Abstract
Bacterial community structure and the predominant nitrifying activities and populations in each compartment of a three-compartment activated sludge system were determined. Each compartment was originally inoculated with the same activated sludge community entrapped in polyethylene glycol gel granules, and ammonium nitrogen was supplied to the system in an inorganic salts solution at a rate of 5.0 g of N liter of granular activated sludge−1 day−1. After 150 days of operation, the system was found to comprise a series of sequential nitrifying reactions (K. Noto, T. Ogasawara, Y. Suwa, and T. Sumino, Water Res. 32:769–773, 1998), presumably mediated by different bacterial populations. Activity data showed that all NH4-N was completely oxidized in compartments one and two (approximately half in each), but no significant nitrite oxidation was observed in these compartments. In contrast, all available nitrite was oxidized to nitrate in compartment three. To study the microbial populations and communities in this system, total bacterial DNA isolated from each compartment was analyzed for community structure based on the G+C contents of the component populations. Compartment one showed dominant populations having 50 and 67% G+C contents. Compartment two was similar in structure to compartment one. The bacterial community in compartment three had dominant populations with 62 and 67% G+C contents and retained the 50% G+C content population only at a greatly diminished level. The 50% G+C content population from compartment one hybridized strongly with amo (ammonia monooxygenase) and hao (hydroxylamine oxidoreductase) gene probes from Nitrosomonas europaea. However, the 50% G+C content population from compartment two hybridized strongly with the hao probe but only weakly with the amo probe, suggesting that the predominant ammonia-oxidizing populations in compartments one and two might be different. Since different activities and populations come to dominate in each compartment from an identical inoculum, it appears that the nitrification processes may be somewhat incompatible, resulting in a series of sequential reactions and different communities in this three-compartment system.
In order to prevent eutrophication, wastewater containing ammonium nitrogen from a variety of human activities should not be released into environmental waters until nitrogen levels are reduced to acceptable levels. Biological nitrogen removal processes, which are basically a combination of nitrification and denitrification, are widely used for this purpose. These two incompatible biochemical processes are carried out by physiologically different groups of organisms. The nitrification process is mediated by two different kinds of chemolithotrophic bacterial groups, ammonia oxidizers and nitrite oxidizers. The former are responsible for oxidation of ammonia to nitrite, and the latter are responsible for oxidation of nitrite to nitrate. Because of the slow growth rates and poor yields of the organisms involved, nitrification is generally regarded as the rate-limiting step in the nitrogen removal process. Thus, process engineers continuously search for the most efficient, optimal, and stable way to maintain the populations and biological activities of nitrifiers in wastewater treatment systems. It also follows that developing a better understanding of the biology and ecology of the microbial populations in biological reactor systems is key to developing and optimizing efficient and economic reactor systems in general.
Noto et al. (23) proposed and demonstrated a novel nitrification process with three sequentially connective, equal-volume compartments containing activated sludge populations embedded in a polyethylene glycol matrix. In those experiments, inorganic synthetic medium containing ammonium nitrogen was supplied to the reactor at 5.0 g of N liter of granules−1 day−1. After 150 days of operation, a series of sequential nitrifying reactions was observed in the system, with half of the ammonium nitrogen load being oxidized to nitrite in the first compartment and the remaining half being oxidized in the second compartment. Significant nitrite oxidation was observed solely in the third compartment. The ammonia oxidation rate in the first two compartments of this system ultimately reached 6.8 g of N liter of granules−1 day−1. In a parallel experiment with a single-compartment reactor with ammonium nitrogen similarly supplied at 5.0 g of N liter of granules−1 day−1, the ammonia oxidation rate did not exceed 2.7 g of N liter of granules−1 day−1. Thus, the overall ammonia oxidation rate of the three-compartment system was more than 2.5 times that of the single-compartment system (23). We hypothesized that different bacterial populations, which were responsible for different and possibly incompatible nitrification reactions, were dominant in each compartment and that segregation of the ammonia and nitrite oxidation reactions enhanced the overall nitrification rate for the system.
