Abstract
Paediatric heart transplant recipients (HTRs) have reduced exercise capacity, physical activity (PA), health-related quality of life (HRQoL), and self-efficacy towards PA. Exercise interventions have demonstrated improvements in exercise capacity and functional status in adult HTRs, with a specific emerging interest in the role of high-intensity interval training (HIIT). Studies of exercise interventions in paediatric HTRs have been limited and nonrandomized to date. HIIT has not yet been evaluated in paediatric HTRs. We thus seek to evaluate the safety and feasibility of a randomized crossover trial of a 12-week, home-based, video game–linked HIIT intervention using a cycle ergometer with telemedicine and remote physiological monitoring capabilities (MedBIKE) in paediatric HTRs. The secondary objective is to evaluate the impact of the intervention on (1) exercise capacity, (2) PA, (3) HRQoL and self-efficacy towards PA, and (4) sustained changes in secondary outcomes at 6 and 12 months after intervention. After a baseline assessment of the secondary outcomes, participants will be randomized to receive the MedBIKE intervention (12 weeks, 36 sessions) or usual care. After the intervention and a repeated assessment, all participants will cross over. Follow-up assessments will be administered at 6 and 12 months after the MedBIKE intervention. We anticipate that the MedBIKE intervention will be feasible and safely yield sustained improvements in exercise capacity, PA, HRQoL, and self-efficacy towards PA in paediatric HTRs. This study will serve as the foundation for a larger, multicentre randomized crossover trial and will help inform exercise rehabilitation programmes for paediatric HTRs.
Résumé
La tolérance à l’effort, le niveau d’activité physique (AP), le score de la qualité de vie liée à la santé (QVLS) ainsi que l’auto-efficacité à la pratique d’une AP se trouvent diminués chez les patients pédiatriques ayant reçu une transplantation cardiaque. Il a été montré que les exercices physiques permettent d’améliorer la tolérance à l’effort ainsi que le statut fonctionnel chez les patients adultes ayant reçu une transplantation cardiaque. D’ailleurs, le rôle de l’entraînement par intervalles de haute intensité (EIHI) suscite depuis peu un nouvel intérêt à cet égard. Les études réalisées à ce jour sur les programmes d’activité physique chez les patients pédiatriques ayant reçu une transplantation cardiaque sont toutefois peu nombreuses et ne reposent pas sur une répartition aléatoire. De plus, l’EIHI n’a pas encore été évalué chez ce groupe de patients. La présente étude a donc pour objectif d’évaluer la faisabilité et l’innocuité d’un essai clinique croisé à répartition aléatoire d’une durée de 12 semaines chez des patients pédiatriques ayant reçu une transplantation cardiaque. Le programme d’activité physique prendra la forme d’un EIHI à la maison au moyen d’un jeu vidéo et d’une bicyclette ergométrique permettant une assistance et une surveillance des données physiologiques à distance (MedBIKE). Les objectifs secondaires de l’étude consistent à évaluer les effets du programme sur : 1) la tolérance à l’effort; 2) le niveau d’AP; 3) la QVLS ainsi que l’auto-efficacité à la pratique d’une AP; et 4) le maintien des améliorations relatives aux critères d’évaluation se-condaires à 6 et 12 mois. Après une évaluation initiale des critères d’évaluation secondaires, les participants seront répartis aléatoirement dans le groupe suivant le programme à l’aide du vélo MedBIKE (36 séances réparties sur 12 semaines) ou dans le groupe recevant le traitement usuel. Tous les participants changeront ensuite de groupe, et une nouvelle évaluation des critères d’évaluation se-condaires sera effectuée. Les évaluations de suivi auront lieu 6 et 12 mois après la fin du programme. On s’attend à ce que ce dernier soit sûr, facile à suivre et accompagné d’améliorations soutenues de la tolérance à l’effort, du niveau d’AP, de la QVLS et de l’auto-efficacité à la pratique d’une AP chez les patients pédiatriques ayant reçu une transplantation cardiaque. Cette étude servira de modèle à un essai clinique croisé, multicentrique, à répartition aléatoire de plus grande envergure. Elle permettra aussi de générer des renseignements utiles pour les programmes de réadaptation destinés aux patients pédiatriques ayant reçu une transplantation cardiaque.
Heart transplantation remains the cornerstone of long-term management for children with advanced, medically refractive heart failure. Post-transplant outcomes have drastically improved across eras such that the mean graft survival is now more than 18 years in paediatric heart transplant recipients (HTRs) and over 25 years for infant HTRs.1 With improved short- and medium-term outcomes, focus has shifted towards optimizing long-term survival and reducing transplant-associated morbidities. This includes strategies aimed at optimizing cardiorespiratory fitness, physical activity (PA), cardiovascular health, and health-related quality of life (HRQoL) in paediatric HTRs.2
Paediatric and adult HTRs demonstrate reduced exercise capacity compared with the general population.2, 3, 4, 5, 6, 7, 8, 9 In adult HTRs, reduced peak oxygen consumption (VO2peak), an important indicator of cardiorespiratory fitness, is associated with reduced post-transplant survival.10 The mechanism of reduced post-transplant cardiorespiratory fitness is multifactorial and includes central and peripheral etiologies.2, 3, 4, 5 Early post-transplant recovery in paediatric HTRs is associated with improvements in cardiac function, peripheral vascular resistance, skeletal muscle mass, and functional capacity,2,6,11, 12, 13 while heart rate response and exercise capacity improve more gradually.2,6,11, 12, 13 Unfortunately, exercise capacity has been observed to plateau and eventually decline in paediatric HTRs, remaining less than the general population.11 Moreover, paediatric HTRs experience lower levels of PA and perceived health status after transplant relative to peers, with one Canadian study demonstrating a mean duration of moderate-to-vigorous PA of only 7.6 minutes/d in paediatric HTRs.14,15 Reduced exercise capacity has been associated with reduced HRQoL16, 17, 18 and self-efficacy towards PA,19, 20, 21 the latter reflecting an individual’s belief in their ability to participate in PA.
