophilicity, and metabolic vulnerability. Pleasingly, all derivatives had improved plasma stability over the SuTEx probes (Table 1).
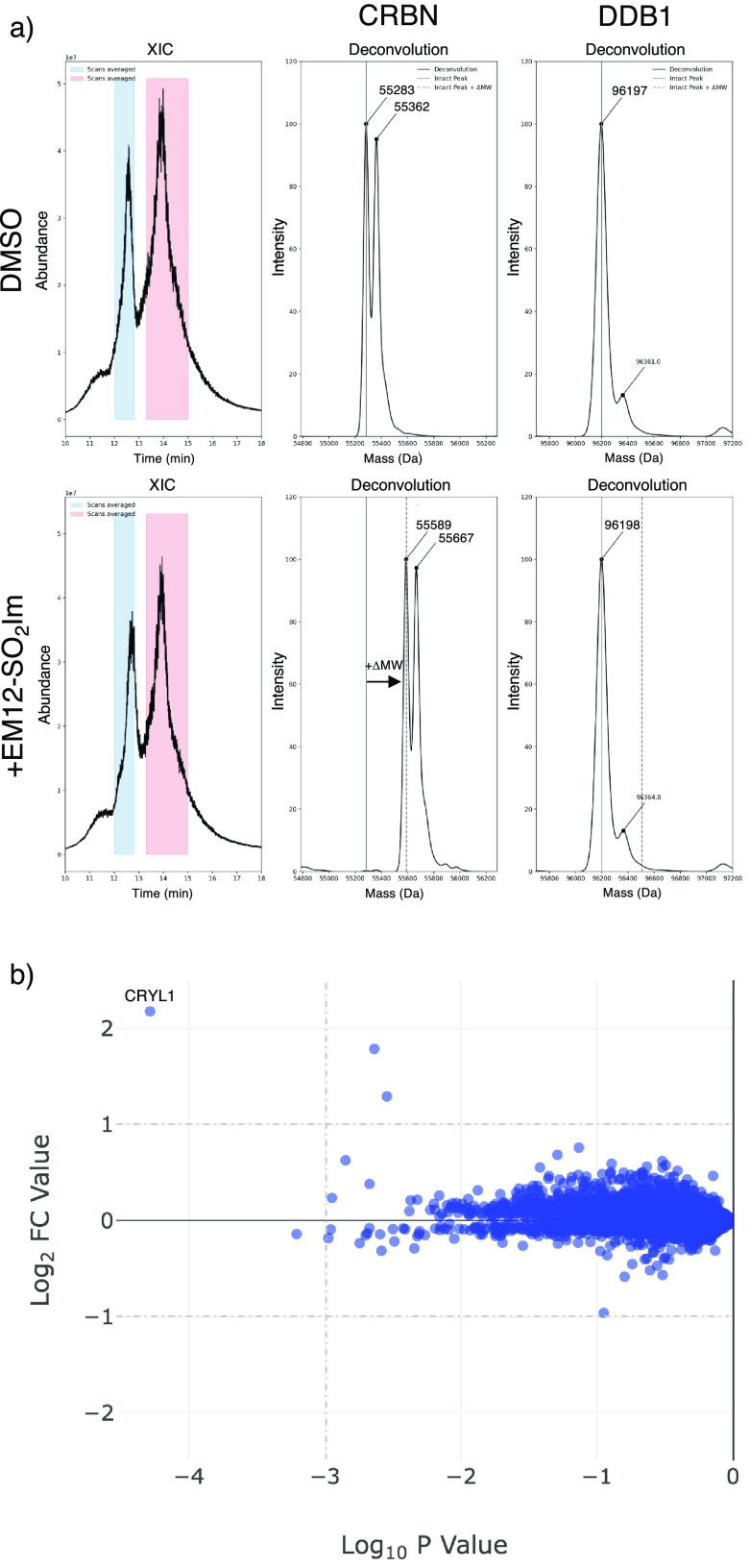
Broadly, there was a moderate correlation between CRBN potency and plasma stability across the set, reflecting a potential for nonspecific electrophilicity-driven events (Figure 2). However, EM12-SO2F and EM12-SO2Im were clear outliers, and the latter noticeably possessed similar plasma stability to the parent EM12 but with considerably improved CRBN potency. Intact protein MS showed a range of labeling efficiencies across the analogues, and those that modified CRBN to a measurable extent did so in a 1:1 ratio, and no labeling of DDB1 was observed. Representative intact MS data for EM12-SO2Im are shown in Figure 3a. The proximity of the EM12-SO2Im warhead to His353 was supported by computational modeling of the CRBN complex, and peptide mapping MS confirmed that His353 was the exclusively labeled site (SI).
