Abstract
Background
Normative data for the effect of cardiopulmonary bypass (CPB) on coronary artery Doppler velocities by transesophageal echocardiography in paediatric patients with congenital heart disease (CHD) are lacking. The objective of the study was to prospectively examine the effects of CPB on coronary artery flow patterns by transesophageal echocardiography before and after CPB in children with CHD.
Methods
All cases undergoing CHD surgery at the Hospital for Sick Children, Toronto, were eligible. The excluded cases included Norwood operation, heart transplantation, or weight <2.5 kg. Coronary Dopplers and coronary flow reserve (CFR) for the right coronary artery (RCA) and left anterior descending (LAD) were obtained. Multivariable analyses using linear regression models were performed, adjusted for age and cross-clamp time.
Results
From May 2017 to June 2018, 69 children (median age at surgery: 0.7 years, interquartile range [IQR]: 0.4-3.7 years; median weight: 7.4 kg, IQR: 5.8-13.3 kg) were included. They were grouped into shunt lesions (N = 26), obstructive lesions (N = 26), transposition of the great arteries (N = 5), and single ventricle (N = 12). N = 39 (57%) were primary repairs, and 56 (81%) had 1 CPB run. For RCA and LAD peak velocities, there was an increase from pre- to post-CPB in RCA peak 39 cm/s (IQR: 30-54 cm/s) to 65 cm/s (IQR: 47-81 cm/s), P < 0.001, mean CFR 1.52 (IQR: 1.25-1.81), and LAD peak 49 cm/s (IQR: 39-60 cm/s) to 70 cm/s (IQR: 52-90 cm/s), P < 0.001, mean CFR 1.48 (IQR: 1.14-1.77).
Conclusions
Coronary flow velocities increase from pre- to post-CPB in congenital heart lesions. CFR is consistent across all lesions but is relatively low compared with the adult population.
Résumé
Contexte
On ne dispose pas de données normatives sur les effets de la dérivation cardiopulmonaire (DCP) sur le débit coronarien mesuré au moyen d’une échocardiographie transœsophagienne Doppler chez des enfants présentant une cardiopathie congénitale. L’objectif de l’étude était d’examiner de manière prospective les effets de la DCP sur le débit coronarien avant et après l’intervention chez des enfants présentant une cardiopathie congénitale.
Méthodologie
Tous les enfants ayant subi une intervention chirurgicale pour une cardiopathie congénitale à l’Hospital for Sick Children de Toronto étaient admissibles à l’étude, à l’exception de ceux ayant subi une intervention de Norwood ou une transplantation cardiaque, de même que les enfants pesant moins de 2,5 kg. Les résultats du test Doppler et la réserve coronarienne pour l’artère coronaire droite (ACD) et la branche antérieure de l’artère coronaire gauche (ACG) ont été obtenus. Des analyses multivariées ont été réalisées au moyen de modèles de régression linéaire, avec correction en fonction de l’âge et du temps de clampage total.
Résultats
Entre mai 2017 et juin 2018, 69 enfants (âge médian au moment de la chirurgie : 0,7 an, intervalle interquartile (IIQ) : 0,4-3,7 ans; poids médian : 7,4 kg, IIQ : 5,8-13,3 kg) ont été inclus dans l’étude. Les sujets ont été répartis en quatre groupes : shunts (n = 26), lésions obstructives (n = 26), permutation des gros vaisseaux (n = 5) et ventricule unique (n = 12). Chez 39 sujets (57 %), il s’agissait d’une réparation primitive, et 56 enfants (81 %) avaient déjà subi une DCP. Les vitesses maximales dans l’ACD et dans la branche antérieure de l’ACG ont augmenté après la DCP, passant de 39 cm/s (IIQ : 30-54 cm/s) à 65 cm/s (IIQ : 47-81 cm/s), p < 0,001; réserve coronarienne moyenne : 1,52 (IIQ : 1,25-1,81) pour l’ACD, et de 49 cm/s (IIQ : 39-60 cm/s) à 70 cm/s (IIQ : 52-90 cm/s), p < 0,001; réserve coronarienne moyenne : 1,48 (IIQ : 1,14-1,77) pour la branche antérieure de l’ACG.
Conclusions
Le débit coronarien augmente après une DCP dans les cas de lésions cardiaques congénitales. La réserve coronarienne est constante dans tous les types de lésions, mais elle est relativement faible comparativement à celle de la population adulte.
Transthoracic coronary artery assessment by 2D, colour, and pulse wave Doppler is frequently used to define coronary artery anatomy and physiology in congenital cardiac lesions (such as transposition of the great arteries [TGA], anomalous coronary artery from pulmonary artery, and coronary fistulae) and in acquired cardiac lesions (eg, Kawasaki’s disease).1, 2, 3, 4, 5 Normal coronary flow is defined as biphasic, low velocity with a diastolic peak, and only antegrade flow. “Normal” baseline flow velocities in the left coronary artery are generally accepted between 30 and 60 cm/s depending on the age of the patient, either invasively, or by echocardiography.10, 6, 7, 8, 9 Studies have shown that coronary artery velocities in children decrease with age and heart rate11 and that coronary flow velocities in infants and children are higher than those in adults.12 Interestingly, in neonates, coronary velocities show a linear increase with age and left ventricular (LV) mass.13
The impact of congenital heart surgery on intraoperative coronary blood flow has not been well studied. Cardiopulmonary bypass (CPB) and intracoronary injection of cardioplegia influence coronary physiology and result in coronary vasodilatation.14 After congenital heart surgery, flow in the left anterior descending coronary artery (LAD) increases immediately after CPB and is dependent on cardioplegia dosage.15 In addition, cardioplegia is known to alter vasomotor function, both in the microvasculature and in the epicardial coronary bed, which can lead to myocardial dysfunction.16 In children undergoing cardiac surgery requiring coronary artery manipulation, alterations in coronary flow patterns can identify important coronary artery stenoses. Our group previously demonstrated that, in patients undergoing the arterial switch operation for TGA, increased coronary peak systolic velocity was associated with a need for surgical revision, and flow reversal in the left coronary artery was associated with major adverse outcomes.17,18
The primary objective of this study was to examine the effects of CPB on right and left coronary artery flow patterns and coronary velocities, and coronary flow reserve (CFR) before and after CPB in patients with a spectrum of congenital heart lesions, measured by transesophageal echocardiography (TEE). A secondary aim was to evaluate coronary flow abnormalities associated with adverse postoperative cardiac outcomes.
