Abstract
Wide complex tachycardia (WCT) is an infrequently encountered condition in paediatric patients and may be due to a variety of causes including supraventricular tachycardia with aberrant conduction, ventricular activation via an accessory pathway, ventricular pacing, or ventricular tachycardia. Immediate tachycardia termination is required in haemodynamically unstable patients. After stabilization or in those with haemodynamically tolerated WCT, a careful review of electrocardiographic tracings and diagnostic manoeuvres are essential to help elucidate the cause. Subacute and chronic management for WCT will depend on the underlying cause as well as features of the patient and the tachycardia presentation. This article will review the epidemiology, potential causes, and management of WCT in children. A detailed review of the pathophysiology, differential diagnosis, and diagnostic and treatment options is provided to enable the reader to develop a practical approach to managing this condition in young patients.
Graphical abstract
Résumé
La tachycardie à complexes QRS larges est rare en pédiatrie et peut avoir diverses causes, notamment une tachycardie supraventriculaire avec trouble de la conduction, l’activation ventriculaire par une voie accessoire, une stimulation ventriculaire ou une tachycardie ventriculaire. La suppression immédiate de la tachycardie est primordiale lorsque l’état hémodynamique du patient est instable. Une fois l’état du patient stabilisé, ou en cas de tachycardie à complexes QRS larges tolérée sur le plan hémodynamique, l’examen minutieux des tracés électrocardiographiques et des manœuvres diagnostiques est crucial pour en élucider la cause. La prise en charge des cas subaigus et chroniques de tachycardie à complexes QRS larges dépend de sa cause sous-jacente ainsi que des caractéristiques du patient et du tableau clinique de la tachycardie. Cet article porte sur l’épidémiologie, les causes possibles et la prise en charge de la tachycardie à complexes QRS larges chez les enfants. Un examen approfondi de la physiopathologie, du diagnostic différentiel et des options diagnostiques et thérapeutiques est présenté pour permettre au lecteur d’élaborer une approche pratique pour la prise en charge de cette affection chez leurs jeunes patients.
Tachyarrhythmias are defined as fast, abnormal heart rhythms that can present as either a narrow or wide complex arrhythmia. Though often mistakenly thought to be synonymous with ventricular tachycardia (VT), wide complex tachycardia (WCT) in the paediatric population can be secondary to supraventricular tachycardia (SVT). SVT is the most common type of tachycardia seen in paediatric patients, with an estimated incidence between 1 in 250 and 1 in 1000 patients.1 SVT with a wide QRS complex must be in the differential when considering a WCT in the younger patient. A wide QRS complex in the setting of SVT may be due to several causes including an underlying bundle branch block, rate-related bundle branch block, slowing within the distal His-Purkinje system and myocardium in the setting of electrolyte abnormalities or drug toxicity, antegrade conduction via an accessory pathway (AP), or a paced ventricular rhythm. The mechanisms of WCT will be discussed further below. In contrast, VTs are much less common in paediatric patients, with rates between 1 and 8 per 100,000 children.2 VT can occur in children in the setting of a structurally normal heart and may be an idiopathic and self-limited arrhythmia. Young patients with structural heart disease, inherited arrhythmia syndromes, or acquired cardiomyopathies have a higher incidence of VT and are more likely to present with a tachyarrhythmia that can result in significant haemodynamic compromise and arrhythmia recurrence. This article will aim to review the causes of WCT in paediatric patients, highlight acute management strategies including diagnostic testing and both noninvasive and invasive treatment options, and identify long-term management and follow-up goals.
Definition of WCT in Paediatric Patients
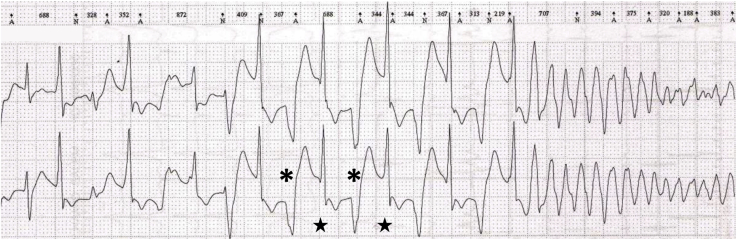
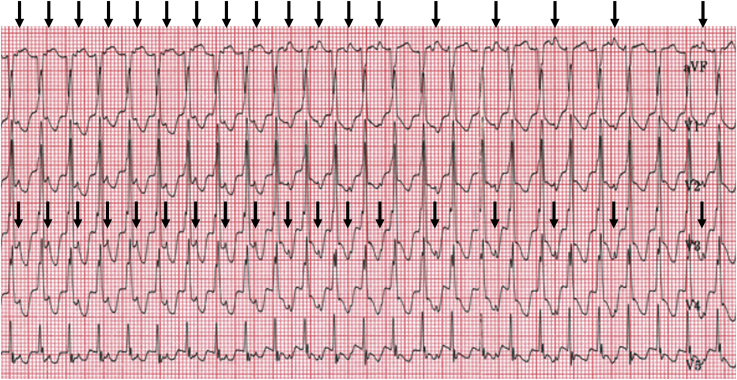
QRS width is age dependent. Therefore, the definition of WCT is also dependent on age with normal QRS width being much narrower in infancy as compared with adolescents or adults. An example of a narrow-appearing, but wide complex rhythm in an infant is provided in Figure 1. In order for a tachycardia to be classified as wide complex, the QRS duration should be greater than the 98th percentile for age (Table 1).
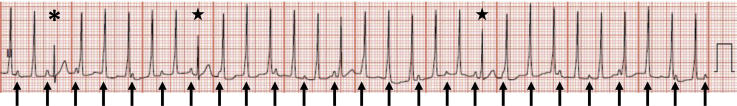
Figure 1.
Accelerated idioventricular rhythm with ventriculoatrial dissociation in an infant. The QRS duration of the ventricular tachycardia beats is 80 milliseconds. The arrows indicate sinus P waves, which are dissociated from the QRS complexes. The asterisk indicates a sinus capture beat. The stars demonstrate fusion beats.
Table 1.
Age dependent ECG upper limit of normal (98th percentile) for paediatric heart rate and QRS width
| 0-1 mo | 1-3 mo | 3-6 mo | 6-12 mo | 1-3 y | 3-5 y | 5-8 y | 8-12 y | 12-16 y | 16-19 y | |
|---|---|---|---|---|---|---|---|---|---|---|
| Heart rate (beats/min), male | 192 | 187 | 165 | 194 | 155 | 123 | 113 | 101 | 99 | 107 |
| Heart rate (beats/min), female | 216 | 200 | 191 | 187 | 178 | 124 | 115 | 110 | 107 | 105 |
| QRS duration (ms), male | 85 | 77 | 85 | 86 | 88 | 92 | 98 | 103 | 111 | 126 |
| QRS duration (ms), female | 79 | 77 | 78 | 80 | 85 | 88 | 95 | 99 | 106 | 112 |
Adapted from: Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA. New normal limits for the paediatric electrocardiogram. Eur Heart J 2001;22:702-11; and Rijnbeek PR, van Herpen G, Bots ML, et al. Normal values of the electrocardiogram for ages 16-90 years. J Electrocardiol 2014;47:914-2.
