Abstract
Background
Acute kidney injury (AKI) is a common complication after cardiovascular surgery in children, noted in approximately 40% of children undergoing cardiopulmonary bypass (CPB). We sought to determine the risk factors including inflammatory and vascular endothelial markers associated with AKI in children undergoing cardiac surgery.
Methods
A secondary analysis of a prospective observational cohort study of paediatric patients with a cardiac defect requiring CPB and a weight of >2.5 kg was performed. AKI was defined as a 1.5 times increase from the preoperative value in serum creatinine or an absolute increase by ≥0.3 mg/dL (≥26.5 μmol/L). Plasma inflammatory markers (interleukin [IL]-1a, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, and tumour necrosis factor α) and vascular endothelial markers (vascular endothelial growth factor, von Willebrand factor, regulated on activation, normal T-cell expressed and secreted, granulocyte macrophage colony-stimulating factor, monocyte chemoattractant protein-1, platelet-derived growth factor, and microparticles) were assessed at 5 perioperative time points. Associations with AKI were found using generalized linear regression models adjusted for repeated measures.
Results
A total of 207 patients were assessed, of whom 56% (n = 116) were male. Thirty-three percent (n = 68) developed AKI. In univariable analyses, adverse outcomes significantly related to the presence of AKI included increased intensive care unit stay (3.0 vs 5.6 hours, P < 0.001). In multivariable analysis, independent factors that were significantly associated with AKI included longer duration of CPB (111 vs 154 minutes, P < 0.001) and lower preoperative creatinine. Inflammatory and vascular endothelial biomarkers were not associated with AKI.
Conclusions
AKI remains a prevalent problem after cardiac surgery, and renal ischemia related to longer bypass time potentially plays a key role in the etiology. Inflammatory and vascular endothelial biomarkers were not significantly related to AKI.
Graphical abstract
Résumé
Contexte
L’insuffisance rénale aiguë (IRA) est une complication fréquente qui survient chez les enfants après une intervention chirurgicale cardiovasculaire. Environ 40 % des enfants chez qui une circulation extracorporelle (CEC) est mise en place durant l’intervention présentent ultérieurement une IRA. Nous avons tenté de définir les facteurs de risque, y compris les marqueurs inflammatoires et endothéliaux vasculaires, qui sont associés à l’IRA chez les enfants qui subissent une intervention chirurgicale cardiaque.
Méthodologie
Nous avons réalisé une analyse secondaire d’une étude de cohorte observationnelle prospective menée auprès d’enfants qui étaient atteints d’une anomalie cardiaque nécessitant une CEC et qui pesaient plus de 2,5 kg. L’IRA était définie comme une hausse du taux de créatinine sérique par un facteur de 1,5 par rapport à la valeur préopératoire ou comme une augmentation absolue de ≥ 0,3 mg/dL (≥ 26,5 μmol/l). Les marqueurs inflammatoires plasmatiques (interleukine [IL]-1a, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, facteur de nécrose tumorale alpha) et les marqueurs endothéliaux vasculaires (facteur de croissance de l’endothélium vasculaire, facteur de von Willebrand, chimiokine exprimée et sécrétée après l’activation des lymphocytes T normaux, facteur de stimulation des granulocytes et macrophages, protéine chimiotactique des monocytes-1, facteur de croissance dérivé des plaquettes, microparticules) ont été évalués à 5 moments périopératoires différents. Les associations avec l’IRA ont été établies au moyen de modèles de régression linéaire généraux, qui ont été ajustés pour tenir compte des mesures répétées.
Résultats
L’évaluation a porté sur 207 patients, dont 56 % (n = 116) étaient des garçons, et une IRA a été observée chez 33 % (n = 68) d’entre eux. Les résultats d’analyses univariées ont montré que les issues indésirables associées de façon significative à la présence d’une IRA comprenaient un séjour prolongé à l’unité de soins intensifs (3,0 c. 5,6 heures, p < 0,001). Dans les analyses multivariées, les facteurs indépendants associés de façon significative à une IRA comprenaient une CEC prolongée (111 c. 154 minutes, p < 0,001) et un faible taux de créatinine préopératoire. Les biomarqueurs inflammatoires et endothéliaux vasculaires n’ont pas été associés à l’IRA.
Conclusions
L’IRA demeure un problème répandu après une intervention chirurgicale cardiaque. L’ischémie rénale associée à une CEC prolongée joue potentiellement un rôle clé dans son étiologie. Par ailleurs, les biomarqueurs inflammatoires et endothéliaux vasculaires n’ont pas été associés de façon significative à l’IRA.
Paediatric acute kidney injury (AKI) is a common complication after cardiovascular surgery,1 although 98% of cases resolve within 48 hours.2 The reported incidence of AKI after cardiopulmonary bypass (CPB) has been variable due to heterogeneity between studies regarding the patient population and the definition of AKI used.2 A recent paediatric study showed that 42% of those who had CPB developed AKI,2 which has been shown to be associated with increased hospital mortality, duration of ventilation, and hospital stay.3,4 Further, hypertension and chronic kidney disease have been shown to be common in children 5 years after undergoing cardiac surgery,1 and this may be related to AKI leading to chronic kidney injury,5, 6, 7 which has been shown in adult populations in multiple settings, including cardiac surgery.8,9
Reported factors associated with developing AKI after cardiac surgery in children have included patient age less than 2 years and longer duration of CPB.2 A meta-analysis identified associated factors for AKI in this scenario to include younger age, lower preoperative creatinine, longer surgery time, longer CPB time, longer aortic cross-clamping time, cyanotic heart disease, univentricular heart, vasopressor use, cardiac reoperation, and postoperative sepsis.10 A better understanding of which paediatric patients are at highest risk of AKI may inform further research and the development of targeted preventive strategies.11
The etiology of AKI after cardiac surgery is multifactorial, including systemic inflammatory response to CPB, renal ischemia, oxidative stress, microemboli, and reperfusion injury.12 In CPB, aorta cross-clamping and nonpulsatile blood flow contribute to the inflammatory responses and oxidative stress injury, which may induce AKI.13 The pathogenesis of AKI involves a complex interplay and crosstalk between damaged/injured renal epithelial cells, the vascular endothelium, and inflammatory cells.14 Many novel biomarkers involved in myocardial injury, renal tubular injury, and inflammation have been studied, raising the possibility of improved risk stratification for AKI.15, 16, 17 Serum cytokines as biomarkers for AKI have been studied in paediatric cardiac surgery patients.12,14,16 Vascular endothelial biomarkers, including microparticles, have been studied in adults.13 To our knowledge, microparticles have not been studied in the paediatric population. Other vascular markers, including regulated on activation, normal T-cell expressed and secreted (RANTES), have been shown to be produced by renal tubular cells and play a key role in ischemia-reperfusion injury.18 Therefore, we sought to determine clinical and biomarker factors associated with AKI in paediatric patients undergoing cardiac surgery with CPB, including plasma inflammatory and vascular endothelial biomarkers.