Materials and Methods
Study design and patient population
This prospective study was carried out at the Hospital for Sick Children, Toronto, Canada. Patients who underwent CPB for surgical correction of congenital heart lesions between May 8, 2017, and June 26, 2018, were included after parental informed consent. We excluded patients who underwent stage 1 palliation for hypoplastic left heart syndrome with either a Norwood procedure or a hybrid procedure, patients who underwent heart transplantation, patients with anomalous pulmonary or aortic origin of a coronary artery, patients where a TEE was contraindicated (less than 2.5 kg, recent upper gastrointestinal surgery) and operations where TEE was not routinely performed (patent arterial duct ligation, aortic coarctation, and vascular ring), or if informed consent was not obtained. Cases were excluded if the TEE coronary Dopplers were not obtained either before or after CPB or if the tracings were inadequate for analysis. In all cases, standard del Nido cardioplegia was used.19
Patients were divided into the following 4 subcategories: (1) shunt lesions (atrial or ventricular septal defects [VSDs], atrioventricular septal defect, and total anomalous pulmonary venous drainage); (2) left or right outflow tract obstruction; (3) TGA; and (4) single ventricle circulations (patients with hypoplastic right or left ventricles who were on the single ventricle palliative route after stage 1 palliation). Patient demographics were obtained from the patient’s chart. Adverse outcome was defined as death, or a composite of ST changes or ventricular tachycardia/fibrillation, or use of extracorporeal membrane oxygenation (ECMO) postoperatively to the time of hospital discharge.
The study was approved by the hospital’s research ethics board.
Demographic data
Clinical and operative data were obtained for each case up until hospital discharge. Relevant variables are represented in Table 1.
Table 1.
Demographic and clinical data of all cases, and the 4 subgroups
| Demographics and clinical characteristics | N | Total | N | Shunt | N | Obstruction | N | TGA | N | Single ventricle |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 69 | 34 (49) | 26 | 9 (35) | 26 | 10 (38) | 5 | 5 (100) | 12 | 10 (83) |
| Gestational age at birth (wk) | 21 | 38 ± 3 | 8 | 38 ± 3 | 5 | 39 ± 3 | 3 | 39 ± 2 | 5 | 38 ± 3 |
| Age at surgery (y) | 69 | 0.7 (0.4-3.7) | 26 | 0.7 (0.4-2.3) | 26 | 1.1 (0.5-9.1) | 5 | 0.1 (0.1-0.1) | 12 | 2.8 (0.7-3.4) |
| Weight at surgery (kg) | 69 | 7.4 (5.8-13.3) | 26 | 6.3 (5.2-11.8) | 26 | 10.2 (6.6-21.7) | 5 | 4.1 (3.9-4.1) | 12 | 12.9 (6.8-13.6) |
| BSA at the time of surgery (m2) | 66 | 0.4 (0.3-0.6) | 26 | 0.3 (0.3-0.5) | 24 | 0.5 (0.3-0.8) | 5 | 0.2 (0.2-0.2) | 11 | 0.6 (0.3-0.7) |
| Type of repair | 69 | 26 | 26 | 5 | 12 | |||||
| Primary repair | 39 (57) | 23 (88) | 11 (42) | 5 (100) | 0 (0) | |||||
| Reoperation | 30 (43) | 3 (12) | 15 (58) | 0 (0) | 12 (100) | |||||
| Coronaries were manipulated on first operation | 21 | 2 (10) | 3 | 0 (0) | 10 | 1 (10) | 0 | 0 (0) | 8 | 1 (13) |
| Coronaries manipulated intraoperatively | 69 | 8 (12) | 26 | 0 (0) | 26 | 2 (8) | 5 | 5 (100) | 12 | 1 (8) |
| X clamp time (min) | 59 | 79 (48-110) | 25 | 62 (47-106) | 26 | 75 (59-105) | 5 | 127 (107-128) | 3 | 59 (0-79) |
| Bypass time (min) | 67 | 102 (65-136) | 25 | 82 (70-123) | 26 | 110 (75-167) | 5 | 154 (138-183) | 11 | 84 (49-122) |
| Circulatory arrest | 68 | 5 (7) | 26 | 2 (8) | 26 | 1 (4) | 5 | 1 (20) | 11 | 1 (9) |
| Circulatory arrest time (min) | 5 | 24 (15-32) | 2 | 15 (15-33) | 1 | 4 (4-4) | 1 | 24 (24-24) | 1 | 32 (32-32) |
| Number of bypass runs | 69 | 26 | 26 | 5 | 12 | |||||
| 1 | 56 (81) | 22 (85) | 22 (85) | 2 (40) | 10 (83) | |||||
| 2 | 9 (13) | 2 (8) | 4 (15) | 2 (40) | 1 (8) | |||||
| Missing | 4 (6) | 2 (8) | 0 (0) | 1 (20) | 1 (8) | |||||
| VT/VF noted in OR | 65 | 1 (2) | 25 | 0 (0) | 26 | 1 (4) | 3 | 0 (0) | 11 | 0 (0) |
| ST segment changes noted in OR | 65 | 4 (6) | 25 | 3 (12) | 26 | 0 (0) | 3 | 1 (33) | 11 | 0 (0) |
| Chest left open | 68 | 6 (9) | 26 | 0 (0) | 26 | 2 (8) | 5 | 4 (80) | 11 | 0 (0) |
| Duration of ICU stay (d) | 63 | 2.0 (1.0-3.0) | 25 | 2.0 (1.0-2.0) | 22 | 2.0 (1.0-5.0) | 5 | 4.0 (4.0-9.0) | 11 | 2.0 (1.0-3.0) |
| Duration of hospital stay (d) | 66 | 6.0 (4.0-9.0) | 26 | 5.0 (4.0-6.0) | 24 | 5.0 (4.0-11.0) | 5 | 24.0 (8.0-25.0) | 11 | 9.0 (6.0-11.0) |
Data are presented as n (%) unless otherwise indicated.
BSA, body surface area; ICU, intensive care unit; OR, operating room; TGA, transposition of the great arteries; VF, ventricular fibrillation; VT, ventricular tachycardia.
Transesophageal echocardiography assessment before and after CPB
All pre- and post-CPB TEEs were performed by a staff echocardiographer (LEN, LM, AD, MKF) along with an advanced echocardiography fellow (CTM, SD, MAV, Xiaoling Zhang). Patients underwent a pre-CPB TEE in the operating room after induction of anaesthesia, before sternal opening. Standard 4, 3, and 2 chamber and transgastric views were obtained to qualitatively assess ventricular function. The heart rate and rhythm were recorded at the time of the TEE, in order to define systole and diastole. Both atrioventricular and semilunar valves were interrogated with colour Doppler. A qualitative assessment of ventricular function was performed and graded as normal, mild, or moderately to severely reduced. Atrioventricular and semilunar valve regurgitation was qualitatively assessed as none, mild, or moderate to severe. Coronary artery imaging was obtained from the short axis for the right coronary artery (RCA), left main coronary artery, LAD, and circumflex arteries as per our previously published protocols.8 For all coronaries, a 2D image of the vessel was obtained, and for the RCA and left main coronary artery, the connection to the aortic sinus was obtained. Colour and pulse wave Dopplers were obtained for each coronary artery when possible, with at least 3 consecutive heart beats and optimal image quality. Peak and mean velocities were recorded as well as the presence of flow reversal. A second TEE was performed after CPB to assess the results of the surgical repair as per our hospital protocol. In addition to the usual TEE variables, coronary arteries were reassessed by 2D, colour, and pulse wave Doppler. Similarly, a qualitative assessment of ventricular and valvar function was recorded.
The timing of coronary artery pulse wave Doppler was measured according to the electrocardiogram tracing, with systolic flow defined as the time interval from the onset of the QRS to the end of the T-wave and diastole defined as the end of the T-wave to the onset of the QRS. Analysis of coronary artery imaging was performed offline (Syngo Dynamics V10; Siemens Medical Solutions, Malvern, PA) by a single reviewer (CM, blinded to clinical outcomes, but aware of cardiac diagnosis, coronary anatomy, and type of surgical repair). In cases of diagnostic uncertainty, the case was reviewed by a second investigator (LEN).