Physiology and Pathophysiology of QRS Width
A normal, narrow QRS complex results from activation of the rapidly conducting His-Purkinje system once an electrical impulse has passed through the atrioventricular (AV) node. The impulse then moves briskly through the Purkinje network, typically translating into a well-coordinated ventricular contraction and a narrow QRS complex. A ventricular impulse originating from within the ventricular myocardium, an AP, or a pacemaker results in a less simultaneous ventricular activation and a wider QRS complex. A supraventricular beat encountering a delay or block in the His-Purkinje system will also result in a wide QRS complex and is referred to as aberrant conduction. In addition, myocardial disease, electrolyte abnormalities, and drugs may also affect timing of ventricular depolarization and result in a widened QRS complex.
Electrocardiogram Analysis and Differential Diagnosis of WCT in Children
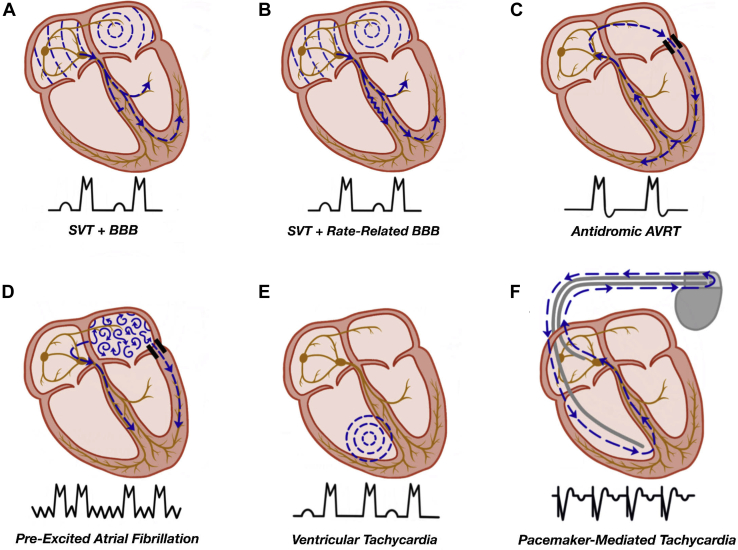
The differential diagnosis for WCTs includes SVT with aberrant conduction, antegrade conduction via an AP, tachycardias in the setting of ventricular pacing, and VT, in addition to the aforementioned electrolyte disturbances, drug effects, and myocardial ischemia. Table 2 summarizes the potential causes of a WCT, and a diagrammatic representation of the common WCTs in children is provided in Figure 2.
Table 2.
Wide complex tachycardia diagnoses in paediatric patients
| Differential diagnosis of wide complex tachycardias in children |
|---|
| Usual-complex tachycardia with baseline conduction abnormality |
| SVT with rate-related aberrancy |
| Pre-excited tachycardia |
| Antidromic tachycardia |
| SVT with bystander accessory pathway (including pre-excited atrial fibrillation) |
| Ventricular pacing |
| Tracked atrial rhythm |
| Pacemaker-mediated tachycardia |
| Ventricular tachycardia |
| Monomorphic VT |
| Idiopathic VT—outflow tract VT or fascicular VT |
| Other monomorphic VT |
| Bidirectional VT |
| Polymorphic VT |
| Torsades de pointes |
| Ventricular fibrillation |
| Other causes and mimickers of aberrancy |
| Electrolyte abnormalities |
| Drug ingestion |
| Myocardial ischemia |
SVT, supraventricular tachycardia; VT, ventricular tachycardia.
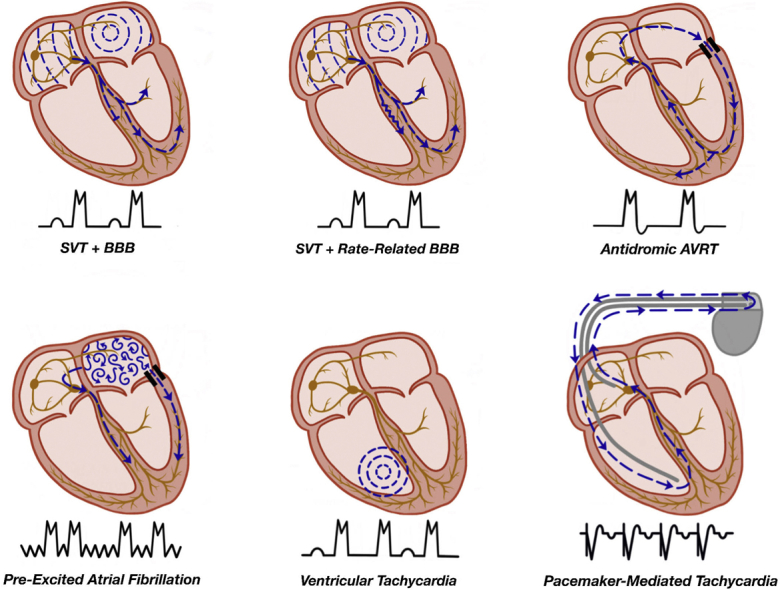
Figure 2.
Mechanisms of wide complex tachycardia. (A) SVT with an underlying bundle branch block (BBB). (B) SVT with a rate-related bundle branch block. (C) Antidromic tachycardia as seen in a patient with ventricular pre-excitation, with the antegrade limb of the circuit being the accessory pathway and the retrograde limb the AV node. (D) Pre-excited atrial fibrillation with antegrade conduction via the accessory pathway and the AV node, leading to an irregularly irregular wide complex tachycardia. (E) Ventricular tachycardia with VA dissociation due to the absence of retrograde AV nodal conduction. (F) Pacemaker-mediated tachycardia with a ventricular paced beat followed by retrograde conduction via the AV node. The retrograde atrial activity is sensed by the pacemaker with subsequent ventricular pacing to complete the reentrant circuit. AV, atrioventricular; AVRT, atrioventricular reciprocating tachycardia; SVT, supraventricular tachycardia; VA, ventriculoatrial.
Supraventricular tachycardia with aberrant conduction
WCTs in children are most commonly caused by SVT with aberrant conduction due to a baseline aberrancy or a rate-related aberrancy only in tachycardia. Patients with an abnormal baseline QRS morphology such as a bundle branch block, referred to as a “usual-complex” tachycardia, can occur in the setting of a rapid supraventricular rhythm (Fig. 2A). This occurs because the patient’s intrinsic (“usual”) QRS complex reflects the baseline abnormality of the His-Purkinje system. Sinus tachycardia in a patient with an underlying right bundle branch block (RBBB), for example, will appear wide, as the RBBB morphology would be expected to persist during tachycardia (Fig. 3). Rate-related aberrancy, or a rate-related bundle branch block, is due to differences in the relative refractory periods of the bundle branches (Fig. 2B). This phenomenon may resolve spontaneously after the onset of tachycardia (Fig. 4).
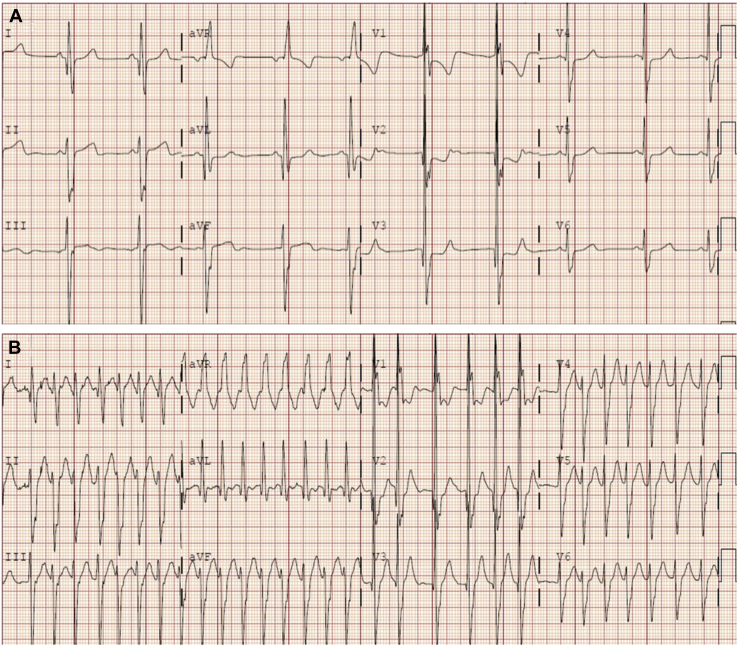
Figure 3.
