Abstract
Background
CKD of unknown etiology (CKDu) disproportionately affects young people in Central America who lack traditional CKD risk factors (diabetes and hypertension) and has instead been variably linked to heat stress, occupational and environmental exposures, nephrotoxic medications, and/or genetic susceptibility. This study aimed to estimate the prevalence of CKD and identify risk factors for traditional CKD and CKDu in Nicaragua.
Methods
Surveys and assessment for CKD markers in urine and serum were performed in 15–59 year olds in households of the León municipality of Nicaragua. The survey included questions on demographics, health behaviors, occupation, and medical history. Participants with CKD were subdivided into traditional CKD and suspected CKDu based on history of diabetes, hypertension, or other specified conditions. A multinomial logistic regression model was used to identify factors associated with traditional CKD and suspected CKDu, compared to the non-CKD reference group.
Results
In 1795 study participants, CKD prevalence was 8.6%. Prevalence in males was twofold higher than females (12% vs 6%). Of those with CKD, 35% had suspected CKDu. Both traditional CKD and CKDu were associated with male sex and increasing age. Traditional CKD was associated with a family history of CKD, history of urinary tract infections, and lower socioeconomic status, while CKDu was associated with drinking well water and a lower body mass index.
Conclusions
Both traditional CKD and CKDu are significant burdens in this region. Our study supports previous hypotheses of CKDu etiology and emphasizes the importance of CKD screening.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03381-1.
Keywords: CKDu, Mesoamerican nephropathy, Risk factors, Nicaragua
Background
Chronic kidney disease (CKD) is expectedto be the fifth leading cause of mortality worldwide by 2040 [1]. CKD prevention requires increased awareness and identification of the underlying causes, which may vary globally. In some regions, in particular Central America and Southeast Asia, unexpectedly high rates of CKD affect young populations without traditional risk factors, a condition commonly known as CKD of unknown etiology (CKDu) [2]. Epidemiological studies have described CKDu as a form of chronic interstitial nephritis without hematuria or proteinuria, most commonly affecting males from lowlands working in agriculture [3, 4].
The etiology of CKDu is unknown but is likely to be multifactorial. Leading theories include heat stress, recurrent volume depletion, and hyperuricemia worsened by consumption of fructose [5–7]. Uric acid is released from muscle during strenuous labor and can crystallize in the urine causing tubular damage [8]. Other hypothesized contributors include agrochemical or heavy metal exposures, which could occur during work or from the environment, such as well water contamination [4, 9–12]. Nephrotoxic medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and diuretics are commonly used for many symptoms, including dysuria and lower abdominal pain colloquially thought to be due to urinary tract infection [13, 14]. Tropical infectious diseases such as leptospirosis, hantavirus, or malaria have also been proposed as potential contributors to CKDu [15–17]. Finally, a genetic predisposition to CKDu has been hypothesized due to familial clustering [18].
The prevalence of CKDu is challenging to estimate due to a lack of large community-based studies in Central America. In Nicaragua, the mortality from CKD is high and increasing, at least partly attributed to CKDu, highlighting the need for further studies to define its prevalence and risk factors [19]. This study aimed to estimate the prevalence of CKD and to identify possible risk factors for traditional and non-traditional CKD in an area of Nicaragua where the presence of CKDu has been established [4].
Methods
Setting and participants
Our study took place in the municipality of León, with a population of 204,000 at the time of this study [20]. Households were randomly selected from the León Health and Demographic Surveillance System (LHDSS), which is designed to be representative of the entire municipality, and household members aged 15 to 59 years old were recruited between May and August 2014 [21]. Household visits occurred on weekends to limit absences due to work. Data were collected by staff of the Research Center of Health, Work and Environment (CISTA), the Center for Demography and Health Research (CIDs) and a previously trained group of medical students from Universidad Nacional Autonoma de Nicaragua, León (UNAN-León). The current analysis excluded participants who did not have a serum creatinine sample collected.
Procedures
Data were collected through a standardized questionnaire, physical measurements, and collection of blood and urine samples. Weight was measured with a digital scale, calibrated daily, and height with a measuring rod. These values were used to calculate body mass index (BMI).
Serum creatinine, blood urea nitrogen (BUN), and uric acid were measured in the national laboratory of the Nicaraguan Ministry of Health (MINSA) using the Roche Cobas Integra 400 Plus. The instrument was calibrated for creatinine analysis using isotope dilution mass spectrometry reference standards. Urine samples were analyzed through dipstick testing (Combur test). Participants with abnormal results were routed to local medical services provided by MINSA for further care.
Variable definitions
Socioeconomic level was based on the World Bank’s Development Research group, using the daily per capita income, calculated in United States dollars ($) in 2005 [22]. Categories include extremely poor (< $1.25), poor ($1.25—$2.00), and not poor (≥ $2.01) [22].
Current and previous occupations were classified using the United Nations' International Standard Industrial Classification of All Economic Activities [23]. The cumulative years of working in any agricultural occupation were calculated. For the subgroup of females who worked in agriculture, work tasks were reviewed in detail to evaluate for the performance of hard manual labor.
CKD awareness was considered positive if the participant indicated a personal history of CKD. NSAID or diuretic use was defined as self-reported use for more than one consecutive week.
Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epidemiology Collaboration 2021 Equation [24]. Stages of CKD were defined based on Kidney Disease Improving Global Outcomes (KDIGO) guidelines [25].