CFR was calculated from both LAD and RCA peak and mean velocities by determining the ratio of post-CPB velocity to pre-CPB velocity, as previously validated by both transthoracic and TEE studies.20,21
Statistical analysis
Continuous variables were summarized in terms of medians and interquartile ranges (IQRs), with between-group differences assessed using Wilcoxon rank-sum tests. Dichotomous and polytomous variables were summarized in terms of the number and proportion of patients in each stratum, with between-group differences assessed using Fisher’s exact tests. Changes in pre-to-post coronary velocities were assessed using Wilcoxon signed-rank tests. Multivariable analyses of factors associated with coronary flow velocity and gradients were conducted using linear regression models and adjusted for age and cross-clamp time, which were selected for inclusion a priori to account for operative complexity.
Results
Two hundred and seventy-five families were approached in the preoperative clinic between May 8, 2017, and June 26, 2018. Of those, a total of 117 (43%) patients consented to the study. Two operative cases were cancelled after the pre-CPB TEE findings, after discussion with the surgeon. The first was a case with Noonan’s syndrome and pulmonary stenosis, where the outflow gradient was not severe by TEE under general anaesthetic, and the second case was a congenitally corrected TGA for double switch, where the LV function was severely reduced, which contrasted with the preoperative transthoracic echocardiogram.
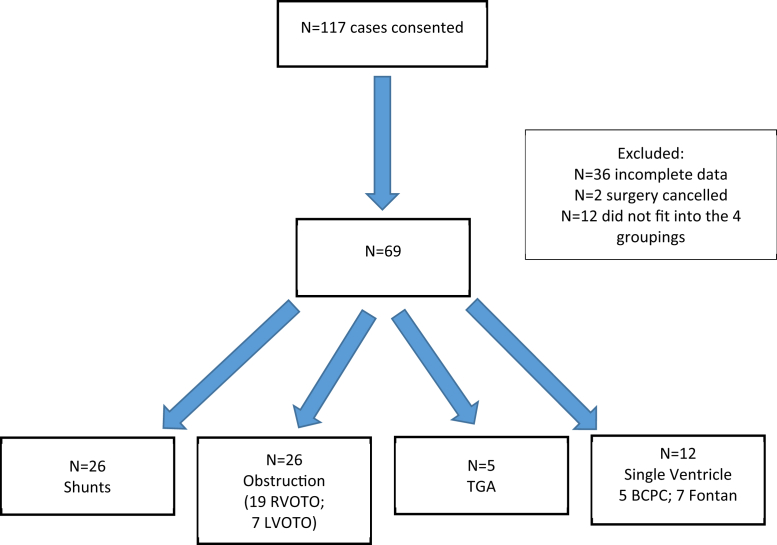
Of the initial 117 patients, 36 patients were excluded because of insufficient data. A further 12 patients did not fit into the 4 diagnostic categories (Fig. 1).
Figure 1.
Coronary TEE study patient inclusion/exclusion. BCPC, bidirectional Glenn; LVOTO, left outflow tract obstruction; RVOTO, right outflow tract obstruction; TEE, transesophageal echocardiography; TGA, transposition of the great arteries.
Therefore, a total of 69 patients were included in the final analysis (Table 1). There were 26 patients (38%) in the shunt group, 26 patients (38%) in the obstruction group, 5 patients (7%) in the TGA group, and 12 patients (17%) in the single ventricle group.
The coronary arteries were surgically manipulated in 7 cases: 5 arterial switch operations and 2 Ross procedures. Two patients underwent postoperative cardiac catheterization for suspected coronary stenosis. One patient had undergone an arterial switch operation for TGA. During this child’s arterial switch operation, the RCA button was revised twice, with noted increased velocity and electrocardiogram changes. The child returned to the operating room at day 12 for a revision of the RCA button. The other patient had a mechanical aortic valve, Konno, and mitral valve replacement with a left internal mammary artery coronary bypass graft to the RCA after accidental transection during the procedure (no revision was required). Two of the 5 cases of TGA required coronary revision during the hospital admission. One case was previously described. The second case had a Taussig-Bing anomaly with 1L2RCx anatomy. On postoperative day 3, a coronary angiogram demonstrated occlusion at the RCA/circumflex artery junction, and a thrombus was surgically removed. Coronary revision was not required in any of the other groups.
The median age at surgery was 0.7 years (range: 0.1-17.8 years, IQR: 0.4-3.7 years). The median weight at surgery was 7.4 kg (IQR: 5.8-13.3 kg). As expected, CPB time differed significantly between the groups, with the shortest bypass time in the shunt group and the longest bypass time in the TGA group. The median cross-clamp time was longest in the TGA patients (127 minutes, IQR: 107-128 minutes) followed by obstruction (75 minutes, IQR: 59-105 minutes) and shunt patients (62 minutes, IQR: 47-106 minutes), with the shortest time among the single ventricle patients (59 minutes, IQR: 0-79 minutes), although there was no statistically significant difference between the groups (P = 0.13). Nine cases (13%) required a secondary CPB run due to a residual lesion requiring surgical intervention. Preoperatively, no cases were on milrinone or norepinephrine, and 6 (9%) cases were on epinephrine, compared with 64 (93%) cases on milrinone, 18 (26%) on norepinephrine, and 56 (81%) cases on epinephrine after CPB. Delayed sternal closure occurred in 9% patients (n = 6). Ventricular fibrillation occurred in the operating room in 1 patient, and ST segment changes, either elevation or depression, were noted in 6% (n = 4). No patients required ECMO, and there were no deaths at the last follow-up.
There were statistically significant differences in intensive care stay (overall 2.0 days, IQR: 1-3 days, P = 0.01 between groups) and in total hospital stay (6 days, IQR: 4-9 days, P = 0.006 between groups) with longer stay in the TGA and single ventricle groups.
Pre- and post-CPB TEE coronary artery results
There were no complications related to either the pre- or post-CPB TEEs. Table 2 depicts the results for all coronary artery variables. The circumflex artery was the least frequently visualized (in 19 [28%] patients preoperatively and 22 [32%] intraoperatively), followed by the left main coronary artery (in 27 [39%] of patients pre- and intraoperatively).
Table 2.