Wide complex tachycardia in a patient with a baseline conduction abnormality. (A) Baseline electrocardiogram (ECG) showing sinus rhythm and a right bundle branch block in a child with congenital heart disease. (B) The same patient’s ECG during an atrial tachycardia showing a QRS complex similar to baseline. The rhythm is irregular due to variable atrioventricular conduction of the atrial tachycardia.
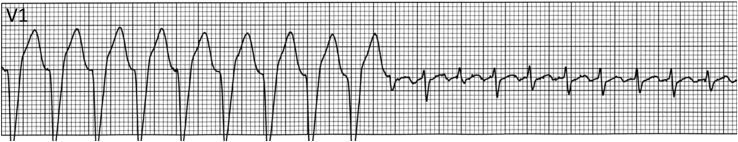
Figure 4.
Supraventricular tachycardia (SVT) with rate-related aberrancy in a child with tachycardia. A wide complex tachycardia with rate-related left bundle branch block (rhythm strip from lead V1) transitioning to a narrow complex tachycardia; diagnostic of SVT. Reproduced with permission.11
Supraventricular tachycardia with antegrade accessory pathway conduction
Ventricular pre-excitation refers to early ventricular activation from an AP with antegrade conduction. Because the earliest conduction to the ventricle is via the AP rather than the AV node, ventricular activation does not initially use the His-Purkinje system and therefore results in a wide QRS complex.
In antidromic AV reciprocating tachycardia, the antegrade limb of the tachycardia circuit is the AP, whereas the retrograde limb is most commonly the AV node (Fig. 2C). This type of AP-mediated tachycardia is relatively uncommon, occurring in fewer than 5% of patients with ventricular pre-excitation. A specific type of antegrade conducting AP is an atriofascicular pathway (also commonly referred to as a “Mahaim fiber”). Patients with atriofascicular pathways rarely show evidence of pre-excitation in sinus rhythm, but the associated tachycardias are exclusively wide complex.
An AP can also serve as a “bystander” during a tachycardia in which it is not a critical component of the circuit but allows for pre-excitation of the ventricle. An important example of this is atrial fibrillation in the presence of an AP with antegrade conduction (Fig. 2D). The pathognomonic appearance of pre-excited atrial fibrillation is an irregularly irregular WCT with each wide complex beat having a similar axis (Fig. 5). Pre-excited atrial fibrillation is an especially important tachycardia for a paediatric cardiologist to immediately recognize, because treatment with adenosine or other AV nodal blocking agents can result in degeneration to ventricular fibrillation.
Figure 5.
Pre-excited atrial fibrillation. The characteristic features of an irregularly irregular wide complex tachycardia, variable QRS duration, and consistent QRS axis are shown. The degree of ventricular pre-excitation is a reflection of fusion between atrial wavefronts conducting via the atrioventricular node and the accessory pathway. Reproduced with permission.11
Ventricular tachycardia and other ventricular arrhythmias
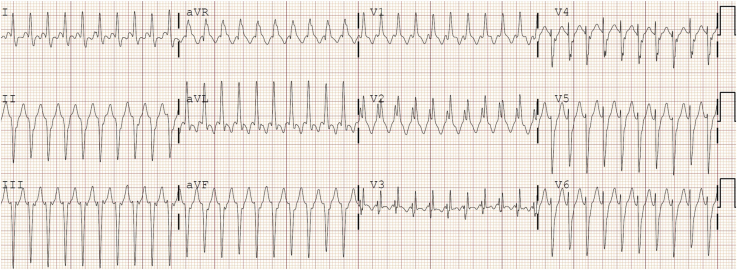
Cardiac impulses originating from the ventricular myocardium generate a wide QRS complex as the wave of depolarization moves directly cell-to-cell rather than through the rapidly conducting His-Purkinje system (Fig. 2E). The site of origin of a monomorphic VT may be roughly ascertained based on the specific QRS morphology seen. Several specific types of VT deserve mention when considering WCT in children. Idiopathic VTs are those occurring in patients without other heart disease. Right ventricular outflow tract (RVOT) or left ventricular outflow tract VTs are the most common idiopathic VTs (Fig. 6). This tachycardia will have an inferior axis and either a left bundle branch block (LBBB) or RBBB pattern depending on the outflow tract origin. Left ventricular (LV) fascicular VT, also known as verapamil-sensitive VT or Belhassen VT, is also a common idiopathic VT. This tachycardia most commonly has a superior axis and an RBBB morphology (Fig. 7). Finally, accelerated idioventricular rhythm is a wide complex rhythm observed in paediatric patients and frequently in neonates. It is characterized by a wide complex rhythm slightly faster than the sinus rate (10%-20% faster). It is typically well tolerated and unlikely to require treatment (Fig. 1).
Figure 6.
Right ventricular outflow tract ventricular tachycardia. This tachycardia typically has an inferior axis and left bundle branch block morphology. The arrows indicate P waves, which are best seen in the right precordial leads, and therefore demonstrate ventriculoatrial dissociation.
Figure 7.
Left ventricular posterior fascicular ventricular tachycardia. The electrocardiogram demonstrates a superior QRS axis and right bundle branch block morphology. Note the characteristic sharp initial deflection of the QRS (indicative of activation of the fascicular tissue) and relatively narrow appearance of this wide complex tachycardia.
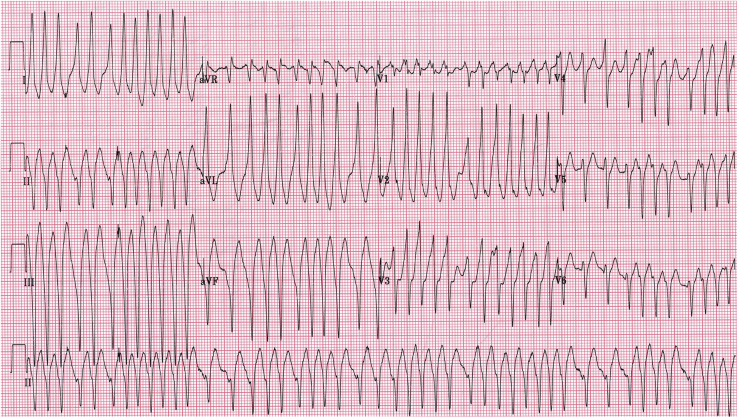
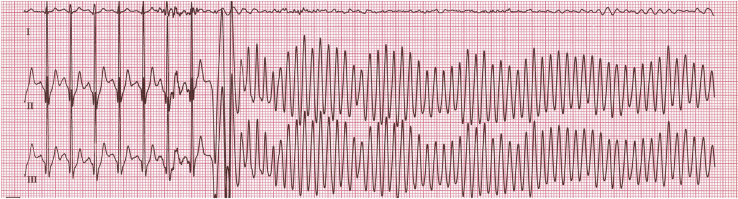
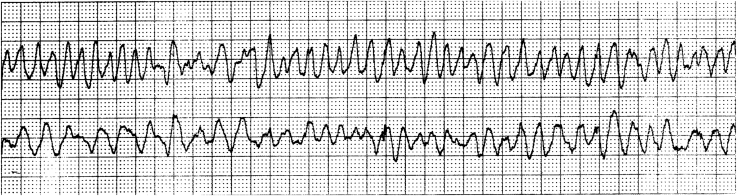
Important malignant VTs include bidirectional VT, which is a rare tachycardia that is critical to recognize. Bidirectional VT has an alternating QRS axis (Fig. 8) and is associated with catecholaminergic polymorphic VT (CPVT) and digitalis toxicity. Polymorphic VT and ventricular fibrillation (VF) should be recognized immediately. Polymorphic VT, in which a changing QRS morphology with some organization is seen, can rapidly deteriorate into VF. Torsades de pointes “twisting of the points” is a specific type of polymorphic VT associated with QT prolongation that has a characteristic appearance of “twisting” around an isoelectric line (Fig. 9). VF is a disordered, life-threatening ventricular arrhythmia during which the heart does not beat effectively (Fig. 10). In paediatric patients, polymorphic VT and VF are often associated with inherited myocardial disease, channelopathies, and acquired cardiomyopathies.