Traditionally, ammonia oxidizers have been enumerated by most-probable-number methods. However, this approach is somewhat inaccurate and is very time-consuming, requiring weeks of incubation for these slow-growing populations. Thus, improved methodologies are desirable for more rapid and precise analysis of these and other slow-growing or fastidious populations. DNA-based molecular approaches provide some advantage in this regard, since total bacterial community DNA can be directly extracted from samples, preserving the relative proportions of the various populations in the original samples, and subsequently analyzed by a variety of methods (8, 15, 27). For example, comparison of 16S ribosomal DNA sequences has permitted determination of the phylogenetic relationships among cultured ammonia-oxidizing bacteria (6, 24, 31, 32, 33, 38). Based on this information, oligonucleotide probes and PCR have been used to study ammonia-oxidizing populations in natural environments (5, 7, 13, 17, 18, 20, 22, 34, 35, 36). Functional probes specific for ammonia-oxidizing bacteria (based on the amo gene) have also been used to study these organisms (5, 12, 28). These methods focus mainly on the specific detection and monitoring of ammonia-oxidizing populations, not on studying this functional group in the context of the entire microbial community.
The objective of this study was to analyze both the total microbial community structure and the ammonia-oxidizing populations in each compartment of the three-compartment system. We used two molecular methods with different principles: G+C content-based fractionation of total bacterial community DNA coupled with hybridization analyses using functional probes from the energy production pathway of the ammonia-oxidizing bacterium Nitrosomonas europaea. This approach allowed characterization of the overall microbial community structure in each compartment and monitoring the ammonia-oxidizing populations present. We used gene probes for the two consecutive functional genes amo and hao from the ammonia oxidation pathway of N. europaea to analyze predominant ammonia-oxidizing populations in this system without isolation or cultivation.
MATERIALS AND METHODS
Three-compartment nitrogen removal reactor system.
The construction of the three-compartment fluidized bed reactor system has been described in detail elsewhere (23). The compartments of this reactor were connected serially in a cascade mode to prevent backward flow of effluent from each compartment. Each compartment (600-ml working volume) was loaded with 20% (vol/vol) municipal activated sludge embedded in a polyethylene glycol matrix as described previously (29). Inorganic synthetic wastewater, which was comprised, per liter of tap water, of NH4Cl (1,910 mg), NaHCO3 (1,170 mg), Na2HPO4 · 12H2O (116 mg), NaCl (51 mg), KCl (24 mg), CaCl2 · 2H2O (24 mg), and MgSO4 · 7H2O (84 mg), was continuously supplied to the system at the NH4-N loading rate of 5.0 g of N liter of granular activated sludge−1 day−1 with a hydraulic retention time of 0.1 day. The system was maintained at 8.8 mg of dissolved oxygen liter−1, 20°C, and pH 7.5 to 8.0. There was no loss of sludge granules from any compartment during the course of these experiments, so sludge retention time can be assumed to have approached infinity.
Isolation of bacterial community DNA and community profile analysis.
Granular activated sludge samples were taken from each compartment of the reactor on day 139 of operation, when the overall nitrification reactions of the reactor were stabilized, as demonstrated by the method of Noto et al. (23). For comparative purposes, sewage sludge samples were also obtained from aerobic, anoxic (i.e., lacking oxygen), and anaerobic (i.e., lacking oxygen, nitrite, and nitrate) vessels of a municipal wastewater treatment plant in Japan (a so-called A2/O system, comprising an aerobic-anaerobic alternating bioreactor for the removal of nitrogen and phosphorus). Total bacterial community DNA was isolated from the sludge samples by a modification of the direct lysis method described by Holben (11). Briefly, 5 g (wet weight) of granular activated sludge samples was homogenized with a Dounce homogenizer, and then the cells were lysed in 20 ml of lysis buffer by a combination of high heat (70°C), high concentration of detergent (1% sodium dodecyl sulfate), and physical disruption (reciprocal shaking at high speed with glass beads) as described previously (11). The liberated DNA was then purified on ethidium bromide-cesium chloride equilibrium density gradients and concentrated as described previously (11). The purified sludge community DNA was then fractionated, based on G+C content, on bisbenzimidazole-cesium chloride equilibrium density gradients as described by Holben and Harris (10). After reaching equilibrium, the gradients were pumped through a spectrophotometric flow cell for DNA quantitation and fractionated into 120 fractions for density determination and hybridization analyses as described previously (10). DNA quantitation and density data were integrated and are presented as a histogram or profile of the bacterial community in terms of relative abundance versus G+C content of DNA.
Hybridization analyses.