Cardiac rehabilitation is an established secondary prevention strategy in adults with cardiovascular disease,22, 23, 24, 25, 26, 27, 28 and, while underutilized, it has demonstrated decreased hospital readmission and improved long-term outcomes in adult HTRs.29, 30, 31, 32 As such, both the International Society for Heart and Lung Transplantation (ISHLT) and the Canadian Society of Transplantation/Canadian Network for Rehabilitation and Exercise for Solid Organ Transplant Optimal Recovery (CAN-RESTORE) recommend cardiac rehabilitation for all HTRs.33,34 Despite this recommendation, studies of exercise interventions in paediatric HTRs remain significantly lacking,33 and those studies that have been performed are limited by factors such as a lack of randomization and variable study outcomes.33,35, 36, 37
Although most exercise rehabilitation strategies in HTRs have focused on moderate-intensity continuous exercise (MICE),32 there is emerging evidence in adult HTRs that high-intensity interval training (HIIT) may yield superior improvements in cardiorespiratory fitness.32,38, 39, 40 HIIT consists of short, intense bursts of exercise interspersed with short breaks. One randomized controlled trial demonstrated HIIT to be safe compared with MICE and to bestow superior improvements in exercise capacity.41 This same group also demonstrated a reduced incidence and progression of cardiac allograft vasculopathy after HIIT as well as a trend of improvement in physical components of HRQoL.42 HIIT programmes in adult HTRs have shown at least equivalent improvements on the burden of depression, anxiety, and HRQoL compared with MICE.43 Unfortunately, HIIT interventions in paediatric HTRs have not been studied to date.
Cardiac rehabilitation and exercise programmes can be either facility or home based. Facility-based programmes can be impractical and inaccessible to patients and present additional time and financial burdens.44 This is particularly relevant in the Canadian care model in which paediatric heart centres provide regionalized care serving many patients living in remote communities with limited space and funding in hospital settings.44,45 Home-based programmes are a more practical alternative, although they sacrifice real-time supervision. Paediatric studies of such programmes have been limited by small sample sizes and relied on interval assessments of participant progress.18,46, 47, 48, 49, 50 Specifically, studies of home-based exercise interventions with real-time physiological monitoring in paediatric HTRs remain scarce.
Thus, we now seek to evaluate the safety and feasibility of a randomized crossover trial evaluating a home-based exercise intervention with remote physiological monitoring in paediatric HTRs using MedBIKE, a telemedicine-equipped and video game–linked cycle ergometer.
Study Design and Methods
This study is a single-centre randomized crossover feasibility trial. Potential participants and their families will be approached by a clinical team member. They will then be screened for eligibility by the dedicated research coordinator and clinical investigator by a chart review. Participant inclusion and exclusion criteria are described in Table 1. Patients younger than 10 years were not included as they were felt to be less likely to use the MedBIKE system safely and effectively, follow technical and exercise-related directions, and assist in troubleshooting should difficulties arise. Written consent and assent will be obtained.
Table 1.
Participant inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
BP, blood pressure; HIIT, high-intensity interval training; HTR, heart transplant recipient; HTx, heart transplant.
Primary objective
The primary objective is the assessment of the feasibility and safety of a randomized crossover trial evaluating a 12-week, home-based MedBIKE HIIT intervention in paediatric HTRs. To evaluate feasibility, a dedicated research coordinator will track all study details, changes, and inquiries, and will record the time needed to complete each stage of participation as outlined in Table 2. On the basis of our experience in the congenital heart disease population51 and a telemedicine exercise intervention in paediatric HTRs,37 we will consider the approach feasible if >66% of 10- to 18-year-old HTRs are eligible, enrolment of eligible participants is >60%, and >80% of MedBIKE intervention sessions are completed, with a dropout rate of <10%. Assessments will be deemed feasible if each outcome measure has >80% complete data.
Table 2.
