Abstract
Introduction
Mediport use as a clinical option for the administration of chimeric antigen receptor T cell (CAR T cell) therapy in patients with B-cell malignancies has yet to be standardized. Concern for mediport dislodgement, cell infiltration, and ineffective therapy delivery to systemic circulation has resulted in variable practice with intravenous administration of CAR T cell therapy. With CAR T cell commercialization, it is important to establish practice standards for CAR T cell delivery. We conducted a study to establish usage patterns of mediports in the clinical setting and provide a standard of care recommendation for mediport use as an acceptable form of access for CAR T cell infusions.
Methods
In this retrospective cohort study, data on mediport use and infiltration rate was collected from a survey across 34 medical centers in the Pediatric Real-World CAR Consortium, capturing 504 CAR T cell infusion routes across 489 patients. Data represents the largest, and to our knowledge sole, report on clinical CAR T cell infusion practice patterns since FDA approval and CAR T cell commercialization in 2017.
Results
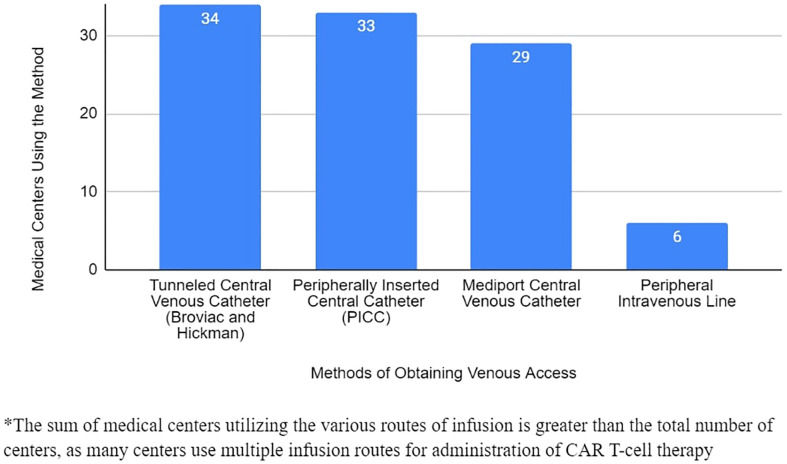
Across 34 sites, all reported tunneled central venous catheters, including Broviac® and Hickman® catheters, as accepted standard venous options for CAR T cell infusion. Use of mediports as a standard clinical practice was reported in 29 of 34 sites (85%). Of 489 evaluable patients with reported route of CAR T cell infusion, 184 patients were infused using mediports, with no reported incidences of CAR T cell infiltration.
Discussion/Conclusion
Based on current clinical practice, mediports are a commonly utilized form of access for CAR T cell therapy administration. These findings support the safe practice of mediport usage as an accepted standard line option for CAR T cell infusion.
Keywords: chimeric antigen receptor T cell, immunotherapy, cancer, immune cell engineering, mediport, implanted catheter
Introduction
Chimeric antigen receptor T cell (CAR T cell) therapy is an approach to adoptive cell therapy that has reformed management of relapsed and refractory B-cell acute lymphoblastic leukemia (B-ALL) in children and young adults. Clinical studies using CAR T cell targeting the canonical B cell marker, CD19, have demonstrated complete remission rates of 70-90% in B-ALL, driving FDA approval for patients <26 years with refractory B-ALL or disease in ≥2nd relapse (1–5).
CAR T cell products are personalized therapies whose production requires leukapheresis to procure a T cell product, followed by T cell activation, then CAR integration via genomic engineering, most commonly achieved in the clinical setting using lentiviral or retroviral transduction (2). CAR T cells are then expanded ex vivo in the presence of cytokines prior to patient infusion (6). Commercial CAR T cell production is highly specialized and extremely costly, nearing half a million dollars per patient (7). Additionally, the therapeutic window for cell infusion is limited. Due to the complex nature of CAR T cell manufacturing and the high-risk patient population, it is vital to ensure successful systemic delivery of the clinical CAR T cell product and avoid clinical processes that could risk compromising the product.
Currently, there is no standard recommendation for acceptable venous access infusion for the administration of CAR T cell therapy. Current options for infusion include tunneled venous catheters (Broviacs® and Hickmans®), mediports, peripherally inserted central catheter (PICC), and peripheral intravenous (IV) catheters (8). Tunneled venous catheters with external line access, such as Broviacs® and Hickmans®, are functional line options, but in clinical practice are more often placed for allogeneic hematopoietic stem cell transplantation (HSCT) and are not usually present in patients receiving upfront leukemia therapy or CAR T cell therapy. While PICC lines can be placed for the administration of CAR T cell therapy, PICCs have demonstrated significant risk of complications, including central-line associated infections, catheter occlusion, and thrombosis (deep vein thrombosis and pulmonary embolism) (9).
