Summary
Background
In VISION, the prostate-specific membrane antigen (PSMA)-targeted radioligand therapy lutetium (177Lu) vipivotide tetraxetan ([177Lu]Lu-PSMA-617; 177Lu-PSMA-617) improved radiographic progression-free survival and overall survival when added to protocol-permitted standard of care (SoC) in patients with metastatic castration-resistant prostate cancer (mCRPC). Here, we report additional health-related quality-of-life (HRQoL), pain, and symptomatic skeletal event (SSE) results.
Methods
In the international, open-label, phase 3 VISION study, patient were randomised 2:1 to receive intravenous infusions of 177Lu-PSMA-617 7·4 GBq (200 mCi) plus SoC or SoC alone every 6 weeks for up to six cycles. Eligible patients had PSMA-positive mCRPC previously treated with at least one androgen receptor pathway inhibitor and one or two taxanes. Time to first SSE was a key secondary endpoint. Other secondary endpoints included HRQoL assessed with the Functional Assessment of Cancer Therapy–Prostate (FACT-P) and the EuroQol 5-dimension 5-level (EQ-5D-5L) instruments, and pain assessed with the Brief Pain Inventory–Short Form (BPI-SF). Outcomes were analysed in intention-to-treat populations. This trial is registered on ClinicalTrials.gov, NCT03511664 (active, not recruiting).
Findings
Patients were randomly assigned to treatment between June 4, 2018 and October 23, 2019. Time to first SSE or death was delayed in subgroups with (hazard ratio [HR], 0·49; 95% confidence interval [CI]: 0·36, 0·68) and without (HR, 0·50; 95% CI: 0·37, 0·68) concurrent use of bone-targeted therapy. Time to worsening was delayed in the 177Lu-PSMA-617 plus SoC group (n = 385) versus SoC alone (n = 196) for FACT-P total score (HR, 0·46; 95% CI: 0·35, 0·61) and subdomains, BPI-SF pain intensity score (HR, 0·45; 95% CI: 0·33, 0·60) and EQ-5D-5L utility score (HR, 0·49; 95% CI: 0·40, 0·62). Rates of haematologic and renal toxicity were low in patients receiving 177Lu-PSMA-617.
Interpretation
The radioligand 177Lu-PSMA-617 improves quality of life and delays SSEs in patients with advanced mCRPC.
Funding
This work was supported by Advanced Accelerator Applications, a Novartis Company.
Introduction
Despite significant therapeutic advances in recent years, metastatic castration-resistant prostate cancer (mCRPC) remains an incurable and fatal disease.1,2 In the past two decades, several new classes of therapy shown to provide survival benefit in patients with mCRPC have been approved. These include taxanes (docetaxel and cabazitaxel), androgen receptor pathway inhibitors (ARPIs, such as abiraterone and enzalutamide), immunotherapy (sipuleucel-T), and bone-targeted radionuclide therapy (radium-223).1
Patients with progressive mCRPC after treatment with ARPIs and taxanes have limited further treatment options and are at high risk of impaired health-related quality of life (HRQoL), morbidity, and mortality.3 mCRPC and its treatment are associated with significant impairment of physical, emotional, and functional well-being,4 HRQoL is therefore an important outcome to consider for treatment decision-making in patients with mCRPC, in addition to efficacy, safety, and tolerability.
Symptomatic skeletal events (SSEs), such as fracture, spinal cord compression, and pain, have a significant negative impact on HRQoL in patients with bone metastasis.5,6 Bone metastases are common in mCRPC, occurring in up to 90% of patients,7 and bone-targeted therapies like bisphosphonates (zoledronic acid) or denosumab are the mainstay of treatment for the prevention of SSEs.1,2 Delaying the time to SSEs regardless of bone-targeted therapy use is therefore an important aspect of the efficacy of anti-cancer therapy in patients with mCRPC and bone metastasis.
Prostate-specific membrane antigen (PSMA) is a transmembrane glutamate carboxypeptidase with highly upregulated expression in prostate cancer cells and restricted expression in non-prostate-cancer cells.8 Radioligand therapy selectively targets cancer cells via specific cell-surface proteins, while sparing most normal tissues.9 Lutetium (177Lu) vipivotide tetraxetan (also known as [177Lu]Lu-PSMA-617; 177Lu-PSMA-617) is a high-affinity PSMA-targeted small-molecule radioligand therapy that delivers β-particle radiation specifically to PSMA-expressing cells and their surrounding microenvironment.10
The kidneys and bone marrow are recognised as dose-limiting organs for therapeutic radiopharmaceuticals. In patients receiving 177Lu-PSMA-617, the kidneys are exposed to radiation because urinary excretion is the principal route of elimination of 177Lu-PSMA-617 and also because PSMA is expressed in proximal tubular cells. The bone marrow is at risk of toxicity because of radiation-induced myelosuppression. Patients with severe haematological and renal complications could require interventions that negatively impact their quality of life.
In the phase 2 TheraP trial, the prostate-specific antigen (PSA) response rate was higher in patients treated with 177Lu-PSMA-617 than in those receiving cabazitaxel, and a smaller proportion of patients experienced grade 3 or 4 adverse events in the 177Lu-PSMA-617 group.11 In the alternate primary endpoints of the phase 3 VISION trial, 177Lu-PSMA-617 prolonged radiographic progression-free survival (rPFS; hazard ratio [HR], 0·40; 99·2% confidence interval [CI]: 0·29, 0·57; p<0·001) and overall survival (OS, 0·62; 95% CI: 0·52, 0·74; p<0·001) when added to protocol-permitted standard of care (SoC) in patients with advanced PSMA-positive mCRPC.12
Top-level prespecified HRQoL and pain results from VISION have been briefly reported, with time to worsening in Functional Assessment of Cancer Therapy–Prostate (FACT-P) total score (HR, 0·54; 95% CI: 0·45, 0·66) and Brief Pain Inventory–Short Form (BPI-SF) pain intensity score (HR, 0·52; 95% CI: 0·42, 0·63) favoring the addition of 177Lu-PSMA-617 to SoC.12 These analyses included clinical disease progression or death as a composite endpoint together with HRQoL and pain deterioration. Here, we report detailed new post hoc non-composite analyses of HRQoL and pain in VISION, together with new results on SSEs, and haematological and renal parameters.
Methods
Study design and participants
VISION was an open-label, international, randomised phase 3 trial of the efficacy and safety of 177Lu-PSMA-617 in patients with progressive PSMA-positive mCRPC previously treated with at least one ARPI and one or two taxane-containing regimens, as previously described.12 Eligible patients had progressive mCRPC with at least one metastatic lesion on baseline computed tomography (CT), magnetic resonance imaging (MRI), or bone scan imaging. Eligible patients had PSMA-positive mCRPC, defined as at least one PSMA-positive metastatic lesion and no PSMA-negative lesions per protocol criteria.12 PSMA-positive tumor status was determined by gallium (68Ga) gozetotide (also known as [68Ga]Ga-PSMA-11; 68Ga-PSMA-11) positron emission tomography (PET)-CT imaging during screening and evaluated by independent central review. Radiographic imaging was evaluated in accordance with Response Evaluation Criteria in Solid Tumors 1·1 (RECIST 1·1) and Prostate Cancer Working Group 3 (PCWG3) criteria.
Additional inclusion criteria were: 18 years or older, Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2, life expectancy of at least 6 months, and adequate hepatic, renal, and bone marrow function. Patients were ineligible if they were candidates for additional chemotherapy at screening or required treatments that were not permitted under the study protocol.12
This study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice, and with any applicable local regulations. All participants provided written informed consent before study entry.
Randomisation and masking
In this open-label study, patients were randomised 2:1 using an interactive response system to receive either 177Lu-PSMA-617 plus protocol-permitted SoC (177Lu-PSMA-617 group) or protocol-permitted SoC alone (control group) using a permuted block scheme. Randomisation was stratified by baseline lactate dehydrogenase level (≤260 IU/L or >260 IU/L), presence of liver metastases (yes or no), ECOG performance status (0/1 or 2), and inclusion of ARPI in protocol-permitted SoC (yes or no).