Each of the 120 gradient fractions for a particular sludge sample was split into four subsamples. Subsamples of fractions 1 to 120 for any gradient were then spotted onto a nitrocellulose hybridization membrane (BA-S; Schleicher & Schuell, Inc., Keene, N.H.) for subsequent hybridization analysis. Briefly, the DNA subsamples were diluted 1:4 with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), denatured by boiling for 10 min, and then spotted onto membranes with a Bio-Rad (Hercules, Calif.) dot blot manifold. The membranes were placed on filter paper pads soaked with 1.5 M NaCl–0.5 M NaOH twice for 10 min each time, placed on filter paper pads soaked with 1.5 M NaCl–0.5 M Tris (pH 8) twice for 10 min each time, and finally fixed with a Stratalinker UV 2400 cross-linker (Stratagene, La Jolla, Calif.) according to the manufacturer’s specifications. The functional gene probes corresponded to bases 34 to 825 of the amo gene and bases 73 to 1014 of the hao gene of N. europaea (19, 26) and were kindly provided by D. Arp, Oregon State University. Gene probe DNA was purified for labeling by PCR amplification of the cloned sequences and was labeled with [α-32P]dCTP by nick translation with a Boehringer Mannheim Biochemicals (Indianapolis, Ind.) nick translation kit according to the manufacturer’s directions. Hybridization reaction and wash conditions were as described previously (9). Hybridization signals were quantified with an Ambis 100 Radioisotopic Imaging System (Scanalytics, Billerica, Mass.).
RESULTS
Community profile analysis.
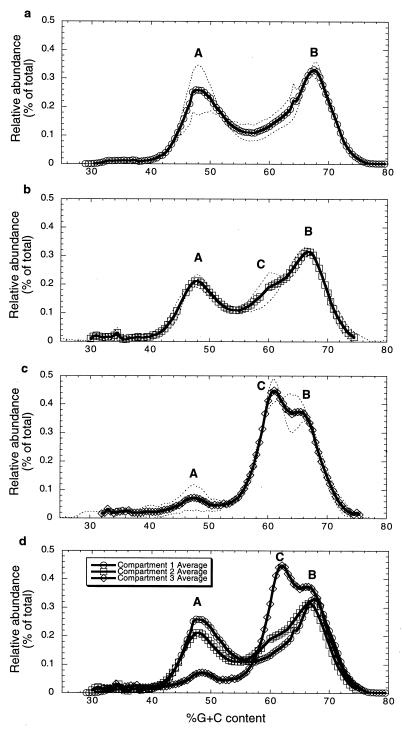
The community profile analysis showed that the first compartment, in which vigorous ammonia oxidation was found, was dominated by two peaks (A and B) representing populations having G+C contents of about 48 and 67%, respectively (Fig. 1a). The second compartment, in which vigorous ammonia oxidation was also observed, was likewise dominated by populations having 48 and 67% G+C contents (Fig. 1b), although the 48% G+C content populations may have been different in these two compartments (see below). Compartment two also contained a minor peak at about 61% G+C content (peak C) which appeared as a shoulder on the leading edge of peak B (Fig. 1b). The third compartment, in which high nitrite oxidation activity was observed, was dominated by organisms having 61% G+C content (peak C) and also had a significant peak at about 67% G+C content (peak B) (Fig. 1c).
FIG. 1.
Community profile analysis of the three-compartment system based on cesium chloride-bisbenzimidazole gradient fractionation of total community DNA from each compartment. (a) Compartment one (○). (b) Compartment two (□). (c) Compartment three (◊). (d) Overlay of profiles for all three compartments. Broken lines represent the profiles of individual samples; solid lines represent the average values for two replicate samples.
The community profiles for the three compartments were overlaid for direct comparison (Fig. 1d). Replicate analyses of community DNA obtained from each compartment were performed and produced very similar results, indicating that the community profile differences observed were compartment specific (data not shown). Peak A was much smaller in the third compartment than in other two compartments, peak B was present in approximately the same levels in all three compartments, and peak C was absent or present at low levels in compartment one, present at low levels in compartment two, and dominant in compartment three (Fig. 1d). Thus, peak A predominated when ammonia oxidation activity was higher, and peak C predominated in the compartment showing high nitrite oxidation activity. It has been reported that the G+C content of chemolithotrophic ammonia oxidizers is 46 to 56% (2, 16, 37) and that the G+C content of terrestrial nitrite oxidizers is 58 to 61% (1, 21, 37). Our community profile findings are consistent with these values in that peak A presumably represents the ammonia-oxidizing populations and peak C presumably represents the nitrite-oxidizing populations.