Materials and Methods
Study design and population
We performed a prospective observational cohort study of paediatric patients (0-18 years old) with a cardiac defect (acquired or congenital) who underwent repair with CPB between July 2011 and November 2014 at the Hospital for Sick Children, Toronto, Ontario, Canada. The primary aim of the original Clinical Assessment of Thrombosis in Children after Heart surgery (CATCH) study was to determine the prevalence of postoperative thrombosis, and associated physiological, pharmacological, and clinical factors increasing thrombosis risk. The current study was a secondary analysis aimed at determining factors associated with AKI. Patients were enrolled in the CATCH study at the time of their presurgical consultation usually 1-14 days before their index operation. Inclusion criteria were cardiovascular surgery with CPB and patient weight greater than 2.5 kg. Patients who required multiple operations to correct their defects were excluded from repeat enrolment, meaning if a patient had multiple surgeries, only the first surgery a patient had was included. The CATCH study was approved by the Hospital for Sick Children’s Research Ethics Board, and parental and/or patient written consent/assent was obtained for all participants. A separate approval with waiver of consent was obtained for the AKI secondary analysis.
Acute kidney injury
Creatinine values were not obtained as part of the CATCH study data collection, and for the current study, these were abstracted from the clinical record. Participants were eligible for inclusion in the secondary analysis if they had preoperative creatinine assessed within 90 days before their index operation and postoperative creatinine assessed within 7 days after the index operation. The preoperative creatinine value used was that closest in time to surgery, and the postoperative value used was the highest value after but within 7 days of surgery. The presence of AKI was defined as a 1.5-fold increase in serum creatinine (SCr) or an absolute increase by ≥0.3 mg/dL (≥26.5 μmol/L) between these 2 time points.19
Preoperative data
For the CATCH study, demographic information (age, sex, ethnicity, and weight), previous hospitalizations, catheterization and surgical interventions, cardiac and noncardiac complications, and preoperative clinical status (systemic oxygen saturation, echocardiographic findings such as ejection fraction and valve function) were abstracted from the medical record. In addition, data regarding congenital malformations, chromosomal abnormalities, and the presence of noncardiac disease (renal dysfunction/failure, infection, liver failure, pulmonary oedema, and neurological abnormalities) were obtained. All medications were recorded.
Preoperative clinical laboratory assessments
Results of preoperative laboratory investigations, including complete blood cell counts (red blood cell count, haemoglobin, haematocrit, platelet count, white blood cell counts, and differential) and related indices (mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, and mean platelet volume) were captured. Preoperative polycythaemia, as indicated by haematocrit, was used as a surrogate indicator of cyanosis burden (as opposed to a single oxygen saturation measurement). International normalized ratio and levels of activated partial thromboplastin and hepatic transaminases were captured. All included laboratory testing was performed on site using validated techniques and standard operating procedures.
Surgical data
Underlying cardiac anatomy and the type of surgical procedure were categorized according to the International Nomenclature for Congenital Heart Surgery. The Aristotle score was used as a measure of surgical complexity.20,21 Study coordinators present in the operating room monitored the entire surgery and collected data from the anaesthesia, perfusion, and nursing records, including the duration of aortic cross clamping, duration and number of CPB cycles, and use and duration of deep hypothermic circulatory arrest. Selected medications (steroids, vasopressin, antifibrinolytic agents, and antithrombin) used intraoperatively and during the first 24 postoperative hours were recorded.
Outcomes
For all patients, a detailed review of all surgical complications and in-hospital outcomes was performed. Outcomes captured included anaemia, bleeding, infections (catheter infections, urinary tract infection, wound infection, sepsis, and endocarditis), cardiac complications (cardiopulmonary arrest, low cardiac output syndrome, cardiogenic shock, pericardial effusion and cardiac tamponade, and pericarditis), pulmonary hypertension, multiorgan failure, necrotizing enterocolitis, neurological injury, and seizures. In addition, use of extracorporeal membrane oxygenation (ECMO), catheter interventions, surgical reoperations, length of intensive care unit (ICU) stay (hours), and length of hospital stay (hours) were recorded.
Inflammation and vascular endothelial biomarkers
A comprehensive panel of inflammatory markers (interleukin [IL]-1a, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, tumour necrosis factor α [TNF-α], and high-sensitivity C-reactive protein) and vascular endothelial markers (vascular endothelial growth factor, von Willebrand factor, RANTES, granulocyte macrophage colony-stimulating factor, monocyte chemoattractant protein-1, platelet-derived growth factor, and microparticles) were assessed at 5 perioperative time points (induction, 5 minutes into CPB, end of CPB, 1 day postoperatively, and 3 days postoperatively).
Statistical analysis
Data are presented as means with standard deviation, median with quartiles (25th and 75th percentile), and frequencies as appropriate to the level of measurement and distribution of the values. The characteristics and outcomes of patients with vs without AKI were compared using χ2, Fisher’s exact test, Mantel-Haentzel χ2, Student’s t-test, and Kruskal-Wallis analysis of variance as appropriate. Associations of AKI with serial measures of inflammatory markers, vascular markers, and microparticles were sought using mixed linear regression analyses using a compound symmetry covariance structure. Before multivariable analyses, multiple imputation was used to impute missing values. Multivariable associations of preoperative and intraoperative variables with AKI were sought using multivariable logistic regression analysis. To determine associations of preoperative and intraoperative factors with AKI, together with selected postoperative factors (such as ECMO, catheter reinterventions, and reoperations) with the absolute change from the preoperative to the highest postoperative creatinine level (as a continuous measure of kidney injury), multivariable linear regression analysis was performed. All statistical analyses were performed using SAS statistical software v9.4 (SAS Institute, Cary, NC).