Figure 8.
Bidirectional ventricular tachycardia with alternating axes, as demonstrated by the asterisks and stars, followed by degeneration into ventricular fibrillation in a patient with catecholaminergic polymorphic ventricular tachycardia.
Figure 9.
Initiation of torsades de pointes with characteristic “twisting” appearance around the isoelectric line. This patient had idiopathic ventricular fibrillation thought to be due to intermediate-coupled premature ventricular contractions as described by Li et al.35 Reproduced with permission.35
Figure 10.
Coarse ventricular fibrillation characterized by a disorganized, low-amplitude, and polymorphic appearance.
Electrocardiogram differentiation of SVT from VT
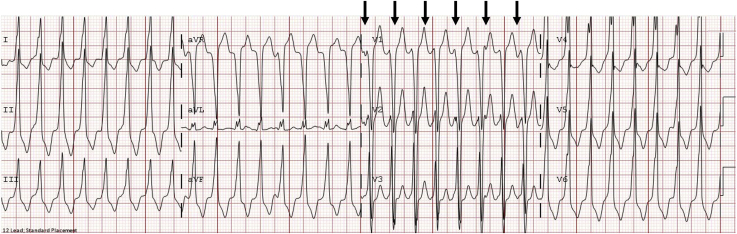
Detailed evaluation of a 12-lead electrocardiogram (ECG) not only suggests the etiology of the tachycardia but can also direct the most appropriate next steps in the management. Importantly, 12- or 15-lead ECGs provide more comprehensive data than a single lead rhythm strip. The QRS duration, for example, may appear normal in one lead and wider in another, as seen in Figure 11, where the QRS complex in lead V6 appears narrow during the ventricular beats. This difference in QRS width in different leads may be due to there being an isoelectric component of the QRS in some leads and not others. Although several algorithms for identifying the etiology of a WCT exist, the diagnostic accuracy of such algorithms is only 66%-69% in paediatric patients compared with up to 96%-98% accuracy in the adult population.3 Thus, careful examination of the ECG by a paediatric cardiologist or electrophysiologist can be exceptionally valuable.
Figure 11.
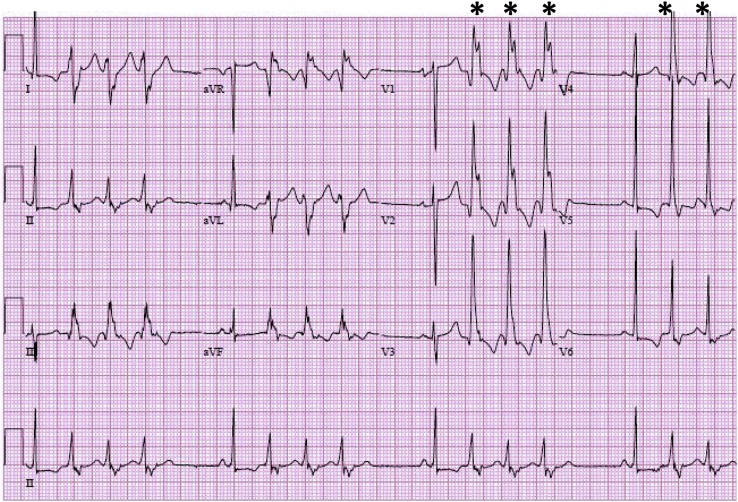
Ventricular tachycardia with concordance. The QRS complexes of the ventricular ectopic beats (indicated by asterisks) are monophasic with the same polarity in the precordial leads (positive concordance), suggesting a ventricular origin.
Comparison of the ECG with a prior ECG in sinus rhythm (if available) is essential to determine the patient’s baseline QRS morphology or assess for ventricular pre-excitation. Identification of the P waves during the WCT may reveal AV dissociation with a slower atrial rate than ventricular rate, which highly suggests VT. However, a 1:1 P:QRS relationship does not exclude VT as it is not uncommon for young children to have robust AV node conduction, allowing for 1:1 retrograde conduction during VT. When available, P-wave identification may be enhanced with an oesophageal electrode or cardiac pacing wires in postoperative cardiac patients. The presence of sinus capture beats is consistent with VT. These appear as intermittent early narrower beats during the WCT and may be preceded by obvious P waves. They are due to occasional normal capture of the conduction system by a sinus beat during VT (Fig. 1). Fusion beats also appear as intermittent narrower beats during the WCT and are due to normal conduction to the ventricular myocardium by a sinus beat occurring nearly simultaneously with a beat of VT, leading to a fused QRS morphology (Fig. 1).
In addition to AV dissociation and sinus capture or fusion beats, other findings suggestive of a ventricular origin for the tachycardia can be deduced by careful examination of the QRS complex itself when it is different from the baseline QRS morphology. A QRS complex that is atypical of a RBBB or LBBB pattern, one with extreme axis deviation, or one with a very prolonged QRS duration is more likely to be ventricular in origin. Lastly, the finding of precordial concordance (V1-V6 demonstrating monophasic QRS complexes with the same polarity) strongly suggests VT (Fig. 11), although the absence of concordance is not diagnostic of SVT. ECG features suggestive of VT are summarized in Table 3.
Table 3.
Features on ECG suggestive of a ventricular tachycardia diagnosis
| ECG features suggestive of VT |
|---|
| Diagnostic features |
| AV dissociation (with ventricular rate greater than atrial rate) |
| Sinus capture or fusion beats |
| Suggestive features |
| QRS duration >90 ms in infants; 160 ms in young adults |
| Concordance |
| QRS complex atypical for a right or left bundle branch block pattern in a patient with a structurally normal heart |
AV, atrioventricular; ECG, electrocardiogram; VT, ventricular tachycardia.