Figure 3.
Mass spectrometry studies of EM12-SO2Im. (a) Intact protein MS of the CRBN/DDB1 complex treated with EM12-SO2Im. The mass shift of 306 Da is commensurate with sulfonylation. (b) Quantitative MS proteomics of MOLT4 cells treated with EM12-SO2Im showing no reductions in protein abundance as expected.
Generally, compounds that efficiently modified the sensor loop of His353 were also potent CRBN binders in the NanoBRET assay (Table 1). For example, the electron deficient CF3-substituted sulfonyl imidazole and pyrazole probes potently engaged CRBN, but these compounds suffered from a lower plasma stability. Conversely, the more electron-rich 4-Me substituted imidazole and pyrazole congeners were significantly weaker CRBN binders than other analogues within the series and did not label the protein, which may also explain their higher plasma stability (due to low intrinsic reactivity). Similarly, within the nucleobase series, the more electron rich sulfonyl cytosine present within EM12-SO2-C translated to low binding potency, high plasma stability, and lower labeling efficiency than the thymine and uracil derivatives.
EM12-SO2Im clearly emerged as the outstanding compound from this study due to its high CRBN potency and plasma stability combined with site-specific and efficient labeling of His353, and so the probe was chosen for further profiling. LC-UV-MS1,2 was used to identify EM12-SO2Im metabolites in human plasma. Glutarimide hydrolysis was the only metabolite produced in human plasma to any significant extent, as seen for EM12, while hydrolysis of the sulfonyl imidazole motif was produced in only trace amounts (SI). To understand neosubstrate degradation capacity, a previously published quantitative MS proteomics methodology18 was used to confirm that EM12-SO2Im did not change protein levels in the MOLT4 line (Figure 3b), as shown for EM12-SO2F, and the electrophilic compound was not cytotoxic in these cells either. Importantly, from a drug development perspective, EM12-SO2Im was stable not only in human plasma but also in human liver microsomes (HLM) and human hepatocytes (HHeps) (Figure 4).
Figure 4.
Summary of the effects of incorporating the sulfonyl imidazole electrophile into EM12.
Although aryl sulfonyl fluorides have proven utility as chemical probes, their application in drug discovery has been hindered due to their low plasma stability. Attenuation of intrinsic electrophilicity through the incorporation of an electron donating oxygen atom to provide the fluorosulfate electrophile delivers a warhead suitable for in vivo use.19,20 Sulfonyl triazoles enable further fine-tuning of the leaving group but also suffer from low plasma stability. Here, we have presented an alternative strategy to enhance metabolic stability while retaining potent binding site-selective covalent labeling effectiveness by employing alternative sulfonyl heterocycles.
The attractive features of the sulfonyl imidazole warhead described in this study support its application in covalent drug discovery. Additionally, the imidazole leaving group, similarly to SuFEx-derived fluoride,21 is safe in vivo up to high exposure levels.22 The underlying driving force behind the sulfonyl transfer to His353 is worthy of further mechanistic investigation. Imidazole is approximately 10-fold less basic than 4-methylimidazole, which is consistent with differences in their leaving group abilities demonstrated here.23 The lack of CRBN labeling by the 4-Me imidazole derivative EM12-SO2Im(4-Me) corresponds to the formation of a relatively stable sulfonylated histidine adduct following the exchange reaction, which is locked in place through efficient binding within the TBD. Analogously, tosylation has long been used to protect the histidine imidazole nitrogen during peptide synthesis due to its relatively high stability and resilience to various reaction conditions.24 Furthermore, the apparently elevated nucleophilicity of His353, a residue which is highly conserved across species, may reflect its involvement in the endogenous function of CRBN, possibly as a catalytic nucleophile, proton acceptor–donor, or as a site of post-translational modification.9
Histidine has been hitherto underexploited in covalent drug discovery despite its preponderance in protein active sites and proximity to small molecule ligands.11 Additionally, histidine is frequently an acquired mutation in cancer, providing a gain in pH sensing to mutant proteins,25 which may present a targetable vulnerability using sulfonyl exchange chemistry. The warheads reported in this study could equally be incorporated into CRBN-mediated PROTACs to enhance catalytic degradation efficiencies and pharmacodynamic profiles.26 Sulfonylation extends beyond protein labeling because 2′-OH RNA modification using sulfonyl imidazole and related species was demonstrated recently,27 and we speculate that such electrophiles may enable the development of RNA targeting drugs in the future. We hope that this study will expand the chemical toolkit to aid the design of small molecule covalent modulators for a variety of therapeutic purposes.