Results
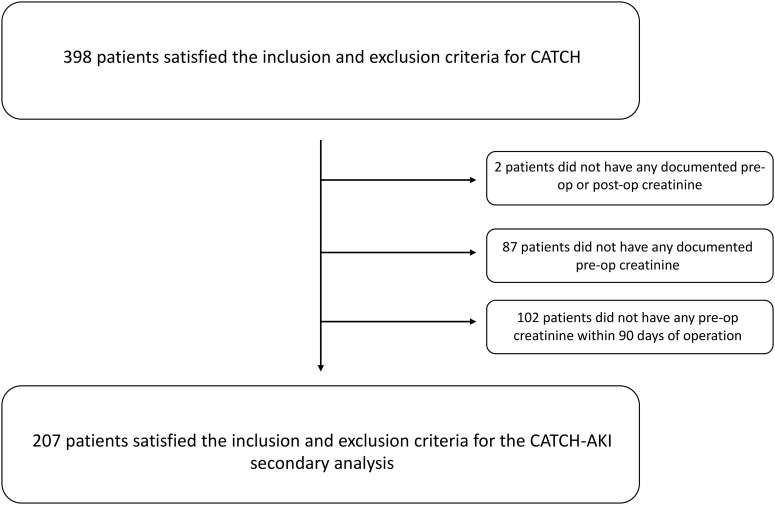
The main CATCH study enrolled 398 participants. After the AKI study inclusion criteria were applied, 207 patients were included in this secondary analysis (Fig. 1). The study had 56% male (n = 116) and 44% female patients (n = 91). The median age was 2 years (interquartile range [IQR]: 0.1-2.1 years). The mean CPB time was 125 ± 65 minutes. The mean aortic cross-clamp time was 86 ± 47 minutes. In terms of past medical history, 66% (n = 137) had no previous cardiac surgery. The mean number of previous cardiac catheterizations was 0.87 ± 1.16 (Table 1). The types of cardiac surgeries related to the cardiac diagnoses can be found in Table 2. The 2 most common surgeries were double outlet right ventricle/transposition of the great arteries repair (n = 37, 18%) and tetralogy of Fallot repair (n = 26, 13%).
Figure 1.
Study population. AKI, acute kidney injury; CATCH, Clinical Assessment of Thrombosis in Children after Heart surgery.
Table 1.
Characteristics of the study population
| Variable | All (n = 207) | Without AKI (n = 139) | With AKI (n = 67) | P value |
|---|---|---|---|---|
| Male sex, n (%) | 116 (56) | 78 (56) | 38 (56) | 0.98 |
| Age (y), median (IQR) | 2 (0.1-2.1) | 0.4 (0.1-2.8) | 0.5 (0.3-1.7) | 0.28 |
| Any chromosomal abnormality, n (%) | 32 (15) | 18 (13) | 14 (21) | 0.16 |
| Major congenital noncardiac abnormality, n (%) | 30 (14) | 19 (14) | 11 (16) | 0.64 |
| Previous cardiac surgery, n (%) | ||||
| 0 | 137 (66) | 96 (69) | 68 (60) | 0.13∗ |
| 1 | 45 (22) | 29 (21) | 16 (24) | |
| 2 | 15 (7) | 9 (6) | 6 (9) | |
| 3 | 10 (5) | 5 (4) | 5 (7) | |
| Previous cardiac catheterizations, n (%) | ||||
| 0 | 11 (54) | 78 (56) | 33 (49) | 0.008∗ |
| 1 | 46 (22) | 35 (25) | 11 (16) | |
| 2 | 26 (13) | 15 (11) | 11 (16) | |
| 3 | 14 (7) | 9 (6) | 5 (7) | |
| 4 | 10 (5) | 2 (1) | 8 (12) |
AKI, acute kidney injury; IQR, interquartile range.
Mantel-Haenszel statistic.
Table 2.
Incidence of acute kidney injury (AKI) by the type of cardiac surgery
| Variables | Total cases (n = 207) |
No AKI (n = 139) |
AKI (n = 68) |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Aortic defect repair | 18 | 9 | 11 | 61 | 7 | 39 |
| Atrial septal defect repair | 4 | 2 | 4 | 100 | 0 | 0 |
| Atrioventricular septal defect repair | 9 | 4 | 4 | 44 | 5 | 56 |
| Coronary artery bypass | 3 | 1 | 2 | 67 | 1 | 33 |
| DORV/TGA repair | 37 | 18 | 25 | 68 | 12 | 32 |
| Heart transplant | 1 | 0 | 0 | 0 | 1 | 100 |
| LVOT repair/AV repair/replacement | 11 | 5 | 11 | 5 | 3 | 27 |
| Mitral valve repair/replacement | 8 | 4 | 6 | 75 | 2 | 25 |
| Pulmonary circulation repair | 7 | 3 | 3 | 43 | 4 | 57 |
| Pulmonary venous repair | 10 | 5 | 9 | 9 | 1 | 10 |
| RVOT repair/PV replacement | 5 | 2 | 5 | 100 | 0 | 0 |
| Norwood (stage 1) | 9 | 4 | 7 | 78 | 2 | 22 |
| Cavopulmonary shunt (stage 2) | 16 | 8 | 13 | 18 | 3 | 19 |
| Hybrid (stage 2) | 5 | 2 | 2 | 40 | 3 | 60 |
| Fontan (stage 3) | 14 | 7 | 9 | 64 | 5 | 36 |
| Tetralogy of Fallot repair | 26 | 13 | 12 | 46 | 14 | 54 |
| Tricuspid valve repair/replacement | 3 | 1 | 2 | 67 | 1 | 33 |
| Ventricular septal defect repair | 21 | 10 | 17 | 81 | 4 | 19 |
AV, atrioventricular; DORV, double outlet right ventricle; LVOT, left ventricular outflow tract, PV, pulmonary valve; RVOT, right ventricular outflow tract; TGA, transposition of the great arteries.
A total of 32 patients (15%) had a chromosomal abnormality, 30 (14%) had a major congenital noncardiac abnormality (Table 1), 62 (30%) had preoperative cyanosis (oxygen saturation <85%), 20 (10%) had a preoperative infection, 13 (6%) had preoperative arrhythmia, and only 5 (2%) had preoperative renal dysfunction. In terms of preoperative medications, 67 (32%) were on diuretics, 39 (19%) were on antibiotics, and 39 (19%) were on prostaglandin (Table 3). The preoperative complete inflammatory markers and vascular cytokines can be found in Table 4.
Table 3.