Mediports are implanted venous access ports, typically placed under the skin on the upper chest, used to administer medications, fluids, blood products, and chemotherapy. Benefits of mediports include their ease of access, decreased risk of infection compared to tunneled venous catheters, and improved quality of life (10–12) (13). Challenges associated with mediports include risk of thrombosis, subcutaneous port movement, port blockage, and difficulty of device usage in young children (8). Mediports are commonly placed for management of upfront leukemia and although not always present at relapse, they are often present in patients who meet indications for clinical CAR T cell therapy. While there are many benefits to mediports, due to the highly personalized and expensive nature of CAR T cell therapy there is a theoretical concern of infiltration. HSCT serves as the paradigmatic example of adoptive cellular transfer, and common pediatric practice is to avoid mediport use for HSCT cell infusions, to ensure more direct systemic stem cell delivery (14, 15). Mediport infiltration can occur if the implanted needle dislodges from the port, which could result in the therapy entering the extravascular space. Further concern is that the loss of therapy may go unnoticed because the port is subcutaneous. These theoretical concerns have resulted in variable practice in line administration in real world settings, as practitioners debate the relative risks and benefits of placing new PICC lines or utilizing existing mediports for CAR T cell infusion.
In this study we conducted a survey of medical centers in the Pediatric Real-World CAR Consortium (PRWCC) to establish usage patterns of mediports in the clinical commercial CAR T cell setting to provide an evidence-based standard of care recommendation for mediport use for CAR T cell infusions.
Methods
In this retrospective cohort study data on the usage of mediports and occurrence of infiltration was collected from a two-tiered survey distributed to the thirty-four medical centers in the PRWCC. The first survey collected aggregate data at the site level on the method(s) of venous access used for CAR T cell therapy infusion in clinical practice ( Supplemental Figure 1 ). Included was reporting on incidence of infiltration for sites that used mediports and peripheral intravenous devices for administration. Incidence of infiltration was retrospectively reported based on clinical assessments at PRWCC centers via patient, nursing and/or physician reporting, and clinical documentation of surrounding edema, erythema, and/or pain at the mediport site during and after the infusion administration.
The second survey collected patient specific data from individual sites on the total number of patients treated with CAR T cell therapy within the study timeframe, as well as the form of venous access utilized for each infusion ( Supplemental Figure 2 ). Data represents clinical practice patterns over the first five years of commercial CAR T cell therapy usage since FDA approval in August 2017 through September 1st, 2022.
Results
In this study, retrospective data were collected across 489 patients from 34 PRWCC sites. A two-tiered survey approach was applied ( Table 1 ). The first tier of the survey included four questions regarding the methods of venous access utilized in each medical center for CAR T cell therapy administration ( Supplemental Figure 1 ) The survey specifically inquired about the use of tunneled central venous catheters, peripherally inserted central catheters, mediport central venous catheters, and peripheral intravenous catheters. For centers using mediports and peripheral intravenous catheters, there was a follow-up question regarding the number of incidences of therapy infiltration. The second tier of the survey collected data on total number of Tisagenlecleucel infused patients between August 2017 – September 1st, 2022, as well as the route of administration for each infusion ( Supplemental Figure 2 ).
Table 1.
PRWCC medical centers surveyed on line utilization for clinical CART delivery (N = 34).
| Ann & Robert H. Lurie Children’s Hospital of Chicago | Medical College of Wisconsin |
| Children’s Health Orange County | Memorial Sloan Kettering Cancer Center |
| Children’s Hospital at Montefiore | Phoenix Children’s Hospital |
| Children’s Hospital of Philadelphia | Rady Children’s Hospital San Diego |
| Children’s Mercy Hospital (Kansas) | Riley Children Health, Indiana University Health |
| Children’s National Hospital | St. Louis Children’s Hospital |
| Cincinnati Children’s Medical Center | Texas Children’s Cancer Center |
| City of Hope National Medical Center | UCSF Benioff Children’s Hospital |
| Columbia University Medical Center | University of Arizona |
| Cook Children’s Hospital | University of California Los Angeles |
| Dana Farber/Boston Children’s Hospital | University of Colorado, Anschutz Medical Campus |
| Duke Children’s Hospital & Health Center | University of Florida Health |
| Helen DeVos Children’s Hospital | University of Minnesota Medical School |
| Hospital for Sick Children | University of Texas Southwestern Medical Center |
| John Hopkins All Children’s Hospital | University of Wisconsin |
| Johns Hopkins Sidney Kimmel Comprehensive Cancer Center | Winship Cancer Institute, Emory |
| Lucile Packard Children’s Hospital Stanford | Yale University and Yale New Haven Children’s Hospital |
All 34 responding sites reported using tunneled central venous catheters, including Broviac® and Hickman® lines, as accepted standard line options for CAR T cell infusion. Use of mediports as a standard clinical practice was reported in 29 of 34 sites (85%). Of the sites using mediports, there were no incidences of CAR T cell therapy infiltration reported. One site reported exclusive use of mediports for CAR T cell therapy infusion. Notably, 6 of 34 centers (18%) also reported using peripheral intravenous lines for administration of CAR T cell therapy ( Figure 1 ). Sites using peripheral intravenous catheters reported no incidences of CAR T cell therapy infiltration.