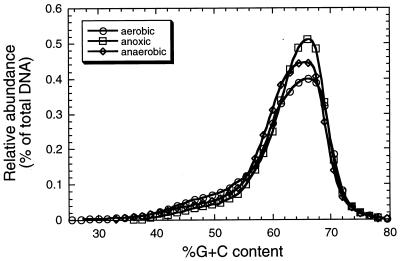
We also compared the community profiles of the three-compartment system to those of a conventional (A2/O) sewage treatment plant system. As shown in Fig. 2, a single large peak at approximately 66% G+C content dominated the microbial community of activated sludge from aerobic, anoxic, and anaerobic points in the waste stream processing of the municipal sewage treatment plant. Such a result might be expected for such systems, where heterotrophic bacteria dominate, since similar community profiles were observed for microbial communities from arable soil samples (10). In fact, it has been reported that the G+C content of many aerobic, heterotrophic bacterial populations which predominate in soil and sediment systems is in the range of 60 to 70% (10). These findings also suggest that the populations represented by peak B in the three-compartment system likely represent heterotrophic bacteria present throughout the three-compartment system.
FIG. 2.
Community profile analysis of a conventional A2/O sewage treatment plant. Samples representing aerobic, anoxic, and anaerobic points in the waste stream processing were analyzed. Only every third datum point is shown for the sake of clarity of the profiles.
Hybridization analyses.
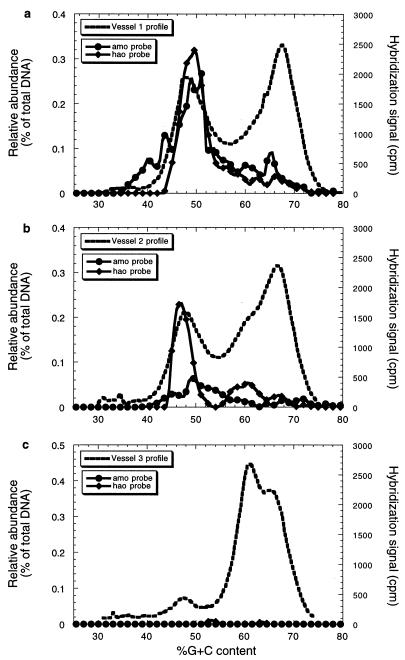
The hao gene probe exhibited strong hybridization to peak A but not to peak B or C, indicating that peak A in the first and second compartments represents the ammonia-oxidizing populations (Fig. 3a and b). This result is in good agreement with the activity measurements for the three compartments (23), especially when interpreted in light of the community profile data indicating relative levels. However, the amo gene probe hybridized strongly to peak A in the first compartment but only weakly to peak A in the second compartment under conditions of identical stringency (Fig. 3a and b), suggesting that the predominant ammonia-oxidizing populations in the first and second compartments are different. Neither probe showed significant hybridization to the DNA from the third compartment (Fig. 3c).
FIG. 3.
Hybridization analysis of fractionated microbial community DNA from the three-compartment system with amo and hao gene probes from N. europaea. Hybridization data are shown relative to the community profile data for compartment one (a), compartment two (b), and compartment three (c). Only every third datum point is shown for the sake of clarity.
DISCUSSION
In an earlier paper it was demonstrated that the overall nitrification rate for an activated sludge process can be significantly increased by partitioning a bioreactor into three compartments without increasing the total volume of the system (23). Although each compartment was initially inoculated with identical municipal activated sludge granules and hence microbial communities, the overall result of this arrangement was a series of sequential incompatible nitrifying reactions in each compartment which consequently enhanced the overall ammonia oxidation rate of the system. We hypothesized that different community structures developed in each compartment through acclimation and optimization to influents having different compositions in this cascading flowthrough system.
The objective of this study was to compare the microbial community structure in each compartment by use of a combination of two DNA-based approaches: total community profile analysis and functional gene probe hybridization. Of special interest was a comparison of the ammonia-oxidizing populations in the first two compartments, since the ammonium nitrogen carried over from the first compartment was completely oxidized in the second. That observation raised the intriguing possibility that different ammonia-oxidizing populations, presumably having different affinities, sensitivities, or other physiological features related to ammonia and nitrite (and/or nitrate), might play a key role in enhancing the overall nitrification rate in this system. Presumably, the one-way communication between the three compartments in the model system is key to the segregation of the somewhat incompatible nitrification processes and the concomitant segregation of the original granular activated sludge inoculum, which was identical for all three compartments at time zero, into three distinctly different communities.