Pre-, intra-, and postoperative risk factors for acute kidney injury (AKI)
| Variable | n | No AKI | n | AKI | P value |
|---|---|---|---|---|---|
| Preoperative medical status, n (%) | |||||
| Cyanosis (oxygen saturation <85%) | 139 | 34 (24) | 68 | 28 (41) | 0.02 |
| Arrhythmia | 139 | 11 (8) | 68 | 2 (3) | 0.23 |
| Ventilated | 139 | 9 (6) | 68 | 7 (10) | 0.44 |
| Lung collapse | 139 | 5 (4) | 68 | 8 (12) | 0.04 |
| Pulmonary oedema | 139 | 17 (12) | 68 | 3 (4) | 0.09 |
| Infection | 139 | 13 (9) | 68 | 7 (10) | 0.83 |
| Renal dysfunction/failure | 139 | 5 (4) | 68 | 0 (0) | 0.14 |
| Preoperative medications, n (%) | |||||
| Diuretics | 139 | 38 (27) | 68 | 29 (43) | 0.03 |
| ACEi | 139 | 15 (11) | 68 | 5 (7) | 0.62 |
| Antibiotics | 139 | 28 (20) | 68 | 11 (16) | 0.50 |
| Aspirin | 139 | 9 (6) | 68 | 10 (15) | 0.06 |
| Beta-blockers | 139 | 9 (6) | 68 | 5 (7) | 0.78 |
| Digoxin | 139 | 5 (4) | 68 | 0 (0) | 0.18 |
| Iron | 139 | 8 (6) | 68 | 6 (9) | 0.41 |
| Prostaglandin | 139 | 33 (24) | 68 | 6 (9) | 0.01 |
| Blood composition | |||||
| Haemoglobin (g/L), mean ± SD | 139 | 141 ± 26 | 68 | 143 ± 28 | 0.73 |
| Platelet count (×109/L), mean ± SD | 135 | 325 ± 131 | 65 | 347 ± 134 | 0.27 |
| White blood cell count (×109/L), mean ± SD | 139 | 11.1 ± 5.7 | 68 | 10.5 ± 5.1 | 0.49 |
| Neutrophils (×109/L), median (IQR) | 63 | 5.5 (3.8, 7.7) | 30 | 3.1 (2.2, 6.2) | 0.003 |
| Lymphocyte (×109/L), mean ± SD | 63 | 4.5 ± 2.8 | 30 | 5.5 ± 3.2 | 0.15 |
| Blood pH, mean ± SD | 135 | 7.38 ± 0.1 | 67 | 7.38 ± 0.1 | 0.91 |
| Biochemistry, n (%) | |||||
| Elevated alanine transaminase (>2× normal values) | 137 | 1 (1) | 67 | 7 (10) | 0.002 |
| Elevated aspartate transaminase (>3× normal values) | 138 | 15 (11) | 68 | 11 (16) | 0.3 |
| Operative factors | |||||
| Total number of CPB cycles, n (%) | 139 | 68 | 0.19∗ | ||
| One | 100 (72) | 41 (60) | |||
| Two | 24 (17) | 17 (25) | |||
| Three | 8 (6) | 6 (9) | |||
| Four | 7 (5) | 4 (6) | |||
| Total CPB time (min), mean ± SD | 139 | 111 ± 56 | 68 | 154 ± 73 | <0.001 |
| Total number of cross-clamp cycles, n (%) | 139 | 68 | 0.55 | ||
| None | 11 (8) | 6 (9) | |||
| One | 118 (85) | 54 (79) | |||
| Two | 10 (7) | 8 (12) | |||
| Total cross-clamp time (min), mean ± SD | 128 | 76 ± 36 | 62 | 106 ± 58 | <0.001 |
| Deep hypothermic circulatory arrest, n (%) | 139 | 21 (15) | 68 | 13 (19) | 0.47 |
| Circulatory arrest time (min), mean ± SD | 21 | 24 ± 13 | 13 | 18 ± 9 | 0.12 |
| Cerebral perfusion, n (%) | 139 | 12 (9) | 68 | 5 (7) | 1 |
| Modified ultrafiltration, n (%) | 139 | 129 (93) | 68 | 65 (96) | 0.56 |
| Any intraoperative steroids, n (%) | 139 | 41 (30) | 68 | 13 (19) | 0.12 |
| Steroids day before surgery, n (%) | 139 | 29 (21) | 68 | 6 (9) | 0.03 |
| Steroids in CPB prime, n (%) | 139 | 38 (27) | 68 | 9 (13) | 0.03 |
| Extubated in OR, n (%) | 139 | 35 (25) | 68 | 9 (13) | <0.05 |
| Total time with chest open (h), median (IQR) | 139 | 4 (2.9, 5.7) | 68 | 4.1 (4.1, 9.1) | <0.001 |
| Chest left open in ICU, n (%) | 139 | 25 (18) | 68 | 14 (12) | 0.66 |
| Total time intubated (h), median (IQR) | 139 | 27.4 (7.0, 96.4) | 68 | 56.8 (21, 129.2) | 0.004 |
| Catheter reintervention, n (%) | 136 | 3 (2) | 68 | 5 (7) | 0.12 |
| Postoperative ECMO, n (%) | 138 | 3 (2) | 67 | 6 (9) | 0.07 |
| Surgical reoperation, n (%) | 139 | 9 (6) | 68 | 7 (10) | 0.34 |
| Length of ICU stay (h), median (IQR) | 138 | 3.0 (1.1, 7.0) | 67 | 5.6 (2.9, 12.8) | <0.001 |
| Length of hospital stay (d), median (IQR) | 137 | 7.9 (4.9, 15.1) | 67 | 11.1 (7.2, 29.8) | <0.001 |
| Postoperative RBC transfusions, n (%) | 139 | (22) | 68 | 3 (5) | 0.04 |
| Elevated preoperative lactate (mmol/L), median (IQR) | 136 | 2.8 (2.4, 4.3) | 68 | 3.3 (2.3, 5.1) | 0.67 |
| Postoperative outcomes, n (%) | |||||
| Low cardiac output syndrome | 139 | 5 (4) | 68 | 6 (9) | 0.18 |
| Cardiorespiratory insufficiency | 139 | 11 (8) | 68 | 11 (16) | 0.07 |
| Cardiogenic shock | 139 | 0 (0) | 68 | 1 (1) | 0.33 |
| Pulmonary hypertension | 139 | 4 (3) | 68 | 2 (3) | 1 |
| Cardiac arrest | 139 | 3 (2) | 68 | 5 (7) | 0.12 |
| Arrhythmia | 139 | 31 (22) | 68 | 19 (28) | 0.4 |
| Pneumothorax | 139 | 4 (3) | 68 | 0 (0) | 0.31 |
| Chylothorax | 139 | 13 (9) | 68 | 16 (24) | 0.006 |
| Lung collapse | 139 | 11 (8) | 68 | 11 (16) | 0.07 |
| Cardiac tamponade | 139 | 1 (1) | 68 | 0 (0) | 1 |
| Pleural effusion | 139 | 7 (5) | 68 | 6 (9) | 0.29 |
| Pericarditis | 139 | 9 (6) | 68 | 6 (9) | 0.54 |