Procedures
In addition to SoC, patients in the 177Lu-PSMA-617 group received intravenous infusions of 177Lu-PSMA-617 7·4 GBq (200 mCi) every 6 weeks for four cycles, plus two optional additional cycles (up to six in total) that could be administered in patients tolerating therapy and showing evidence of response, at the investigator’s discretion. One dose of 177Lu-PSMA-617 could be reduced or delayed by up to 4 weeks at the investigator’s discretion.12
Tumour assessments (CT, MRI and bone scan imaging) were done every 8 weeks for the first 24 weeks, then every 12 weeks. SoC with or without 177Lu-PSMA-617 continued until radiographic disease progression, unacceptable toxicity, determined lack of clinical benefit, or until a prohibited treatment was deemed necessary. Patients who completed 177Lu-PSMA-617 continued to receive SoC. Investigator-determined SoC could include approved hormonal treatments (such as abiraterone and enzalutamide), bisphosphonates, radiation therapy, denosumab, glucocorticoids, hydration, and analgesics. Cytotoxic chemotherapy, systemic radioisotopes (e.g. radium-223), immunotherapy, and investigational drugs were not allowed.12
Patient-reported outcomes (PROs) comprised the FACT-P, BPI-SF, and EuroQol 5-dimension 5-level (EQ-5D-5L) instruments. Questionnaires were completed electronically by patients alone or with assistance during a face-to-face interview at baseline (before or at randomisation), on the first day of each cycle and at end-of-treatment, but not during follow-up. The cycle length was 6 weeks for cycles 1–6 (SoC with or without 177Lu-PSMA-617) and 12 weeks for cycle 7 onwards (continuation of SoC).
Haematology and clinical chemistry parameters were assessed every week during cycle 1, every other week during subsequent cycles, every 12 weeks after cycle 6, and at end-of-treatment, but not during subsequent follow-up. Haematological adverse events were monitored throughout treatment and were defined as occurring from first administration up to and including 30 days after the last dose of study treatment or before subsequent anticancer treatment, whichever occurred first. Adverse events and haematological abnormalities were based on Common Terminology Criteria for Adverse Events (CTCAE) grading.
Outcomes
The alternate primary endpoints of VISION were rPFS and OS. Key secondary endpoints included time to first SSE or death, defined as the time from randomisation to first new pathological bone fracture, spinal cord compression, tumor-related orthopedic surgical intervention, requirement for radiation therapy to relieve bone pain, or death from any cause, whichever occurred first. SSEs were monitored throughout the study up to and including end-of-treatment, but not during subsequent follow-up. Additional secondary endpoints included the safety profile of 177Lu-PSMA-617 and patient-reported HRQoL and pain outcomes. PROs and SSEs were analysed according to assigned treatment group in the PFS analysis set, comprising patients who were randomised after implementation of the measures designed to reduce the drop-out rate in the control group (on or after March 5, 2019). Details of these measures, which included enhanced study site education, have been published elsewhere.12 Safety was analysed according to treatment received in the safety analysis set, comprising all patients who received at least one dose of randomised treatment.
The FACT-P consists of two parts: Functional Assessment of Cancer Therapy–General (FACT-G; 27 items) and Prostate Cancer Subscale (PCS; 12 items). The FACT-P total score (range, 0–156) is the sum of the scores (39 items) with higher scores indicating better HRQoL. The five subscales are: physical well-being, social/family well-being, emotional well-being, functional well-being, and PCS. The EQ-5D-5L generates a preference-based health-state utility score (EQ-5D utility index; range, –0·594 to 1) consisting of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and an overall health-state score based on a visual analogue scale (EQ-5D VAS; range, 0–100). The BPI-SF assesses pain intensity (range 0–10, no pain to worse pain), and how pain interferes with daily activities (range 0–10, no interference to complete interference). BPI-SF pain intensity is a mean of four individual scales: worst pain intensity, least pain intensity, mean pain intensity, and current pain.
Definitions of change in FACT-P and BPI-SF were based on established minimal clinically important differences: 6–10 points for FACT-P total score, 1–2 points for FACT-P pain related subscale, 5–9 points for FACT-P trial-outcome index, 2–3 points for FACT-P other subscales and an increase of at least 30% or at least 2 points for BPI-SF scales and subscales.13,14 Time to worsening in FACT-P was pre-defined as the time from randomisation to a decrease of at least 10 points from baseline for total score, at least 2 points from baseline for pain-related subscale, at least 9 points from baseline for trial-outcome index or at least 3 points from baseline for other subscales. Time to worsening in EQ-5D-5L utility score was pre-defined as the time from randomisation to any decrease or no change from baseline. Time to worsening in BPI-SF scales and subscales was pre-defined as the time from randomisation to an increase of at least 30% or at least 2 points from baseline.
Statistical analysis
All analyses presented in this manuscript were performed as prespecified in the statistical analysis plan, other than those described as post hoc. In prespecified analyses of BPI-SF, FACT-P and EQ-5D, the endpoint was the composite of worsening in score, clinical disease progression (excluding radiographic and PSA progression), or death due to any cause.12 Clinical disease progression was assessed by investigators based on marked escalation in cancer-related pain, immediate need for initiation of new anticancer treatment, surgical or radiological intervention, or marked deterioration in ECOG performance status higher or equal to Grade 3. In post hoc analyses of PROs, only worsening in score was considered an event. In additional post hoc analyses, time to worsening in EQ-5D-5L was defined as the time from randomisation to a decrease in utility score of at least 0·10 points from baseline15, and time to worsening in BPI-SF scales was defined as the time from randomisation to an increase of at least 2 points from baseline. Prespecified analyses for time to improvement after worsening in BPI-SF pain intensity scale was defined as the time from worsening to occurrence of a score below or equal to the baseline value.
Efficacy outcomes were analysed in intention-to-treat populations; HRQoL outcomes were analysed in the intention-to-treat subset randomised on or after March 5, 2019. VISION was powered for rPFS and OS, as previously described12. For post hoc subgroup analyses of time to first SSE (with or without inclusion of death in the definition) in subgroups of patients receiving or not receiving bone-targeted agents, HRs and associated CIs were estimated using the previously described stratified Cox regression model.12 Median, percentiles, and associated CIs were estimated using the Kaplan-Meier method. For patient-reported outcomes, the prespecified method of statistical comparison was the Wald chi-square test from the stratified Cox proportional-hazards model, stratified by baseline lactate dehydrogenase level (≤260 U/mL or >260 U/mL), presence of liver metastases (yes or no), ECOG Performance Status (0–1 or 2), and inclusion of ARPI in protocol-permitted SoC at the time of randomisation (yes or no). The Cox model was also used to estimate HRs and associated CIs. Median, percentiles, and associated CIs were estimated using the Kaplan-Meier method. SAS version 9·4 was used for statistical analyses.
HRQoL and pain time-to-worsening endpoints were prespecified as excluded from study-wide type I error control; analyses used an unadjusted two-sided α of 0·05; sensitivity analyses were not prespecified. Multiplicity was also uncontrolled in post hoc analyses. All p values presented herein are therefore nominal, descriptive, and non-inferential.
Patients without an event were censored at the date of their last PRO assessment; patients without evaluable post-baseline data were censored at randomisation. The approach to missing items for each HRQoL and pain scale was detailed in the statistical analysis plan and followed the instrument developer’s guidelines. No methods for imputation of missing data were prespecified.
This trial is registered on ClinicalTrials.gov, NCT03511664.
Role of the funding source
The study funder had a role in study design, data analysis and interpretation, and writing of the report, but no role in data collection.
Results
Between June 4, 2018 and October 23, 2019, 831 patients were randomised, of whom 581 were randomised to the 177Lu-PSMA-617 group (n = 385) or control group (n = 196) on or after March 5, 2019, as previously reported (figure 1).12 These 581 patients were included in analyses of HRQoL, pain, and time to first SSE. Safety analyses included the 734 patients who received at least one dose of randomised treatment (177Lu-PSMA-617 group, n = 529; control group, n = 205), as previously reported (figure 1).12 Baseline characteristics were balanced between the groups (appendix p 2). The median duration of exposure to treatment has previously been reported.12
Figure 1: CONSORT diagram.