Measurement of feasibility outcomes throughout data collection
| Feasibility outcome | Outcome measure | Free text |
|---|---|---|
| Participant recruitment | ||
| Number of 10- to 18-year-old HTRs encountered | ||
| Eligible participants | ||
| Refused to participate | ||
| Reason for refusal to participate | ||
| Ineligible | ||
| Missed | ||
| Required MD consult to clarify eligibility | ||
| Baseline assessment (adequate data obtained) | ||
| CPET | ||
| Accelerometry | ||
| CSAPPA (self-efficacy) | ||
| PedsQL (HRQoL) | ||
| Personal Attributes Questionnaire | ||
| Scheduling changes needed for baseline assessment | ||
| MedBIKE intervention | ||
| Total number of sessions completed per participant | ||
| Number of sessions rescheduled per participant | ||
| Participant dropout | ||
| Time per exercise session and parent burden (qualitative) | ||
| Follow-up assessments #1 & #2 (completed individually) | ||
| Completed assessment | ||
| CPET | ||
| Accelerometry | ||
| CSAPPA (self-efficacy) | ||
| PedsQL (HRQoL) | ||
| Personal Attributes Questionnaire | ||
| Scheduling changes needed for baseline assessment | ||
CPET, cardiopulmonary exercise test; CSAPPA, Children’s Self-Perceptions of Adequacy in and Predilection for Physical Activity; HRQoL, health-related quality of life; HTR, heart transplant recipient.
To address safety, all adverse events during assessments or MedBIKE intervention will be recorded and will result in immediate termination of the assessment/intervention with evaluation by a medical professional as needed. Adverse effects include profound desaturation (>10% for more than 1 minute), chest pain, electrocardiogram (ECG) changes consistent with ischemia, development of arrhythmia, and bike-related injuries.
Secondary objectives
Secondary objectives include evaluation of the impact of the MedBIKE HIIT intervention on (1) exercise capacity, (2) PA, (3) HRQoL and self-efficacy towards PA, and (4) sustained changes in all secondary outcomes at 6 and 12 months after intervention.
To evaluate changes in exercise capacity, VO2peak will be compared after intervention with baseline. Additional measures to address this will include the VO2 at anaerobic threshold, oxygen pulse, peak power output (PPO), ventilation/carbon dioxide production slope, heart rate reserve, and heart rate recovery at 1 and 3 minutes. This will be measured via the cycle ergometer cardiopulmonary exercise test (CPET) with 12-lead ECG, preceding spirometry conducted according to standard guidelines and a 10-W ramp protocol.52 A test will be considered maximal if 2 of the following 3 criteria are met: respiratory exchange ratio (RER) >1.1, maximal heart rate (HR) >85% predicted,53 or maximal rating of perceived exertion (RPE) >18, while considering impaired heart rate responses in HTRs.54,55 VO2peak will be converted to age, weight, and sex-specific norms and a percent predicted value.56
Changes in PA will be assessed using ActiGraph GT3X+ accelerometers (ActiGraph, LLC, Pensacola, FL) for 7 consecutive days. Previous studies have demonstrated that participants must wear an accelerometer for 10 hours/d for 4 days, including one weekend day for children ≥5 years old.57 Thus, wearing the device for 7 days is likely sufficient to account for imperfect compliance.58,59 The Physical Activity Questionnaire for Children (10-12 years) or Adolescents (13-18 years) will be used to evaluate PA.60, 61, 62
