Abstract
Background
A hallmark feature of children with congenital heart disease (CHD) is exercise intolerance. Whether a home-based resistance training intervention improves muscle oxygenation (as measured by tissue oxygenation index, TOI) and exercise tolerance (O2 reserve) during aerobic exercise in children with CHD compared with healthy children is unknown.
Methods
We report findings for 10 children with CHD (female/male: 4/6; mean ± standard deviation age: 13 ± 1 years) and 9 healthy controls (female/male: 5/4; age: 12 ± 3 years). Children with CHD completed a 12-week home-based exercise programme in addition to 6 in-person sessions. Exercise tolerance was assessed with a peak exercise test. Vastus lateralis TOI was continuously sampled during the peak O2 test via near-infrared spectroscopy.
Results
There was a medium effect (Cohen’s d = 0.67) of exercise training on lowering TOI at peak exercise (pre: 30 ± 16 %total labile signal vs post: 20 ± 13 % total labile signal; P = 0.099). Exercise training had a small effect (Cohen’s d = 0.23) on increasing O2 reserve by 1.6 mL/kg/min (pre: 27.2 ± 5.7 mL/kg/min vs post: 29.4 ± 8.8 mL/kg/min; P = 0.382). There was also a small effect (Cohen’s d = 0.27) of exercise on peak heart rate (pre: 175 ± 23 beats/min vs post: 169 ± 21 beats/min; P = 0.18). TOI, O2 reserve, and heart rate were generally lower than healthy control participants.
Conclusions
Our findings indicate that home-based resistance training may enhance skeletal muscle oxygen extraction (lower TOI) and subsequently O2 reserve in children with CHD.
Résumé
Contexte
L'une des manifestations caractéristiques de la cardiopathie congénitale chez les enfants est l'intolérance à l'effort. Il n'est pas clair si un entraînement musculaire à la maison permet d'améliorer l'oxygénation musculaire (selon l'indice d'oxygénation tissulaire, ou TOI pour tissue oxygenation index) et la tolérance à l'effort (réserve de consommation d'oxygène [O2]) lors d'un exercice aérobique chez les enfants atteints d'une cardiopathie congénitale, comparativement aux enfants en bonne santé.
Méthodologie
Les résultats présentés concernent 10 enfants atteints d’une cardiopathie congénitale (filles/garçons : 4/6; âge moyen ± écart-type : 13 ans ± 1 an) et neuf enfants témoins en bonne santé (filles/garçons : 5/4; âge : 12 ans ± 3 ans). Les enfants atteints d'une cardiopathie congénitale ont participé à un programme d'exercices à la maison de 12 semaines, en plus d'assister en personne à six séances. La tolérance à l'effort a été évaluée au moyen de l'épreuve d'effort maximal. Le TOI du muscle vaste externe a été mesuré de façon continue pendant le test du O2 max par spectroscopie proche infrarouge.
Résultats
Le programme d'exercices a entraîné un effet modéré (valeur d de Cohen = 0,67) sur la réduction du TOI au moment de l'effort maximal (pré-entraînement : signal labile total de 30 ± 16 % vs post-entraînement : signal labile total de 20 ± 13 % ; p = 0,099). Le programme d'exercices a eu un effet léger (valeur d de Cohen = 0,23) sur l'augmentation de la réserve de O2, soit de 1,6 ml/kg/min (pré-entraînement : 27,2 ± 5,7 ml/kg/min vs post-entraînement : 29,4 ± 8,8 ml/kg/min; p = 0,382). On a également observé un effet léger (valeur d de Cohen = 0,27) sur la fréquence cardiaque maximale (pré-entraînement : 175 ± 23 battements/minute vs post-entraînement : 169 ± 21 battements/minute; p = 0,18). Le TOI, la réserve de O2 et la fréquence cardiaque étaient généralement inférieurs comparativement aux témoins en bonne santé.
Conclusions
Nos résultats montrent qu'un entraînement musculaire à la maison pourrait améliorer la capacité d'extraction de l'oxygène par les muscles squelettiques (TOI inférieur) et ultimement la réserve de O2 chez les enfants atteints d'une cardiopathie congénitale.
