Abstract
Atrial fibrillation (AF) can occur predominantly associated with right atrial (RA) lesions in congenital heart disease, particularly when the RA cavity is dilated. RA electrical potentials occasionally appear organized during AF. We clearly mapped such areas circumscribed by an intra-atrial re-entrant circuit during an isoproterenol infusion, in a patient with a repaired tetralogy of Fallot, using an ultrahigh-density mapping system and its beat acceptance criteria function. Ablation of areas inside the re-entrant circuit successfully eliminated the AF. Our experience indicated that a macro-re-entrant tachycardia was a driver as well as a trigger of AF of this right-sided origin.
Résumé
Chez les patients atteints d’une cardiopathie congénitale, la fibrillation auriculaire (FA) peut souvent survenir en association avec des lésions auriculaires droites (AD), en particulier lorsque la cavité AD est dilatée. Lors d’une FA, il peut arriver que les potentiels électriques AD semblent normaux. Chez un patient ayant une tétralogie de Fallot réparée, nous avons clairement cartographié des zones délimitées par un circuit de réentrée intra-auriculaire lors d’une perfusion d’isoprotérénol, et ce, à l’aide d’un système de cartographie à très haute densité et de ses critères d’acceptation liés aux battements cardiaques. L’ablation des régions se trouvant dans le circuit de réentrée a permis d’éliminer la FA avec succès. Notre expérience a démontré qu’une tachycardie macroréentrante avait été un facteur déterminant et même un déclencheur de la FA, laquelle est apparue à droite.
Novel Teaching Points.
-
•
The area circumscribed by an intra-atrial re-entrant circuit in the RA was detected during AF in a patient with a repaired tetralogy of Fallot.
-
•
The beat acceptance criteria function of the ultrahigh-density mapping system (Rhythmia; Boston Scientific) enabled the detection of the re-entrant circuit during AF.
-
•
Ablation in the area inside the re-entrant circuit successfully eliminated the AF.
-
•
This case report indicated that a macro-re-entrant tachycardia was a driver as well as a trigger of AF with a right-sided origin.
Is standard pulmonary venous isolation (PVI) mandatory for non-longstanding atrial fibrillation (AF) in congenital heart disease (CHD) associated with a volume overload of the right atrium (RA) but not to the left? The mechanism of AF could be associated with damaged RA tissue.1 However, PVI has been the principal procedure of catheter ablation even without a left atrial overload.2 An AF origin on the right side, if any, would require a different strategy other than PVI.
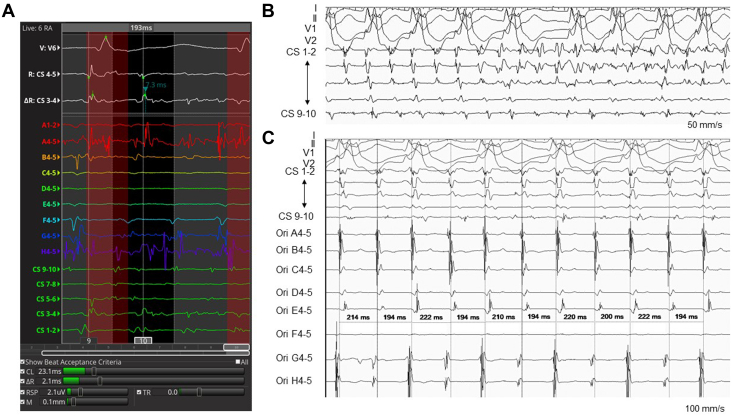
There may be areas of organized electrical activity in the RA during AF in CHD.2 Intra-atrial re-entrant tachycardia (IART) is known to often precede, trigger, or coincide with AF in this setting.1 To investigate these phenomena, an ultrahigh-density mapping system may be useful, possessing the following 6 unique beat acceptance criteria (Fig. 1A): (1) a stable cycle length (CL); (2) stable timing difference between 2 reference electrodes; (3) respiration gating; (4) stable catheter motion; (5) stable catheter electrogram recordings as compared with the adjacent points; and (6) the tracking quality.3
Figure 1.
Intracardiac electrocardiograms. (A) Mapping potential with an organized pattern. Shown in the lower column are the beat acceptance criteria using the CL (window set as 193 ± 40 ms), a stable timing difference between 2 reference electrodes (ΔR), respiration gating (RSP), stable catheter motion (M), and tracking quality (TR). The stability of electrograms recorded by the catheter (S) was not used as a criterion. (B) A shift from the intra-atrial re-entrant tachycardia to AF. (C) Recorded potentials with an organized pattern during AF while on an isoproterenol infusion. AF, atrial fibrillation; CL, cycle length; CS, coronary sinus; Ori, Orion mapping catheter.