The community profile analysis showed clear-cut differences in community structure when compartments one and two, where ammonia oxidation predominantly occurred, were compared to compartment three, where nitrite oxidation predominated. While this analysis showed that the total community structures in the first two compartments were relatively similar at a coarser phylogenetic level, a difference in the specific dominant ammonia-oxidizing populations in each compartment was clearly indicated by molecular probing with the amo gene probe. This finding is perhaps not surprising, since community profile analysis is a fairly low-resolution technique based on the G+C content of the entire genome, which resolves groups of related bacteria at about the genus level and higher (10), while gene probe hybridization analysis relies on homology between relatively small internal regions of a structural gene and is thus expected to have a greater resolution, based on localized differences in the DNA sequence. Diversity in the nucleotide sequence of amoA has been demonstrated at both the intergeneric (12, 14) and the interspecific (30) levels. Based on those studies, the degree of sequence divergence observed between species ranges up to 24% (i.e., 76% homology). The hybridization conditions used in these experiments required approximately 85% homology to allow hybridization. Thus, the amoA gene homology between the predominant ammonia oxidizers in the first and second compartments was estimated to be less than 85%, suggesting that different species and possibly different genera of ammonia oxidizers were predominant in the first and second compartments. By contrast, the hao gene from ammonia oxidizers appeared to be more highly conserved, as indicated by the comparable hybridization signals observed for the first and second compartments.
Microbial community profile analysis could also be used to infer the ratio between autotrophic nitrifier biomass and heterotrophic biomass. Based on our results, it appears that peak A (48% G+C content) and peak B (67% G+C content) most likely represented autotrophic ammonia oxidizers and heterotrophs, respectively. Since the inorganic synthetic wastewater supplied to the three-compartment system did not contain any organic substances, all organic material in the system was produced by autotrophic metabolism (4). Thus, after 139 days of continuous operation, all heterotrophic metabolism and biomass in the system were supported by the autotrophic community. Assuming that the amounts of total genomic DNA in both autotrophic and heterotrophic cells were similar, the biomasses of autotrophic ammonia oxidizers and heterotrophs would be comparable, as might be expected if heterotrophic productivity were limited by autotrophic production of organic substances. These ideas are compatible with models developed by Rittmann and colleagues (3, 25) and also with the findings of Wagner and co-workers (35), who showed that ammonia oxidizers can constitute up to 20% of the bacterial biomass in a wastewater treatment plant receiving high influent concentrations of ammonia. In contrast, in the A2/O sewage treatment plant system, all three compartments had very similar bacterial community structures dominated by G+C contents representative of heterotrophic bacterial populations. It seems likely that the high levels of organic matter in this “native” system supported very large populations of heterotrophic organisms, limiting the ability of community profile analysis to detect the less dominant nitrifying populations based on the relative abundance of their DNA, with its more unique G+C content. Nonetheless, this study provides a first glimpse of the total bacterial community structure of a working sewage treatment plant in a single analysis.
The combination of these two molecular methods, which are mechanistically different and provide different levels of resolution, allowed simultaneous analysis at the total community level (overall community structure) and the population level (predominant ammonia oxidizers). This approach also has several technical advantages for detecting specific populations of interest. (i) Since the G+C content of bacteria is characteristic at about the genus level and higher (10) and the total community DNA was fractionated based on G+C content, we reduced the complexity of the DNA being probed and increased the relative abundance of the specific target DNA (amo and hao genes) in individual sample fractions. (ii) Nonspecific hybridization of total community DNA to gene probes specific for phylogenetic or functional groups of interest can be revealed if the probes hybridize to areas in the community profile representing G+C contents not normally associated with the organisms of interest. This same rationale might also reveal the presence of genes or sequences of interest in different or unexpected phylogenetic groups in the community. (iii) The use of multiple gene probes for a pathway of interest can provide useful information regarding the population diversity of phylogenetic or functional groups of interest. (iv) Direct molecular detection eliminates the necessity of culturing organisms to facilitate monitoring; this advantage is particularly relevant when the populations of interest are difficult to grow or are unculturable.
ACKNOWLEDGMENT
We thank D. Arp, Oregon State University, for providing the amo and hao gene probe clones.