To evaluate changes in HRQoL and attitudes towards PA, 2 questionnaires will be administered: the pediatric quality of life and the Children’s Self-Perceptions of Adequacy in and Predilection for Physical Activity Scale. Both questionnaires are validated, readable at a grade 5 level, and take 10-15 minutes to complete.63,64 The pediatric quality of life has been used in studies of paediatric HTRs,65, 66, 67, 68 and the Children’s Self-Perceptions of Adequacy in and Predilection for Physical Activity Scale has been administered in the paediatric cardiac population.19,69
Participant Timeline and Randomization
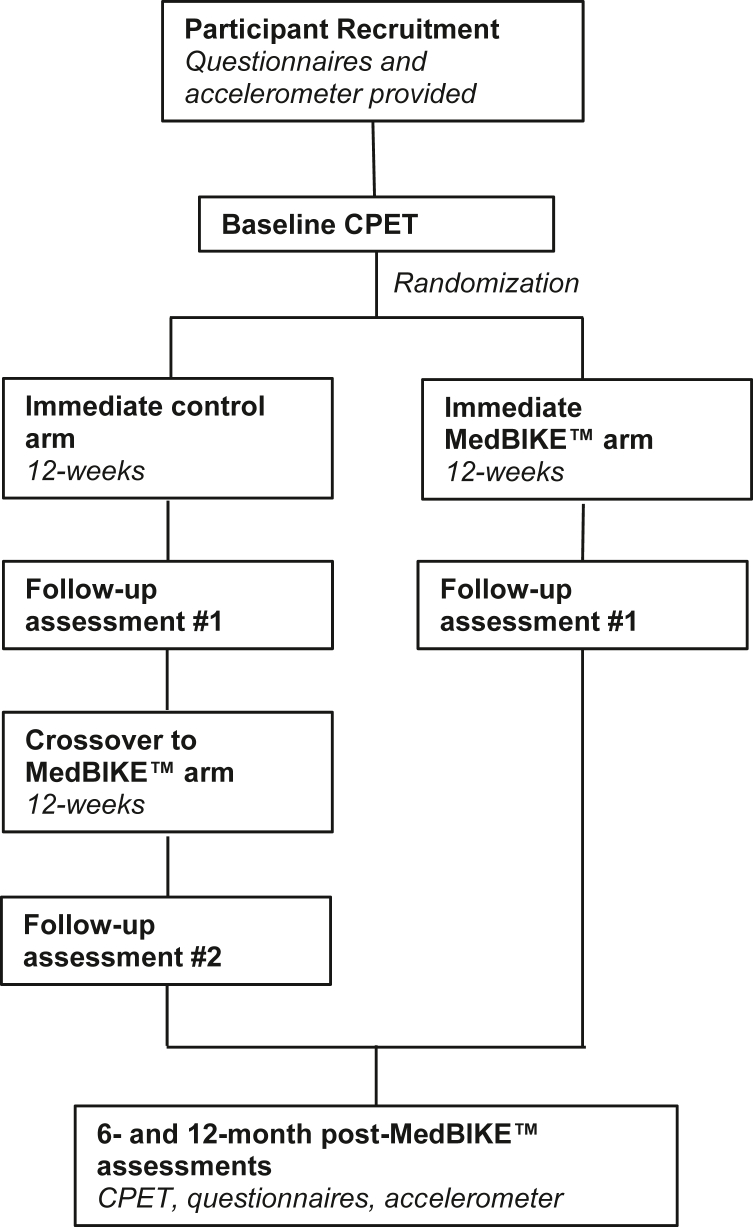
After recruitment, baseline measurements of secondary outcomes will be obtained. Participants will then be randomized via a 1:1 allocation ratio with permuted blocks of randomly varied sizes to either the immediate MedBIKE arm or the immediate control arm for 12 weeks, followed by a repeat assessment. Participants in the control arm will then cross over and complete the 12-week MedBIKE HIIT programme, followed by a repeat assessment at the completion of the intervention (Fig. 1). All postintervention assessments will be administered 3-7 days after the MedBIKE intervention to allow adequate recovery. Accelerometers will be programmed to commence counts 24 hours after assessment to avoid bias from fatigue after the CPET. Participants will return for final assessments at 6 and 12 months after intervention to evaluate sustained changes in secondary outcomes.
Figure 1.
Participant timeline. CPET, cardiopulmonary exercise test.
MedBIKE
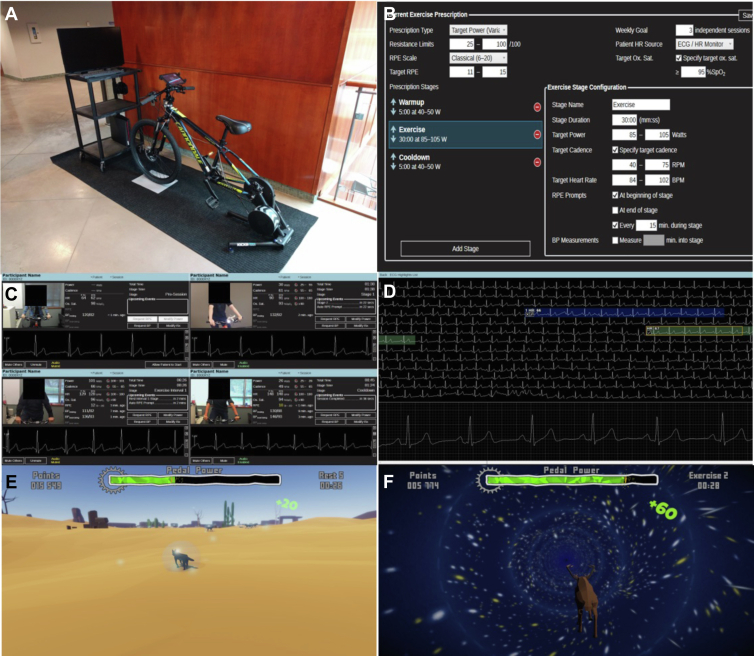
MedBIKE (Fig. 2) is a custom-made remote cycle ergometer developed in collaboration with the Advanced Man-Machine Interface Laboratory at the University of Alberta. It is a networked, video game–linked device that allows real-time interaction with up to 4 participants per session. The video game is interactive, and the user accrues in-game currency, allowing them to “purchase” new animal avatars throughout the 12-week programme. Music matching the cadence at which the participant should cycle is played throughout the exercise session. During high-intensity intervals, the complex, navigable environment is replaced by a simple tunnel to ensure that the participant is engaged and to help prevent steering the handlebars during high exertion. Medical supervision occurs via a live 2-way audiovisual feed, providing face-to-face communication as well as continuous 3-lead ECG and oximetry monitoring. MedBIKE allows live remote modulation of participant workload throughout each session by the supervising health care professional as well as communication of RPE by the participant via a built-in tablet. The safety and feasibility of MedBIKE for home-based HIIT interventions has been established in the Fontan population51 and has received Health Canada approval (ITA no. 330806) for its use in the paediatric HTR population. This clinical trial is currently registered (NCT05451979).
Figure 2.