Preoperative and intraoperative TEE findings at each time point and for each diagnostic category
| TEE findings | N | Total | N | Shunt | N | Obstruction | N | TGA | N | Single ventricle | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative LMCA | 19 | 4 | 5 | 3 | 7 | 0.05 | |||||
| Visualized | 7 (37) | 0 (0) | 1 (20) | 3 (100) | 3 (43) | ||||||
| Unable to visualize | 12 (63) | 4 (100) | 4 (80) | 0 (0) | 4 (57) | ||||||
| Intraoperative LMCA | 22 | 6 | 7 | 4 | 5 | 0.003 | |||||
| Visualized | 7 (32) | 1 (17) | 0 (0) | 4 (100) | 2 (40) | ||||||
| Unable to visualize | 15 (68) | 5 (83) | 7 (100) | 0 (0) | 3 (60) | ||||||
| Preoperative LAD | 67 | 25 | 26 | 5 | 11 | 0.14 | |||||
| Visualized | 64 (96) | 25 (100) | 25 (96) | 4 (80) | 10 (91) | ||||||
| Unable to visualize | 3 (4) | 0 (0) | 1 (4) | 1 (20) | 1 (9) | ||||||
| Intraoperative LAD | 67 | 26 | 26 | 4 | 11 | 0.04 | |||||
| Visualized | 62 (93) | 24 (92) | 26 (100) | 4 (100) | 8 (73) | ||||||
| Unable to visualize | 5 (7) | 2 (8) | 0 (0) | 0 (0) | 3 (27) | ||||||
| Preoperative RCA | 67 | 25 | 25 | 5 | 12 | 0.16 | |||||
| Visualized | 53 (79) | 16 (64) | 21 (84) | 5 (100) | 11 (92) | ||||||
| Unable to visualize | 14 (21) | 9 (36) | 4 (16) | 0 (0) | 1 (8) | ||||||
| Intraoperative RCA | 68 | 25 | 26 | 5 | 12 | 0.97 | |||||
| Visualized | 54 (79) | 19 (76) | 21 (81) | 4 (80) | 10 (83) | ||||||
| Unable to visualize | 14 (21) | 6 (24) | 5 (19) | 1 (20) | 2 (17) | ||||||
| Preoperative circumflex | 27 | 9 | 9 | 3 | 6 | 1.00 | |||||
| Visualized | 1 (4) | 0 (0) | 1 (11) | 0 (0) | 0 (0) | ||||||
| Unable to visualize | 26 (96) | 9 (100) | 8 (89) | 3 (100) | 6 (100) | ||||||
| Intraoperative circumflex | 27 | 8 | 9 | 3 | 7 | 0.13 | |||||
| Visualized | 2 (7) | 0 (0) | 0 (0) | 1 (33) | 1 (14) | ||||||
| Unable to visualize | 25 (93) | 8 (100) | 9 (100) | 2 (67) | 6 (86) | ||||||
| Preoperative LAD: flow reversal | 63 | 4 (6) | 25 | 1 (4) | 24 | 2 (8) | 4 | 0 (0) | 10 | 1 (10) | 0.74 |
| Intraoperative LAD: flow reversal | 62 | 9 (15) | 24 | 3 (13) | 26 | 5 (19) | 4 | 1 (25) | 8 | 0 (0) | 0.52 |
| Preoperative LAD: peak vs diastolic (cm/s) | 65 | 49 (39-60) | 26 | 49 (43-62) | 25 | 49 (36-62) | 4 | 44 (27-55) | 10 | 35 (27-55) | 0.45 |
| Intraoperative LAD: peak vs diastolic (cm/s) | 64 | 70 (52-90) | 26 | 71 (52-81) | 26 | 70 (55-93) | 4 | 53 (27-95) | 8 | 49 (33-87) | 0.63 |
| Preoperative LAD: mean vs diastolic (cm/s) | 65 | 24 (19-32) | 26 | 24 (22-33) | 25 | 23 (20-32) | 4 | 17 (14-24) | 10 | 22 (19-39) | 0.59 |
| Intraoperative LAD: mean vs diastolic (cm/s) | 64 | 37 (30-49) | 26 | 36 (31-48) | 26 | 38 (32-52) | 4 | 29 (19-30) | 8 | 36 (22-52) | 0.56 |
| Preoperative RCA: flow reversal | 53 | 5 (9) | 16 | 3 (19) | 21 | 2 (10) | 5 | 0 (0) | 11 | 0 (0) | 0.45 |
| Intraoperative RCA: flow reversal | 54 | 12 (22) | 19 | 2 (11) | 21 | 5 (24) | 4 | 1 (25) | 10 | 4 (40) | 0.28 |
| Preoperative RCA: peak vs diastolic (cm/s) | 53 | 39 (30-54) | 17 | 39 (30-52) | 21 | 41 (32-54) | 5 | 24 (23-30) | 10 | 41 (34-60) | 0.32 |
| Intraoperative RCA: peak vs diastolic (cm/s) | 55 | 65 (47-81) | 20 | 74 (54-90) | 21 | 56 (43-63) | 4 | 58 (24-74) | 10 | 72 (64-89) | 0.03 |
| Preoperative RCA: mean vs diastolic (cm/s) | 53 | 22 (16-29) | 17 | 25 (15-27) | 21 | 22 (19-29) | 5 | 16 (16-17) | 10 | 17 (15-33) | 0.49 |
| Intraoperative RCA: mean vs diastolic (cm/s) | 55 | 34 (26-42) | 20 | 34 (29-43) | 21 | 28 (23-37) | 4 | 31 (17-44) | 10 | 36 (33-42) | 0.22 |
| Preoperative rhythm | 69 | 26 | 26 | 5 | 12 | 1.00 | |||||
| Sinus | 68 (99) | 26 (100) | 25 (96) | 5 (100) | 12 (100) | ||||||
| Missing | 1 (1) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | ||||||
| Intraoperative rhythm | 69 | 26 | 26 | 5 | 12 | 0.06 | |||||
| Sinus | 57 (83) | 24 (92) | 19 (73) | 4 (80) | 10 (83) | ||||||
| Paced | 4 (6) | 2 (8) | 1 (4) | 1 (20) | 0 (0) | ||||||
| Other | 8 (12) | 0 (0) | 6 (23) | 0 (0) | 2 (17) | ||||||
| Preoperative LV function | 69 | 26 | 26 | 5 | 12 | – | |||||
| Normal | 69 (100) | 26 (100) | 26 (100) | 5 (100) | 12 (100) | ||||||
| Intraoperative LV function | 69 | 26 | 26 | 5 | 12 | 0.04 | |||||
| Normal | 60 (87) | 22 (85) | 24 (92) | 2 (40) | 12 (100) | ||||||
| Mildly reduced | 7 (10) | 3 (12) | 2 (8) | 2 (40) | 0 (0) | ||||||
| Moderately to severely reduced | 2 (3) | 1 (4) | 0 (0) | 1 (20) | 0 (0) | ||||||
| Preoperative RV function | 63 | 26 | 26 | 5 | 6 | 1.00 | |||||
| Normal | 62 (98) | 25 (96) | 26 (100) | 5 (100) | 6 (100) | ||||||
| Mildly reduced | 1 (2) | 1 (4) | 0 (0) | 0 (0) | 0 (0) | ||||||
| Intraoperative RV function | 63 | 26 | 26 | 5 | 6 | 0.02 | |||||
| Normal | 56 (89) | 23 (88) | 25 (96) | 2 (40) | 6 (100) | ||||||
| Mildly reduced | 7 (11) | 3 (12) | 1 (4) | 3 (60) | 0 (0) | ||||||
| Preoperative heart rate (beats/min) | 69 | 102 ± 22 | 26 | 108 ± 22 | 26 | 96 ± 23 | 5 | 122 ± 15 | 12 | 97 ± 14 | 0.04 |
| Intraoperative heart rate (beats/min) | 69 | 122 ± 24 | 26 | 123 ± 24 | 26 | 122 ± 26 | 5 | 137 ± 11 | 12 | 113 ± 24 | 0.31 |
| Preoperative systolic blood pressure (mm Hg) | 68 | 74 ± 12 | 26 | 73 ± 12 | 26 | 74 ± 12 | 5 | 60 ± 9 | 11 | 79 ± 11 | 0.03 |
| Intraoperative systolic blood pressure (mm Hg) | 66 | 76 ± 12 | 25 | 77 ± 11 | 25 | 77 ± 12 | 5 | 62 ± 3 | 11 | 80 ± 14 | 0.03 |
| Preoperative diastolic blood pressure (mm Hg) | 68 | 39 ± 8 | 26 | 38 ± 8 | 26 | 40 ± 9 | 5 | 33 ± 6 | 11 | 40 ± 7 | 0.29 |
| Intraoperative diastolic blood pressure (mm Hg) | 66 | 42 ± 8 | 25 | 41 ± 5 | 25 | 43 ± 10 | 5 | 34 ± 4 | 11 | 43 ± 7 | 0.06 |
| Preoperative O2 saturation (%) | 68 | 94 ± 8 | 26 | 97 ± 5 | 26 | 95 ± 9 | 5 | 94 ± 3 | 11 | 84 ± 8 | <0.001 |
| Intraoperative O2 saturation (%) | 66 | 98 ± 5 | 25 | 99 ± 3 | 25 | 99 ± 3 | 5 | 99 ± 1 | 11 | 91 ± 7 | <0.001 |
Data are presented as n (%) unless otherwise indicated.