Case definitions
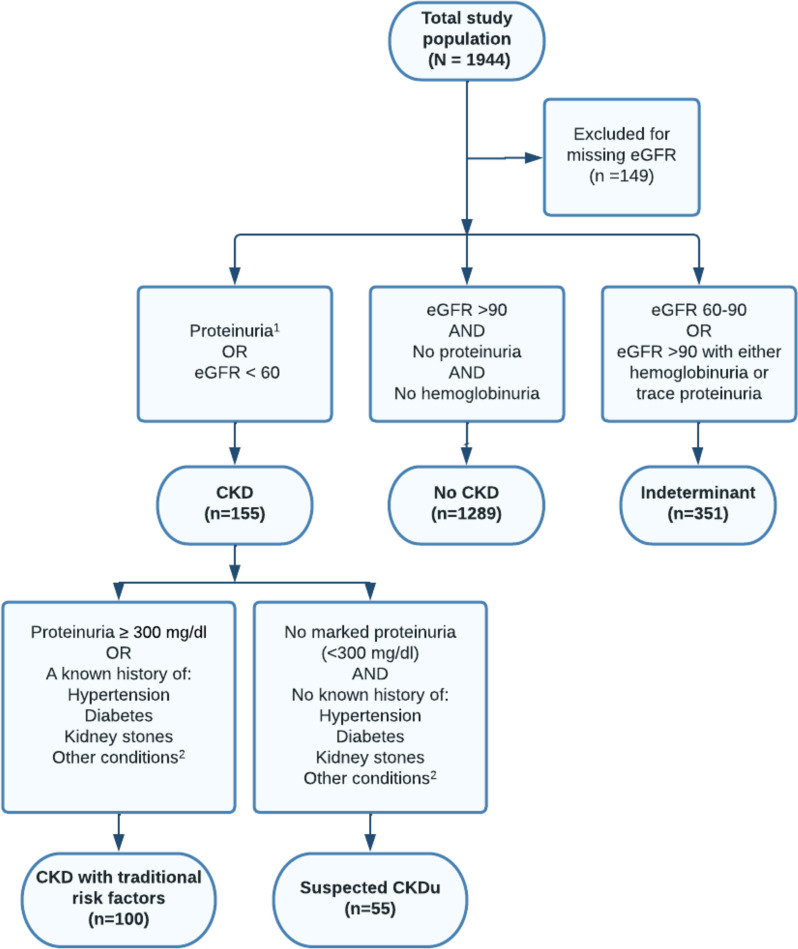
The Pan American Health Organization’s (PAHO) clinical case definition for CKDu includes individuals aged 2–59, diagnosed with CKD by KDIGO guidelines and without exclusion criteria including diabetes or hypertension, urologic pathology, primary glomerulopathy, hematologic or autoimmune disease, repeated exposure to contrast media or phosphate-containing products, or hematuria or significant proteinuria indicating a different underlying cause [26]. Participants were classified as suspected CKDu if they matched the PAHO criteria as closely as possible based on the available data, with self-reported diabetes or hypertension considered sufficient to exclude participants from the CKDu classification. The study case definitions included: (a) no CKD, (b) CKD with traditional risk factors, (c) suspected CKDu, and (d) indeterminant CKD. The study case definitions are further illustrated in Fig. 1.
No CKD: Study participants with an eGFR ≥ 90 ml/min/1.73m2 and dipstick negative proteinuria and hemoglobinuria.
CKD with traditional risk factors (traditional CKD): Study participants with 1) proteinuria ≥ 300 mg/dl on urine dipstick or 2) participants with an eGFR ≤ 60 ml/min/1.73m2 who have one or more of the traditional risk factors for CKD including hypertension (or who were taking blood pressure medications), diabetes, hyperglycemia, or kidney stones. Participants with eGFR ≤ 60 ml/min/1.73m2 with hemoglobinuria or glucosuria were also included in this group.
Suspected CKDu: Study participants with eGFR ≤ 60 ml/min/1.73m2without the following features: (1) proteinuria ≥ 300 mg/dl or hemoglobinuria on urine dipstick, (2) reported traditional risk factors for CKD [26, 27].
Indeterminant CKD: For the purpose of comparison between groups that clearly have impaired kidney function and those that do not, comparative analyses excluded participants with (1) eGFR between 60 and 90 ml/min/1.73m2 without proteinuria (2) eGFR ≥ 90 ml/min/1.73m2 with hemoglobinuria or trace proteinuria.
Fig. 1.
Study participant classification and sample sizes. Footnotes: eGFR units are ml/min/1.73m2. 1. Proteinuria is defined by urine dipstick. 2. Other conditions include taking blood pressure medication, glucosuria, hyperglycemia, known history of urologic, hematologic disease, genetic or hereditary kidney disease, or autoimmune disease
Analysis
Continuous variables were described in medians and quartiles while categorical variables were described in proportions. Proportions were calculated excluding participants with missing data for that particular covariate. Participants categorized as indeterminant for CKD were included in the denominator for CKD prevalence estimates but were excluded from analyses comparing traditional CKD and suspected CKDu. Covariates were compared between the following groups: no CKD, traditional CKD, and suspected CKDu. Nonparametric tests were used due to small sample size in the CKD groups. Continuous variables were compared with Kruskal Wallis test and categorical variables were compared with Fisher’s Exact test. Significance values were set at α = 0.05.
Multinomial logistic regression was used to evaluate the odds ratio (OR) and 95% confidence intervals (CI) for associations with CKD. Sex, age, and socioeconomic status have been shown to be associated with CKD and were selected as covariates for the model. Variables found to be significant in the univariate analysis that had at least a 15% prevalence were added individually to the model with the aforementioned prioritized variables and evaluated. Variables with a p-value < 0.05 were included in a full model which was reduced by backward elimination. If covariates were strongly correlated, then the covariate with the smaller p-value was chosen. The ORs for age and BMI were calculated in the model as a per-year or per-unit increase respectively, but then exponentiated to report the OR for a 10-year increase in age and a 5-unit increase in BMI. A variance inflation factor was calculated in the full model to ensure there was no influence of multicollinearity. All analyses were conducted using R language for statistical computing (version 4.2.1) [28].
In separate sensitivity analyses, we evaluated the impact of (1) including the indeterminant CKD group as an additional group in comparative analyses or (2) including the indeterminant CKD group as part of the no CKD group.
Human subjects research protections
This study was approved by the ethics committee of the medical sciences at UNAN-León, and analysis of deidentified data was determined exempt from full review by the Duke University Institutional Review Board. Written informed consent was obtained from each adult participant, and both parental consent and participant assent were obtained for those < 18 years old.
Results
After excluding 149 participants with missing eGFR, the study population included 1795 participants (Fig. 1). Females made up 60% of the population, and the median age was 36 (Table 1).
Table 1.