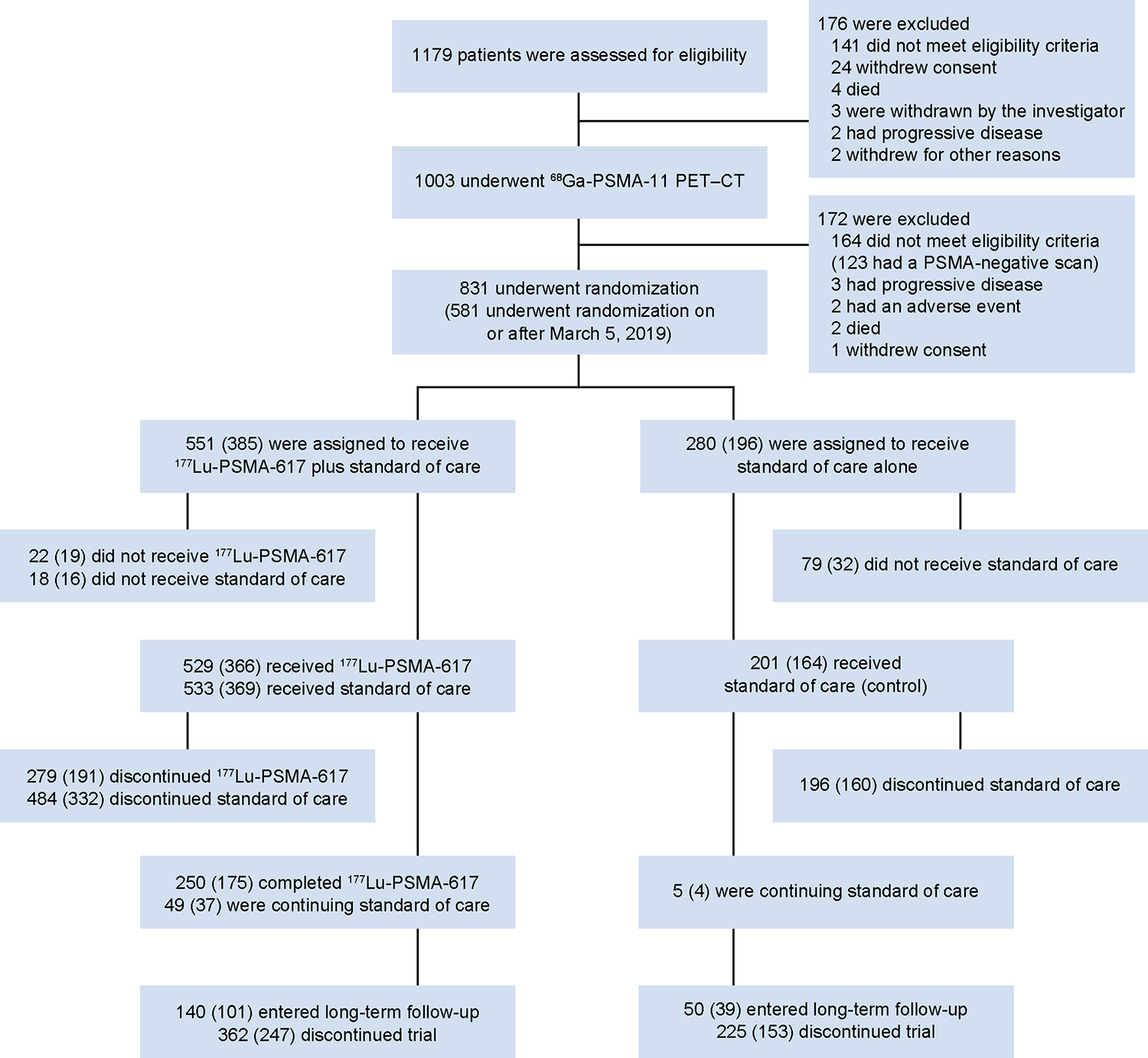
CT=computed tomography. PET=positron emission tomography. PSMA=prostate-specific membrane antigen. The number in parentheses indicate the numbers of patients who underwent randomisation after the enhanced trial-site education measures implemented on or after March 5, 2019, as reported in Methods.
Among patients remaining on study treatment, questionnaire completion rates were high (appendix p 3). Among patients remaining in the study, PRO completion rates were similar in the 177Lu-PSMA-617 group and the control group throughout study treatment. However, the study drop-out rate was higher in the control group than the 177Lu-PSMA-617 group. Questionnaire completion rates were similar at the end-of-treatment visit, with data available for approximately 45% of patients in both groups (appendix p 3). Reasons for non-completion of PRO questionnaires are shown in the appendix (p 4). Mean PRO scores at baseline and changes from baseline at each study visit are summarized in the appendix (p 5).
The time to worsening in FACT-P total score and all subscales was delayed in the 177Lu-PSMA-617 group compared with the control group, in both prespecified and post hoc analyses (figure 2A and table 1). The largest differences between the 177Lu-PSMA-617 and control groups were for FACT-P total score and the physical, emotional, and functional well-being subscales (table 1). The median follow-up time for FACT-P total score was 4·37 months (range, 0·0–22·3) in the 177Lu-PSMA-617 group and 0·76 months (range, 0·0–19·8) in the control group.
Figure 2: Time to worsening in FACT-P total score (A), BPI-SF pain intensity scale (B), and EQ-5D-5L utility score (C).
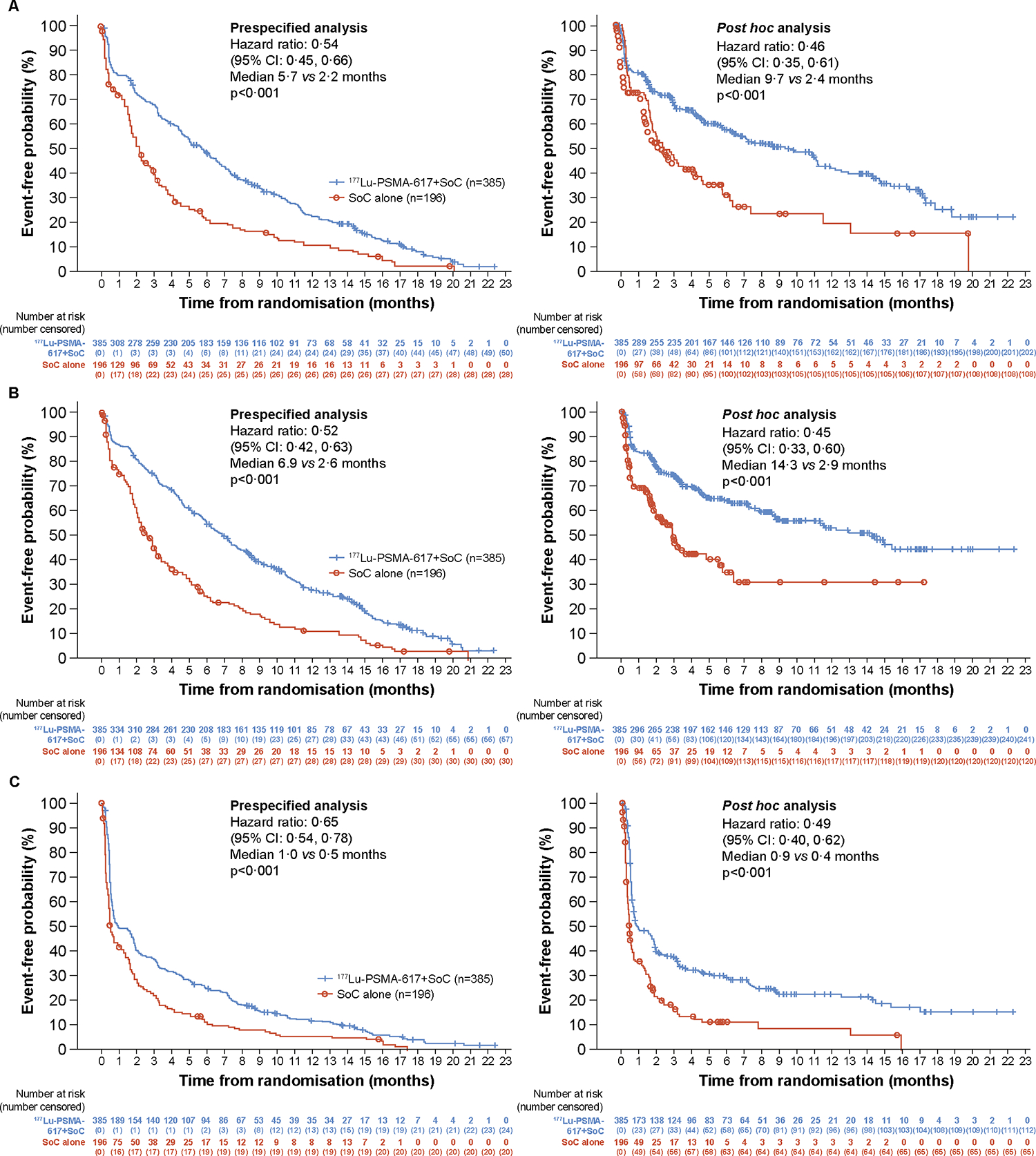
BPI-SF=Brief Pain Inventory – Short Form. CI=confidence interval. EQ-5D-5L=EuroQol 5-dimension 5-level. FACT-P=Functional Assessment of Cancer Therapy–Prostate. PSMA=prostate-specific membrane antigen. SoC=standard of care. Testing was two-sided using the Cox model (Wald Chi-square test). All p values are nominal, descriptive, and non-inferential. Analyses in the 581 patients randomised after measures were implemented on or after March 5, 2019. (A) Time to worsening of 10 points or greater in FACT-P total score, clinical disease progression, or death (prespecified analysis) and time to worsening of 10 points or greater in FACT-P total score (post hoc analysis); (B) Time to worsening of 30% or greater or 2 points or greater in BPI-SF pain intensity, clinical disease progression, or death (prespecified analysis) or time to worsening of 30% or greater or 2 points or greater in BPI-SF pain intensity (post hoc analysis); (C) Time to worsening in EQ-5D-5L utility score was defined as the time to the first occurrence of worsening in utility score relative to baseline (no change or any decrease).