Acknowledgments
We thank Wuxi for compound synthesis, measuring compound stability in human plasma, microsomes, and hepatocytes, and performing metabolite identification studies in human plasma. J.A.M. acknowledges generous support from the NIH (CA233800, CA247671, and U24DK116204), The Mark Foundation for Cancer Research, and the Massachusetts Life Science Center.
Glossary
Abbreviations
- CRBN
cereblon
- HHep
human hepatocytes
- HLM
human liver microsomes
- IMiD
immunomodulatory imide drug
- MS
mass spectrometry
- PROTAC
proteolysis targeting chimera
- SAR
structure–activity relationship
- SuDEx
sulfonyl diazole exchange
- SuFEx
sulfur(VI) fluoride exchange
- TBD
thalidomide binding domain
- TCI
targeted covalent inhibitor
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00371.
Methods for determining human plasma stability (and metabolite identification), human liver microsome stability, human hepatocyte stability, cellular CRBN NanoBRET engagement assay, quantitative MS proteomics, CRBN labeling and peptide mapping MS; EM12-SO2Im/CRBN computational binding model, synthetic procedures, and associated characterization data for new compounds (PDF)
Author Contributions
J.T.C. and R.P.N. are co-first authors. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): The CPD receives research funding from Deerfield.
Special Issue
Published as part of ACS Medicinal Chemistry Lettersvirtual special issue “Exploring Covalent Modulators in Drug Discovery and Chemical Biology”.
Supplementary Material
References
- Gehringer M.; Laufer S. A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62, 5673–5724. 10.1021/acs.jmedchem.8b01153. [DOI] [PubMed] [Google Scholar]
- Daryaee F.; Zhang Z.; Gogarty K. R.; Li Y.; Merino J.; Fisher S. L.; Tonge P. J. A quantitative mechanistic PK/PD model directly connects Btk target engagement and. Chem. Sci. 2017, 8, 3434–3443. 10.1039/C6SC03306G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Sabnis Y.; Zhao Z.; Zhang T.; Buhrlage S. J.; Jones L. H.; Gray N. S. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 2013, 20, 146–59. 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. H. Design of next-generation covalent inhibitors: Targeting residues beyond cysteine. Annu. Rep. Med. Chem. 2021, 56, 95–134. 10.1016/bs.armc.2020.10.001. [DOI] [Google Scholar]
- Jones L. H.Rational Targeting of Active Site Tyrosine Residues using Sulfonyl Fluoride Probes. In 248th ACS National Meeting, San Francisco, American Chemical Society, 2014. [DOI] [PubMed]
- Dong J.; Krasnova L.; Finn M. G.; Sharpless K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem., Int. Ed. Engl. 2014, 53, 9430–48. 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- Narayanan A.; Jones L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659. 10.1039/C5SC00408J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain P. P.; Hamann L. G. Development of targeted protein degradation therapeutics. Nat. Chem. Biol. 2019, 15, 937–944. 10.1038/s41589-019-0362-y. [DOI] [PubMed] [Google Scholar]
- Cruite J. T.; Dann G. P.; Che J.; Donovan K. A.; Ferrao S.; Ficarro S. B.; Fischer E. S.; Gray N. S.; Huerta F.; Kong N. R.; Liu H.; Marto J. A.; Metivier R. J.; Nowak R.ła. P.; Zerfas B. L.; Jones L. H. Cereblon covalent modulation through structure-based design of histidine targeting chemical probes. RSC Chem. Biol. 2022, 3, 1105–1110. 10.1039/D2CB00078D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. R.; Novick S.; Matyskiela M. E.; Chamberlain P. P.; de la Peña A. H.; Zhu J.; Tran E.; Griffin P. R.; Wertz I. E.; Lander G. C. Molecular glue CELMoD compounds are regulators of cereblon conformation. Science 2022, 378, 549–553. 10.1126/science.add7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che J.; Jones L. H. Covalent drugs targeting histidine - an unexploited opportunity?. RSC Med. Chem. 2022, 13, 1121–1126. 10.1039/D2MD00258B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold G.; Fischer E. S.; Thomä N. H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4(CRBN) ubiquitin ligase. Nature 2016, 532, 127–30. 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- Dann G. P.; Liu H.; Nowak R. P.; Jones L. H. Cereblon target validation using a covalent inhibitor of neosubstrate recruitment. Methods Enzymol 2023, 681, 155–167. 10.1016/bs.mie.2022.08.056. [DOI] [PubMed] [Google Scholar]