MedBIKE. (A) MedBIKE with a linked tablet and telemedicine system. (B) Built-in software on the supervisor’s end allows remote design and modulation of the exercise regimen. (C) A live audiovisual feed with oximetry and electrocardiography data is available for up to 4 participants at a time. (D) A more detailed telemetry analysis is available. (E, F) Video game screenshots demonstrating the navigable environment during noninterval periods and the “worm-hole” tunnel to promote focus and less steering during high-intensity intervals. Reproduced with permission from Khoury et al.51
Intervention
Participants will have a MedBIKE installed in their home within 2 weeks of the baseline CPET. An orientation session and the first exercise session will take place the same day. The participant will then start a 12-week, 3 times weekly HIIT programme. All sessions will be scheduled by the research coordinator with at least 24 hours between sessions and supervised by a physician or exercise specialist (exercise physiologist or kinesiologist with a physician-investigator available on call). The programme involves a 5-minute warm-up, four 2-minute high-intensity intervals interspersed with 2-minute breaks, and a 5-minute cooldown (total 24 minutes). Participants are instructed to cycle at 50 rpm during the warm-up, breaks, and cooldown and at 90 rpm during exercise intervals, while the supervisor remotely modulates bike resistance. Power output will be determined by the cycling cadence and the set resistance. The HIIT programme will be designed according to the PPO generated in the baseline CPET, with HIIT intervals set at 80%-90% PPO and breaks <40% PPO. The participant will submit an RPE54 after each interval via an integrated tablet. Resistance for subsequent intervals will be adjusted based on the RPE (goal 16-18) and the HR response (goal 80%-90% baseline HRmax). Further modifications to the HIIT protocol (such as the length of the intervals and the breaks) will be made based on factors such as the participant’s HR response to exercise and recovery during breaks, and global perceived difficulty of the intervention.
If a session is cancelled, participants will proceed with the next scheduled session. If ≥2 consecutive sessions or ≥3 in a 2-week period are cancelled, compliance will be reviewed. If an issue arises limiting participation, the participant’s protocol will be paused for up to 2 weeks. If the participant cannot resume after this time, they will be considered to have dropped out. As per the intention-to-treat analysis, we will attempt to include dropped-out participants in the postintervention assessments. After completion of the intervention, MedBIKE will be uninstalled and returned to the study team.
Analysis plan
We plan to recruit 12 participants (approximately 60% of potentially eligible paediatric HTRs) to evaluate the primary objective of feasibility and safety. The proportion of all 10- to 18-year-old HTRs transplanted at our centre who are eligible and enrolled will be determined. The completion rates of baseline assessments, MedBIKE sessions, and postintervention assessments will also be assessed, as outlined in Table 2. If possible, we intend to recruit more than the minimum of 12 participants to better address secondary outcomes. To assess secondary objectives, the Wilcoxon signed-rank test will be used to evaluate pre- and post-MedBIKE intervention changes in VO2peak, PA, HRQoL, and PA self-efficacy. We will also evaluate sustained changes in the secondary objectives at 6 and 12 months after the MedBIKE intervention (Fig. 2). This assessment will inform subsequent trial designs permitting longer-term follow-up.
Safety monitoring
Although no major adverse events directly attributable to exercise interventions in the paediatric cardiac population have been reported to date,18,44,70, 71, 72 and all participants in the proposed study should have no exercise restrictions imposed by their primary cardiologist, contingency plans are in place should an event occur. Participant guardians will be instructed to be present and to supervise their child for each session. In the event that the supervisor or guardian find the participant acutely unwell or potential adverse events occur such as profound desaturation (>10% for more than 1 minute), chest pain, ECG changes consistent with ischemia, development of an arrhythmia (including supraventricular and ventricular ectopy and tachycardia), cardiac arrest, and MedBIKE-related injuries, the study will be terminated and the participant will be instructed to seek immediate medical care or call paramedics as appropriate. If the network connection is interrupted during a session, the session will pause until the connection is re-established with available trouble-shooting strategies. In the event the connection cannot be successfully re-established in a timely fashion (<15 minutes), the session will be rescheduled for another date. A physician investigator will be available during all MedBIKE sessions. The research team will convene monthly to review study safety and progress. If multiple adverse events occur, the trial will be suspended and the protocol will be re-evaluated.
Limitations
This study is designed as a feasibility trial that will inform a future larger, multicentre randomized crossover trial. As a result, the anticipated sample size is not powered to address the secondary objectives. Moreover, although the planned number of participants is rather small for a randomized trial, maintaining a randomized study design approach will facilitate implementation of larger-scale future studies. Technical troubleshooting may be required for the MedBIKE system, and this may result in rescheduling of sessions and prolongation of the exercise intervention. This may be particularly difficult for participants who live remotely. As participants are responsible for ECG lead application and oximeter placement for sessions, there is a possibility of excess artefact or suboptimal signals that could limit remote monitoring. Participants may experience clinically important events such as graft rejection or the need for procedures after recruitment, which may delay or interrupt interventions and result in protocol deviations to accommodate the participant’s clinical needs.
Anticipated Results and Conclusions
We anticipate that the proposed home-based MedBIKE HIIT programme will be feasible and safely yield sustained improvements in exercise capacity, PA, HRQoL, and self-efficacy towards PA in paediatric HTRs. The findings will inform a larger, multicentre home-based exercise intervention in paediatric HTRs. Moreover, this programme may potentially benefit other at-risk paediatric populations, including other solid-organ transplant recipients. Importantly, this novel exercise design has direct clinical applicability and can form the basis of a home-based paediatric cardiac rehabilitation programme. Through this work, we aim to make substantial advances in the paediatric cardiac rehabilitation knowledge base and enhance the long-term health and well-being of this patient population.
Acknowledgments
Ethics Statement
Ethics approval received from the University of Alberta Health Research Ethics Board.
Patient Consent
The authors confirm that patient consent is not applicable to this article.