| Pericardial effusion | 139 | 1 (1) | 68 | 0 (0) | 1 |
| Pulmonary oedema | 139 | 8 (6) | 68 | 4 (6) | 1 |
| Anaemia | 139 | 2 (1) | 68 | 1 (1) | 1 |
| Bleeding | 139 | 9 (4) | 68 | 4 (6) | 0.74 |
| Pulmonary haemorrhage | 139 | 3 (2) | 68 | 3 (4) | 0.4 |
| Renal failure | 139 | 1 (1) | 68 | 1 (1) | 0.56 |
| Dialysis | 139 | 0 (0) | 68 | 3 (4) | 0.04 |
| Phrenic nerve/neurological injury | 139 | 3 (2) | 68 | 6 (9) | 0.07 |
| Necrotizing enterocolitis | 139 | 0 (0) | 68 | 1 (1) | 0.33 |
| Endocarditis | 139 | 1 (1) | 68 | 0 (0) | 1 |
| Infection | 139 | 20 (14) | 68 | 19 (28) | 0.02 |
| Sepsis | 139 | 3 (2) | 68 | 4 (6) | 0.23 |
| Upper respiratory tract infection | 139 | 7 (5) | 68 | 5 (7) | 0.51 |
| Urinary tract infection | 139 | 2 (1) | 68 | 1 (1) | 1 |
| Wound infection | 139 | 7 (5) | 68 | 2 (3) | 0.73 |
| Catheter infection | 139 | 1 (1) | 68 | 0 (0) | 1 |
ACEi, angiotensin converting enzyme inhibitor; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; OR, operating room; pH, potential of hydrogen; SD, standard deviation.
Numbers apply unless otherwise indicated by numbers in rows of the table.
Mantel-Haenszel statistic.
Table 4.
Preoperative inflammatory and vascular endothelial markers stratified by acute kidney injury (AKI)
| Variables | No AKI, median (IQR) | AKI, median (IQR) | P value |
|---|---|---|---|
| CRP (mg/L) | 0.5 (0.3-1.4) | 0.4 (0.3-0.9) | 0.86 |
| TNF-α (ng/mL) | 10.4 (7.8-15.2) | 10.0 (8.0-15.1) | 0.94 |
| IL-1a (pg/mL) | 0.3 (0.0-0.8) | 0.2 (0.0-1.0) | 0.95 |
| IL-1b (pg/mL) | 0.5 (0.3-0.7) | 0.5 (0.3-0.7) | 0.94 |
| IL-2 (pg/mL) | 3.4 (1.9-5.0) | 3.1 (1.6-5.3) | 0.5 |
| IL-4 (pg/mL) | 1.4 (0.8-2.0) | 1.4 (1.0-2.2) | 0.35 |
| IL-6 (pg/mL) | 4.2 (3.2-6.1) | 4.3 (2.9-6.5) | 0.97 |
| IL-8 (pg/mL) | 12.4 (9.5-15.4) | 12.5 (9.2-16.3) | 0.71 |
| IL-10 (pg/mL) | 1.0 (0.5-1.5) | 1.0 (0.6-1.5) | 0.85 |
| IL-12p70 (pg/mL) | 3.0 (1.9-4.9) | 3.1 (1.8-5.7) | 0.86 |
| Microparticles (×106/mL) | 15.7 (9.4-23.7) | 16.5 (8.6-21.2) | 0.66 |
| Endothelial microparticles | 0.01 (0.01-0.02) | 0.01 (0.0-0.03) | 0.96 |
| Leukocyte microparticles | 0.6 (0.3-1.0) | 0.6 (0.4-0.8) | 0.63 |
| Platelet microparticles | 7.4 (4.1-14.7) | 8.1 (4.7-13.4) | 0.88 |
| GM-CFS (pg/mL) | 26.5 (20.1-38.0) | 23.0 (23.1-38.4) | 0.27 |
| MCP-1 (pg/mL) | 23.0 (17.5-29.0) | 24.9 (18.7-33.5) | 0.08 |
| PDGF (pg/mL) | 100.4 (50.2-175.1) | 113.5 (54.3-194.8) | 0.45 |
| RANTES (ng/mL) | 2703 (2159-3120) | 2873 (2285-3339) | 0.09 |
| VEGF (pg/mL) | 7.01 (4.1-11.7) | 7.4 (4.7-15.5) | 0.18 |
CRP, C-reactive protein; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukin; IQR, interquartile range; MCP-1, monocyte chemoattractant protein-1; PDGF, platelet-derived growth factor; RANTES, regulated on activation, normal T-cell expressed and secreted; TNF-α, tumour necrosis factor α; VEGF, vascular endothelial growth factor.
The characteristics of the study population by AKI status are noted in Table 1. Baseline SCr was obtained within 1 week before the surgery for 67% (n = 129) of patients and within 30 days before the surgery for 84% (n = 176) of patients (see Fig. 1). The mean preoperative creatinine level was 35 ± 15 μmol/L, and the mean peak creatinine level at a median of 1 day after surgery was 50 ± 32 μmol/L. The median absolute increase was 9 mmol/L (IQR: 3-18 mmol/L), and the median percent increase was 128% (IQR: 109%-159%). AKI, as defined above, occurred in 33% (n = 68) of patients within 7 days of surgery. Of the patients with AKI, 37 had a ≥150% increase in creatinine but not a ≥26.5 μmol/L absolute increase, 30 patients had both, and 1 patient with a preoperative creatinine of 117 μmol/L had an absolute increase of 39 μmol/L but only a 133% increase.
The median age in the AKI group was 0.51 years (IQR: 0.28-1.71 years) vs 0.44 years (IQR: 0.07-2.79 years) in the non-AKI group. A younger age was not significantly related to AKI (P = 0.28). The mean CPB time was longer in the AKI group at 154 ± 73 minutes vs 111 ± 56 minutes in the non-AKI group. A comorbid genetic abnormality was not significantly associated with AKI (21% in AKI vs 13% in non-AKI, P = 0.16), nor was having Down syndrome (10% vs 7%, P = 0.44). Having DiGeorge syndrome was a significant risk factor for AKI (7% vs 1%, P = 0.04). The number of previous cardiac surgeries was not significantly associated with AKI (P = 0.13). The number of previous cardiac catheterizations was a significant risk factor for AKI (P = 0.008). This information can be found in Table 2.