REFERENCES
- 1.Bock E, Koops H-P, Möller U C, Rudert M. A new facultatively nitrite oxidizing bacterium, Nitrobacter vulgaris sp. nov. Arch Microbiol. 1990;153:105–110. [Google Scholar]
- 2.Dodson M, Mangan J, Watson S W. Comparison of deoxyribonucleic acid homologies of six strains of ammonia-oxidizing bacteria. Int J Syst Bacteriol. 1983;33:521–524. [Google Scholar]
- 3.Furumai H, Rittmann B E. Advanced modeling of mixed populations of heterotrophs and nitrifiers considering the formation and exchange of soluble microbial products. Water Sci Technol. 1992;26:493–502. [Google Scholar]
- 4.Glover H E. The relationship between inorganic nitrogen oxidation and organic carbon production in batch and chemostat cultures of marine nitrifying bacteria. Arch Microbiol. 1985;142:45–50. [Google Scholar]
- 5.Hastings R C, Ceccherini M T, Miclaus N, Saunders J R, Bazzicalupo M, McCarthy A J. Direct molecular biological analysis of ammonia oxidizing bacterial populations in cultivated soil plots treated with swine manure. FEMS Microbiol Ecol. 1997;23:45–54. [Google Scholar]
- 6.Head I M, Hiorns W D, Embley T M, McCarthy A J, Saunders J R. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J Gen Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 7.Hiorns W D, Hastings R C, Head I M, McCarthy A J, Saunders J R, Pickup R W, Hall G H. Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of nitrosospiras in the environment. Microbiology. 1995;141:2793–2800. doi: 10.1099/13500872-141-11-2793. [DOI] [PubMed] [Google Scholar]
- 8.Holben W E, Tiedje J M. Applications of nucleic acid hybridization in microbial ecology. Ecology. 1988;69:561–568. [Google Scholar]
- 9.Holben W E, Schroeter B M, Calabrese V G M, Olsen R H, Kukor J K, Biederbeck V O, Smith A E, Tiedje J M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992;58:3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holben W E, Harris D. DNA-based monitoring of total bacterial community structure in environmental samples. Mol Ecol. 1995;4:627–631. doi: 10.1111/j.1365-294x.1995.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 11.Holben W E. Isolation and purification of bacterial community DNA from environmental samples. In: Hurst C H, Knudsen G R, McInerny M J, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C: American Society for Microbiology Press; 1997. pp. 431–436. [Google Scholar]
- 12.Holmes A J, Costello A, Kindstrom M E, Murrell J C. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett. 1995;132:203–208. doi: 10.1016/0378-1097(95)00311-r. [DOI] [PubMed] [Google Scholar]
- 13.Hovanec T A, DeLong E F. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl Environ Microbiol. 1996;62:2888–2896. doi: 10.1128/aem.62.8.2888-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klotz M G, Norton J M. Sequence of an ammonia monooxygenase subunit A-encoding gene from Nitrosospira sp. NpAV. Gene. 1995;163:159–160. doi: 10.1016/0378-1119(95)00392-j. [DOI] [PubMed] [Google Scholar]
- 15.Knight I T, Holben W E, Tiedje J M, Colwell R R. Nucleic acid hybridization techniques for detection, identification, and enumeration of microorganisms in the environment. In: Levin M A, Seidler R J, Rogul M, editors. Microbial ecology: principles, methods, and applications. New York, N.Y: McGraw-Hill Book Co.; 1992. pp. 65–91. [Google Scholar]
- 16.Koops H-P, Harms H. Deoxyribonucleic acid homologies among 96 strains of ammonia-oxidizing bacteria. Arch Microbiol. 1985;141:214–218. doi: 10.1007/BF00408061. [DOI] [PubMed] [Google Scholar]
- 17.Kowalchuk G A, Stephen J R, deBoer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaig A E, Embley T M, Prosser J I. Molecular analysis of enrichment cultures of marine ammonia oxidizers. FEMS Microbiol Lett. 1994;120:363–368. doi: 10.1111/j.1574-6968.1994.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 19.Mctavish H, Fuchs J A, Hooper A B. Sequence of the gene coding for ammonia monooxygenase in Nitrosomonas europaea. J Bacteriol. 1993;175:2436–2444. doi: 10.1128/jb.175.8.2436-2444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobarry B K, Wagner M, Urbain V, Rittman B E, Stahl D A. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol. 1996;62:2156–2162. doi: 10.1128/aem.62.6.2156-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro E, Fernandez M P, Grimont F, Clays-Josserand A, Bardin R. Genomic heterogeneity of the genus Nitrobacter. Int J Syst Bacteriol. 1992;42:554–560. [Google Scholar]