Total study population demographics
| Demographics | Total study population (N = 1795) |
|---|---|
| Sex | |
| Male | 714 (40%) |
| Female | 1081 (60%) |
| Median age, years (IQR) | 36 (25–47) |
| Age categories | |
| < 20 years old | 210 (12%) |
| 20–39 years old | 872 (49%) |
| 40–59 years old | 713 (40%) |
| Socioeconomic levela | |
| Extremely poor | 595 (33%) |
| Poor | 462 (26%) |
| Not poor | 721 (41%) |
| Zone | |
| Rural | 619 (34%) |
| Urban | 1176 (66%) |
| Illiterate | 136 (7%) |
| Last grade level completed, median (IQR)b | 9 (6–12) |
Abbreviations: IQR Interquartile range
aSocioeconomic level based on the World Bank’s Development Research group. Missing socioeconomic status on 17 participants
bMissing grade level for 124 participants
CKD prevalence
The overall prevalence of CKD was 8.6% (Table 2). Males had a higher prevalence of CKD than females (12.5% vs. 6.1%). CKD was based on proteinuria alone in 30% of cases. Stage 3 CKD was most common (46% of CKD), while Stages 4 and 5 CKD were less common (16% and 8% respectively) with a much higher proportion of males in these higher stages. Of the 155 participants with CKD, 100 (65%, or 5.6% overall prevalence) had high-grade proteinuria or traditional CKD risk factors while 55 (35%, or 3.1% overall prevalence) were classified as suspected CKDu.
Table 2.
Prevalence of CKD and CKD awareness
| Overall, n (%) N = 1795 |
Males, n (%) n = 714 |
Females, n (%) n = 1081 |
|
|---|---|---|---|
| Population with CKD | 155 (8.6%) | 89 (12.5%) | 66 (6.1%) |
| Stage of CKDa | |||
| Proteinuria with eGFR > 60b | 47 (30.3%) | 18 (20.2%) | 29 (43.9%) |
| Stage 3 | 71 (45.8%) | 39 (43.8%) | 32 (48.5%) |
| Stage 4 | 25 (16.1%) | 21 (23.6%) | 4 (6.1%) |
| Stage 5 | 12 (7.7%) | 11 (12.4%) | 1 (1.5%) |
| Self-reported history of CKD (CKD Awareness) | 34 (21.9%) | 22 (24.7%) | 12 (18.2%) |
| CKD awareness in each stage of CKD | |||
| Proteinuria with eGFR > 60b | 3 (6.4%) | 1 (5.6%) | 2 (6.9%) |
| Stage 3 | 16 (22.5%) | 7 (17.9%) | 9 (28.1%) |
| Stage 4 | 7 (28%) | 6 (28.6%) | 1 (25.0%) |
| Stage 5 | 8 (66.7%) | 8 (72.7%) | 0 |
| CKD category | |||
| CKD with traditional risk factors | 100 (64.5%) | 52 (58.4%) | 48 (72.7%) |
| Suspected CKDu | 55 (35.5%) | 37 (41.6%) | 18 (27.3%) |
Abbreviations: CKD Chronic kidney disease, eGFR Estimated glomerular filtration rate, CKDu Chronic kidney disease of unknown etiology
aCKD stages by Kidney Disease Improving Global Outcomes (KDIGO)
beGFR in ml/min/1.73 m2
Only 22% of participants with CKD reported a personal history of CKD (Table 2). Although awareness increased at higher stages of CKD, 4 participants with Stage 5 CKD and 18 with Stage 4 CKD did not report a history of CKD. Awareness was low among participants with both traditional CKD (25%) and suspected CKDu (16%).
Comparison of groups with no CKD, CKD from traditional causes, and suspected CKDu
The groups differed significantly in most demographic variables. We observed a higher proportion of males in both the traditional CKD group (52%) and the suspected CKDu group (67%) compared to the non-CKD group (40%) (Table 3). The CKD groups were older (median age 48 years for traditional CKD and 44 years in CKDu) than the non-CKD group (33 years) and higher proportions classified as extremely poor, or poor compared to the non-CKD group. Illiteracy rate was 20% in the CKDu group versus 13% in the traditional CKD group and 6% in the non-CKD group. A higher proportion of the suspected CKDu group lived in rural zones, and 38% obtained water from a well compared to 29% in the traditional CKD group and 19% in the non-CKD group. Reported daily water intake was highest in the suspected CKDu group, but soda intake, current alcohol use, and history of drug use did not differ between groups. NSAID and diuretic use was highest in the traditional CKD group. Exercise and eating fresh foods were rare overall.
Table 3.
Comparison of demographics, health behaviors, medical history, and occupation by CKD category (no CKD, CKD with traditional risk factors, and suspected CKDu)
| Characteristics | No CKD (n = 1289) | CKD with traditional risk factors (n = 100) | Suspected CKDu group (n = 55) |
P value |
|---|---|---|---|---|
| Demographics | ||||
| Sex | < 0.001a * | |||
| Male | 513 (39.8%) | 48 (48.0%) | 37 (67.3%) | |
| Female | 776 (60.2%) | 52 (52.0%) | 18 (32.7%) | |
| Age, median (IQR) | 33 (23–43) | 48 (37–54) | 44 (34–55) | < 0.001b * |
| Socioeconomic level | 0.029a * | |||
| Extremely poor | 421 (33.0%) | 43 (43.0%) | 24 (43.6%) | |
| Poor | 328 (25.7%) | 29 (29.0%) | 13 (23.6%) | |
| Not poor | 527 (41.3%) | 28 (28.0%) | 16 (29.1%) | |
| Illiterate | 75 (5.8%) | 13 (13.0%) | 11 (20.0%) | < 0.001a * |
| Highest grade level completed, median (IQR)c | 9 (6–12) | 6 (3–11) | 6 (4–9) | < 0.001a * |
| Rural Zone | 447 (34.7%) | 37 (37.0%) | 24 (43.6%) | 0.352a |
| Well water sourced | 242 (18.8%) | 29 (29.0%) | 21 (38.2%) | < 0.001a * |
| Health behaviors | ||||
| Daily water intake (L), median (IQR) | 2 (1–3) | 2 (1–4) | 3 (2–4) | 0.003b * |
| Daily soda intake (L), median (IQR) | 0.25 (0–0.5) | 0 (0 – 0.5) | 0 (0–0.5) | 0.129b |
| Alcohol consumption in last day | 80 (6.2%) | 2 (2.0%) | 7 (12.7%) | 0.078b |
| History of smoking | 285 (22.1%) | 27 (27.0%) | 19 (34.5%) | 0.061a |