Table 1:
Statistical analysis of time to worsening in patient-reported outcome scores
| Prespecified analysis | Post hoc analysis | |||
|---|---|---|---|---|
| 177Lu-PSMA-617 plus SoC (n = 385) | SoC alone (n = 196) | 177Lu-PSMA-617 plus SoC (n = 385) | SoC alone (n = 196) | |
|
| ||||
| FACT-P | ||||
| Total score | ||||
| Median time to worsening, months (95% CI) | 5·7 (4·8, 6·6) | 2·2 (1·8, 2·8) | 9·7 (6·9, 11·5) | 2·4 (1·8, 4·1) |
| HR (95% CI; p value) | 0·54 (0·45, 0·66; < 0·001) | 0·46 (0·35, 0·61; < 0·001) | ||
| Pain-related subscale | ||||
| Median time to worsening, months (95% CI) | 4·6 (3·4, 5·0) | 1·8 (1·6, 2·1) | 5·9 (4·6, 9·1) | 1·8 (1·6, 2·3) |
| HR (95% CI; p value) | 0·55 (0·45, 0·66; < 0·001) | 0·55 (0·42, 0·71; < 0·001) | ||
| Trial outcome index | ||||
| Median time to worsening, months (95% CI) | 6·0 (5·0, 6·9) | 2·2 (1·8, 2·9) | 11·0 (7·5, 12·0) | 2·9 (1·8, 4·1) |
| HR (95% CI; p value) | 0·56 (0·46, 0·68; < 0·001) | 0·48 (0·36, 0·63; < 0·001) | ||
| Prostate cancer subscale | ||||
| Median time to worsening, months (95% CI) | 3·9 (3·3, 4·6) | 1·8 (1·6, 2·3) | 4·8 (3·4, 6·4) | 1·8 (1·6, 2·9) |
| HR (95% CI; p value) | 0·62 (0·51, 0·76; < 0·001) | 0·59 (0·46, 0·76; < 0·001) | ||
| Physical well-being | ||||
| Median time to worsening, months (95% CI) | 4·7 (3·9, 6·1) | 1·8 (1·6, 2·3) | 7·6 (4·8, 10·2) | 1·8 (1·6, 2·9) |
| HR (95% CI; p value) | 0·51 (0·42, 0·62; < 0·001) | 0·44 (0·34, 0·58; < 0·001) | ||
| Social/family well-being | ||||
| Median time to worsening, months (95% CI) | 5·9 (5·1, 6·9) | 2·9 (2·3, 3·5) | 14·3 (8·8, NE) | 7·4 (5·8, NE) |
| HR (95% CI; p value) | 0·55 (0·45, 0·67; < 0·001) | 0·83 (0·58, 1·17; 0·29) | ||
| Emotional well-being | ||||
| Median time to worsening, months (95% CI) | 6·6 (5·7, 7·3) | 2·4 (1·9, 3·0) | 11·4 (8·9, 16·4) | 4·4 (2·4, 6·2) |
| HR (95% CI; p value) | 0·52 (0·43, 0·63; < 0·001) | 0·47 (0·35, 0·64; < 0·001) | ||
| Functional well-being | ||||
| Median time to worsening, months (95% CI) | 4·6 (3·3, 5·2) | 2·0 (1·6, 2·6) | 6·4 (4·4, 8·5) | 2·1 (1·6, 3·0) |
| HR (95% CI; p value) | 0·61 (0·51, 0·74; < 0·001) | 0·53 (0·41, 0·69; < 0·001) | ||
|
| ||||
| BPI-SF | ||||
| Pain intensity | ||||
| Median time to worsening, months (95% CI) | 6·9 (6·0, 7·7) | 2·6 (2·1, 3·1) | 14·3 (9·0, NE) | 2·9 (2·0, 5·5) |
| HR (95% CI; p value) | 0·52 (0·42, 0·63; < 0·001) | 0·45 (0·33, 0·60; < 0·001) | ||
| Pain interference | ||||
| Median time to worsening, months (95% CI) | 5·9 (5·0, 6·8) | 2·9 (2·3, 3·5) | 9·5 (6·5, 14·4) | 4·4 (1·7, 6·4) |
| HR (95% CI; p value) | 0·62 (0·51, 0·75; < 0·001) | 0·60 (0·45, 0·80; < 0·001) | ||
| Worst pain intensity | ||||
| Median time to worsening, months (95% CI) | 5·0 (4·2, 5·9) | 2·0 (1·7, 2·2) | 11·3 (7·2, 14·0) | 2·3 (1·7, 3·8) |
| HR (95% CI; p value) | 0·51 (0·42, 0·63; < 0·001) | 0·49 (0·37, 0·65; < 0·001) | ||
|
| ||||
| EQ-5D-5L | ||||
| Utility score | ||||
| Median time to worsening, months (95% CI) | 1·0 (0·7, 1·8) | 0·5 (0·4, 1·0) | 0·9 (0·7, 1·7) | 0·5 (0·4, 0·5) |
| HR (95% CI; p value) | 0·65 (0·54, 0·78; < 0·001) | 0·49 (0·40, 0·62; < 0·001) | ||
BPI-SF=Brief Pain Inventory–Short Form. CI=confidence interval. EQ-5D-5L=EuroQol 5-dimension 5-level. FACT-P=Functional Assessment of Cancer Therapy–Prostate. HR=hazard ratio. All p values are nominal, descriptive, and non-inferential. Analyses in the 581 patients randomised after measures were implemented on or after March 5, 2019.
The time to worsening in all BPI-SF scales was delayed in the 177Lu-PSMA-617 group compared with the control group, in both prespecified and post hoc analyses (figure 2B and table 1). Results of post hoc analyses of time to worsening in BPI-SF scales using a single threshold of an increase of at least 2 points from baseline were similar to results of analyses using the prespecified threshold of an increase of least 30% or at least 2 points from baseline (appendix p 6). A summary of the number of patients with increases of at least 30% and/or at least 2 points from baseline and the reasons for censoring are shown in the appendix (p 7). The median follow-up time for BPI-SF pain intensity scale was 4·14 months (range, 0·0–22·3) in the 177Lu-PSMA-617 group and 0·66 months (range, 0·0–17·2) in the control group. The time to improvement after worsening in BPI-SF pain intensity scale was shorter in the 177Lu-PSMA-617 arm than the control arm (appendix p 8).
The time to worsening in EQ-5D-5L utility score was delayed in the 177Lu-PSMA-617 group compared with the control group, in both prespecified and post hoc analyses (figure 2C and table 1). The time to worsening in EQ-5D-5L utility score of 0·10 points was also delayed in the 177Lu-PSMA-617 group compared with the control group, both when clinical disease progression or death were included in the definition and when not included (appendix p 9). EQ-VAS values by visit are summarised in the appendix (p 10).