- 22.Neijdat A, Abeliovich A. Detection of Nitrosomonas spp. by polymerase chain reaction. FEMS Microbiol Lett. 1994;120:191–194. doi: 10.1111/j.1574-6968.1994.tb07029.x. [DOI] [PubMed] [Google Scholar]
- 23.Noto K, Ogasawara T, Suwa Y, Sumino T. Complete oxidation of high concentrations of ammonia by retaining incompatible nitrification activities in a 3-vessel system. Water Res. 1998;32:769–773. [Google Scholar]
- 24.Pommerening-Röser A, Rath G, Koops H-P. Phylogenetic diversity within the genus Nitrosomonas. Syst Appl Microbiol. 1996;19:344–351. [Google Scholar]
- 25.Rittmann B E, Regan J M, Stahl D A. Nitrification as a source of soluble organic substrate in biological treatment. Water Sci Technol. 1994;30:1–8. [Google Scholar]
- 26.Sayavedra-Soto L A, Hommes N G, Arp D J. Characterization of the gene encoding hydroxylamine oxidoreductase in Nitrosomonas europaea. J Bacteriol. 1994;176:504–510. doi: 10.1128/jb.176.2.504-510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayler G S, Layton A C. Environmental applications of nucleic acid hybridization. Annu Rev Microbiol. 1990;44:625–648. doi: 10.1146/annurev.mi.44.100190.003205. [DOI] [PubMed] [Google Scholar]
- 28.Sinigalliano C D, Kuhn D N, Jones R D. Amplification of the amoA gene from diverse species of ammonium-oxidizing bacteria and from an indigenous bacterial population from seawater. Appl Environ Microbiol. 1995;61:2702–2706. doi: 10.1128/aem.61.7.2702-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumino T, Nakamura H, Mori N, Kawaguchi Y. Immobilization of nitrifying bacteria by polyethlene glycol prepolymer. J Ferment Bioeng. 1992;73:37–42. [Google Scholar]
- 30.Suwa Y, Noto K, Sumino T, Urushigawa Y. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Sequence of the gene encoding a subunit of ammonia monooxygenase, amoA, from an (NH4)2SO4-sensitive ammonia-oxidizing bacterium, strain JL21, isolated from activated sludge, abstr. Q-257; p. 430. [Google Scholar]
- 31.Suwa Y, Sumino T, Noto K. Phylogenetic relationships of activated sludge isolates of ammonia oxidizers with different sensitivities to ammonium sulfate. J Gen Appl Microbiol. 1997;43:373–379. doi: 10.2323/jgam.43.373. [DOI] [PubMed] [Google Scholar]
- 32.Teske A, Alm E, Regan J M, Toze S, Rittmann B R, Stahl D A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Utaker J B, Bakken L, Jiang Q Q, Nes I F. Phylogenetic analysis of seven new isolates of ammonia-oxidizing bacteria based on 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:549–559. [Google Scholar]
- 34.Voytek M A, Ward B B. Detection of ammonium-oxidizing bacteria of the beta subclass of the class Proteobacteria in aquatic samples with the PCR. Appl Environ Microbiol. 1995;61:1444–1450. doi: 10.1128/aem.61.4.1444-1450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner M, Rath G, Amann R, Koops H-P, Schleifer K-H. In situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol. 1995;18:251–264. [Google Scholar]
- 36.Ward B B, Voytek M A, Witzel K-P. Phylogenetic diversity of ammonia oxidizers investigated by specific PCR amplification. Microb Ecol. 1997;33:87–96. doi: 10.1007/s002489900011. [DOI] [PubMed] [Google Scholar]
- 37.Watson S W, Mandel M. Comparison of the morphology and deoxyribonucleic acid composition of 27 strains of nitrifying bacteria. J Bacteriol. 1971;107:563–569. doi: 10.1128/jb.107.2.563-569.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woese C R, Weisburg W G, Paster B J, Hahn C M, Tanner R S, Krieg N R, Koops H-P, Harms H, Stackebrandt E. The phylogeny of purple bacteria: the beta subdivision. Syst Appl Microbiol. 1984;5:327–336. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]