| Median years smoking | 8 (3–19) | 22 (13–35) | 24 (16–35) | < 0.001b * |
| History of drug consumption | 121 (9.4%) | 12 (12.0%) | 6 (10.9%) | 0.562a |
| Exercise ≥ 2 times weekly | 256 (19.9%) | 14 (14.0%) | 7 (12.7%) | 0.192a |
| Eat fruits, vegetables, or salads ≥ 2 times weekly | 769 (59.9%) | 60 (60.0%) | 23 (41.8%) | 0.030a * |
| Medical history | ||||
| NSAID use | 346 (26.8%) | 37 (37.0%) | 16 (29.1%) | 0.083a |
| Diuretic use | 49 (3.8%) | 13 (13.0%) | 2 (3.6%) | < 0.001a * |
| Hypertension | 142 (11.0%) | 53 (53.0%) | 0 | < 0.001a * |
| Diabetes | 52 (4.0%) | 22 (22.0%) | 0 | < 0.001a * |
| Kidney stones | 67 (5.2%) | 18 (18.0%) | 0 | < 0.001a * |
| Arthritis | 106 (8.2%) | 27 (27.0%) | 8 (14.5%) | < 0.001a * |
| Malaria | 128 (9.9%) | 15 (15.0%) | 7 (12.7%) | 0.196a |
| Urinary tract infection | 631 (49.0%) | 65 (65.0%) | 28 (50.1%) | 0.008a * |
| Family history of CKD | 312 (24.2%) | 42 (42.0%) | 16 (29.1%) | 0.003a * |
| Occupation | ||||
| Current agricultural occupatione | 86 (6.7%) | 11 (11.0%) | 16 (29.1%) | < 0.001a * |
| History of working in agriculture | 228 (17.7%) | 27 (27.0%) | 25 (45.5%) | < 0.001a * |
| Males | 152 (66.7%) | 24 (88.9%) | 22 (88.0%) | < 0.001a * |
| Females | 76 (33.3%) | 3 (11.1%) | 3 (12.5%) | 0.411a |
| Years worked, median(IQR) | 10 (5–20) | 13 (9–30) | 20 (8–32) | 0.042b * |
| History of fainting at work | 136 (10.6%) | 17 (17.0%) | 6 (10.9%) | 0.139a |
| History of heat exhaustion | 445 (34.5%) | 42 (42.0%) | 15 (27.2%) | 0.163a |
Abbreviations: CKD Chronic kidney disease, CKDu Chronic kidney disease of unknown etiology, IQR Interquartile range, L Liters, NSAID Non-steroidal anti-inflammatory drug
All percentages calculated from non-missing data. Less than 5% of each group is missing data unless otherwise indicated
ap-value calculated with Fisher’s Exact Test
bp-value calculated with Kruskal Wallis test
c92 participants missing grade level
dThe remainder of participants obtained water from a public water system with interior plumbing with the exception of 4 participants in the No CKD group who had a different water source that did not fit either of these categories (i.e. river, purchasing drinking water, ect)
eOf those with a current occupation in agriculture, all were male except 4 females in the no CKD group
* significant finding at p-value < 0.05
Drinking well water was reported in 296 participants in the included groups. Almost half (47%) of participants using well water reported no treatment, and 48% reported using only chlorination treatment for their well water. Only 14 (5%) reported boiling, using a household filter, or other treatment to their drinking water. Type of water treatment did not differ significantly between the group with traditional CKD, CKDu, and no CKD (p = 0.85). Most of the wells (175, 75% of wells with data available) were hand-dug, while 25% were machine-drilled, and this did not differ by CKD category. There were 113 (39%) wells reported to be close to agricultural fields, which did not differ between groups (p = 0.92). The most common reported crops were peanuts (44), corn (21), sugar cane (19), sesame (15), and yuca (16). This did not differ between CKD categories except sugar cane, which was proportionally more common in the traditional CKD group than the others (p = 0.045).
The distribution of diabetes, hypertension, and kidney stones history largely reflects the study design (Table 3). Self-reported history of urinary tract infections (UTIs) was more common in the traditional CKD (65%) group than the suspected CKDu (50%) and the non-CKD groups (49%). Participants with a history of UTIs had a median of one infection in the past year, and a notable 41% of males reported a history of UTIs. Family history of CKD was highest in the traditional CKD group and almost equivalent in the suspected CKDu and non-CKD group.
The most common current occupation was homemaker for females (42% of females) and agricultural worker or student for males (18% and 17% of males, respectively). The suspected CKDu group had the highest proportions currently working in agriculture and with a lifetime history of working in agriculture compared to the other groups (Table 3). The suspected CKDu group also had the longest median time of working in agriculture (20 years). History of fainting or experiencing heat exhaustion at work did not differ between groups. Among 112 females with a history of working in agriculture, there were 3 with traditional CKD and 3 with suspected CKDu. Many females (66) performed tasks that included cutting crops or wood, with the largest number cutting cotton. Other tasks females performed commonly were seeding and weeding. Self-reported history of heat exhaustion was similar between male and female agricultural workers.
Median BMI was highest in the traditional CKD group (28) and lowest in the suspected CKDu group (24) (Table 4). Overall, there was a high prevalence of overweight, obese, or extremely obese individuals. The CKDu group had the highest uric acid levels in both males and females. When results were stratified by CKD stage, uric acid was still higher in the CKDu group, although the difference was no longer significant (Supplemental Figs. 1 and 2).
Table 4.