Funding Sources
This work was funded by Big Gifts for Little Lives (RES0055267), Canadian Donation and Transplantation Research Program (CDTRP) Innovation Grant (RES0055178), and Evans Family Research Grant in Pediatric Cardiovascular Research (RES0055125). Funding sources had no role in the design of the study, data collection, analysis, or interpretation.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Rossano J.W., Singh T.P., Cherikh W.S., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-second pediatric heart transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1028–1041. doi: 10.1016/j.healun.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson S., Su J.A., Szmuszkovicz J.R., Johnson R., Sargent B. Exercise capacity following pediatric heart transplantation: a systematic review. Pediatr Transplant. 2017;21(5) doi: 10.1111/petr.12922. [DOI] [PubMed] [Google Scholar]
- 3.Tucker W.J., Brubaker P.H., Haykowsky M.J. Improving exercise capacity in recent heart transplant recipients. Circulation. 2019;139:2212–2214. doi: 10.1161/CIRCULATIONAHA.119.039845. [DOI] [PubMed] [Google Scholar]
- 4.Tucker W.J., Beaudry R.I., Samuel T.J., et al. Performance limitations in heart transplant recipients. Exerc Sport Sci Rev. 2018;46:144–151. doi: 10.1249/JES.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 5.Masarone D., Melillo E., Petraio A., et al. Exercise-based rehabilitation strategies in heart transplant recipients: focus on high-intensity interval training. Clin Transplant. 2021;35 doi: 10.1111/ctr.14143. [DOI] [PubMed] [Google Scholar]
- 6.Abarbanell G., Mulla N., Chinnock R., Larsen R. Exercise assessment in infants after cardiac transplantation. J Heart Lung Transplant. 2004;23:1334–1338. doi: 10.1016/j.healun.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Christos S.C., Katch V., Crowley D.C., et al. Hemodynamic responses to upright exercise of adolescent cardiac transplant recipients. J Pediatr. 1992;121:312–316. doi: 10.1016/s0022-3476(05)81213-2. [DOI] [PubMed] [Google Scholar]
- 8.Hsu D.T., Garofano R.P., Douglas J.M., et al. Exercise performance after pediatric heart transplantation. Circulation. 1993;88(Pt 2):II238–I242. [PubMed] [Google Scholar]
- 9.Nixon P.A., Fricker F.J., Noyes B.E., et al. Exercise testing in pediatric heart, heart-lung, and lung transplant recipients. Chest. 1995;107:1328–1335. doi: 10.1378/chest.107.5.1328. [DOI] [PubMed] [Google Scholar]
- 10.Yardley M., Havik O.E., Grov I., et al. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transplant. 2016;30:161–169. doi: 10.1111/ctr.12672. [DOI] [PubMed] [Google Scholar]
- 11.Davis J.A., McBride M.G., Chrisant M.R., et al. Longitudinal assessment of cardiovascular exercise performance after pediatric heart transplantation. J Heart Lung Transplant. 2006;25:626–633. doi: 10.1016/j.healun.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Dipchand A.I., Manlhiot C., Russell J.L., et al. Exercise capacity improves with time in pediatric heart transplant recipients. J Heart Lung Transplant. 2009;28:585–590. doi: 10.1016/j.healun.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Singh T.P., Gauvreau K., Rhodes J., Blume E.D. Longitudinal changes in heart rate recovery after maximal exercise in pediatric heart transplant recipients: evidence of autonomic re-innervation? J Heart Lung Transplant. 2007;26:1306–1312. doi: 10.1016/j.healun.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Dietitians of Canada; Canadian Paediatric Society; College of Family Physicians of Canada; Community Health Nurses of Canada; Secker D Promoting optimal monitoring of child growth in Canada: using the new WHO growth charts. Can J Diet Pract Res. 2010;71:e1–3. doi: 10.3148/71.1.2010.54. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay M.S., Carson V., Chaput J.P., et al. Canadian 24-hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2016;41(suppl 3):S311–S327. doi: 10.1139/apnm-2016-0151. [DOI] [PubMed] [Google Scholar]
- 16.Atz A.M., Zak V., Mahony L., et al. Longitudinal outcomes of patients with single ventricle after the Fontan procedure. J Am Coll Cardiol. 2017;69:2735–2744. doi: 10.1016/j.jacc.2017.03.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H.J., Jae S.Y., Choo J., et al. Mediating effects of exercise capacity on the association between physical activity and health-related quality of life among adolescents with complex congenital heart disease. Am J Hum Biol. 2019;31 doi: 10.1002/ajhb.23297. [DOI] [PubMed] [Google Scholar]
- 18.Tikkanen A.U., Oyaga A.R., Riano O.A., Alvaro E.M., Rhodes J. Paediatric cardiac rehabilitation in congenital heart disease: a systematic review. Cardiol Young. 2012;22:241–250. doi: 10.1017/S1047951111002010. [DOI] [PubMed] [Google Scholar]
- 19.Banks L., Rosenthal S., Manlhiot C., et al. Exercise capacity and self-efficacy are associated with moderate-to-vigorous intensity physical activity in children with congenital heart disease. Pediatr Cardiol. 2017;38:1206–1214. doi: 10.1007/s00246-017-1645-2. [DOI] [PubMed] [Google Scholar]
- 20.Bar-Mor G., Bar-Tal Y., Krulik T., Zeevi B. Self-efficacy and physical activity in adolescents with trivial, mild, or moderate congenital cardiac malformations. Cardiol Young. 2000;10:561–566. doi: 10.1017/s1047951100008829. [DOI] [PubMed] [Google Scholar]