Once a suspected diagnosis of VT is made, examination of the QRS complex pattern and axis can suggest the site of origin in a patient with a structurally normal heart. A relatively common and benign VT origin is the RVOT. This type of VT manifests as WCT with an LBBB morphology and inferior axis (positive QRS in leads II, III, and aVF) in a stable patient (Fig. 6). LV fascicular VT manifests with somewhat narrower QRS complexes, a short RS interval, and an RBBB pattern. The axis in LV fascicular VT can vary depending on which fascicle is involved. The posterior fascicle is most commonly involved and results in a left axis deviation (Fig. 7). Other VTs also exhibit characteristic ECG findings, and expeditious diagnosis is important in order to provide optimal directed management. Torsades de pointes (Fig. 9), bidirectional VT (Fig. 8), and pre-excited atrial fibrillation (Fig. 5) all have characteristic ECG findings, as previously described.
WCT due to ventricular pacing
Ventricular pacing nearly always generates a wide QRS complex. SVTs including sinus tachycardia will be wide complex in a patient dependent on ventricular pacing. One specific pacemaker-related arrhythmia is pacemaker-mediated tachycardia, which results from the retrograde atrial conduction of a paced ventricular beat (typically through the AV node, Fig. 2F). The retrograde atrial impulse is then sensed and tracked by the pacemaker, creating the tachycardia circuit (Fig. 12). Pacemaker-mediated tachycardia should be considered in patients with dual chamber pacemakers with sustained regular ventricularly paced tachycardia.
Figure 12.
Pacemaker-mediated tachycardia. Ventricular paced beats conduct retrograde to the atrium, with subsequent ventricular tracking of the retrograde atrial activation, continuing the tachycardia circuit. The arrows indicate the retrograde atrial activation. The black triangles indicate the pacing spikes.
Acute Management Approach
The immediate goals of WCT management are to stabilize the patient, determine the cause of the tachycardia, and treat any reversible causes. Thus, the initial step in management should be to assess the patient’s stability. An unstable patient is defined as one who exhibits signs of cardiorespiratory compromise and/or shock. The Pediatric Advanced Life Support (PALS) framework should be followed in first evaluating for a pulse, and if absent, commencing cardiopulmonary resuscitation.4 The most cautious approach to an unstable patient in a WCT is to assume VT as an etiology.
If a pulse is present but a patient is unstable, it is imperative to recognize that the patient could decompensate quickly. Defibrillator pads should be attached as soon as possible, and emergent synchronized cardioversion should be performed (with 2 J/kg if pulseless and 0.5-1 J/kg if a pulse is present).
Intravenous (IV) access, blood pressure monitoring, and cardiac monitoring should also be initiated quickly. The PALS algorithm should be followed with subsequent cardiopulmonary resuscitation and shock with 4 J/kg if pulseless, or repeat synchronized cardioversion with 2 J/kg if a pulse is present.4 Should the patient continue in an unstable WCT, either amiodarone 5 mg/kg slow bolus over 20-60 minutes or lidocaine 1 mg/kg can be administered intravenously or intraosseously.
Acute medical management in the haemodynamically stable patient
In a stable patient, time allows for the important step of refining the diagnosis in order to deliver the most appropriate treatment. A personal history should be obtained with particular attention to the presence of congenital heart disease, inherited arrhythmia or cardiomyopathy, prior palpitations, syncope, poor feeding, irritability, medications or possible ingestions, and exercise intolerance. QT-prolonging medications can cause ventricular arrhythmias; thus, it is important to elucidate a history of antiarrhythmic medications, nonsedating antihistamines, macrolide antibiotics, certain opioids (such as methadone), antipsychotics, antiepileptics, and antidepressants. Noncardiac diseases causing hypokalaemia or hypomagnesaemia can also result in acquired QT prolongation and VT. A family history of sudden death, syncope, seizures, channelopathy, or cardiomyopathy can also be helpful in identifying the underlying etiology. The physical examination should focus on the cardiac examination and any evidence of haemodynamic instability or respiratory compromise.
Additional laboratory testing is warranted in patients presenting with WCT, with specific tests guided by the history, clinical presentation, and differential diagnosis. Testing should exclude potential causes of ventricular arrhythmias including acute inflammation (eg, myocarditis), metabolic or electrolyte disturbances, and drug toxicity.5
Initial medical management of stable patients with WCT should also follow PALS recommendations unless otherwise directed by an electrophysiologist. Initial interventions can include vagal manoeuvres and pharmacotherapy. Resuscitation equipment including a defibrillator should be made available in case haemodynamic compromise develops.
It is reasonable to try to perform vagal manoeuvres in stable patients with undifferentiated WCT, and especially for those with a high index of suspicion for SVT with aberrancy or RVOT VT. Vagal manoeuvres may be therapeutic by terminating tachycardia or provide clues to diagnosis if transient antegrade or retrograde AV nodal block is achieved. A 12-lead rhythm strip should be recorded during vagal manoeuvres, as subtle findings such as changes in the ventriculoatrial (VA) relationship may provide clues to the diagnosis, as described below in the case of using adenosine.
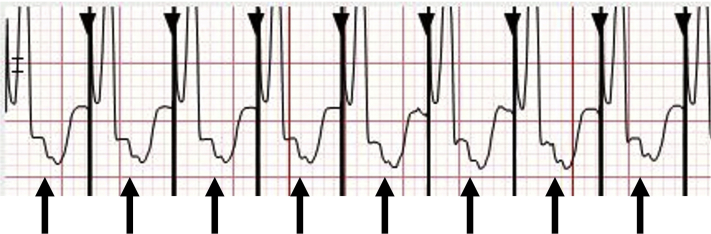
Adenosine can be safely administered to transiently block AV nodal conduction in the case of undifferentiated regular WCT and may yield both therapeutic and diagnostic effects.6 Adenosine should never be administered to a patient with an irregularly irregular WCT. Resuscitation equipment, and specifically a defibrillator, should always be present when administering adenosine, particularly in the case of WCT. A 12-lead rhythm strip should also be acquired at the time of adenosine effect in order to detect changes in the VA relationship. Proper administration of an adequate dose of adenosine will achieve AV nodal blockade and terminate an SVT that depends on the AV node and may also terminate idiopathic outflow tract VTs. AV nodal blockade with continuation of an atrial tachycardia will establish the mechanism of SVT with aberrant conduction as being due to an atrial tachycardia. In VT with 1:1 VA conduction, adenosine can lead to brief termination of the retrograde AV nodal conduction to give VA dissociation without tachycardia termination, which underscores the importance of 12-lead rhythm strip documentation during adenosine administration (Fig. 13). If WCT continues despite adenosine causing VA block and VA dissociation, a diagnosis of VT or junctional ectopic tachycardia with an underlying bundle branch block can be made.
Figure 13.
Ventriculoatrial (VA) dissociation with adenosine. The arrows indicate the P waves. There is initially 1:1 VA conduction during the wide complex tachycardia. With adenosine administration, there is gradual VA prolongation followed by VA dissociation with continuation of the tachycardia. This finding is consistent with a diagnosis of ventricular tachycardia. The differential diagnosis for this pattern is junctional ectopic tachycardia in a patient with an underlying bundle branch block, as could be seen after surgery for congenital heart disease.
If vagal manoeuvres and adenosine are unsuccessful in restoring sinus rhythm, then additional pharmacologic therapy should be considered. There are no strong data to support the use of one antiarrhythmic over another in the initial treatment of undifferentiated WCT or sustained VT in children; choice of therapy may therefore be guided by the likely tachycardia mechanism, patient age, and presence or absence of structural heart disease and ventricular dysfunction.4 Options for initial therapy include IV amiodarone, procainamide, lidocaine, and esmolol. Oral antiarrhythmic therapy could be considered in cases of relatively slower or intermittent tachycardias if there are no concerns regarding haemodynamic instability but may result in longer times to achieving tachycardia control. Synchronized direct current cardioversion with appropriate sedation is also an option for acute tachycardia termination.