On univariate analysis, preoperative cyanosis (41% vs 24%, P < 0.03), lung collapse (12% vs 4%, P < 0.04), preoperative elevated alanine transaminase (ALT) (10% vs 1%, P < 0.002), preoperative diuretic use (43% vs 27%, P < 0.03), preoperative prostaglandin use (9% vs 24%, P < 0.02), and preoperative steroid use (9% vs 21%, P < 0.03) were significantly related to AKI. This can be found in Table 3.
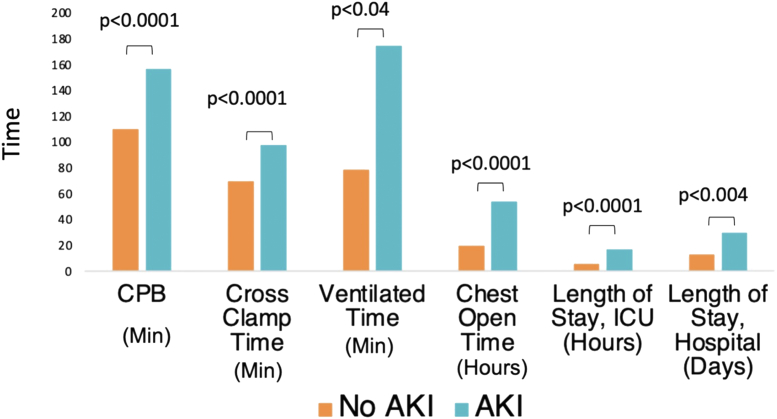
Adverse outcomes significantly related to the presence of AKI included increased total time of an open chest (median: 5.2 vs 4.0 hours, P < 0.001), increased ventilation time (56.8 vs 27.4 hours, P < 0.01), increased ICU stay (3.0 vs 5.6 hours, P < 0.001), and increased overall hospital stay (266 vs 190 hours, P = 0.04) (Fig. 2).
Figure 2.
Comparison of AKI and non-AKI cohorts for significant ischemia variables and relevant clinical outcomes in terms of median times. AKI, acute kidney injury; CPB, cardiopulmonary bypass; ICU, intensive care unit.
No aspect of the complete blood cell count was significantly associated with AKI. The biochemistry results showed that an increased preoperative ALT (median: 31 U/mL in non-AKI vs 29 U/mL in AKI, P = 0.02) was significantly associated with AKI in univariate analysis (Table 3).
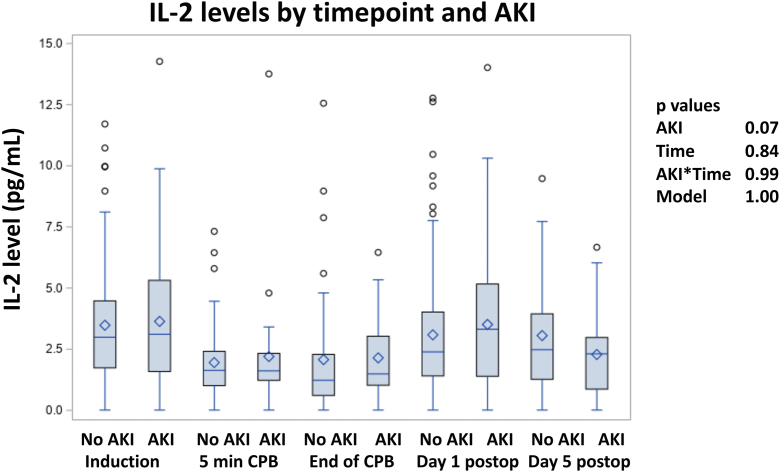
None of the inflammatory cytokines, vascular markers, or microparticle-type levels assessed preoperatively (at the time of induction) were significantly associated with AKI (Table 4). In mixed linear regression analysis also incorporating levels assessed at the additional time points of 5 minutes into CPB, end of CPB, and postoperative days 1 and 3, no marker was significantly associated with AKI over all the time points, including an interaction term of AKI with time (data not shown). Figure 3 for time-related values for IL-2 by AKI status is provided as a representative of the observed time course for the markers assessed.
Figure 3.
Interleukin-2 (IL-2) levels at all time points. AKI, acute kidney injury; CPB, cardiopulmonary bypass.
In multivariable logistic regression analysis for AKI, only a lower preoperative creatinine level (parameter estimate: −0.048, standard error: 0.015; P < 0.001) and a longer total duration of CPB (parameter estimate: 0.0109, standard error: 0.027; P < 0.001) were independently associated with an increased probability of AKI (model c-statistic 0.78). In a multivariable linear regression analysis for the absolute change in creatinine, a higher preoperative aspartate transaminase level, longer total duration of cardiopulmonary artery bypass, cardiac catheter reinterventions, and ECMO were all independently associated with a greater increase in creatinine (Table 5).
Table 5.
Multivariable logistic regression analysis for AKI and multivariable linear regression analysis for the absolute change in creatinine
| Variable | Parameter estimate | Standard error | P value |
|---|---|---|---|
| Multivariable logistic regression analysis for AKI∗ | |||
| Lower preoperative creatinine (per mg/dL) | −0.048 | 0.015 | <0.001 |
| Longer total duration of CPB (per 10 min) | 0.109 | 0.027 | <0.001 |
| Multivariable linear regression analysis for absolute change in creatinine† | |||
| Higher preoperative AST level (per 10 U/mL) | 0.32 | 0.03 | <0.001 |
| Longer total duration of CPB (per 10 min) | 0.87 | 0.23 | <0.001 |
| Catheter reintervention | 23.3 | 7.8 | 0.004 |
| ECMO | 27.2 | 7.4 | <0.001 |
AKI, acute kidney injury; AST, aspartate transaminase; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation.
Not including repeated measures of inflammatory and vascular markers and microparticles.
Model c-statistic = 0.78.
Adjusted model R2= 0.52; note that preoperative creatinine was not significant in this model.