- 21.Moola F., McCrindle B.W., Longmuir P.E. Physical activity participation in youth with surgically corrected congenital heart disease: devising guidelines so Johnny can participate. Pediatr Child Health. 2009;14:167–170. doi: 10.1093/pch/14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbert J.D., Martin B.J., Haykowsky M.J., et al. Cardiac rehabilitation referral, attendance and mortality in women. Eur J Prev Cardiol. 2015;22:979–986. doi: 10.1177/2047487314545279. [DOI] [PubMed] [Google Scholar]
- 23.Leon A.S., Franklin B.A., Costa F., et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 24.Mampuya W.M. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2:38–49. doi: 10.3978/j.issn.2223-3652.2012.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin B.J., Arena R., Haykowsky M., et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88:455–463. doi: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Martin B.J., Hauer T., Arena R., et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126:677–687. doi: 10.1161/CIRCULATIONAHA.111.066738. [DOI] [PubMed] [Google Scholar]
- 27.Mezzani A., Hamm L.F., Jones A.M., et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 28.Piepoli M.F., Corra U., Adamopoulos S., et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol. 2014;21:664–681. doi: 10.1177/2047487312449597. [DOI] [PubMed] [Google Scholar]
- 29.Bachmann J.M., Shah A.S., Duncan M.S., et al. Cardiac rehabilitation and readmissions after heart transplantation. J Heart Lung Transplant. 2018;37:467–476. doi: 10.1016/j.healun.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum A.N., Kremers W.K., Schirger J.A., et al. Association between early cardiac rehabilitation and long-term survival in cardiac transplant recipients. Mayo Clin Proc. 2016;91:149–156. doi: 10.1016/j.mayocp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Uithoven K.E., Smith J.R., Medina-Inojosa J.R., Squires R.W., Olson T.P. The role of cardiac rehabilitation in reducing major adverse cardiac events in heart transplant patients. J Card Fail. 2020;26:645–651. doi: 10.1016/j.cardfail.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Anderson L., Nguyen T.T., Dall C.H., et al. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janaudis-Ferreira T., Mathur S., Deliva R., et al. Exercise for solid organ transplant candidates and recipients: a joint position statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation. 2019;103:e220–e238. doi: 10.1097/TP.0000000000002806. [DOI] [PubMed] [Google Scholar]
- 34.Costanzo M.R., Dipchand A., Starling R., et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 35.Deliva R.D., Hassall A., Manlhiot C., et al. Effects of an acute, outpatient physiotherapy exercise program following pediatric heart or lung transplantation. Pediatr Transplant. 2012;16:879–886. doi: 10.1111/petr.12003. [DOI] [PubMed] [Google Scholar]
- 36.Patel J.N., Kavey R.E., Pophal S.G., et al. Improved exercise performance in pediatric heart transplant recipients after home exercise training. Pediatr Transplant. 2008;12:336–340. doi: 10.1111/j.1399-3046.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen A.C., Ramirez F.D., Rosenthal D.N., et al. Healthy hearts via live videoconferencing: an exercise and diet intervention in pediatric heart transplant recipients. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dall C.H., Snoer M., Christensen S., et al. Effect of high-intensity training versus moderate training on peak oxygen uptake and chronotropic response in heart transplant recipients: a randomized crossover trial. Am J Transplant. 2014;14:2391–2399. doi: 10.1111/ajt.12873. [DOI] [PubMed] [Google Scholar]
- 39.Haykowsky M., Taylor D., Kim D., Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734–739. doi: 10.1111/j.1600-6143.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 40.Nytroen K., Rustad L.A., Aukrust P., et al. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12:3134–3142. doi: 10.1111/j.1600-6143.2012.04221.x. [DOI] [PubMed] [Google Scholar]
- 41.Nytroen K., Rolid K., Andreassen A.K., et al. Effect of high-intensity interval training in de novo heart transplant recipients in Scandinavia. Circulation. 2019;139:2198–2211. doi: 10.1161/CIRCULATIONAHA.118.036747. [DOI] [PubMed] [Google Scholar]
- 42.Nytroen K., Rustad L.A., Erikstad I., et al. Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32:1073–1080. doi: 10.1016/j.healun.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Dall C.H., Gustafsson F., Christensen S.B., et al. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: a randomized, crossover trial. J Heart Lung Transplant. 2015;34:1033–1041. doi: 10.1016/j.healun.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Rhodes J., Ubeda Tikkanen A., Jenkins K.J. Exercise testing and training in children with congenital heart disease. Circulation. 2010;122:1957–1967. doi: 10.1161/CIRCULATIONAHA.110.958025. [DOI] [PubMed] [Google Scholar]
- 45.Martin B.J., Ross D.B., Aklabi M.A., et al. Post-operative outcomes in children undergoing Fontan palliation in a regionalized surgical system. Pediatr Cardiol. 2017;38:1654–1662. doi: 10.1007/s00246-017-1710-x. [DOI] [PubMed] [Google Scholar]
- 46.Rhodes J., Curran T.J., Camil L., et al. Sustained effects of cardiac rehabilitation in children with serious congenital heart disease. Pediatrics. 2006;118:e586–e593. doi: 10.1542/peds.2006-0264. [DOI] [PubMed] [Google Scholar]