LAD, left anterior descending; LMCA, left main coronary artery; LV, left ventricular; RCA, right coronary artery; RV, left ventricular; TEE, transesophageal echocardiography; TGA, transposition of the great arteries.
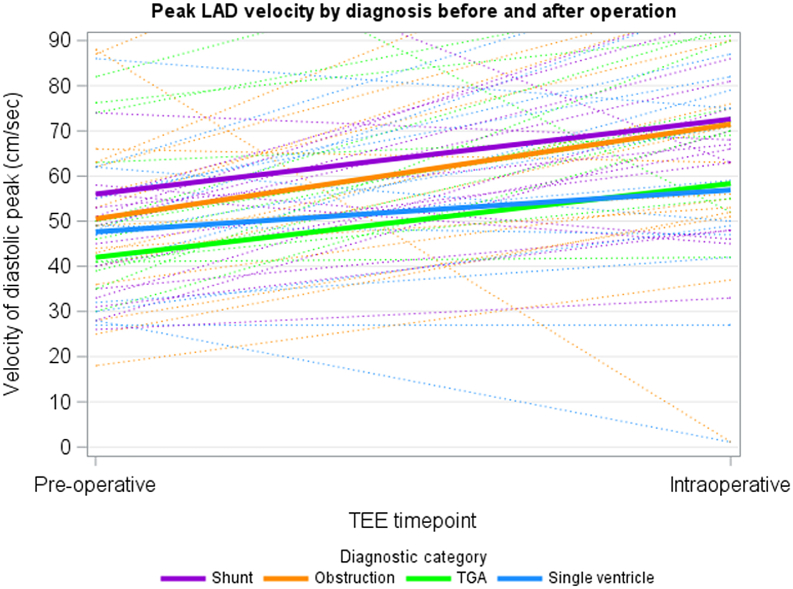
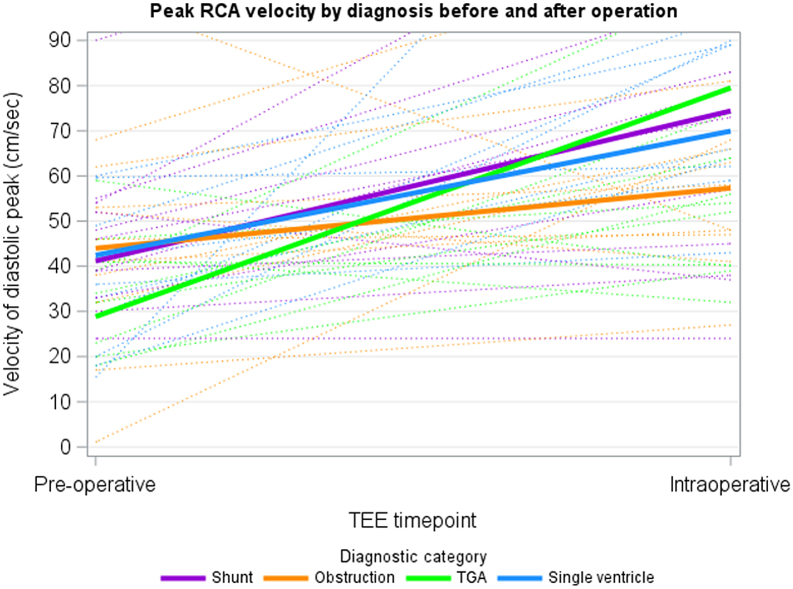
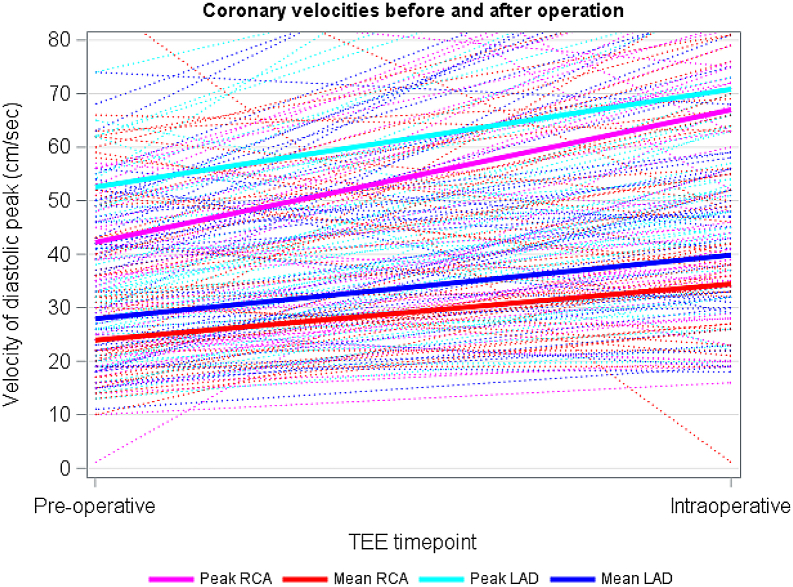
The LAD was adequately imaged in 93% (n = 64) of patients. Flow reversal was noted in 6% (n = 4) preoperatively. Figures 2 and 3 demonstrate peak LAD and RCA velocities by diagnostic category, respectively. Multivariable linear regression adjusted for age and cross-clamp time showed no statistically significant difference in pre-post peak or mean LAD or RCA velocity change between the 4 groups, although the preoperative RCA and LAD velocity was lowest in the TGA group, with the highest change in velocity for the RCA pre- and intraoperatively (Table 3). Figure 4 depicts the overall peak and mean RCA and LAD velocities for the entire cohort. The velocities all increased from pre- to post-CPB assessment. Figure 5 depicts the change in RCA and LAD Dopplers by TEE before and after CPB.