SSEs occurred in 60/385 patients (16%) in the 177Lu-PSMA-617 group and 34/196 patients (17%) in the control group (table 2). The predominant types of SSE were radiation to relieve bone pain, followed by spinal cord compression (table 2). The median follow-up time for SSEs was similar between the treatment groups (table 3). Among the 310/581 (53%) patients not receiving bone-targeted therapy at baseline, the median time to first SSE or death was delayed in the 177Lu-PSMA-617 group compared with the control group (table 3 and appendix p 1). Among the 271/581 (47%) patients who were receiving bone-targeted therapy as part of SoC at baseline, the median time to first SSE or death was delayed in the 177Lu-PSMA-617 group compared with the control group (Table 3 and appendix p 1). Time to first SSE was also delayed in the 177Lu-PSMA-617 group compared with the control group in a post hoc analysis of time to first SSE that did not include death as an event, in the concurrent use of bone-targeted agent subgroups (appendix p 11).
Table 2:
Types of symptomatic skeletal event and reasons for censoring
|
177Lu-PSMA-617 plus SoC (n = 385) n (%) |
SoC alone (n = 196) n (%) |
|
|---|---|---|
|
| ||
| Number of events (SSE or death) | 256 (66) | 137 (70) |
| SSEsa | 60 (16) | 34 (17) |
| Symptomatic pathological bone fracture | 13 (3) | 1 (1) |
| Spinal cord compression | 5 (1) | 11 (6) |
| Radiation to relieve bone pain | 42 (11) | 21 (11) |
| Tumour-related orthopaedic surgical intervention | 1 (1) | 1 (1) |
| Deaths | 196 (51) | 103 (53) |
|
| ||
| Number of patients censored | 129 (34) | 59 (30) |
| Reason for censoring | ||
| Censored at last study visit (on or before EOT visit) | 129 (34) | 57 (29) |
| No evaluable data (censored at randomisation) | 0 | 2 (1) |
EOT=end of treatment. PSMA=prostate-specific membrane antigen. SoC=standard of care. SSE=symptomatic skeletal event.
SSE are captured on the adverse event, concurrent surgical/therapeutic procedures, and radiotherapy case report form pages. A patient can be counted in more than one SSE category (symptomatic pathological bone fracture, spinal cord compression, radiation to relieve bone pain, tumour-related orthopaedic surgical intervention) if multiple SSE occurred on the same date. A patient can be counted in more than one SSE sub-category (e.g. location of the radiation to relieve bone pain). Analyses in the 581 patients randomised after measures were implemented on or after March 5, 2019.
Table 3:
Subgroup analysis of time to first symptomatic skeletal event or death by concurrent use of bone-targeted agents as part of standard of care
|
177Lu-PSMA-617 plus SoC (n = 385) |
SoC alone (n = 196) |
|
|---|---|---|
|
| ||
| Bone-targeted agents: Yes | ||
| SSE or death, n/N (%) | 115/175 (66) | 66/96 (69) |
| Median time to event, months (95% CI) | 12 (10·0, 14·2) | 7·2 (5·6, 10·2) |
| HR (95% CI) | 0·49 (0·36, 0·68) | |
| Median follow-up time, months (95% CI) | 17·1 (14·6, 17·7) | 16·6 (11·0, NE) |
|
| ||
| Bone-targeted agents: No | ||
| SSE or death, n/N (%) | 141/210 (67) | 71/100 (71) |
| Median time to event, months (95% CI) | 11·5 (9·8, 13·7) | 5·8 (4·1, 9·2) |
| HR (95% CI) | 0·50 (0·37, 0·68) | |
| Median follow-up time, months (95% CI) | 17 (15·6, 17·4) | 19·8 (11·5, NE) |
|
| ||
| Overall | ||
| SSE or death, n/N (%) | 256/385 (66) | 137/196 (70) |
| Median time to event, months (95% CI) | 11·5 (10·3, 13·2) | 6·8 (5·2, 8·5) |
| HR (95% CI) | 0·50 (0·40, 0·62) | |
| Median follow-up time, months (95% CI) | 17 (15·9, 17·3) | 16·9 (11·5, NE) |
ARPI=androgen receptor pathway inhibitor. CI=confidence interval. ECOG=Eastern Cooperative Oncology Group. HR=hazard ratio. N=total number of events. N=total number of patients. NE=not estimable. PSMA=prostate-specific membrane antigen. SoC=standard of care. SSE=symptomatic skeletal event. HR of 177Lu-PSMA-617+SoC vs SoC only is based on stratified Cox Proportional Hazards model stratified for lactate dehydrogenase (≤260 IU/L vs >260 IU/L); presence of liver metastases (yes vs no); ECOG score (0 or 1 vs 2); and inclusion of ARPI in best supportive care/SoC at time of randomisation (yes vs no). Analyses in the 581 patients randomised after measures were implemented on or after March 5, 2019.
During the randomised treatment period, the median safety follow-up time was 14·78 months (range, 0·6–31·5) in the 177Lu-PSMA-617 group and 10·64 months (range, 0·7–27·1) in the control group. Haematologic abnormalities of CTCAE grade 3 or 4 were more frequent in the 177Lu-PSMA-617 group than in the control group, particularly for anemia, low lymphocyte levels, and low platelet counts (appendix p 12). Mean haemoglobin levels and platelet counts remained stable over time (figure 3A–B). Mean and median haemoglobin levels by visit during randomised treatment are shown in the appendix (p 13). A shift table for haemoglobin levels based on CTCAE grade during randomised treatment is shown in the appendix (p 14). Overall, 21/529 patients (4%) had 177Lu-PSMA-617 dose reductions owing to myelosuppression (appendix p 15).
Figure 3: Haemoglobin (A), platelet (B), and creatinine (C) levels during randomised treatment.
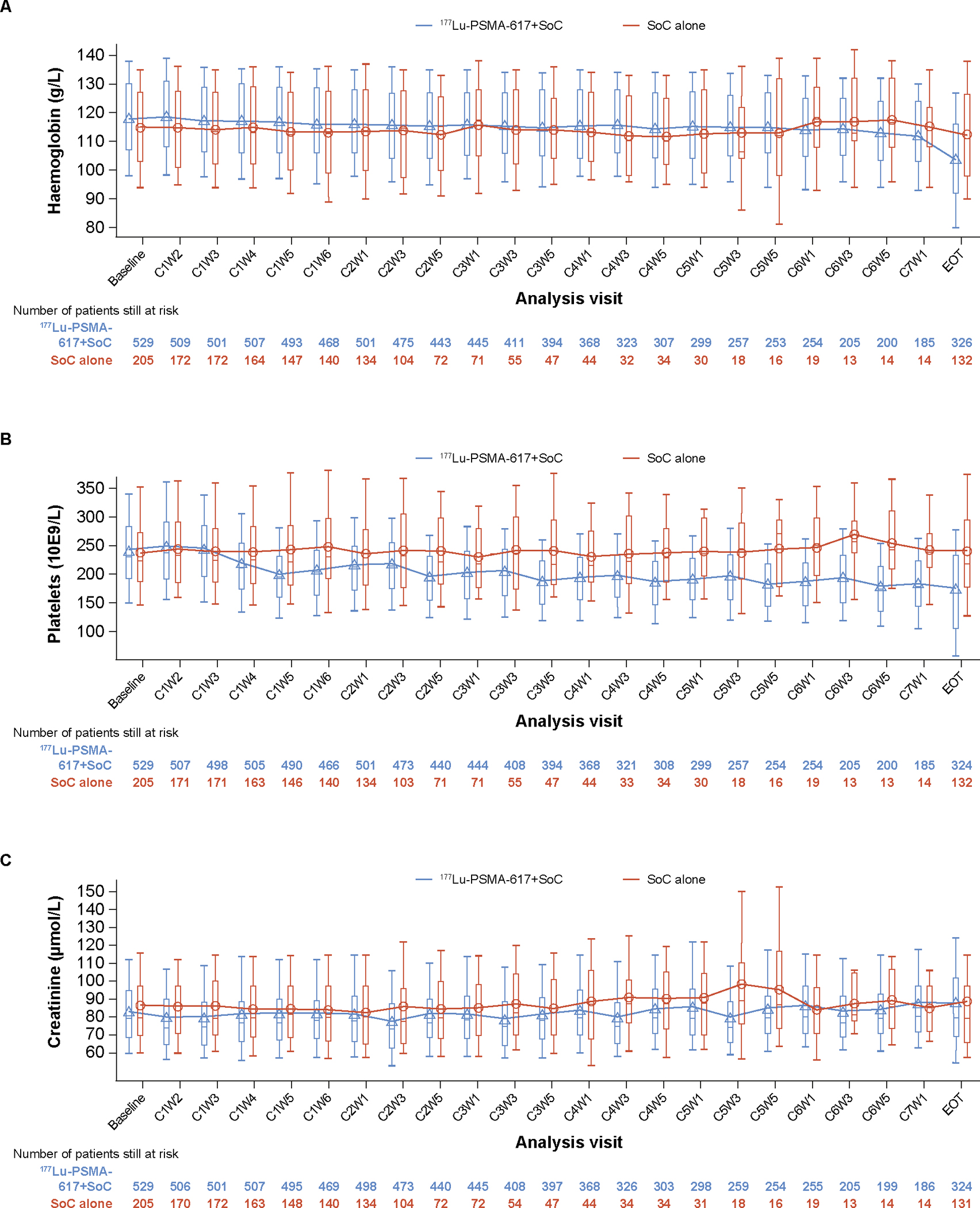
C=cycle. EOT=End of treatment. PSMA=prostate-specific membrane antigen. SoC=standard of care. W=week. Plot shows boxes (25th–75th percentiles) with the median as a horizontal line. The dots in the boxes and joint lines represent the mean values. Whiskers (vertical lines) extend to the 10th–90th percentiles. Values outside this range are not displayed. CxWy: Cycle x Week y Day 1. Cycle visits were scheduled every 6 weeks for the first six cycle visits and then every 12 weeks. This analysis only includes assessments up to the time point at which there are at least 10 patients in each of the treatment groups. Platelet values ≥100 000 109/L at C6W5 were not converted to correct standardized units and were excluded from the analysis. Analyses in the 734 patients who received at least one dose of randomised treatment.