Specific management strategies may be required for certain diagnoses. VTs originating from the RVOT can be treated with IV verapamil or β-blockers. LV fascicular VT (Fig. 7) is classically sensitive to IV verapamil, which should be a first-line therapy for acute termination in this condition in haemodynamically stable children >12 months of age. However, caution is advised when using IV verapamil in the case of undifferentiated WCT, and expert consultation should be sought before administration of this therapy. Stable pre-excited atrial fibrillation is best managed with IV procainamide or elective cardioversion. Additional medications such as ibutilide, flecainide, or propafenone are reasonable but electrophysiology consultation is advisable.7 Direct current cardioversion may be needed in pre-excited atrial fibrillation if pharmacotherapy is ineffective or if the patient is haemodynamically unstable.7
Pacemaker-mediated tachycardia can be terminated by applying a magnet to the device. Magnet application changes the pacing mode to an asynchronous mode in which the ventricle will be paced but will not track the atrium, thereby breaking the tachycardia circuit.8 In cases of ventricular tracking of an atrial arrhythmia, magnet application can abruptly decrease the ventricular pacing rate by changing to an asynchronous, nontracking pacing mode. It is important to be aware that magnet application will turn off defibrillator therapies as long as the magnet is applied.
Management of recurrent torsades de pointes involves magnesium supplementation, correction of serum potassium and calcium levels, and use of temporary pacing to increase the heart rate in cases of bradycardia. Isoproterenol may be used in cases of acquired long QT syndrome (LQTS), whereas β-blocker therapy may be required in congenital LQTS.9 Bidirectional VT thought to be secondary to CVPT should be acutely managed using an IV β-blocker such as esmolol.9
Subacute and Chronic Management of Ventricular Tachycardia
After initial stabilization of WCT, further assessment and specific therapy should be considered. In addition to the etiology of the WCT, long-term management is determined by several factors including the following:
-
•
Tachycardia persistence or recurrence
-
•
Duration of tachycardia episodes
-
•
Symptoms related to the WCT including irritability or poor feeding in infants, syncope, pallor, haemodynamic compromise, or aborted cardiac arrest
-
•
Presence of structural cardiac abnormalities or congenital heart disease
-
•
Evidence of cardiac dysfunction
-
•
Natural history of the condition
The remainder of this article will review the subacute and chronic management of SVT and VT, with a special focus on VT. For further information on the assessment and chronic management of SVT, we refer the reader to resources outlined in the references.7,10,11
Supraventricular tachycardia
The workup for SVT includes an ECG and, most commonly, an echocardiogram. A Holter or event monitor and exercise testing may also be done depending on the history and SVT burden. An ECG during sinus rhythm can identify baseline ventricular pre-excitation or bundle branch block. A Holter monitor may help to establish the frequency and duration of SVT as well as response to therapy in patients with frequent episodes. Exercise testing may reveal ectopy or induce SVT in some patients.
Treatment
Treatment options for SVT in the absence of ventricular pre-excitation includes use of vagal manoeuvres as needed, chronic pharmacologic therapy, or catheter ablation. The approach depends on patient age, symptom severity, SVT frequency, and patient and family preference. Infants and small children are typically managed with pharmacologic therapy, with β-blocker as the usual first-line therapy. Digoxin may be an alternative agent to consider for SVT management in young children. Flecainide, sotalol, and amiodarone are second-line agents that may be used for arrhythmia control.12,13 In children ≥ 5 years or >15 kg, catheter ablation is effective and can be safely performed.14
In patients with ventricular pre-excitation associated with SVT, syncope, or rapidly conducted pre-excited atrial fibrillation, recommended therapy is catheter ablation. Class IC or class III agents can be used in patients with APs who are not candidates for or who decline ablation. Digoxin and verapamil should be avoided in patients with ventricular pre-excitation.
Ventricular tachycardia
WCT due to VT usually requires further evaluation and may require specific therapy. Consultation with a paediatric electrophysiologist is recommended. After initial stabilization and control of VT, the workup is focused on detecting possible underlying cardiac disease. Initial evaluation includes ECG and echocardiography. Additional studies to consider include Holter monitoring, exercise stress testing, cardiac magnetic resonance imaging (MRI), cardiac catheterization with or without endomyocardial biopsy, electrophysiology testing, and genetic testing to evaluate for possible underlying etiologies. Which additional studies need to be performed depends on patient-specific factors, the type of VT, and initial testing results.5,15
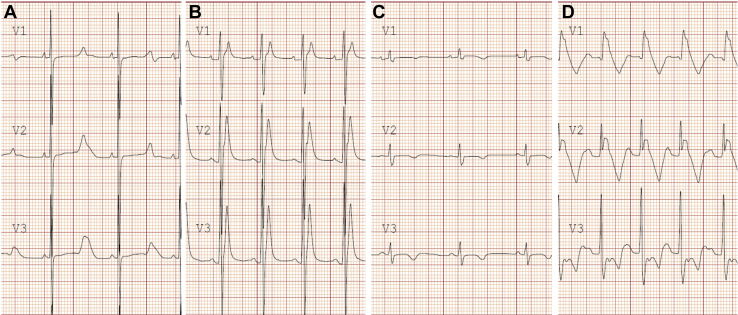
ECG analysis during sinus rhythm may identify certain electrical disorders such as LQTS, Brugada syndrome, ventricular pre-excitation, and arrhythmogenic right ventricular cardiomyopathy (ARVC)/arrhythmogenic cardiomyopathy (ACM) (Fig. 14).
Figure 14.
Electrocardiographic findings in leads V1-V3 in 3 primary channelopathies and arrhythmogenic right ventricular cardiomyopathy (ARVC). (A) Long QT syndrome with prolonged QT interval and abnormal T-wave morphology; (B) short QT syndrome with severely shortened QT interval and peaked T waves; (C) ARVC with low voltage QRS complexes and T-wave inversion in the right precordial leads; (D) Brugada syndrome with coved (type I) ST-segment elevation with T-wave inversion in the right precordial leads.
An echocardiogram can identify structural heart disease and evaluate cardiac function. In the absence of congenital heart disease, the study should focus on looking for tumours, masses, or signs of cardiomyopathy including wall thickness and chamber size, systolic and diastolic function, and ventricular strain.
Holter monitoring allows for assessment of arrhythmia burden and quantification of the number and duration of VT episodes. In addition, it can aid in distinguishing monomorphic from polymorphic ventricular ectopy and can be helpful in evaluating response to ablation or medication therapy.
Exercise testing can be useful in elucidating adrenergic-sensitive ventricular arrhythmias such as CPVT. Effectiveness of therapy in CPVT and LQTS can also be assessed with exercise stress testing. In patients with frequent ventricular ectopy, suppression with exercise may be consistent with, but not indicative of, a good prognosis, whereas worsening of ventricular ectopy may indicate a less favourable prognosis.16 Of note, some patients with RVOT VT and fascicular VT may have worsening of ectopy burden with exercise.