Figure 2.
Linear regression models of peak LAD velocities by diagnostic category (shunt N = 26, obstruction N = 26, TGA N = 5, single ventricle N = 12). LAD, left anterior descending; TEE, transesophageal echocardiography; TGA, transposition of the great arteries.
Figure 3.
Linear regression models of peak RCA velocities by diagnostic category (shunt N = 26, obstruction N = 26, TGA N = 5, single ventricle N = 12). RCA, right coronary artery; TEE, transesophageal echocardiography; TGA, transposition of the great arteries.
Table 3.
Multivariable regression results of the effect of diagnostic category on absolute change in RCA and LAD branch velocities, after controlling for age at surgery and cross-clamp time
| Outcome | Diagnostic group | Estimate (95% CI) | P value |
|---|---|---|---|
| Absolute change in RCA peak velocity | Shunt | Reference | |
| Obstruction | −17.59 (−40.87 to 5.7) | 0.14 | |
| TGA | 8.33 (−28.13 to 44.79) | 0.65 | |
| Single ventricle | −2.21 (−40.77 to 36.34) | 0.91 | |
| Absolute change in RCA mean velocity | Shunt | Reference | |
| Obstruction | −6.31 (−20.6 to 7.99) | 0.39 | |
| TGA | 17.31 (−5.07 to 39.7) | 0.13 | |
| Single ventricle | 3.87 (−19.8 to 27.54) | 0.75 | |
| Absolute change in LAD peak velocity | Shunt | Reference | |
| Obstruction | 5.4 (−8.71 to 19.5) | 0.45 | |
| TGA | 0.3 (−29.63 to 30.23) | 0.98 | |
| Single ventricle | 1.63 (−33.8 to 37.06) | 0.93 | |
| Absolute change in LAD mean velocity | Shunt | Reference | |
| Obstruction | 4.42 (−5.07 to 13.91) | 0.36 | |
| TGA | −3.8 (−23.93 to 16.33) | 0.71 | |
| Single ventricle | −4.25 (−28.08 to 19.57) | 0.73 |
The table can be interpreted as follows: compared with the shunt group, the absolute difference between pre- and intraoperative RCA peak velocity was 17.59 cm/s lower in the obstruction group ([95% CI: 40.87 lower to 5.7 higher], P = 0.14).
CI, confidence interval; LAD, left anterior descending; RCA, right coronary artery; TGA, transposition of the great arteries.
Figure 4.
Linear regression models of peak and mean LAD and RCA velocities among 69 patients, with available data for N = 62 LAD velocities and N = 42 RCA velocities. LAD, left anterior descending; RCA, right coronary artery; TEE, transesophageal echocardiography.
Figure 5.
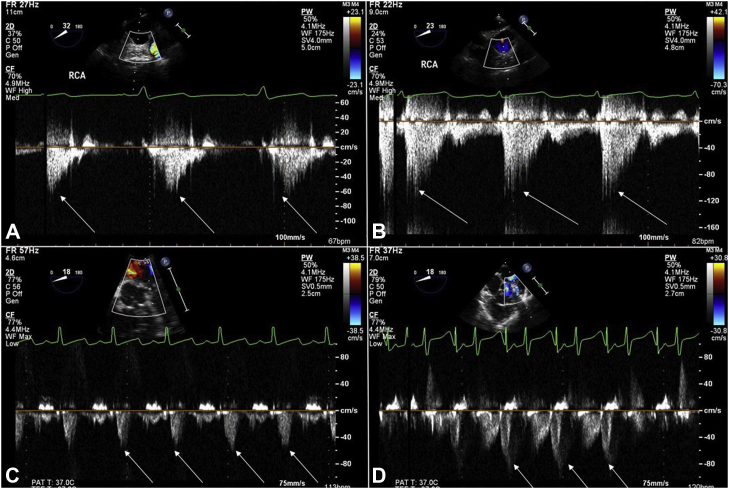
TEE Doppler tracings before and after CPB for the RCA and LAD coronaries. The white arrows in each box depict the peak velocity in the coronary artery. (A) RCA Doppler tracing before CPB in a baby undergoing tetralogy of Fallot repair. (B) RCA Doppler tracing after CPB in a baby after tetralogy of Fallot repair. (C) LAD Doppler tracing before CPB in a baby with Trisomy 21 undergoing VSD closure, (D) LAD Doppler tracing after CPB in a baby with Trisomy 21 after VSD closure. CPB, cardiopulmonary bypass; LAD, left anterior descending; RCA, right coronary artery; TEE, transesophageal echocardiography; VSD, ventricular septal defect.
The CFR measurements were similar in all groups, with a mean LAD CFR of 1.48 (IQR: 1.14-1.77) and a mean RCA CFR of 1.52 (IQR: 1.25-1.81) from peak velocities. The small group of TGA cases had a higher CFR in the RCA by both peak measurement (2.16, IQR: 1.05-6.87, P = 0.53) and mean measurement (2.02, IQR: 1.15-6.00 P = 0.29), but neither reached statistical significance (Table 4).
Table 4.
Estimated intraoperative assessment of coronary flow reserve (CFR) using post-CPB/pre-CPB coronary flow velocities
| Diagnostic category | N | Overall (N = 69) | N | Shunt (N = 26) | N | Obstruction (N = 26) | N | TGA (N = 5) | N | SV (N = 12) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFR LAD peak | 62 | 1.48 (1.14-1.77) | 26 | 1.41 (1.10-1.67) | 25 | 1.55 (1.14-1.86) | 3 | 1.20 (1.00-1.73) | 8 | 1.43 (1.23-1.84) | 0.69 |
| CFR LAD mean | 62 | 1.61 (1.15-2.00) | 26 | 1.52 (1.19-1.77) | 25 | 1.67 (1.25-2.16) | 3 | 1.36 (1.11-1.71) | 8 | 1.41 (1.29-1.68) | 0.72 |
| CFR RCA peak | 42 | 1.52 (1.25-1.81) | 13 | 1.73 (1.27-2.00) | 16 | 1.30 (0.89-2.00) | 4 | 2.16 (1.05-6.87) | 9 | 1.59 (1.48-1.82) | 0.53 |
| CFR RCA mean | 42 | 1.48 (1.05-2.00) | 13 | 1.65 (0.97-2.00) | 16 | 1.27 (0.93-1.62) | 4 | 2.02 (1.15-6.00) | 9 | 1.6 (1.31-2.13) | 0.29 |
Values depicted as median (IQR).
CPB, cardiopulmonary bypass; IQR, interquartile range; LAD, left anterior descending; RCA, right coronary artery; SV, single ventricle; TGA, transposition of the great arteries.
Flow reversal was noted in the RCA in 5 cases preoperatively and in 12 cases after CPB: in the LAD in 4 cases before CPB and in 9 cases after CPB. This was seen most frequently in the RCA in the shunt group preoperatively (N = 3 [19%]) and in the single ventricle group after CPB (N = 4 [40%]). Flow reversal in the LAD was most prevalent in the obstructed patients (N = 2 [8%]) and the TGA group (N = 1 [25%]) after CPB. The composite outcome occurred in 5 of 69 (7%) cases, with 1 patient experiencing ventricular tachycardia/fibrillation in the operating room and 4 with ST segment changes (Table 1). Flow reversal was not associated with the composite outcome of ventricular tachycardia/fibrillation, ST changes, or ECMO.