The incidence of creatinine abnormalities of CTCAE grade 3 or above was low, and similar between the 177Lu-PSMA-617 group and the control group (appendix p 12). Mean creatinine levels remained stable over time (figure 3C). Data on adverse events have been previously published.12
Discussion
In the VISION trial, addition of 177Lu-PSMA-617 to SoC delayed the time to worsening of HRQoL and pain in patients with mCRPC previously treated with at least one ARPI and one or two taxanes. Worsening was delayed across all PROs, and all subscales and domains, with the greatest difference in median time to event in BPI-SF pain intensity and FACT-P total score. HRs were in the range of 0·45 to 0·60 for all instruments, and similar delays in time to worsening in score, clinical disease progression, or death were detected in prespecified and post hoc analyses. These results complement the previously reported primary efficacy endpoints of prolonged rPFS and extended OS with 177Lu-PSMA-617 in the VISION trial.12
VISION aimed to investigate 177Lu-PSMA-617 as an add-on to a broad range of SoC therapies that could safely be combined with investigational radioligand therapy.12 Protocol-permitted SoC therefore did not include chemotherapy or radium-223, and patients who were candidates for a second taxane regimen at baseline were ineligible.12 Drugs that were investigational at the time the study was designed were also not included in protocol-permitted SoC (e.g. olaparib and other poly [ADP-ribose] polymerase inhibitors).12
Time to HRQoL worsening was delayed with 177Lu-PSMA-617 on both the FACT-P and EQ-5D-5L, with similar HRs. For FACT-P total score, the threshold for worsening of a decrease of at least 10 points from baseline is well documented.13 Unlike the prostate cancer-specific FACT-P instrument, the EQ-5D is generic, with no established cut-off and potentially low sensitivity in patients with advanced prostate cancer.14 Although previous studies have used the EQ-5D to complement prostate-cancer specific instruments, patients were at an earlier stage of disease than in the extensively pre-treated VISION population.16,17 Owing to the potentially low sensitivity of EQ-5D in the study population, worsening was pre-defined as no change or any deterioration in utility score from baseline, leading to rapid apparent worsening. However, deterioration was also rapid using a post hoc EQ-5D worsening threshold of 0·10 points in utility score, with similar HRs. These results contrast with those obtained using the more reliable and robust FACT-P and BPI-SF, for which definitions of worsening were based on well established thresholds.13,14
FACT-P and BPI-SF outcomes improved with cabazitaxel treatment in patients with advanced mCRPC in a prospective real-world study.18 In the AFFIRM trial of enzalutamide after docetaxel, FACT-P total score and subscales improved versus placebo in patients with progressive mCRPC.13 These findings suggest that therapies that delay disease progression are associated with maintained HRQoL. In VISION, worsening in BPI-SF scales and subscales was defined as an increase in score of at least 30% or at least 2 points from baseline. This is the established clinically meaningful threshold used in several clinical trials in patients with prostate cancer, as recently reviewed.14 The COU-AA-302 study used a slightly different definition of an increase of at least 30% from baseline at two consecutive evaluations.19 In VISION, the majority of patients meeting the definition of BPI-SF worsening had at least a 2-point increase in score from baseline. Post hoc analyses of time to BPI-SF worsening using only the 2-point threshold confirmed the results using the 2-point or 30% threshold.
Pain in patients with mCRPC can be attributed largely to bone metastases, lumbosacral invasion, and nerve root compression, and is associated with shortened OS.20 Bone metastases are frequent,21 leading to pain, SSEs,22 disability, impaired HRQoL,7,23 and mortality.24,25 SSEs had a significant negative impact on HRQoL and pain in a large cohort study in patients with CRPC and bone metastasis.5,6 In the CARD study, cabazitaxel delayed the time to first SSE and improved pain response versus abiraterone/enzalutamide in patients with mCRPC, but did not delay the time to worsening in FACT-P total score.16 In COMET-1, cabozantinib delayed the time to first SSE in patients with mCRPC versus prednisone.4 In VISION, the median time to first SSE or death was delayed in the 177Lu-PSMA-617 group versus control,12 and spinal cord compression, the most deleterious SSE, was numerically less frequent. In previous studies, bone-targeted agents (denosumab or zoledronic acid) led to a decreased risk of fracture in patients with mCRPC and bone metastasis.26–28 In the present analyses, time to SSE or death was delayed in the 177Lu-PSMA-617 group compared versus control regardless of bone-targeted therapy use.
Adverse events in the “myelosuppression” and “renal effects” groupings were more frequent during randomised treatment with 177Lu-PSMA-617 plus Soc than with SoC alone, but were mainly low-grade.12 Haematology and clinical chemistry results provide additional evidence for an acceptable haematological and renal safety profile. Although haematological abnormalities of CTCAE grade 3 or above were more frequent with 177Lu-PSMA-617 than without, mean haemoglobin levels and platelet counts remained stable during randomised treatment. The incidence of creatinine abnormalities of CTCAE grade 3 or above was low and similar between groups, and mean levels remained stable. This safety profile supports 177Lu-PSMA-617 use in often elderly patients with late-stage disease29 and potentially in younger patients with earlier-stage disease.
Ongoing phase 3 trials are investigating whether 177Lu-PSMA-617 provides therapeutic benefit earlier in the treatment sequence than in the present study. PSMAfore (NCT04689828) is assessing the efficacy and safety of 177Lu-PSMA-617 treatment versus change of ARPI in taxane-naïve patients with mCRPC. PSMAddition (NCT04720157) is assessing the efficacy and safety of 177Lu-PSMA-617 plus SoC versus SoC alone in patients with metastatic hormone-sensitive prostate cancer. SPLASH (NCT04647526) is assessing the efficacy and safety another PSMA-targeted radioligand therapy known as 177Lu-PSMA-I&T versus abiraterone or enzalutamide in patients with mCRPC. ECLIPSE (NCT05204927) will compare of 177Lu-PSMA-I&T versus hormone therapy in patients with mCRPC. All these studies except SPLASH will also investigate HRQoL and/or pain as PROs.
PRO questionnaires were completed during study treatment, but not after disease progression or discontinuation of study treatment in VISION. This is an important limitation, because potential subsequent effects of study treatment on HRQoL and pain were not captured. This limitation is shared by many phase 3 trials in patients with CRPC. The VISION population was heavily pre-treated with short remaining life expectancy, so deterioration in HRQoL and pain was likely to manifest within the follow-up time. Death and disease progression were included in the prespecified composite endpoints of HRQoL and pain worsening, which were therefore assumed to have worsened in patients who did not complete questionnaires because they died or experienced disease progression. The results support the conclusion that there was no adverse effect of 177Lu-PSMA-617 on HRQoL or pain, despite the limitation that data were collected only during study treatment. Another limitation of VISION was the exclusion of chemotherapy and systemic radiotherapy from SoC because their safety profile had not been established in combination with 177Lu-PSMA-617.