Cardiac MRI provides a detailed assessment of cardiac structure, function, and fibrosis. It is particularly important in the diagnosis of ARVC/ACM as MRI findings comprise part of the diagnostic criteria. MRI can also identify tissue abnormalities such as late gadolinium enhancement that can correlate with areas of fibrosis or scarring and serve as a nidus for ventricular arrhythmias in patients with myocarditis or hypertrophic cardiomyopathy.17 Cardiac tumours are typically diagnosed by an echocardiogram, though cardiac MRI can provide additional features to distinguish the type of tumour.18
Cardiac catheterization for haemodynamic and angiographic data may be helpful in patients with congenital heart disease, as declining haemodynamic conditions may worsen arrhythmia burden. If indicated, interventional or surgical therapy along with medication use may be required for patients in whom haemodynamically significant lesions contribute to an increased arrhythmia burden. In addition, coronary angiography may help to delineate structural coronary artery issues, particularly in the setting of lesions requiring coronary manipulation such as arterial switch operation.
An intracardiac electrophysiology study with catheter ablation may be performed as a therapeutic option in patients with documented sustained VT. An electrophysiology study to determine the arrhythmia mechanism alone is rarely required in paediatrics.14
Finally, genetic testing may be used to confirm a molecular diagnosis of LQTS, short QT syndrome, CPVT, Brugada syndrome, hypertrophic cardiomyopathy, or ARVC/arrhythmogenic ventricular cardiomyopathy, with the yield of genetic testing varying by diagnosis. If a pathogenic or likely pathogenic variant is identified, cascade family screening can be employed to identify other affected family members.19
Treatment
The decision to treat ventricular arrhythmias is typically determined by age, symptoms, specific diagnosis, arrhythmia burden, and haemodynamic impact of the arrhythmia. Options can include observation, medication, catheter ablation, and/or implantable cardioverter-defibrillator (ICD) implantation. Chronic management options based on the subtype of VT are detailed below.
Idiopathic VT
Nonsustained or well-tolerated asymptomatic episodes of sustained outflow tract VT in patients without other heart disease or ventricular dysfunction may not require treatment.20 However, therapy is indicated in the presence of ventricular dysfunction or in symptomatic patients. In patients with high-risk features for induction of polymorphic VT or VF such as a history of syncope, rapid VT, frequent VT, and ventricular ectopy with a short coupling interval, alternative diagnoses including arrhythmogenic ventricular cardiomyopathy, CPVT, tumours, or myocarditis should be considered. First-line pharmacotherapy options include β-blockers and calcium channel blockers. Class IC agents can be used as an alternative agent in cases of treatment failure. Class III agents may be considered in patients with severe ventricular dysfunction when catheter ablation is not feasible. Catheter ablation is indicated for patients who fail medical therapy, are unable to tolerate medications, and have severe ventricular dysfunction, or in symptomatic patients >15 kg who prefer ablation over medication.14,21
Symptomatic LV fascicular VT is an indication for ablation if the family chooses. IV verapamil is effective for acute tachycardia termination in 90% of patients, though oral verapamil is somewhat less efficacious for chronic management with a 20% failure rate. Alternative management options include β-blockers and class III antiarrhythmic agents. The success rate of catheter ablation is approximately 77%-90% with a recurrence rate of approximately 20%.5,21,22
Ventricular tachycardia in infancy
VT in infancy is rare. Idiopathic VT in the setting of a structurally normal heart typically carries a good prognosis with or without treatment and has a high likelihood of spontaneous resolution by 1-2 years of age.5,23,24 If pharmacotherapy is used, treatment is usually with β-blockers or class IC antiarrhythmic agents.
Infantile VT may also be associated with ventricular tumours and myocarditis. Cardiac tumours can present as incessant VT and may be due to isolated hamartomas (Purkinje cell tumours), rhabdomyomas, or histiocytoid or lymphocytoid tumours. Incessant VT may cause tachycardia-induced cardiomyopathy, and mortality has been reported in a small subset of these patients. Treatment options include use of antiarrhythmic agents until resolution of rhabdomyoma or resection of the tumour.24,25 Rarely, catheter ablation may be indicated.
Accelerated idioventricular rhythm
Accelerated idioventricular rhythm is generally considered to be benign. Treatment is rarely indicated, particularly in the absence of symptoms, ventricular dysfunction, or underlying arrhythmogenic condition. In patients with a substantial burden of ectopy and ventricular dysfunction, treatment with medication or catheter ablation may rarely be indicated.26 In the presence of congenital heart disease, significant haemodynamic compromise has been reported.27
Ventricular tachycardia associated with cardiomyopathies
Dilated, hypertrophic, restrictive, LV noncompaction, and ACMs can cause both monomorphic and polymorphic VT. Sustained monomorphic VTs are frequently due to scar-related re-entry and rarely from bundle branch re-entry or a focal tachycardia mechanism. Management is best determined by electrophysiology or cardiomyopathy experts and may include an ICD, antiarrhythmic medications, and/or catheter ablation. Various guidelines exist to optimize management of these patients.15,28,29
Ventricular tachycardia associated with cardiac channelopathies
In patients with cardiac channelopathies, medical therapy is targeted to the underlying diagnosis. The 2013 Expert Consensus Statement on Inherited Primary Arrhythmia Syndromes provides specific guidance on the management of these conditions.30 Typical therapies for LQTS include β-blockers, cardiac sympathetic denervation, and/or ICD therapy. CPVT is treated with β-blockers and/or flecainide and exercise restriction. Cardiac sympathetic denervation and ICD implantation are possible secondary treatments in CPVT. Brugada syndrome with documented VT is an indication for ICD implantation. Quinidine, a class Ia antiarrhythmic agent, has been shown to suppress ventricular arrhythmias and is currently indicated in Brugada patients with multiple ICD shocks or with a contraindication for ICD implantation.31
Ventricular tachycardia in congenital heart disease
Patients with congenital heart disease are susceptible to monomorphic VT secondary to their underlying anatomic heart defects, surgical scarring, and myocardial changes, resulting in areas of slow conduction within the ventricular myocardium capable of sustaining macroreentry circuits. There is also increased risk for developing polymorphic VT due to ventricular hypertrophy, fibrosis, decline in cardiac function, and heart failure. Specific lesions with particular risk for ventricular arrhythmias include tetralogy of Fallot, transposition of the great arteries after arterial switch operation, and Ebstein’s anomaly. There is a limited role for medications in the treatment of these arrhythmias. Generally, ICD implantation is indicated for a patient with congenital heart disease and sustained VT, but individual patient factors should be considered. Catheter ablation may be an acceptable alternative in some patients.14,32, 33, 34
Conclusions
Although uncommon in paediatric patients, WCTs can indicate a life-threatening condition and comprise an important category of conditions requiring prompt recognition, assessment, and management. Initial management decisions are guided by patient presentation and condition. Haemodynamic stability gives the clinician time to determine the arrhythmia mechanism and thus direct acute management and further therapy. Assessment of the ECG in tachycardia and comparison with the baseline ECG are critical in determining the diagnosis. SVT with aberrant conduction is the most common cause of WCT in a structurally normal heart. If there is diagnostic uncertainty in undifferentiated WCT, it is safest to treat the patient as if VT were the diagnosis. Chronic management and further diagnostic assessment of the patient depend on a number of patient and arrhythmia-specific factors.
Take-Home Messages
-
•
Normal heart rate and QRS duration vary with age in children, with a wide complex QRS having a duration above the 98th percentile for age.
-
•
The most common cause of WCT in children is SVT with aberrant conduction, but VT is an important differential diagnosis.
-
•
In a patient with a structurally normal heart, ECG features suggestive of VT include AV dissociation, sinus capture or fusion beats, wider QRS durations, concordance in the precordial leads, and a QRS complex atypical for an RBBB or LBBB pattern.