Figure 1.
Venous Access Methods for Delivery of CAR T-Cell Therapy Utilized by PRWCC Medical Centers.
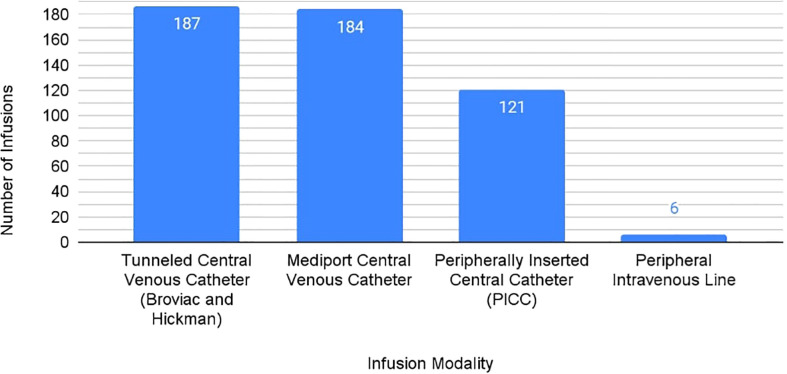
From responding PRWCC sites, 489 patients with a total of 504 infusions were reported with known route of CAR T cell therapy administration. Of these infusions, 187 were administered by central venous catheter (37%), 184 by mediport (37%), 121 by PICC (24%), and 6 with peripheral intravenous catheter (1%) ( Figure 2 ).
Figure 2.
Rates of Infusion Modalities Across PRWCC Sites for CAR T-cell Therapy Administration.
Discussion
Limited data exists describing accepted infusion route for CAR T cell therapy. The paradigmatic use of cell therapy with greatest clinical experience is hematopoietic cell therapy, where practice often avoids mediport use due to high complexity of cell procurement and risk of cell infiltration. In the early days of CAR T cell commercialization, similar practice was adopted by select sites, however, we hypothesized that mediport usage for CAR T cell infusion has since been adopted as a safe accepted practice, with minimal risk of infiltration. Using the PRWCC framework, we conducted a survey of clinical practice for line usage for administration of CAR T cell therapy in children and young adults with B cell malignancies and infiltration rates. We administered a follow-up survey to quantify patient volume infused using different routes of administration. Of the medical centers surveyed, 29 of 34 sites reported routine use of mediports, indicating that mediports are a commonly utilized method of venous access for CAR T cell therapy infusion.
Across surveyed medical centers, 184 CAR T cell products were infused using mediports with no reported instances of infiltration. These findings, which represent clinical practice from Tisagenlecleucel FDA approval in 2017 through September 1st, 2022, suggest that concerns for mediport dislodgement and cell infiltration have not emerged as a clinical challenge. Furthermore, prior studies comparing mediport and PICC line usage for chemotherapy administration in pediatric and adult patients with solid malignancies have found distinct advantages with mediport usage (12, 13). These studies demonstrated fewer post-treatment complications (thrombosis, infection) and improved quality of life with mediport usage, when compared to PICC lines (12, 13). Medical centers have also found increased ease of access and reduced number of needle sticks with mediports (10, 11). These results exhibit the clinical advantages of mediports when compared to other methods of venous access. In the context of CAR T cell therapy, an additional consideration in utilizing a central line is to ensure patients have readily available access in the event that they experience high grade toxicities. Together, these findings support the use of mediports already in place for CAR T cell infusion, as opposed to the placement of a new PICC line, a procedure often requiring anesthesia in pediatric patients, for this purpose.
While our study highlights real-world use of mediports for the delivery of CAR T cell therapy, the possibility of mediport infiltration remains. We therefore highlight the importance of using standard operating procedures for mediport infusions, including appropriate training prior to access and confirmation of blood return prior to infusing the cellular therapy product.