Children with congenital heart disease (CHD) exhibit reduced exercise tolerance compared with healthy peers, predisposing them to cardiovascular disease later in life.1, 2, 3, 4, 5 Mechanisms that contribute to exercise intolerance in children with CHD are not fully understood, resulting in a lack of practical, evidence-based exercise programming for these children. As exercise intolerance is a major predictor for all-cause and cardiovascular mortality, this knowledge gap must be resolved.6, 7, 8
Both central (cardiac) and peripheral (vascular and muscle) factors are known to play a role in reduced exercise tolerance in CHD.9, 10, 11 Structured aerobic exercise training interventions have shown to improve cardiac determinants of O2;1,2,12, 13, 14, 15, 16 however, exercise tolerance generally remains lower compared with healthy age- and sex-matched controls.12 Therefore, the possibility of alterations in O2 delivery to skeletal muscle as a result of impaired blood flow and microvascular dysfunction, as well as reduced O2 utilization at the working muscle, should be considered as a candidate for peripheral determinants of exercise tolerance rather than cardiac factors alone.17
Near-infrared spectroscopy (NIRS) is commonly employed to determine microvascular function in the muscle as it is a direct, reliable, and valid noninvasive measure of muscle oxygenation status at the microvascular level.18,19 Specifically, near-infrared light monitors continuous changes in oxygenated and deoxygenated haemoglobin and myoglobin in the microvasculature (small arterioles, capillaries, and venules).6,8,15,20,21 As such, O2 delivery, availability, and utilization at the muscle can be measured using NIRS. Moalla et al.21 found reduced tissue oxygenation index (TOI), a measure of muscle oxygenation, in the vastus lateralis of children with CHD compared with healthy peers. After a 12-week aerobic exercise intervention, TOI and muscle strength were shown to improve during isometric knee extensor exercise.22 In children with Fontan circulation, both TOI and O2 may also lower at rest and during unloaded cycling compared with healthy controls, suggesting that impaired O2 delivery and the rate of O2 uptake may contribute to reduced exercise tolerance.23 These data support further investigation to evaluate TOI at peak aerobic exercise with the addition of an exercise intervention to determine if impaired TOI in children with CHD can be mitigated.
Structured cardiac rehabilitation programmes and home-based exercise training are well established nonpharmacologic treatments for adults and children with CHD.13, 22, 24, 25, 26, 27 Numerous studies have shown the effectiveness of aerobic exercise training on improving exercise intolerance in children with CHD.1,2,13, 14, 15, 16,22, 24,28,29 However, few of these interventions have incorporated strength training and play-based activities in addition to aerobic exercise that is suitable for children with CHD to participate in regularly outside of a research-based environment.
This study aimed to determine if a 12-week home-based exercise intervention that includes primarily strength training and play activities with supplemental aerobic exercise influences muscle oxygenation (TOI) and exercise tolerance (O2 and heart rate) at peak effort exercise in children with CHD. We tested the primary hypothesis that the 12-week exercise intervention would improve TOI at peak aerobic exercise in children with CHD. The secondary hypothesis was that the exercise intervention would improve peak O2 reserve (peak – nonexercising baseline) and heart rate at peak aerobic exercise in children with CHD, and would improve TOI, O2 reserve, and heart rate compared with healthy age- and sex-matched controls.