-
•
Initial management will depend on patient stability, with haemodynamic instability necessitating immediate management with DC cardioversion or defibrillation.
-
•
Haemodynamic stability allows for additional time to identify the arrhythmia mechanism, which guides the diagnosis and management.
Acknowledgement
We thank Michelle Hopper for her help with the figure illustration (Fig. 2).
Ethics Statement
This review article did not require ethics approval.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Salerno J.C., Seslar S.P. Supraventricular tachycardia. Arch Pediatr Adolesc Med. 2009;163:268–274. doi: 10.1001/archpediatrics.2008.547. [DOI] [PubMed] [Google Scholar]
- 2.Roggen A., Pavlovic M., Pfammatter J.P. Frequency of spontaneous ventricular tachycardia in a pediatric population. Am J Cardiol. 2008;101:852–854. doi: 10.1016/j.amjcard.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Ceresnak S.R., Liberman L., Avasarala K., et al. Are wide complex tachycardia algorithms applicable in children and patients with congenital heart disease? J Electrocardiol. 2010;43:694–854. doi: 10.1016/j.jelectrocard.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Topjian A.A., Raymond T.T., Atkins D., et al. Part 4: pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(suppl 2):469–523. doi: 10.1161/CIR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 5.Crosson J.E., Callans D.J., Bradley D.J., et al. PACES/HRS expert consensus statement on the evaluation and management of ventricular arrhythmias in the child with a structurally normal heart. Heart Rhythm. 2014;11:55–78. doi: 10.1016/j.hrthm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Marill K.A., Wolfram S., DeSouza I.S., et al. Adenosine for wide-complex tachycardia: efficacy and safety. Crit Care Med. 2009;37:2512–2518. doi: 10.1097/CCM.0b013e3181a93661. [DOI] [PubMed] [Google Scholar]
- 7.Brugada J., Katritsis D.G., Arbelo E., et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia: the Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467. [DOI] [PubMed] [Google Scholar]
- 8.Mulpuru S.K., Madhavan M., McLeod C.J., Cha Y.M., Friedman P.A. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol. 2017;69:189–210. doi: 10.1016/j.jacc.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Laksman Z., Barichello S., Roston T.M., Deyell M.W., Krahn A.D. Acute management of ventricular arrhythmia in patients with suspected inherited heart rhythm disorders. JACC Clin Electrophysiol. 2019;5:267–283. doi: 10.1016/j.jacep.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Escudero C., Carr R., Sanatani S. The medical management of pediatric arrhythmias. Curr Treat Options Cardiovasc Med. 2012;14:455–472. doi: 10.1007/s11936-012-0194-5. [DOI] [PubMed] [Google Scholar]
- 11.Escudero C., Blom N.A., Sanatani S. In: Pediatric and Congenital Cardiology, Cardiac Surgery and Intensive Care. Da Cruz E., Ivy D., Jaggers J., editors. Springer; London: 2014. Supraventricular tachycardias; pp. 2937–2969. [Google Scholar]
- 12.Seslar S.P., Garrison M.M., Larison C., Salerno J.C. A multi-institutional analysis of inpatient treatment for supraventricular tachycardia in newborns and infants. Pediatr Cardiol. 2013;34:408–414. doi: 10.1007/s00246-012-0474-6. [DOI] [PubMed] [Google Scholar]
- 13.Hill A.C., Silka M.J., Bar-Cohen Y. A comparison of oral flecainide and amiodarone for the treatment of recurrent supraventricular tachycardia in children. Pacing Clin Electrophysiol. 2019;42:670–677. doi: 10.1111/pace.13662. [DOI] [PubMed] [Google Scholar]
- 14.Philip Saul J., Kanter R.J., Abrams D., et al. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease: Developed in partnership with the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American Academy of Pediatrics (AAP), the American Heart Association (AHA), and the Association for European Pediatric and Congenital Cardiology (AEPC) Heart Rhythm. 2016;13 doi: 10.1016/j.hrthm.2016.02.009. e251-89. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation. 2018;138:272–391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen J.R., Garson A., Gillette P.C., McNamara D.G. Premature ventricular contractions in normal children. J Pediatr. 1978;92:36–38. doi: 10.1016/s0022-3476(78)80066-3. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich M.G., Sechtem U., Schulz-Menger J., et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fournier E., Séguéla P.E., Sauvestre F., et al. Imaging aspects of pediatric cardiac tumors. JACC Cardiovasc Imaging. 2020;13:2245–2253. doi: 10.1016/j.jcmg.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A., et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto M., Niimura I., Shibata T., et al. Long-term course and clinical characteristics of ventricular tachycardia detected in children by school-based heart disease screening. Circ J. 2005;69:273–276. doi: 10.1253/circj.69.273. [DOI] [PubMed] [Google Scholar]
- 21.Wu J., Chen Y., Ji W., et al. Catheter ablation of ventricular tachycardia in the pediatric patients: a single-center experience. Pacing Clin Electrophysiol. 2020;43:37–46. doi: 10.1111/pace.13835. [DOI] [PubMed] [Google Scholar]
- 22.Collins K.K., Schaffer M.S., Liberman L., et al. Fascicular and nonfascicular left ventricular tachycardias in the young: an international multicenter study. J Cardiovasc Electrophysiol. 2013;24:640–648. doi: 10.1111/jce.12105. [DOI] [PubMed] [Google Scholar]
- 23.Wang S., Zhu W., Hamilton R.M., et al. Diagnosis-specific characteristics of ventricular tachycardia in children with structurally normal hearts. Heart Rhythm. 2010;7:1725–1731. doi: 10.1016/j.hrthm.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Levin M.D., Stephens P., Tanel R.E., Vetter V.L., Rhodes L.A. Ventricular tachycardia in infants with structurally normal heart: a benign disorder. Cardiol Young. 2010;20:641–647. doi: 10.1017/S1047951110000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake C.Y., del Nido P.J., Alexander M.E., et al. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol. 2011;58:1903–1909. doi: 10.1016/j.jacc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Perry J.C. Ventricular tachycardia in neonates. Pacing Clin Electrophysiol. 1997;20(Pt 2):2061–2064. doi: 10.1111/j.1540-8159.1997.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 27.Hagel J., Escudero C., Kirsh J. Unstable accelerated idioventricular rhythm in a neonate with congenital heart disease. HeartRhythm Case Rep. 2016;3:137–140. doi: 10.1016/j.hrcr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin J.A., McKenna W.J., Abrams D.J., et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Cronin E.M., Bogun F.M., Maury P., et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17:2–154. doi: 10.1016/j.hrthm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Belhassen B., Glick A., Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 32.Zeppenfeld K., Schalij M.J., Bartelings M.M., et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.723551. [DOI] [PubMed] [Google Scholar]
- 33.Morwood J.G., Triedman J.K., Berul C.I., et al. Radiofrequency catheter ablation of ventricular tachycardia in children and young adults with congenital heart disease. Heart Rhythm. 2004;1:301–308. doi: 10.1016/j.hrthm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Walsh E.P. Arrhythmias in patients with congenital heart disease. Card Electrophysiol Rev. 2002;6:422–430. doi: 10.1023/a:1021192526642. [DOI] [PubMed] [Google Scholar]
- 35.Li C.O.Y., Franciosi S., Deyell M.W., Sanatani S. Intermediate-coupled premature ventricular complexes and ventricular tachycardia during exercise recovery. HeartRhythm Case Rep. 2021;7(2):127–130. doi: 10.1016/j.hrcr.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]