This intelligent system was successfully applied in a patient with CHD and detected an RA mechanism.
Case Report
A 47-year-old woman with a repaired tetralogy of Fallot suffered from syncope during AF and IART (Supplemental Fig. S1). The RA and right ventricle were dilated because of severe tricuspid and pulmonary regurgitation.
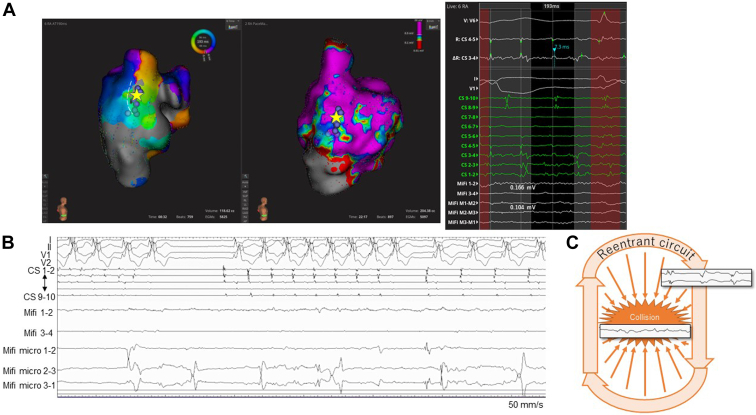
Catheter ablation was carried out with the aid of an ultrahigh-density mapping system (Rhythmia; Boston Scientific). A multipolar basket catheter (IntellaMap Orion catheter; Boston Scientific) and an 8-mm-tip minielectrode-equipped catheter (IntellaTip MiFi XP 8-mm; Boston Scientific, Tokyo, Japan) were used for the mapping and ablation catheter, respectively. An RA voltage map (RA volume 204 mL) was created, and a cavotricuspid isthmus ablation was performed first. Subsequently, IART (CL 194 ms) was induced reproducibly with rapid atrial pacing, and it soon changed to AF (Fig. 1B). The rhythm pattern was relatively organized while on a 0.0025 μg/kg/min continuous isoproterenol infusion; therefore, we mapped the potentials using the beat acceptance criteria while using the coronary sinus potentials as a reference with a window of interest (WOI) setting of 193 ms (Video 1,  view video online). Among the 6 criteria, the stability of the catheter electrograms was excluded, because the electrograms were unstable while mapping the AF. The CL window for the criteria was set at 193 ± 40 ms, but usually was set at WOI ±10 ms (Fig. 1, A and C). Eventually, a clockwise re-entrant circuit was identified on the posterolateral wall of the RA (Fig. 2A; Video 2,
view video online). Among the 6 criteria, the stability of the catheter electrograms was excluded, because the electrograms were unstable while mapping the AF. The CL window for the criteria was set at 193 ± 40 ms, but usually was set at WOI ±10 ms (Fig. 1, A and C). Eventually, a clockwise re-entrant circuit was identified on the posterolateral wall of the RA (Fig. 2A; Video 2,  view video online). The AF terminated while the central area inside the circuit was ablated. An additional line was created from this area to a scarred region around the inferior vena cava while paying attention to the right phrenic nerve (Fig. 2, A and B; Video 3,
view video online). The AF terminated while the central area inside the circuit was ablated. An additional line was created from this area to a scarred region around the inferior vena cava while paying attention to the right phrenic nerve (Fig. 2, A and B; Video 3,  view video online). Neither IART nor AF was further inducible, even while on isoproterenol.
view video online). Neither IART nor AF was further inducible, even while on isoproterenol.
Figure 2.
Catheter positioning and intracardiac electrograms. (A) An activation map showing organized potentials during AF (left) and a voltage map during CS pacing (middle). The intracardiac electrograms (right), together with the yellow star, show the point where the ablation terminated AF. The white broken line shows the projected course of the phrenic nerve identified by diaphragmatic stimulation. The light purple dots indicate the sites of ablation. (B) The intracardiac electrograms when AF was terminated. (C) A possible mechanism of an AF driver with an RA origin; the electrical potentials of the re-entrant circuit collide with that of the inner area and thus sustain the AF. AF, atrial fibrillation; CS, coronary sinus; RA, right atrial.