Table 2 depicts differences in the 4 groups in terms of heart rhythm, ventricular function, blood pressure, heart rate, and oxygen saturations pre- and intraoperatively. All cases had normal LV function preoperatively. Mild, moderate, or severely reduced intraoperative LV function was significantly more prevalent in the TGA group at 60% compared with the other groups (16% in the shunt group, 8% in the obstruction group, and 0% in the single ventricle group, overall P < 0.04 between groups). As expected, the single ventricle group had lower oxygen saturations (85% ± 9%) compared with the other groups, P < 0.001.
Discussion
In a heterogeneous group of children with congenital heart disease undergoing CPB, the flow in the RCA and LAD by TEE increased after CPB surgery. The RCA and LAD resting velocities were similar for each subgroup and are comparable with published normal values in children, both by TEE and by invasive measurements.7,13,18
Overall, the coronary flows were low-velocity, normal biphasic flows, without flow reversal (RCA and LAD both >90%). These findings are similar to our previous intraoperative findings in cases of TGA after arterial switch.17
The effect of CPB on children undergoing cardiac surgery is multifactorial and appears to be different from adults. In a study of 18 infants with VSD and 12 with coarctation of the aorta undergoing CPB surgery, basal coronary flow by transthoracic echocardiography was found to be increased at least 5 days after CPB in children after VSD repair, whereas this was not seen after coarctation.22 It remains unclear whether the main hyperaemic effect is due to endothelial dysfunction or an acute inflammatory reaction.15,23,24
Coronary flow reserve in a variety of congenital cardiac lesions (VSD, TGA, tetralogy of Fallot, hypoplastic left heart syndrome) is lower than in adults.25, 26, 27, 28, 29 These findings are similar to ours, where the CFR in all groups was <2.2. The lower CFR may be due to the observed higher basal coronary flow with a more blunted hyperaemic response.14
We had originally postulated that the shunt lesions would have a different CFR response to an obstructed lesion, but this was not observed in our patients. We would suggest that this is because we were measuring early, immediate post-CPB CFR, and that the effect of CPB is likely to be the dominant mechanism responsible for the increase in coronary flow related to coronary vasodilatation. It would be useful to further assess coronary flow velocities before hospital discharge in a spectrum of congenital heart lesions when the immediate effect on coronary physiology of CPB is likely less important.
Apart from the effects of CPB, there are many other factors that could contribute to alterations in coronary blood flow. In the immediate post-bypass period, there are multiple physiological processes occurring simultaneously, such as changes in mechanical ventilation settings and use of pharmacological vasotropic agents (ie, inotropes and vasodilators). Our data demonstrate that the observed changes in velocities are not associated with adverse outcomes. This contrasts with earlier studies from our group where we looked at the impact of coronary flow velocities and flow patterns on patients undergoing the arterial switch operation.17,18 We observed that in this patient group increased flow velocities are related to problems with the coronary transfer. This has aided in the decision to reintervene, even in the absence of obvious coronary ischemia or regional wall motion abnormalities, to avoid leaving any coronary narrowing or kinking before closing the chest. In our earlier study, coronary flow pattern changes with abnormal flow reversal in the left coronary artery were associated with adverse events in the perioperative period. The mechanism causing flow reversal is not well defined and could be related to microvascular changes in the more distal coronary bed with increased pressure wave reversals related to increased distal resistance. The clinical significance of this phenomenon requires further study based on the outcome data in TGA patients. Unfortunately, the current study likely did not have the necessary power to unveil the effect of flow reversal in the other lesions.
Further studies on myocardial perfusion in patients with different types of congenital heart disease are needed, in particular in lesions where coronary anatomy and physiology is abnormal. Novel echocardiographic techniques such as ultrafast Doppler to study myocardial blood volume may help to further understand the impact of coronary perfusion abnormalities in different congenital lesions.30
Limitations
Heterogeneity of CHD
At the time of study design, the investigators were aware that the types of CHD would range from simple to complex. After the initial analysis, we elected to subdivide the patients into haemodynamic subgroups, realizing that we would have to exclude certain patients who did not fit into 1 of the 4 categories. We felt that it was important to obtain a wide sample of cases to determine if any particular subgroups would benefit from more careful routine assessment of the coronary arteries, such as single ventricle cases.
Incomplete data set
The preoperative TEEs were the most technically challenging. This was in part due to the number of procedures occurring simultaneously in the operating room, such as central line insertion, intubation, urinary catheters, and the urgency to begin the surgical case. We suspect that if there was more allotted time to perform the preoperative TEE, more coronaries would have been imaged with more clarity. The other issue preoperatively, before sternotomy, was the movement of the coronary in and out of the TEE plane, in particular the RCA. This was noted mainly in older children, not in the neonates. Intraoperatively, the heart was more fixed in position and the flows were much easier to obtain. During the intraoperative study, the operator had more time to obtain more accurate images, given that at our institution an intraoperative TEE and/or epicardial echocardiogram is standard of care. We had the most difficulty with the left main coronary ostium and the circumflex coronary arteries. In the case of the left main coronary, it is possible to obtain reasonable Doppler measurements, but this is time consuming, and epicardial echocardiography is superior for the left main coronary artery and the circumflex artery. We calculated CFR using pre- and post-CPB coronary velocities, which is not the standard of using adenosine or other hyperaemic agents. However, we felt that CPB is by definition inducing a hyperaemic response.
Conclusions
Coronary flow velocities in both the RCA and LAD increase from pre- to post-CPB in a wide range of congenital heart lesions and are consistently less than 60-80 cm/s. The calculated CFR by LAD and RCA are similar and consistent across congenital heart lesions, but lower than in adults. These data will help to identify pathologic changes in coronary flow velocities and patterns in children undergoing cardiac surgery.
Acknowledgement
We would like to sincerely thank Dr Xiaoling Zhang for her exceptional assistance to this study.