In conclusion, to our knowledge, we report the first multi-institutional reporting (N=489) on routes of commercial CAR T cell delivery across children and young adults. We describe that current clinical practice in medical centers across the United States indicates that mediports are a commonly utilized method of CAR T cell therapy administration. Of centers that use mediports, 184 patients had CAR T cells delivered using mediports, with no reported instances of therapy infiltration. From these clinically based findings, we can draw support for the obviation of new PICC line insertion for CAR T cell infusion in patients who already have mediports in place. These results support the usage of mediports as a safe, accepted standard line option for the infusion of CAR T cell therapy.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
ME and LS contributed to conception, design, statistical analysis, and writing of the manuscript. MK and DB contributed to database organization and statistical analysis. SP, SJ, KC, CE, NK, CP, PS, MH, ES, SB, JT, MM, JR, CLB, GM, RR, KT, TD, EK, DBS, DS, SO, CC, HP, TP, NS, VH, JS, TL, EF, KM, TQ, JK, JL, VF, and CB contributed to data collection and writing of sections of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We acknowledge PRWCC sites, the Stanford Association of Auxiliaries for Children, the Bass Center for Childhood Cancer and Blood Diseases at Stanford Children’s Health, and the Stanford University Center for Cellular Therapy.
Funding Statement
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
JR has consulted for Novartis. CBo has patents pending on the use of engineered cellular therapies for cancer, and has research support provided by BMS, Merck, and Kiadis pharma. CC has served as the C.M.C. reports honorarium for advisory boards from Bayer, Elephas, Nektar Therapeutics, Novartis and WiCell Research Institute, all of whom had no input in the study design, analysis, manuscript preparation or decision to submit for publication. VH has grant funding from Servier. TL has consulted for Advanced Microbubbles, AI Therapeutics, Bayer, GentiBio, Jazz Pharmaceuticals, MassiveBio, Menarini, Novartis, Pyramid Biosciences, and Treeline Bio. VF has consulted for Adaptimmune. LS served on advisory board for Novartis and CARGO therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1239132/full#supplementary-material
Survey #1.
Survey #2.
References
- 1. Leukemia & Lymphoma Society . Chimeric antigen receptor (CAR) T-cell therapy. Rye Brook, New York: LLS.Org. Available at: www.lls.org/treatment/types-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy. [Google Scholar]
- 2. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. New Engl J Med (2018) 378(5):439–48. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood (2017) 129(25):3322–31. doi: 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood (2019) 134(26):2361–8. doi: 10.1182/blood.2019001641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Portell CA. How does CAR T-cell therapy work in treating cancer Alexandria, Virgina: Cancer.Net; (2021). Available at: https://www.cancer.net/blog/2021-06/how-does-car-t-cell-therapy-work-treating-cancer. [Google Scholar]
- 7. Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin oncology; Off J Am Soc Clin Oncol (2018) 36(32):3192–202. doi: 10.1200/JCO.2018.79.0642 [DOI] [PubMed] [Google Scholar]
- 8. Cancer.Net Editorial Board . Catheters and ports in cancer treatment. Alexandria, Virgina: Cancer.Net; (2020). Available at: https://www.cancer.net/navigating-cancer-care/how-cancer-treated/chemotherapy/catheters-and-ports-cancer-treatment. [Google Scholar]
- 9. Mitbander UB, Geer MJ, Taxbro K, Horowitz JK, Zhand Q, O’Malley ME, et al. Patterns of use and outcomes of peripherally inserted central catheters in hospitalized patients with solid tumors: A multicenter study. Cancer (2022) 128(20), 3681–3690. doi: 10.1002/cncr.34410 [DOI] [PubMed] [Google Scholar]
- 10. Memorial Sloan Kettering Cancer Center . About your implanted port. Manhattan, New York: MSKCC; (2021). Available at: https://www.mskcc.org/cancer-care/patient-education/your-implanted-port. [Google Scholar]
- 11. Cleveland clinic . Implanted ports. Cleveland, Ohio: Myclevelandclinic.org; (2021). Available at: https://my.clevelandclinic.org/health/treatments/21701-implanted-port. [Google Scholar]
- 12. Patel GS, Jain K, Kumar R, Strickland AH, Pellegrini L, Slavotinek J, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological Malignancies. Supportive Care Cancer (2014) 22(1):121–8. doi: 10.1007/s00520-013-1941-1 [DOI] [PubMed] [Google Scholar]
- 13. Zhang H, Li Y, Zhu N, Li Y, Fu J, Liu J. Comparison of peripherally inserted central catheters (PICCs) versus totally implantable venous-access ports in pediatric oncology patients, a single center study. Sci Rep (2022) 12:3510. doi: 10.1038/s41598-022-07584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crocoli A, Martucci C, Persano G, De Pasquale MD, Serra A, Accinni A, et al. Vascular access in pediatric oncology and hematology: state of the art. Children (Basel Switzerland) (2022) 9(1):70. doi: 10.3390/children9010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva S, Reichembach MT, Pontes L, Souza G, Kusma S. Heparin solution in the prevention of occlusions in Hickman® catheters a randomized clinical trial. Rev latino-americana enfermagem (2021) 29:e3385. doi: 10.1590/1518-8345.3310.3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey #1.
Survey #2.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.