Further analyses were performed using Kidney Disease: Improving Global Outcomes (KDIGO) 2012 staging criteria, excluding urine output data that were not collected (results not shown).19 Of the 68 patients with AKI, 38 were classified at stage 1, 23 as stage 2, and 7 as stage 3. Stage 2 and 3 patients were combined and compared separately with stage 0 only patients and stage 0 and 1 patients combined. Given that this decreased the size of the AKI group, thus decreasing the statistical power, P values related to comparisons increased, with no new findings. In addition, the magnitude of difference also decreased for the majority of factors compared.
Discussion
Incidence of AKI
This is one of the largest prospective studies assessing AKI in paediatric patients undergoing cardiac surgery as well as examining serum inflammatory and serum vascular markers. We found an incidence of AKI of 33%. This is similar to the Translational Research in Biomarker Endpoints and Applications in Kidney Disease (TRIBE-AKI) consortium, who found an incidence in a similar population of 42%2 and a meta-analysis that found a pooled estimated incidence of 34%.10
Demographics
In our cohort, a younger age was not associated with AKI. This breaks from multiple other studies that show younger age as a risk factor for AKI.2,22 Our exclusion criteria did omit patients <2.5 kg, but studies like TRIBE-AKI with an older cohort and studies looking at just neonates both did show younger age as a risk factor for AKI.22 A possible explanation for our finding is that younger children have higher blood creatinine levels; thus the absolute level would be higher. This would make it more difficult to be diagnosed with AKI as per our definition. Syndromes (including Down syndrome) and any noncardiac congenital abnormality were not associated with developing AKI. However, DiGeorge syndrome was a risk factor for AKI but had a small sample size.
Case complexity
A new finding from our study is that the number of previous cardiac catheterizations was an independent risk factor for AKI. This was not reported in other studies and is possibly related to an increase in contrast used although this was not looked at in this study. This is likely related to the increased complexity of their cardiac anatomy and is likely a surrogate of complexity in these children. In multiple studies, a higher Risk Adjustment for Congenital Heart Surgery score has been a risk factor for AKI.2,10 In our study, the number of previous cardiac surgeries was not associated with AKI.
Preoperative renal function
Paradoxically, lower preoperative creatinine was significantly associated with AKI. This also was significant on multivariable logistic regression analysis. This has consistently been found in other studies.2,10 An explanation for this may be that this finding might result from the definition of AKI itself, as current criteria mainly focus on relative increases in SCr.23 In patients with lower preoperative SCr levels, a smaller increase in absolute terms is needed to reach a 1.5-fold increase. Therefore, these children are more likely to be caught as having AKI by our criteria. We feel this may be an example of regression towards the mean. On the other hand, children with higher preoperative SCr levels are at risk of developing AKI that remains undetected.
Preoperative medications
Preoperative diuretic use was common throughout our cohort, with 32% of patients on that medication. From univariate analysis, there was a significant difference between patients who developed AKI and those who did not (43% vs 27%, P < 0.03). Postoperative diuretic use is controversial, but preoperative furosemide use has been found to be an independent risk factor for postoperative AKI in cardiac surgery before.24 The specific pathophysiology of this is still not clear. From our study, preoperative antibiotics, which may include nephrotoxic drugs, was not associated with AKI. Of note, the specific antibiotics that are concerning (eg, gentamicin) were not specifically elicited from this study. Previous studies have noted that nephrotoxic medications, including antibiotics, were associated with AKI.2
Preoperative clinical status
Preoperative cyanosis (41% in AKI vs 24% in non-AKI, P = 0.02) was significantly related to AKI in our cohort. Glomerulopathy has been seen and reported as a common feature in cyanotic congenital heart disease.25 The dominant feature appears to be glomerular damage, which directly related to the duration of cyanosis.25 This would put these patients at risk of developing AKI when a significant stressor, like CPB, is applied. This contrasts the fact that patients with preoperative prostaglandin use, commonly used in cyanotic heart conditions, were less likely to have AKI (9% vs 24%, P < 0.01). As was seen, an elevated preoperative ALT was associated with AKI, which may be a proxy for multiorgan dysfunction increasing risk for AKI.
Operative factors
It should not be surprising that the mean CPB time and the mean aortic cross-clamp time were higher in the AKI group. Studies have shown that increasing CPB times is associated with increasing trends of developing AKI.2 Furthermore, patients with >180 minutes of CPB were at 7.5× higher change of developing AKI.2 This is possibly related to the haemolysis, production of free radicals, and the low-flow, low-pressure nonpulsatile perfusion in CPB.12 This finding was also confirmed on multivariable logistic regression analysis (P < 0.001). This highlights that ischemia likely plays a critical role in the etiology of AKI.
Postoperative management
In terms of outcomes associated with AKI, we found that those who developed AKI were more likely to remain on mechanical ventilation for a longer period of time and be in the ICU for longer. The etiology of this association is likely multifactorial, with contributing factors specific to AKI including increased fluid retention, interstitial oedema, and prolongation of sedation secondary to decreased drug elimination because of AKI.2 These may be confounded by variables such as right ventricular dysfunction, right ventricular hypertrophy, and residual cardiac lesions.2 In addition, AKI may also be a colinear factor with other morbidities that further enhance these associations. Patient and procedural factors reflective of case complexity may be further confounding factors.
Inflammatory markers
An interesting finding of this study was that no inflammatory cytokine biomarkers (IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 p70, and TNF-α) at any of the time points described in the study were found to be significantly associated with AKI. The TRIBE-AKI Consortium found that preoperative IL-8 and postoperative TNF-α where significantly related to AKI and greater hospital lengths in children undergoing cardiac surgery and who were >2 years old.16 Another study found that serum IL-6 and IL-8 were early biomarkers of AKI in this same population and were associated with prolonged mechanical ventilation.14 Of note, none of these were found in our study. Because steroid use before the index operation was associated with AKI in this study, it is possible that steroid use may be a confounding factor affecting inflammatory biomarkers.
Vascular markers
One aspect of this study with a high impact was looking at vascular endothelial markers in children who underwent cardiac surgery. Previously, smaller studies have shown that circulating endothelial microparticles are an independent risk factor for developing AKI in patients undergoing cardiac surgery.13 Furthermore, other endothelial vascular markers, such as RANTES, have been shown to mediate ischemia reperfusion injury in the kidney. There is some evidence linking RANTES and the nuclear factor kappa B pathway to AKI.18 Our study did not find an association with any inflammatory, vascular endothelial, or microparticle biomarker and AKI. This applied to not only absolute preoperative values but also trends of the biomarkers.