Two months later, a surgical intervention was carried out for a pulmonary valve replacement, tricuspid valve repair, cryoablation and plication of the RA, and implantation of an epicardial pacemaker system. No IART or AF has recurred for 7 months while on carvedilol despite no PVI being performed.
Discussion
We wondered whether standard PVI was mandatory for non-longstanding AF in younger CHD patients with an RA volume overload but not of the left side. Because PVI could induce a stiff left atrial wall,4 it would be better to avoid injury to the tissue unless absolutely necessary.
A limited number of studies have been reported of successful catheter ablation targeting the RA only.2 Complex fractionated electrograms and a border marked by a scar formation within the RA wall were among the substrates treated, the circumstance being mentioned mainly in the Fontan-type procedure with an atriopulmonary connection form.5 In this approach, multisite ablation is needed. de Groot et al.6 discussed a possible mechanism for how electrical potentials could be organized in AF dominantly associated with the RA. The structure, which differs from the left atrium, involves native and anatomical conduction blocks. In CHD, in general, surgical incisions of the RA and scars at the venous cannulation sites can interfere with the intra-atrial conduction. With these electrical obstacles, the potential activity can be found to be organized even during AF.
It was feasible to extract such potentials in our patient by using the beat acceptance criteria in the high-density mapping system. The device accurately determined the mechanisms of AF generated exclusively from the RA and could clarify where to ablate. To the best of our knowledge, such a finding has not been previously mentioned. On the other hand, this method may have some limitations. In our case, the low-voltage areas were relatively small. We were able to use the coronary sinus potentials as a reference in an acceptable and organized manner. This may not always be the case.
This experience convinced us that the IART functioned not only as a trigger but also as a driver of AF with an RA origin. However, its mechanisms still remained unclear. We could not tell whether this was a temporary alternation between the IART and AF or coexistence of the IART and AF with the IART being superimposed on the AF and with a different degree of organization depending on the proximity to the re-entrant circuit. One possible mechanism was that the electrical potentials of the re-entrant circuit collided with those of the inner area, thus sustaining the AF (Fig. 2C). This was supported by the findings that the AF terminated during the ablation in the central area inside the IART circuit, and neither IART nor AF could be induced after the ablation. However, this is only a hypothesis. Cases in a similar circumstance need to be investigated before the mechanisms of “right-sided AF” can be recognized in detail.
Conclusion
Comprehensive mapping of the atrial potentials illustrated an organized pattern of excitation during AF and identified an ablation target in a patient with CHD. This method was useful for documenting the mechanism of AF associated with a right-sided origin. The role of the IART appeared to be as a driver as well as a trigger.
Acknowledgements
We thank Chinatsu Hamaguchi (Boston Scientific, Japan) for the detailed interpretation and comments on Rhythmia and John Martin for assistance in the preparation of this manuscript.
Ethics Statement
The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national guidelines on human experimentation (Japan) and with the Helsinki Declaration of 1975 (as revised in 2013). This type of retrospective case report was determined not to require approval by the ethics committee.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2022.10.004.
Supplementary Material
References
- 1.Miyazaki A., Negishi J., Hayama Y., et al. Etiology of atrial fibrillation in patients with complex congenital heart disease—for a better treatment strategy. J Cardiol. 2020;76:438–445. doi: 10.1016/j.jjcc.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann V., Laredo M., Abadir S., Mondésert B., Khairy P. Atrial fibrillation in adults with congenital heart disease. Int J Cardiol. 2019;287:148–154. doi: 10.1016/j.ijcard.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 3.Mantziari L., Butcher C., Shi R., et al. Characterization of the mechanism and substrate of atrial tachycardia using ultra-high-density mapping in adults with congenital heart disease: impact on clinical outcomes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy Y.N.V., El Sabbagh A., Packer D., Nishimura R.A. Evaluation of shortness of breath after atrial fibrillation ablation—is there a stiff left atrium? Heart Rhythm. 2018;15:930–935. doi: 10.1016/j.hrthm.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K., Shoda M., Manaka T., Nakanishi T. Successful radiofrequency catheter ablation of atrial fibrillation late after modified Fontan operation. Europace. 2008;10:1012–1014. doi: 10.1093/europace/eun132. [DOI] [PubMed] [Google Scholar]
- 6.de Groot N.M., Zeppenfeld K., Wijffels M.C., et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;3:526–535. doi: 10.1016/j.hrthm.2006.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.