PRO completion rates were similar in the 177Lu-PSMA-617 and control groups among patients remaining on study treatment. The proportion of patients remaining in the study was smaller in control group than the 177Lu-PSMA-617 group owing to the higher drop-out rate, especially after cycle 3 (because the treatment period was shorter in the control group than the 177Lu-PSMA-617 group) and PRO questionnaires were not completed after discontinuation. This is another limitation and means the results should be interpreted with caution. Furthermore, the cycle length was 6 weeks for cycles 1–6 of SoC with or without 177Lu-PSMA-617 and 12 weeks for subsequent SoC cycles. Sustained worsening was not assessed in VISION because of the relatively long interval between PRO assessments (6 or 12 weeks), and because of inevitable declining HRQoL and death in patients with advanced and highly pre-treated mCRPC. Randomisation to open-label SoC alone may itself have adversely affected patients’ HRQoL. The lack of double-blinding and placebo control are limitations shared by studies of other radiopharmaceuticals.30 The impact of the high early drop-out rate in the control group was mitigated by analysing HRQoL and pain outcomes in the PFS analysis set, comprising patients who were randomised after the implementation of measures that reduced the drop-out rate in the control group.12 Collecting HRQoL and pain data for as many patients as possible is important for replication of the present findings in future trials.
In summary, the efficacy of 177Lu-PSMA-617 plus SoC in delaying rPFS and prolonging OS in patients with mCRPC was associated with a longer period without deterioration in patient-reported HRQoL and pain, compared with SoC alone. Low rates of haematologic and renal toxicity were observed with 177Lu-PSMA-617 treatment. These additional findings from VISION strengthen the rationale for adoption of 177Lu-PSMA-617 as a treatment option in patients with mCRPC who have received previous ARPI and taxane treatment.
Supplementary Material
Research in context.
Evidence before this study
177Lu-PSMA-617 is a small-molecule targeted radioligand therapy for the treatment of metastatic castration-resistant prostate cancer (mCRPC). We searched PubMed from January 2016 to September 2021 using the terms “metastatic castration-resistant prostate cancer” and “prostate-specific membrane antigen” and “lutetium” and “clinical trial”. This search detected only small phase 1–2 clinical trials of 177Lu-PSMA-617 in patients with mCRPC. Of these, the only randomised controlled trial was the phase 2 TheraP study, in which 177Lu-PSMA-617 improved prostate specific antigen response compared with cabazitaxel in patients with mCRPC previously treated with ARPIs and/or chemotherapy.
To our knowledge, VISION was the first phase 3 study of 177Lu-PSMA-617. In the primary efficacy outcomes of VISION, 177Lu-PSMA-617 prolonged both radiographic progression-free survival (rPFS) and overall survival (OS) when added to protocol-permitted standard of care (SoC) in patients with PSMA-positive mCRPC previously treated with at least one androgen receptor pathway inhibitor (ARPI) and one or two taxanes.
Added value of this study
Optimal treatment of mCRPC aims not only to extend life and delay disease progression but also to improve or maintain patients’ health-related quality of life (HRQoL). Patients with mCRPC receiving guideline-directed therapy with ARPIs or taxanes have a high risk of impaired HRQoL as well as morbidity and mortality. Bone metastases are common in patients with mCRPC, and symptomatic skeletal events (SSEs) have a significant negative impact.
The results of this new analysis of VISION data demonstrate that 177Lu-PSMA-617 plus SoC also delayed the time to worsening of patient-reported HRQoL and pain, in multiple domains, compared with SoC alone. In post hoc analyses, this finding was extended from the prespecified composite outcome of worsening in FACT-P, EQ-5D-5L and BPI-SF scores or clinical disease progression or death, to worsening in scores only, with similar results. Furthermore, time to first SSE was also delayed in patients treated with 177Lu-PSMA-617 versus SoC alone, regardless of bone-targeted therapy use.
Implications of all the available evidence
These new findings from the VISION study add to the body of evidence showing that addition of 177Lu-PSMA-617 to SoC prolongs rPFS and extends OS, with no adverse effect on patient-reported HRQoL or pain, in patients with mCRPC who have previously received at least one ARPI and one or two taxanes. Patient-reported outcomes were also improved or similar with 177Lu-PSMA-617 versus cabazitaxel in TheraP. The findings of the phase 3 VISION studies and previous phase 2 studies provide a strong rationale for adoption of 177Lu-PSMA-617 as a treatment option in patients with advanced mCRPC.
Acknowledgements
This study was funded by Advanced Accelerator Applications, a Novartis company. The authors thank the patients and their families, and all site investigators, and personnel who participated in the study. Under the direction of the authors, Karim Bensaad PhD from Oxford PharmaGenesis, Oxford, UK, provided medical writing support, which was funded by Advanced Accelerator Applications, a Novartis company, in accordance with Good Publication Practice (GPP3) guidelines (https://www.ismpp.org/gpp3).
Footnotes
Declaration of interests
KF reports consultant or advisory fees from Janssen, Bayer, Astellas, Sanofi, Orion Pharma, CureVac, AstraZeneca, ESSA Pharma, Amgen, Bristol-Myers Squibb, Clovis Oncology, Lilly, Novartis, Daiichi-Sankyo, Pfizer and MSD. KH reports consultant or advisory fees from ABX, AstraZeneca, Advanced Accelerator Applications, a Novartis company, Aktis Oncology, Amgen, Bayer, BTG, Curium, Debiopharm, Endocyte, GE HealthCare, Janssen, Ipsen, Novartis, Pharma15, Siemens Healthineers, Sirtex Medical, SOFIE Biosciences, Theragnostics, and Y-mAbs Therapeutics. BJK reports consultant or advisory fees from Advanced Accelerator Applications, a Novartis company, Astellas, Bayer, Janssen, Terumo, PSFI, and ITM, and research funding from Advanced Accelerator Applications, a Novartis company. KR reports consulting or advisory fees from ABX-CRO, ABX, Advanced Accelerator Applications, a Novartis company, and Bayer. KNC reports consultant or advisory fees from, AstraZeneca, ESSA Pharma, Janssen, Merck, POINT Biopharma, and Roche; and research funding from AstraZeneca, ESSA Pharma, Janssen, Merck, POINT Biopharma, Novartis, Roche, and Arvinas. MJM reports consultant or advisory fees from, Exelixis, Lanctheus, AstraZeneca, Amgen, Daiichi, Convergent Therapeutics, Clarity Pharmaceuticals, Pfizer, Blue Earth Diagnostics and ITM; and research funding from Novartis. OS reports consultant or advisory fees from Advanced Accelerator Applications, a Novartis company, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis Oncology, Constellation, Dendreon, EMD Serono, Fusion, ITM, Merck, Janssen, Myovant, Myriad, Noria Therapeutics, NorthStar, Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix and Theragnostics; reports research funding from Bayer,Endocyte, Merck, InVitae, Constellation, Amgen, Advanced Accelerator Applications, Arvinas, AstraZeneca, Dendreon, Janssen, Lantheus, Tenebio, and Progenics; holds an issued US patent (Saposin C and receptors as targets for treatment of benign and malignant disorders. patent no. 7,166,691); and owns stock in Lilly, GlaxoSmithKline, AbbVie, Cardinal Health, UnitedHealth Group, Clarity Pharmaceuticals, Noria Therapeutics, and Clovis Oncology. STT reports consultant or advisory fees from Sanofi, Medivation, Astellas, Janssen, Genentech, Bayer, Eisai, Abbvie, Tolmar, Seattle Genetics, Amgen, Clovis Oncology, Pfizer, Novartis, Clarity Pharmaceuticals, Genomic Health, POINT Biopharma, Blue Earth, Aikido, Telix, Convergent Therapeutics, EMD Serono, Myovant, Merck; and research funding from Sanofi, Medivation, Astellas, Janssen, Amgen, Genentech, Newlink, BMS, Inovio, AstraZeneca, Aveo, Rexahn, Bayer, Merck, Abbvie, Karyopharm, Endocyte, Clovis Oncology, Seattle Genetics, Novartis, Gilead, and POINT Biopharma; holds a patent pending with Gilead; and owns stock in Alkido.ATK reports consultant or advisory fees from Advanced Accelerator Applications, a Novartis company. NV reports consultant or advisory fees from Genzyme, MSD, Janssen, EISAI, AstraZeneca, Dendreon, Pfizer, Bayer, Seagen, Clovis Oncology, Advanced Accelerator Applications, a Novartis company and Amgen. JC reports consultant or advisory fees from Astellas, Blue Earth Diagnostics, Curium, DS Pharma, GE Healthcare, Isoray, Janssen, Lightpoint Medical, Lantheus, POINT Biopharma, Radiomedix, IBA RadioPharma, Monrol, Novartis, Telix, Sanofi; and research funding from Lantheus, Novartis, and POINT Biopharma. JN reports consultant or advisory fees from Curium, POINT Biopharma, Bayer and Pfizer; and research funding from Advanced Accelerator Applications, a Novartis company, and ABX. XXW reports consultant or advisory fees from Advanced Accelerator Applications, a Novartis company. VSK reports consultant or advisory fees from Clovis Oncology, Pfizer, EMD Serono, Seagen, AstraZeneca, Janssen, Astellas, Seattle Genetics, Dendreon, Guidepoint, GLG and ExpertConnect; and research funding from Janssen, Clovis Oncology, Nektar, Taiho, Merck and Advanced Accelerator Applications, a Novartis company. JDB reports consultant or advisory fees from AstraZeneca, Astellas, Bayer, Cell Centric, Daiichi, Genentech/Roche, Endocyte, Advanced Accelerator Applications, a Novartis Company, Pfizer, GSK, Janssen, Merck Serono, MSD, Sanofi Aventis, Pfizer; and research funding from AstraZeneca, Bayer, CellCentric, Daiichi, GSK, Harpoon, Janssen, Merck Serono, MSD, Pfizer, Sanofi Aventis and Genentech/Roche. RG, MDS, and RAM are employees of Novartis and/or own Novartis stocks/shares. JMB and BC report no conflicts of interest.