- 47.Rhodes J., Curran T.J., Camil L., et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 48.Wittekind S., Mays W., Gerdes Y., et al. A novel mechanism for improved exercise performance in pediatric Fontan patients after cardiac rehabilitation. Pediatr Cardiol. 2018;39:1023–1030. doi: 10.1007/s00246-018-1854-3. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg B., Fripp R.R., Lister G., et al. Effect of physical training on exercise performance of children following surgical repair of congenital heart disease. Pediatrics. 1981;68:691–699. [PubMed] [Google Scholar]
- 50.Moalla W., Maingourd Y., Gauthier R., et al. Effect of exercise training on respiratory muscle oxygenation in children with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:604–611. doi: 10.1097/01.hjr.0000201515.59085.69. [DOI] [PubMed] [Google Scholar]
- 51.Khoury M., Phillips D.B., Wood P.W., et al. Cardiac rehabilitation in the paediatric Fontan population: development of a home-based high-intensity interval training programme. Cardiol Young. 2020;30:1409–1416. doi: 10.1017/S1047951120002097. [DOI] [PubMed] [Google Scholar]
- 52.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e637–e697. doi: 10.1161/CIR.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka H., Monahan K.D., Seals D.R. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 54.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 55.Wasserman K., Hansen J., Sue D., et al. In: Principles of Exercise Testing and Interpretation. Wasserman K., editor. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 56.Cooper D.M., Weiler-Ravell D., Whipp B.J., Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:628–634. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 57.Colley R., Connor Gorber S., Tremblay M.S. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. 2010;21:63–69. [PubMed] [Google Scholar]
- 58.Cain K.L., Sallis J.F., Conway T.L., Van Dyck D., Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health. 2013;10:437–450. doi: 10.1123/jpah.10.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward D.S., Evenson K.R., Vaughn A., Rodgers A.B., Troiano R.P. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37suppl:S582–S588. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 60.Voss C., Duncombe S.L., Dean P.H., de Souza A.M., Harris K.C. Physical activity and sedentary behavior in children with congenital heart disease. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voss C., Dean P.H., Gardner R.F., Duncombe S.L., Harris K.C. Validity and reliability of the Physical Activity Questionnaire for Children (PAQ-C) and Adolescents (PAQ-A) in individuals with congenital heart disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crocker P.R., Bailey D.A., Faulkner R.A., Kowalski K.C., McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Grant-Beuttler M., Jennings J., McCauley C., et al. Development of an electronic version of the Children’s Self-Perceptions of Adequacy in and Predilection for Physical Activity (CSAPPA) Scale. Pediatr Exerc Sci. 2017;29:153–160. doi: 10.1123/pes.2016-0115. [DOI] [PubMed] [Google Scholar]
- 64.Varni J.W., Seid M., Rode C.A. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Garcia Guerra G., Bond G.Y., Joffe A.R., et al. Health-related quality of life after pediatric heart transplantation in early childhood. Pediatr Transplant. 2020;24 doi: 10.1111/petr.13822. [DOI] [PubMed] [Google Scholar]
- 66.Hind T., Lui S., Moon E., et al. Post-traumatic stress as a determinant of quality of life in pediatric solid-organ transplant recipients. Pediatr Transplant. 2021;25 doi: 10.1111/petr.14005. [DOI] [PubMed] [Google Scholar]
- 67.Killian M.O., Triplett K.N., Masood S.S., Boehler J., Mayersohn G.S. Measurement of health-related quality of life in pediatric organ transplantation recipients: a systematic review of the PedsQL transplant module. Qual Life Res. 2020;29:1137–1146. doi: 10.1007/s11136-019-02398-0. [DOI] [PubMed] [Google Scholar]
- 68.McCormick A.D., Schumacher K.R., Zamberlan M., et al. Generalized and specific anxiety in adolescents following heart transplant. Pediatr Transplant. 2020;24 doi: 10.1111/petr.13647. [DOI] [PubMed] [Google Scholar]
- 69.Longmuir P.E., Tyrrell P.N., Corey M., et al. Home-based rehabilitation enhances daily physical activity and motor skill in children who have undergone the Fontan procedure. Pediatr Cardiol. 2013;34:1130–1151. doi: 10.1007/s00246-012-0618-8. [DOI] [PubMed] [Google Scholar]
- 70.Duppen N., Kapusta L., de Rijke Y.B., et al. The effect of exercise training on cardiac remodelling in children and young adults with corrected tetralogy of Fallot or Fontan circulation: a randomized controlled trial. Int J Cardiol. 2015;179:97–104. doi: 10.1016/j.ijcard.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 71.Scheffers L.E., Berg L., Ismailova G., et al. Physical exercise training in patients with a Fontan circulation: a systematic review. Eur J Prev Cardiol. 2021;28:1269–1278. doi: 10.1177/2047487320942869. [DOI] [PubMed] [Google Scholar]
- 72.Sutherland N., Jones B., d'Udekem Y. Should we recommend exercise after the Fontan procedure? Heart Lung Circ. 2015;24:753–768. doi: 10.1016/j.hlc.2015.03.005. [DOI] [PubMed] [Google Scholar]