Health assessment and measurements by CKD category
| Health assessment | No CKD (n = 1289) | CKD with traditional risk factors (n = 100) | Suspected CKDu group (n = 55) | P value |
|---|---|---|---|---|
| BMI categories | 0.046a * | |||
| Underweight (< 18.5) | 46 (3.6%) | 3 (3.0%) | 1 (1.8%) | |
| Normal (18.5 – 24.5) | 427 (33.4%) | 28 (28.0%) | 28 (50.1%) | |
| Overweight (25–30) | 442 (34.6%) | 38 (38.0%) | 14 (25.4%) | |
| Obese (30–34.5) or extremely obese (> 35) | 363 (28.4%) | 33 (33.0%) | 10 (18.1%) | |
| BMI, median (IQR) | 27 (23–31) | 28 (24–31) | 24 (22–28) | 0.004b * |
| Serum uric acid (umol/L), median (IQR) | ||||
| Males (reference range 220–476)c | 321 (274 – 371) | 435 (360—508) | 523 (430 – 589) | < 0.001b * |
| Females (reference range 161–363)c | 243 (206 – 249) | 335 (245 – 400) | 359 (288 – 500) | < 0.001b * |
| Serum BUN (mmol/L), median (IQR) | 3.0 (2.4 – 3.7) | 5.5 (3.9 – 9.0) | 7.1 (5.5 – 9.4) | < 0.001b * |
| Serum Creatinine (umol/L), median (IQR) | 59 (51—69) | 127 (69 – 184) | 172 (127 -236) | < 0.001b * |
| eGFR (ml/min/1.73 m2), median (IQR) | 120 (110–129) | 51 (36–105) | 40 (25–54) | < 0.001b * |
| CKD Stages | < 0.001a * | |||
| Proteinuria with eGFR > 60 ml/min/1.73 m2 | 0 | 39 (39.0%) | 8 (14.5%) | |
| Stage 3 | 0 | 42 (42.0%) | 29 (52.7%) | |
| Stage 4 | 0 | 12 (12.0%) | 13 (23.6%) | |
| Stage 5 | 0 | 7 (7.0%) | 5 (9.1%) | |
| Urine dipstick results | ||||
| Positive leukocyte esterased | 144 (14.1%) | 20 (20.8%) | 12 (24.5%) | 0.035a * |
| Positive nitritese | 44 (4.3%) | 7 (7.3%) | 4 (8.1%) | 0.144a |
| Proteinuriaf | < 0.001a * | |||
| None | 993 (100%) | 39 (41.1%) | 31 (63.2%) | |
| Trace | 0 | 8 (8.4%) | 9 (18.4%) | |
| 30 mg/dl | 0 | 5 (5.3%) | 7 (14.3%) | |
| 100 mg/dl | 0 | 2 (2.1%) | 2 (4.1%) | |
| 300 mg/dl | 0 | 36 (37.9%) | 0 | |
| 1000 mg/dl | 0 | 5 (5.3%) | 0 | |
| Current symptoms | ||||
| High thirst | 676 (52.4%) | 57 (57.0%) | 28 (50.1%) | 0.653a |
| Dysuria | 351 (27.2%) | 28 (28.0%) | 9 (16.4%) | 0.203a |
| Dark urine | 356 (27.6%) | 36 (36.0%) | 8 (14.5%) | 0.015a * |
| Cramps | 241 (18.7%) | 37 (37.0%) | 16 (29.1%) | < 0.001a * |
All percentages calculated from non-missing data. Less than 5% of each group is missing data unless otherwise indicated
Abbreviations: CKD Chronic kidney disease, CKDu Chronic kidney disease of unknown etiology, BMI Body mass index, IQR Interquartile range, BUN Blood urea nitrogen
ap-value calculated with Fisher’s Exact Test
bp-value calculated with Kruskal Wallis test
cReference range from Mayo Clinic Reference Laboratories and units converted [29].
d298 participants missing leukocyte esterase data
e281 participants missing nitrites data
f307 participants missing proteinuria data
*significant finding at p-value < 0.05
A higher proportion of participants in the suspected CKDu group had CKD Stages 4 and 5, with a median eGFR of 40 ml/min/1.73 m2 compared to 51 ml/min/1.73 m2 in the traditional CKD group (Table 4). Among 1137 with available data from dipstick urinalysis, almost half of participants in the traditional CKD group have proteinuria (48%) compared to only 16% in the CKDu group. Urinary leukocyte esterase was common (> 20%) in both CKD groups.
The final multinomial logistic regression model included the prioritized covariates (sex, age, socioeconomic status), plus BMI (as a continuous variable), water source (well or public water system), family history of CKD (yes or no), and history of UTI (yes or no) (Fig. 2). Variance inflation factors were all < 1.2, indicating no collinearity of covariates in the final model. Male sex was associated with increased odds of both traditional CKD (OR 2.38, CI 1.51–3.74) and suspected CKDu (OR 3.36, CI 1.80–6.26). A 10-year increase in age was associated with an increased odds of both traditional CKD (OR 2.25, CI 1.85–2.75) and suspected CKDu (OR 2.18, CI 1.71–2.77). Lower socioeconomic level was associated with higher odds of traditional CKD (OR 1.35, CI 1.04–1.76), but was not significantly associated with suspected CKDu (OR 1.29, CI 0.91–1.82). A family history of CKD was strongly associated with higher odds of traditional CKD (OR 2.04, CI 1.31–3.19) but was not significantly associated with suspected CKDu (OR 1.33, CI 0.71–2.51). Similarly, a history of UTIs were associated with traditional CKD (OR 1.83, CI 1.16–2.89) but not with suspected CKDu (OR 1.18, CI 0.65–2.13). Having a well as opposed to a public water system increased the odds of suspected CKDu (OR 2.26, CI 1.21–4.20) but was not significantly associated with traditional CKD (OR 1.62, CI 0.98–2.68). A five unit increase in BMI was associated with 0.4 lower odds of developing CKDu (OR 0.60; CI 0.43–0.82), while BMI was not significantly associated with traditional CKD (OR 0.96; CI 0.80–1.15).
Fig. 2.
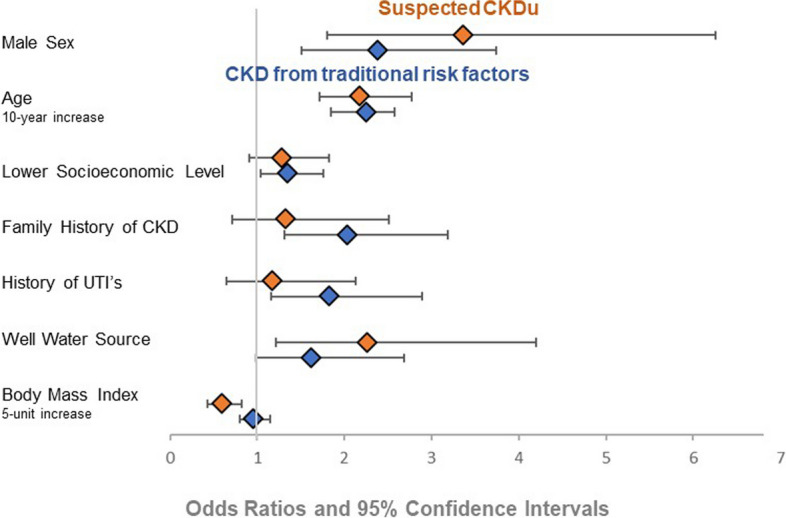
Odds ratios and 95% confidence intervals for the multinomial logistic regression model for CKD with traditional risk factors and suspected CKDu. The reference group is participants with no CKD. Lower socioeconomic status represents a change in one classification per previously defined criteria. Well water source is compared to a public water system. Abbreviations: CKD: chronic kidney disease. CKDu: Chronic kidney disease of unknown etiology. UTI: urinary tract infection. BMI: body mass index
The indeterminant CKD group (n = 351) was excluded from the above analysis, but we performed an analysis for both 1) the indeterminant group included as an outcome and 2) the indeterminant group included in the no CKD group (Tables S1, S2, S3 and S4). We developed a multinomial logistic regression model for both analyses, and found the odds ratios were similar for most variables, but the covariate of agricultural work history remained significant and was included in the final model when the indeterminant group was included as an outcome (Tables S5 and S6). History of agricultural work was protective for traditional CKD (OR 0.52 CI 0.29–0.92) when indeterminant CKD group is included as a separate group (Table S5).