Contributor Information
Karim Fizazi, Department of Cancer Medicine, Institut Gustave Roussy, University of Paris Saclay, Villejuif, France.
Ken Herrmann, Department of Nuclear Medicine, University of Duisburg-Essen, and German Cancer Consortium (DKTK)-University Hospital Essen, Essen, Germany.
Bernd J Krause, Department of Nuclear Medicine, Rostock University Medical Center, Rostock, Germany.
Kambiz Rahbar, Department of Nuclear Medicine, University Hospital Münster, Münster, Germany.
Kim N Chi, Medical Oncology Department, British Columbia Cancer Agency, Vancouver Centre, Vancouver, BC, Canada.
Michael J Morris, Genitourinary Oncology Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Oliver Sartor, Tulane Cancer Center, Tulane University School of Medicine, New Orleans, LA, USA.
Scott T Tagawa, Urology, Hematology and Medical Oncology Department, Weill Cornell Medicine, New York, NY, USA.
Ayse T Kendi, Department of Radiology, Division of Nuclear Medicine, Mayo Clinic, Rochester, MN, USA.
Nicholas Vogelzang, US Oncology Research, Comprehensive Cancer Centers, Las Vegas, NV, USA.
Jeremie Calais, Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, University of California Los Angeles, Los Angeles, CA, USA.
James Nagarajah, Department of Medical Imaging, Radboud University Medical Center, Nijmegen, Netherlands.
Xiao X Wei, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Vadim S Koshkin, Department of Medicine, University of California San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA.
Jean-Mathieu Beauregard, Department of Medical Imaging, CHU de Québec - Université Laval, Quebec City, Canada.
Brian Chang, Radiation Oncology Associates, Parkview Hospital, Fort Wayne, IN, USA.
Ray Ghouse, Advanced Accelerator Applications, a Novartis company, Geneva, Switzerland.
Michelle DeSilvio, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Richard A Messmann, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Johann de Bono, Division of Clinical Studies, The Institute of Cancer Research and Royal Marsden Hospital, London, UK.
Data sharing
Advanced Accelerator Applications, a Novartis company, is committed to sharing patient-level data and supporting clinical documents from eligible studies with bona fide researchers who request it. Data availability for this study is according to the criteria and process described on www.clinicalstudyrequest.com.
References
- 1.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med 2018; 378: 645–57. [DOI] [PubMed] [Google Scholar]
- 2.Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol 2019; 75: 88–99. [DOI] [PubMed] [Google Scholar]
- 3.Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the Advanced Prostate Cancer Consensus Conference 2021. Eur Urol 2022; 82: 115–41. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol 2016; 34: 3005–13. [DOI] [PubMed] [Google Scholar]
- 5.McKay R, Haider B, Duh MS, et al. Impact of symptomatic skeletal events on health-care resource utilization and quality of life among patients with castration-resistant prostate cancer and bone metastases. Prostate Cancer Prostatic Dis 2017; 20: 276–82. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 2015; 26: 368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol 2015; 68: 850–8. [DOI] [PubMed] [Google Scholar]
- 8.Minner S, Wittmer C, Graefen M, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate 2011; 71: 281–8. [DOI] [PubMed] [Google Scholar]
- 9.Rowe SP, Gorin MA, Pomper MG. Imaging of prostate-specific membrane antigen with small-molecule PET radiotracers: from the bench to advanced clinical applications. Annu Rev Med 2019; 70: 461–77. [DOI] [PubMed] [Google Scholar]
- 10.Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med 2019; 60: 517–23. [DOI] [PubMed] [Google Scholar]
- 11.Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021; 397: 797–804. [DOI] [PubMed] [Google Scholar]
- 12.Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021; 385: 1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol 2015; 26: 179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussbaum N, George DJ, Abernethy AP, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate Cancer Prostatic Dis 2016; 19: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fizazi K, Kramer G, Eymard JC, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol 2020; 21: 1513–25. [DOI] [PubMed] [Google Scholar]
- 17.Devlin N, Herdman M, Pavesi M, et al. Health-related quality of life effects of enzalutamide in patients with metastatic castration-resistant prostate cancer: an in-depth post hoc analysis of EQ-5D data from the PREVAIL trial. Health Qual Life Outcomes 2017; 15: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joly F, Oudard S, Fizazi K, et al. Quality of life and pain during treatment of metastatic castration-resistant prostate cancer with cabazitaxel in routine clinical practice. Clin Genitourin Cancer 2020; 18: e510–e6. [DOI] [PubMed] [Google Scholar]
- 19.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014; 66: 815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halabi S, Vogelzang NJ, Kornblith AB, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008; 26: 2544–9. [DOI] [PubMed] [Google Scholar]
- 21.Hussain A, Lee RJ, Graff JN, Halabi S. The evolution and understanding of skeletal complication endpoints in clinical trials of tumors with metastasis to the bone. Crit Rev Oncol Hematol 2019; 139: 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cathomas R, Bajory Z, Bouzid M, et al. Management of bone metastases in patients with castration-resistant prostate cancer. Urol Int 2014; 92: 377–86. [DOI] [PubMed] [Google Scholar]
- 23.Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31: 1119–34. [DOI] [PubMed] [Google Scholar]
- 24.Fizazi K, Massard C, Smith M, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol 2015; 68: 42–50. [DOI] [PubMed] [Google Scholar]
- 25.Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate Cancer. J Clin Oncol 2016; 34: 1652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillessen S, Choudhury A, Rodriguez-Vida A, et al. Decreased fracture rate by mandating bone protecting agents in the EORTC 1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. Journal of Clinical Oncology 2021; 39: 5002. [Google Scholar]
- 27.Saad F, Shore N, Van Poppel H, et al. Impact of bone-targeted therapies in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol 2015; 68: 570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011; 377: 813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer 2019; 116: 116–36. [DOI] [PubMed] [Google Scholar]
- 30.Terrisse S, Karamouza E, Parker CC, et al. Overall survival in men with bone metastases from castration-resistant prostate cancer treated with bone-targeting radioisotopes: a meta-analysis of individual patient data from randomized clinical trials. JAMA Oncol 2020; 6: 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Advanced Accelerator Applications, a Novartis company, is committed to sharing patient-level data and supporting clinical documents from eligible studies with bona fide researchers who request it. Data availability for this study is according to the criteria and process described on www.clinicalstudyrequest.com.