- Jones L. H. Emerging Utility of Fluorosulfate Chemical Probes. ACS Med. Chem. Lett. 2018, 9, 584–586. 10.1021/acsmedchemlett.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm H. S.; Toroitich E. K.; Borne A. L.; Brulet J. W.; Libby A. H.; Yuan K.; Ware T. B.; McCloud R. L.; Ciancone A. M.; Hsu K. L. Global targeting of functional tyrosines using sulfur-triazole exchange chemistry. Nat. Chem. Biol. 2020, 16, 150–159. 10.1038/s41589-019-0404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borne A. L.; Brulet J. W.; Yuan K.; Hsu K. L. Development and biological applications of sulfur-triazole exchange (SuTEx) chemistry. RSC Chem. Biol. 2021, 2, 322–337. 10.1039/D0CB00180E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong N. R.; Liu H.; Che J.; Jones L. H. Physicochemistry of Cereblon Modulating Drugs Determines Pharmacokinetics and Disposition. ACS Med. Chem. Lett. 2021, 12, 1861–1865. 10.1021/acsmedchemlett.1c00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan K. A.; Ferguson F. M.; Bushman J. W.; Eleuteri N. A.; Bhunia D.; Ryu S.; Tan L.; Shi K.; Yue H.; Liu X.; Dobrovolsky D.; Jiang B.; Wang J.; Hao M.; You I.; Teng M.; Liang Y.; Hatcher J.; Li Z.; Manz T. D.; Groendyke B.; Hu W.; Nam Y.; Sengupta S.; Cho H.; Shin I.; Agius M. P.; Ghobrial I. M.; Ma M. W.; Che J.; Buhrlage S. J.; Sim T.; Gray N. S.; Fischer E. S. Mapping the Degradable Kinome Provides a Resource for Expedited Degrader Development. Cell 2020, 183, 1714. 10.1016/j.cell.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Chen Q.; Klauser P. C.; Li M.; Zheng F.; Wang N.; Li X.; Zhang Q.; Fu X.; Wang Q.; Xu Y.; Wang L. Developing Covalent Protein Drugs via Proximity-Enabled Reactive Therapeutics. Cell 2020, 182, 85. 10.1016/j.cell.2020.05.028. [DOI] [PubMed] [Google Scholar]
- Bolding J. E.; Martín-Gago P.; Rajabi N.; Gamon L. F.; Hansen T. N.; Bartling C. R. O.; Strømgaard K.; Davies M. J.; Olsen C. A. Aryl Fluorosulfate Based Inhibitors That Covalently Target the SIRT5 Lysine Deacylase. Angew. Chem., Int. Ed. Engl. 2022, 61, e202204565 10.1002/anie.202204565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth S.; Hüser S.; Roth A.; Degen G.; Diel P.; Edlund K.; Eisenbrand G.; Engel K. H.; Epe B.; Grune T.; Heinz V.; Henle T.; Humpf H. U.; Jäger H.; Joost H. G.; Kulling S. E.; Lampen A.; Mally A.; Marchan R.; Marko D.; Mühle E.; Nitsche M. A.; Röhrdanz E.; Stadler R.; van Thriel C.; Vieths S.; Vogel R. F.; Wascher E.; Watzl C.; Nöthlings U.; Hengstler J. G. Toxicity of fluoride: critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. 10.1007/s00204-020-02725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASF: CLH Report for Imidazole; European Chemicals Agency, 2023; https://echa.europa.eu/documents/10162/9a2b728c-34ac-b4a5-d295-a1ae1b42f613.
- Lenarcik B.; Ojczenasz P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid-base properties. J. Heterocyclic Chem. 2002, 39, 287–290. 10.1002/jhet.5570390206. [DOI] [Google Scholar]
- Sakakibara S.; Fujii T. Synthesis and use of N-im-tosyl-L-histidine. Bull. Chem. Soc. Jpn. 1969, 42, 1466. 10.1246/bcsj.42.1466. [DOI] [PubMed] [Google Scholar]
- White K. A.; Ruiz D. G.; Szpiech Z. A.; Strauli N. B.; Hernandez R. D.; Jacobson M. P.; Barber D. L. Cancer-associated arginine-to-histidine mutations confer a gain in pH sensing to mutant proteins. Sci. Signal 2017, 10, eaam9931. 10.1126/scisignal.aam9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimster N. P. Covalent PROTACs: the best of both worlds?. RSC Med. Chem. 2021, 12, 1452–1458. 10.1039/D1MD00191D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.; Shioi R.; Kool E. T. Sulfonylation of RNA 2’-OH groups. ACS Cent Sci. 2023, 9, 531–539. 10.1021/acscentsci.2c01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.