Ethics Statement
The research reported has adhered to the relevant ethical guidelines.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Freire G., Miller M.S. Echocardiographic evaluation of coronary arteries in congenital heart disease. Cardiol Young. 2015;25:1504–1511. doi: 10.1017/S1047951115002000. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt K.G., Cooper M.J., Silverman N.H., Stanger P. Pulmonary artery origin of the left coronary artery: diagnosis by two-dimensional echocardiography, pulsed Doppler ultrasound and color flow mapping. J Am Coll Cardiol. 1988;11:396–402. doi: 10.1016/0735-1097(88)90108-8. [DOI] [PubMed] [Google Scholar]
- 3.McCrindle B.W., Cifra B. The role of echocardiography in Kawasaki disease. Int J Rheum Dis. 2018;21:50–55. doi: 10.1111/1756-185X.13216. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa M.M., Katina T., Oliveira H.G., Neuenschwander F.E., Oliveira E.C. Doppler echocardiographic features of coronary artery fistula: report of 8 cases. J Am Soc Echocardiogr. 1999;12:149–154. doi: 10.1016/s0894-7317(99)70127-6. [DOI] [PubMed] [Google Scholar]
- 5.Ofili E.O., Labovitz A.J., Kern M.J. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol. 1993;71:3D–9D. doi: 10.1016/0002-9149(93)90128-y. [DOI] [PubMed] [Google Scholar]
- 6.Aoki M., Harada K., Takada G. Normal values for left anterior descending coronary artery flow velocity assessed by transthoracic Doppler echocardiography in healthy children. Tohoku J Exp Med. 2003;199:211–217. doi: 10.1620/tjem.199.211. [DOI] [PubMed] [Google Scholar]
- 7.Kozakova M., Palombo C., Pratali L., et al. Assessment of coronary reserve by transoesophageal Doppler echocardiography. Direct comparison between different modalities of dipyridamole and adenosine administration. Eur Heart J. 1997;18:514–523. doi: 10.1093/oxfordjournals.eurheartj.a015274. [DOI] [PubMed] [Google Scholar]
- 8.Yim D., Mertens L., Honjo O., Nield L.E. Intraoperative echocardiographic coronary artery imaging in congenital and acquired heart disease. Cardiol Young. 2020;30:153–161. doi: 10.1017/S1047951120000116. [DOI] [PubMed] [Google Scholar]
- 9.Kajiya F., Matsuoka S., Ogasawara Y., et al. Velocity profiles and phasic flow patterns in the non-stenotic human left anterior descending coronary artery during cardiac surgery. Cardiovasc Res. 1993;27:845–850. doi: 10.1093/cvr/27.5.845. [DOI] [PubMed] [Google Scholar]
- 10.Sunyecz I.L., McCallinhart P.E., Patel K.U., et al. Defining coronary flow patterns: comprehensive automation of transthoracic Doppler coronary blood flow. Sci Rep. 2018;8:17268. doi: 10.1038/s41598-018-35572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuoka K., Harada K., Tamura M., Takada G. Left anterior descending coronary artery flow and its relation to age in children. J Am Soc Echocardiogr. 2002;15:69–75. doi: 10.1067/mje.2002.115537. [DOI] [PubMed] [Google Scholar]
- 12.Pesonen E., Liuba P., Aburawi E.H. Review findings included diminished coronary flow reserve after surgery in children with congenital heart disease and inflammation. Acta Paediatr. 2019;108:218–223. doi: 10.1111/apa.14613. [DOI] [PubMed] [Google Scholar]
- 13.Oskarsson G., Pesonen E. Coronary blood flow in healthy neonates: effects of left ventricular function and mass. Pediatr Cardiol. 2004;25:11–16. doi: 10.1007/s00246-002-0377-z. [DOI] [PubMed] [Google Scholar]
- 14.Hiratzka L.F., Eastham C.L., Carter J.G., et al. The effects of cardiopulmonary bypass and cold cardioplegia on coronary flow velocity and the reactive hyperemic response in patients and dogs. Ann Thorac Surg. 1988;45:474–481. doi: 10.1016/s0003-4975(10)64518-3. [DOI] [PubMed] [Google Scholar]
- 15.Davidson H., Punn R., Tacy T.A. Cardioplegia dose effect on immediate postoperative alterations in coronary artery flow velocities after congenital cardiac surgery. Pediatr Cardiol. 2016;37:364–371. doi: 10.1007/s00246-015-1285-3. [DOI] [PubMed] [Google Scholar]
- 16.Long C., Hu X., Zhang J., Xiu R., Guan Y. Changes of microvascular vasomotion and oxygen metabolism during cooling and rewarming period of cardiopulmonary bypass. J Extra Corpor Technol. 2003;35:13–16. [PubMed] [Google Scholar]
- 17.Wong D., Golding F., Hess L., et al. Intraoperative coronary artery pulse Doppler patterns in patients with complete transposition of the great arteries undergoing the arterial switch operation. Am Heart J. 2008;156:466–472. doi: 10.1016/j.ahj.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Nield L.E., Dragulescu A., MacColl C., et al. Coronary artery Doppler patterns are associated with clinical outcomes post-arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging. 2018;19:461–468. doi: 10.1093/ehjci/jex050. [DOI] [PubMed] [Google Scholar]
- 19.Matte G.S., del Nido P.J. History and use of del Nido cardioplegia solution at Boston Children’s Hospital. J Extra Corpor Technol. 2012;44:98–103. [PMC free article] [PubMed] [Google Scholar]
- 20.Meimoun P., Tribouilloy C. Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: a magic tool for the real world. Eur J Echocardiogr. 2008;9:449–457. doi: 10.1093/ejechocard/jen004. [DOI] [PubMed] [Google Scholar]
- 21.Saraste M., Koskenvuo J., Knuuti J., et al. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol. 2001;21:114–122. doi: 10.1046/j.1365-2281.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 22.Aburawi E.H., Berg A., Liuba P., Pesonen E. Effects of cardiopulmonary bypass surgery on coronary flow in children assessed with transthoracic Doppler echocardiography. Am J Physiol Heart Circ Physiol. 2007;293:H1138–H1143. doi: 10.1152/ajpheart.00025.2007. [DOI] [PubMed] [Google Scholar]
- 23.Huk I., Nanobashvili J., Neumayer C., et al. L-Arginine treatment alters the kinetics of nitric oxide and superoxide release and reduces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]
- 24.Sellke F.W., Boyle E.M., Jr., Verrier E.D. Endothelial cell injury in cardiovascular surgery: the pathophysiology of vasomotor dysfunction. Ann Thorac Surg. 1996;62:1222–1228. doi: 10.1016/0003-4975(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly J.P., Raffel D.M., Shulkin B.L., et al. Resting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography. J Thorac Cardiovasc Surg. 1998;115:103–110. doi: 10.1016/s0022-5223(98)70448-9. [DOI] [PubMed] [Google Scholar]
- 26.Harada K., Aoki M., Toyono M., Tamura M. Coronary flow velocity and coronary flow velocity reserve in children with ventricular septal defect. Tohoku J Exp Med. 2004;202:77–85. doi: 10.1620/tjem.202.77. [DOI] [PubMed] [Google Scholar]
- 27.Hauser M., Bengel F.M., Kühn A., et al. Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and Ross operation. Circulation. 2001;103:1875–1880. doi: 10.1161/01.cir.103.14.1875. [DOI] [PubMed] [Google Scholar]
- 28.Hauser M., Bengel F.M., Hager A., et al. Impaired myocardial blood flow and coronary flow reserve of the anatomical right systemic ventricle in patients with congenitally corrected transposition of the great arteries. Heart. 2003;89:1231–1235. doi: 10.1136/heart.89.10.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuyama H., Odagawa Y., Katoh C., et al. Altered myocardial flow reserve and endothelial function late after Kawasaki disease. J Pediatr. 2003;142:149–154. doi: 10.1067/mpd.2003.46. [DOI] [PubMed] [Google Scholar]
- 30.Villemain O., Baranger J., Friedberg M.K., et al. Ultrafast ultrasound imaging in pediatric and adult cardiology: techniques, applications, and perspectives. JACC Cardiovasc Imaging. 2020;13:1771–1791. doi: 10.1016/j.jcmg.2019.09.019. [DOI] [PubMed] [Google Scholar]