Discussion
This study identified a CKD prevalence of 8.6% in the León municipality of Nicaragua, with a twofold higher prevalence among males versus females. This trend is consistent with most prior studies of CKDu in Central America, however, it is notable that many of those studies were focused on agricultural workers [4, 30]. One-third of participants with CKD lacked traditional risk factors, leading to an overall prevalence of suspected CKDu of 3.1% in this young population. The odds of suspected CKDu were 3 times higher for males and 2 times higher for participants with a well drinking water source. Older age and lower BMI were also associated with CKDu.
Our observed prevalence of CKD is similar to a previous report by Lebov et al., who demonstrated a CKD prevalence of 7.5% with marked male predominance four years earlier in the same municipality [31]. Unlike the previous study, our analysis was restricted to participants age 15–59 years old and included albuminuria in the diagnosis of CKD. The observed prevalence of CKD is much higher than expected given the younger age distribution. For example, the observed prevalence of CKD in participants age 20–39 is 10 times higher, and the prevalence in those age 40–59 years is 3 times higher, compared to these populations in the United States National Health and Nutritional Examination Survey (2011–2014) [32].
A low proportion of participants with CKD reported a personal history of CKD. Although it is possible that some cases that we classified as Stage 1–3 CKD would not be confirmed on repeat testing, awareness of CKD was also low among participants with more advanced CKD. This may be due to poor healthcare provider communication or due to inadequate screening. Additional efforts should be taken to support healthcare providers in counseling patients on the diagnosis of CKD, preventive measures against disease progression, and estimated individual risk of kidney failure. Population-level and workplace health education could be considered, particularly in high-risk areas such as Nicaragua, because awareness is an essential component of advocacy for workers’ rights and implementation of better worker conditions [33]. CKD screening should not be limited to only those with traditional risk factors, as 35% of the participants with CKD would not have been screened using this limited approach. Screenings of the entire population in certain areas based on high prevalence in prior studies or suspected risk factors should be considered.
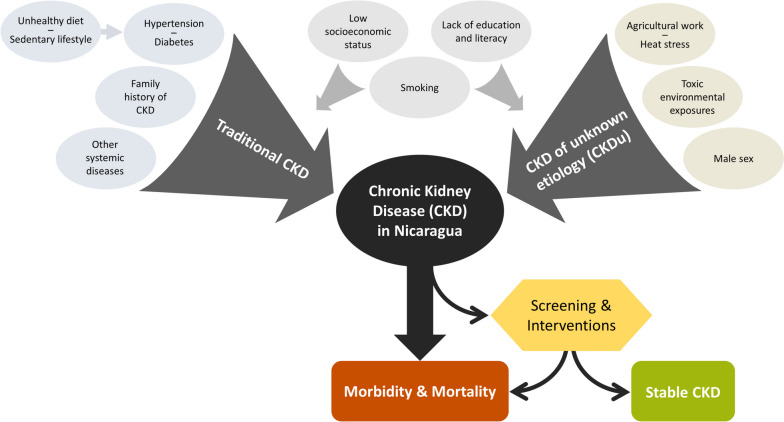
Among participants with CKD in our study, 65% had traditional risk factors and 35% had suspected CKDu. This suggests that this region has two different pathways leading to a public health crisis: both the rise in traditional risk factors, such as diabetes and hypertension, and CKDu (Fig. 3). For each pathway, it is key to determine risk factors, implement preventative programs, and provide early intervention to prevent the devastating mortality and morbidity caused by CKD in Nicaragua.
Fig. 3.
Conceptual map of the contributors to CKD in Nicaragua. Abbreviations: CKD: Chronic kidney disease. CKDu: Chronic kidney disease of unknown etiology
We considered our study findings in light of proposed hypotheses for the etiologies of CKDu. Obtaining drinking water from a well was associated with a remarkable 2.3 times higher odds of suspected CKDu. Many wells (39%) were located near agricultural fields, and participants using wells most commonly did not perform any water treatment or only used chlorine, which would not protect against chemical environmental toxins. In addition, CKDu was associated with a history of agricultural occupation. These findings suggest toxicity from agrochemicals or heavy metals could play a role in CKDu development. Large population-based studies have identified an association of CKDu with agrochemical exposures, and a specific tubulotoxic mechanism has been proposed based on CKDu biopsy pathology [34–36]. Future research should focus on investigation of environmental contaminants.
CKDu was associated with agricultural occupation in univariate analysis, but after adjusting for sex, age, and socioeconomic status, the association of CKDu with agricultural work was no longer significant. Examination of agricultural occupation tasks showed over half of women had a history of heavy manual labor, such as cutting cotton, and a similar proportion of females and males reported a history of heat exhaustion, suggesting that physical demand and heat stress may not completely account for the difference between sexes. We have no information on occupational exposure to agrochemicals, which could differ between sexes due to different tasks, crops, or individual practices (such as wearing personal protective equipment). Hyperuricemia was associated with CKDu, but longitudinal studies are needed to determine if this has a causative role in CKDu.
History of UTI remained a significant risk factor for traditional CKD in the multivariate analysis even when adjusting for multiple other factors. This may be due to the higher prevalence of diabetes in the traditional group, a major UTI risk factor [37]. Dysuria, which is frequently misdiagnosed as a UTI, has previously been associated with CKDu [13, 14]. However, in our study, the CKDu group had the lowest prevalence of current dysuria and had almost identical prevalence of UTI history as the no CKD group. In this population overall, both UTI history and dysuria were surprisingly common and warrant further investigation.