Limitations
This study has a few limitations. First, it was designed as a secondary analysis for a thrombosis study. Therefore, not all variables associated with renal function were assessed. Secondly, the diagnosis of AKI was made retrospectively, and urine output data were not captured; therefore, just one aspect of the KDIGO 2012 definition was used. Moreover, this was a single-centre study; thus we are limited in what can be extrapolated to larger populations. Furthermore, our exclusion criteria removed patients <2.5 kg; therefore, we cannot comment on younger and smaller patients, who are likely to have different renal function than our population. As this study was not originally intended to assess AKI, we were unable to gather preoperative information about urinary structural abnormalities. Lastly, this cohort only consisted of patients where we had both and preoperative and postoperative creatinine and thus is subject to sampling bias with potential for disease severity.
Conclusions
In conclusion, our study reveals that AKI remains a common problem after heart surgery, affecting one-third of paediatric patients. AKI was associated with increased complexity, for example, in patients with an increased number of cardiac catheterizations, increased bypass time, and increased cross-clamp time. Moreover, AKI was associated with adverse clinical outcomes including increased ICU duration and increased hospital stay, as shown in other studies. Importantly, our study did not show any association between inflammatory, vascular endothelial or microparticle biomarkers, and AKI. Independent risk factors for AKI included longer CPB time, which suggests that ischemia, rather than inflammation, plays an important role in the etiology of AKI after paediatric cardiac surgery.
Acknowledgments
Ethics Statement
This study received ethics approval from the SickKids Research Ethics Board with waiver of consent.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
Editorial Disclaimer
Given his role as Associate Editor, Brian McCrindle had no involvement in the peer review of this article and has no access to information regarding its peer review.
References
- 1.Greenberg J.H., Zappitelli M., Devarajan P., et al. Kidney outcomes 5 years after pediatric cardiac surgery: the TRIBE-AKI study. JAMA Pediatr. 2016;170:1071–1078. doi: 10.1001/jamapediatrics.2016.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S., Krawczeski C.D., Zappitelli M., et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg J.H., Devarajan P., Thiessen-Philbrook H.R., et al. Kidney injury biomarkers 5 years after AKI due to pediatric cardiac surgery. Pediatr Nephrol. 2018;33:1069–1077. doi: 10.1007/s00467-018-3888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland S.M., Ji J., Sheikhi F.H., et al. AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8:1661–1669. doi: 10.2215/CJN.00270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw N.J., Brocklebank J.T., Dickinson D.F., Wilson N., Walker D.R. Long-term outcome for children with acute renal failure following cardiac surgery. Int J Cardiol. 1991;31:161–165. doi: 10.1016/0167-5273(91)90211-7. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos K., Diller G.P., Koltsida E., et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–2328. doi: 10.1161/CIRCULATIONAHA.107.734921. [DOI] [PubMed] [Google Scholar]
- 7.Heying R., Seghaye M.C., Grabitz R.G., et al. Mid-term follow-up after multiple system organ failure following cardiac surgery in children. Acta Paediatr. 1999;88:1238–1243. doi: 10.1080/080352599750030356. [DOI] [PubMed] [Google Scholar]
- 8.Ishani A., Nelson D., Clothier B., et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 9.Coca S.G., Singanamala S., Parikh C.R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van den Eynde J., Delpire B., Jacquemyn X., et al. Risk factors for acute kidney injury after pediatric cardiac surgery: a meta-analysis. Pediatr Nephrol. 2022;37:509–519. doi: 10.1007/s00467-021-05297-0. [DOI] [PubMed] [Google Scholar]
- 11.Basu R.K., Chawla L.S., Wheeler D.S., Goldstein S.L. Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol. 2012;27:1067–1078. doi: 10.1007/s00467-011-2024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. doi: 10.1038/nrneph.2017.119. [DOI] [PubMed] [Google Scholar]
- 13.Ma J., Yuan H.X., Chen Y.T., et al. Circulating endothelial microparticles: a promising biomarker of acute kidney injury after cardiac surgery with cardiopulmonary bypass. Ann Transl Med. 2021;9:786. doi: 10.21037/atm-20-7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K.D., Altmann C., Smits G., et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg J.H., Whitlock R., Zhang W.R., et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30:1519–1527. doi: 10.1007/s00467-015-3088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Fontnouvelle C.A., Greenberg J.H., Thiessen-Philbrook H.R., et al. Interleukin-8 and tumor necrosis factor predict acute kidney injury after pediatric cardiac surgery. Ann Thorac Surg. 2017;104:2072–2079. doi: 10.1016/j.athoracsur.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg J.H., Zappitelli M., Jia Y., et al. Biomarkers of AKI progression after pediatric cardiac surgery. J Am Soc Nephrol. 2018;29:1549–1556. doi: 10.1681/ASN.2017090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu T.M., Palanisamy K., Sun K.T., et al. RANTES mediates kidney ischemia reperfusion injury through a possible role of HIF-1alpha and LncRNA PRINS. Sci Rep. 2016;6 doi: 10.1038/srep18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Section 1: introduction and methodology. Kidney Int Suppl (2011) 2012;2:13–18. doi: 10.1038/kisup.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacour-Gayet F., Clarke D., Jacobs J., et al. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg. 2004;25:911–924. doi: 10.1016/j.ejcts.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Lacour-Gayet F., Maruszewski B., Mavroudis C., Jacobs J.P., Elliott M.J. Presentation of the International Nomenclature for Congenital Heart Surgery. The long way from nomenclature to collection of validated data at the EACTS. Eur J Cardiothorac Surg. 2000;18:128–135. doi: 10.1016/s1010-7940(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 22.Morgan C.J., Zappitelli M., Robertson C.M., et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–127.e121. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Algaze C.A., Koth A.M., Faberowski L.W., et al. Acute kidney injury in patients undergoing the extracardiac Fontan operation with and without the use of cardiopulmonary bypass. Pediatr Crit Care Med. 2017;18:34–43. doi: 10.1097/PCC.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H., Liu L., Fan G., et al. Preoperative use of furosemide may increase the incidence of acute kidney injury after coronary artery bypass grafting: a propensity score-matched study. Gen Thorac Cardiovasc Surg. 2021;69:1392–1399. doi: 10.1007/s11748-021-01599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dittrich S., Haas N.A., Buhrer C., et al. Renal impairment in patients with long-standing cyanotic congenital heart disease. Acta Paediatr. 1998;87:949–954. doi: 10.1080/080352598750031608. [DOI] [PubMed] [Google Scholar]