NSAID use did not differ significantly between groups, and diuretic use was highest in the traditional CKD group, which could be due to comorbid hypertension in this population. Overall, this fails to support the hypothesis that NSAID and diuretic use are important contributors to CKDu. Finally, family history was associated with 2 times the odds of traditional CKD but was not associated with CKDu, which does not necessarily support the hypothesis of a genetic contribution to CKDu risk. However, this is very difficult to examine directly since families also share many of the same exposures (drinking water source, socioeconomic status, etc.). Results were qualitatively similar in sensitivity analyses including participants with indeterminant CKD, who were excluded from the primary analyses.
Our study is one of few population-based analysis of CKD in areas endemic for CKDu that performed dual assessment of urine and serum. However, we acknowledge several limitations, including the single timepoint collection of kidney disease markers, when a clinical diagnosis of CKD requires abnormalities for at least 3 months. A large portion of the study population did not undergo urine testing and could have been misclassified. In addition, there is no eGFR equation that has been validated in the Nicaraguan population. We also relied on self-reported data, including history of traditional risk factors. There were no direct inquiries related to exposure to agrochemicals. It was assumed that all those with traditional risk factors who had CKD developed CKD due to these risk factors, when they could have CKDu or CKD from a different cause. The cross-sectional design prevents conclusions about the temporality between exposures and outcomes. Finally, the sample sizes for the individual CKD groups were small, although we were still able to detect important differences between groups.
Conclusions
CKD has an alarmingly high prevalence of 8.6% in the municipality of León, Nicaragua in those less than 60 years old. Among participants with CKD, 35% lack traditional risk factors for disease and may have CKDu. Factors associated with suspected CKDu included male sex, well water source, and lower BMI. CKD from traditional causes was more strongly associated with family history of CKD, history of UTIs, and lower socioeconomic status. This study demonstrates a high burden of CKD from both traditional risk factors and CKDu in this region and emphasizes the importance of CKD screening. Future research should focus on identifying the cause of CKDu in order to implement strategies to prevent disease.
Supplementary Information
Additional file 1: Supplemental Figure 1. Uric Acid by CKD Stage – Males Only. Supplemental Figure 2. Uric Acid by CKD Stage – Females Only. Table S1. Description and comparison of Indeterminant CKD group in demographics, health behaviors, medical history, and occupation. Table S2. Description and Comparison of Indeterminant CKD group in health assessment. Table S3. Sensitivity analysis: Indeterminant CKD group included as no CKD in demographics, health behaviors, medical history, and occupation. Table S4. Sensitivity analysis: Indeterminant CKD group included as no CKD in health assessment. Table S5. Odds ratios and 95% confidence intervals for the multinomial logistic regression model for CKD with traditional risk factors, suspected CKDu and Indeterminant CKD. Table S6. Odds ratios and 95% confidence intervals for the multinomial logistic regression model for CKD with traditional risk factors and suspected CKDu with the indeterminant CKD group counted as no CKD.
Acknowledgements
The authors would like to thank the participants and community leaders for their support during the data collection. We would also like to thank the medical students, drivers, phlebotomists and staff of the Research Centre on Health, Work and Environment (CISTA), and the Center for Demography and Health Research (CIDs), Nicaragua. We thank La Isla Network for administrative assistance. We thank Dr. Eric Monson for assistance with the figures.
Abbreviations
- CKD
Chronic kidney disease
- CKDu
Chronic kidney disease of unknown etiology
- NSAID
Non-steroidal anti-inflammatory drug
- LHDSS
León Health and Demographic Surveillance System
- CISTA
Research Center of Health, Work, and Environment (In Spanish, Centro de Investigación en Salud, Trabajo, y Ambiente)
- CIDS
Center for Demography and Health Research (In Spanish, Centro de Investigación en Demografía y Salud)
- UNAN
Universidad Nacional Autónoma de Nicaragua
- BMI
Body mass index
- RPM
Rotations per minute
- MINSA
Nicaraguan Ministry of Health
- BUN
Blood urea nitrogen
- eGFR
Estimated glomerular filtration rate
- KDIGO
Kidney Disease Improving Global Outcomes
- PAHO
Pan American Health Organization
- UTI
Urinary tract infection
- OR
Odds ratio
- CI
Confidence Interval
Authors’ contributions
This study was conceived by MG and AA and designed by MG, AA, IL. Data collection was performed by MG, AA, IL, AM. The analysis and interpretation of the results was done by AS, LP, CW, SH, NT, and MG. Draft was written by AS and MG. All authors read and approved the final manuscript.
Funding
The study has been supported by a grant from the Dutch National Postcode Lottery provided funding to Solidaridad to support a proportion of the fieldwork costs. The sponsor was not involved in any part of the study design, or the decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the National Autonomous University of Nicaragua, León Committee of Ethics for Biomedical Research. FWA11114523/IRB00003342. Analysis of the de-identified dataset was exempt by Duke University Health System IRB #00112339.
All participants and parents/legal guardians of minor and illiterate participants provided their informed consent to participate in this study. All methods were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayasumana C, Orantes C, Herrera R, et al. Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol Dial Transplant. 2017;32(2):234–241. doi: 10.1093/ndt/gfw346. [DOI] [PubMed] [Google Scholar]
- 3.Wijkström J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62(5):908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.González-Quiroz M, Pearce N, Caplin B, Nitsch D. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? a systematic review and meta-analysis. Clin Kidney J. 2018;11(4):496–506. doi: 10.1093/ckj/sfx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RJ, Wesseling C, Newman LS. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380(19):1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 6.Milagres T, García-Arroyo FE, Lanaspa MA, et al. Rehydration with fructose worsens dehydration-induced renal damage. BMC Nephrol. 2018;19(1):180. doi: 10.1186/s12882-018-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser J, Lemery J, Rajagopalan B, et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. CJASN. 2016;11(8):1472–1483. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roncal-Jimenez C, García-Trabanino R, Barregard L, et al. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on mesoamerican nephropathy. Am J Kidney Dis. 2016;67(1):20–30. doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Holliday MW, Li Q, Bustamante EG, et al. Potential mechanisms involved in chronic kidney disease of unclear etiology. CJASN. 2022;17(9):1293–1304. doi: 10.2215/CJN.16831221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broe MED, Vervaet BA. Is an Environmental Nephrotoxin the Primary Cause of CKDu (Mesoamerican Nephropathy)? PRO. Kidney360. 2020;1(7):591–595. doi: 10.34067/KID.0003172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rango T, Jeuland M, Manthrithilake H, McCornick P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci Total Environ. 2015;518–519:574–585. doi: 10.1016/j.scitotenv.2015.02.097. [DOI] [PubMed] [Google Scholar]
- 12.Jayasumana C, Gajanayake R, Siribaddana S. Importance of Arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 2014;15:124. doi: 10.1186/1471-2369-15-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez-Rubio O, Brooks DR, Amador JJ, Kaufman JS, Weiner DE, Scammell MK. Chronic kidney disease in Nicaragua: a qualitative analysis of semi-structured interviews with physicians and pharmacists. BMC Public Health. 2013;13(1):350. doi: 10.1186/1471-2458-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petropoulos ZE, Laws RL, Amador JJ, et al. Kidney function, self-reported symptoms, and urine findings in Nicaraguan sugarcane workers. Kidney360. 2020;1(10):1042–1051. doi: 10.34067/KID.0003392020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang CW. Leptospirosis renal disease: emerging culprit of chronic kidney disease unknown etiology. Nephron. 2018;138(2):129–136. doi: 10.1159/000480691. [DOI] [PubMed] [Google Scholar]
- 16.Sunil-Chandra NP, Jayaweera JAAS, Kumbukgolla W, Jayasundara MVML. Association of hantavirus infections and leptospirosis with the occurrence of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: a prospective study with patients and healthy persons. Front Cell Infect Microbiol. 2020;10:556737. doi: 10.3389/fcimb.2020.556737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siriwardhana EARIE, Perera PAJ, Sivakanesan R, Abeysekara T, Nugegoda DB, Jayaweera JAAS. Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J Nephrol. 2015;25(3):146–151. doi: 10.4103/0971-4065.140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaranuwatchai S, Chanakul A, Ittiwut C, et al. Whole-exome sequencing solved over 2-decade kidney disease enigma. Nephron. 2021;145(3):311–316. doi: 10.1159/000514293. [DOI] [PubMed] [Google Scholar]
- 19.Ordunez P, Nieto FJ, Martinez R, et al. Chronic kidney disease mortality trends in selected Central America countries, 1997–2013: clues to an epidemic of chronic interstitial nephritis of agricultural communities. J Epidemiol Community Health. 2018;72(4):280–286. doi: 10.1136/jech-2017-210023. [DOI] [PubMed] [Google Scholar]
- 20.Nicaragua: Administrative Division (Departments and Municipalities) - Population Statistics, Charts and Map. https://www.citypopulation.de/en/nicaragua/admin/. Accessed 21 Dec 2022.
- 21.Peña R, Pérez W, Meléndez M, Källestål C, Persson LÅ. The Nicaraguan Health and Demographic Surveillance Site, HDSS-León: A platform for public health research. Scand J Public Health. 2008;36(3):318–325. doi: 10.1177/1403494807085357. [DOI] [PubMed] [Google Scholar]
- 22.Ravallion M, Chen S, Sangraula P. Dollar a Day Revisited. Published online May 2008. 10.1596/1813-9450-4620.
- 23.United Nations, ed. International Standard Industrial Classification of All Economic Activities (ISIC). Rev. 4. United Nations Statistical Papers; 2008. https://unstats.un.org/unsd/publication/seriesm/seriesm_4rev4e.pdf.
- 24.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CKD Evaluation and Management – KDIGO. https://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed 21 Dec 2022.
- 26.“Epidemic of Chronic Kidney Disease in Agricultural Communities in Central America. Case Definitions, Methodological Basis and Approaches for Public Health Surveillance.” Washington, D.C: Pan American Health Organization; 2017. https://iris.paho.org/handle/10665.2/34132.
- 27.Sanchez Polo V, Garcia-Trabanino R, Rodriguez G, Madero M. Mesoamerican Nephropathy (MeN): What we know so far. Int J Nephrol Renovasc Dis. 2020;13:261–272. doi: 10.2147/IJNRD.S270709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. Published online 2022. https://www.R-project.org/.
- 29.Mayo Clinic Laboratories: Test Definition: URIC. Uric acid, serum. https://www.mayocliniclabs.com/api/sitecore/TestCatalog/DownloadTestCatalog?testId=8440. Accessed 1 July 2023.
- 30.Gonzalez-Quiroz M, Smpokou ET, Silverwood RJ, et al. Decline in kidney function among apparently healthy young adults at risk of Mesoamerican nephropathy. JASN. 2018;29(8):2200–2212. doi: 10.1681/ASN.2018020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebov JF, Valladares E, Peña R, et al. A population-based study of prevalence and risk factors of chronic kidney disease in León. Nicaragua Can J Kidney Health Dis. 2015;2:6. doi: 10.1186/s40697-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2016.
- 33.Wegman DH, Apelqvist J, Bottai M, et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health. 2018;44(1):16–24. doi: 10.5271/sjweh.3659. [DOI] [PubMed] [Google Scholar]
- 34.The Chronic Kidney Disease Epidemic in El Salvador: A Cross-Sectional Study. MEDICC Rev. 2019;21(2–3). 10.37757/MR2019.V21.N2-3.7. [DOI] [PubMed]
- 35.Vervaet BA, Nast CC, Jayasumana C, et al. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97(2):350–369. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Orantes Navarro CM, Almaguer López M, Alonso Galbán P, et al. The chronic kidney disease epidemic in El Salvador: the influence of agrochemicals. Rev Cuba Med Trop. 2020;72(2):e531. [DOI] [PubMed]
- 37.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in Patients with Diabetes Mellitus. N Engl J Med. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Figure 1. Uric Acid by CKD Stage – Males Only. Supplemental Figure 2. Uric Acid by CKD Stage – Females Only. Table S1. Description and comparison of Indeterminant CKD group in demographics, health behaviors, medical history, and occupation. Table S2. Description and Comparison of Indeterminant CKD group in health assessment. Table S3. Sensitivity analysis: Indeterminant CKD group included as no CKD in demographics, health behaviors, medical history, and occupation. Table S4. Sensitivity analysis: Indeterminant CKD group included as no CKD in health assessment. Table S5. Odds ratios and 95% confidence intervals for the multinomial logistic regression model for CKD with traditional risk factors, suspected CKDu and Indeterminant CKD. Table S6. Odds ratios and 95% confidence intervals for the multinomial logistic regression model for CKD with traditional risk factors and suspected CKDu with the indeterminant CKD group counted as no CKD.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.