Methods
Participants
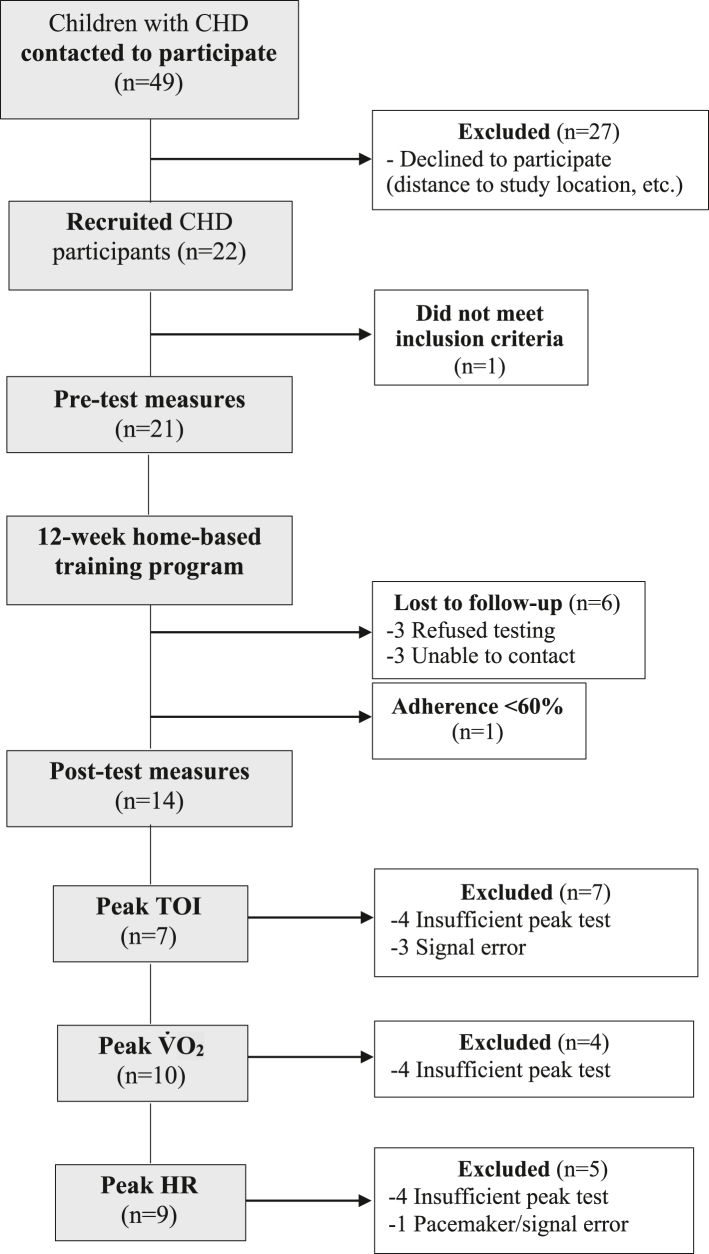
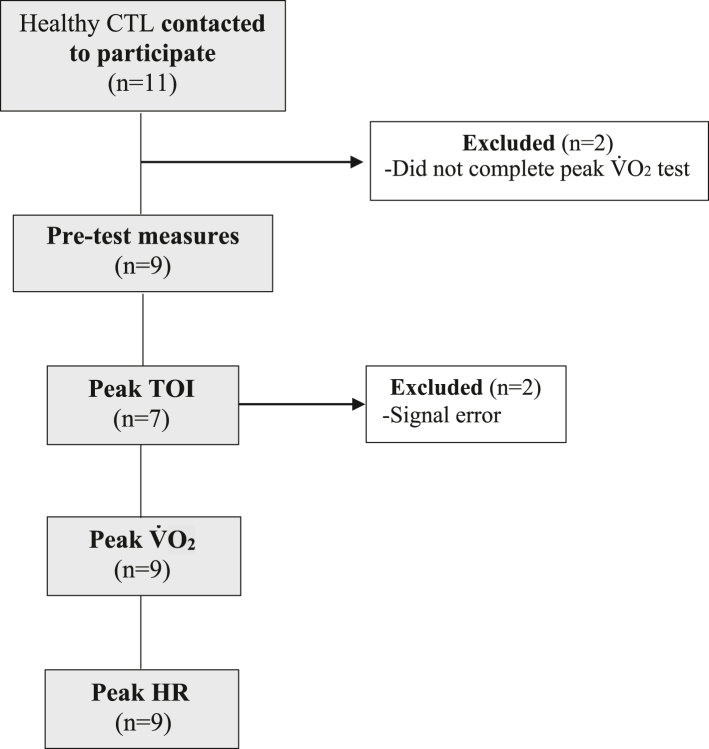
Twenty-one children with CHD between the ages of 9 and 16 were recruited from the Department of Pediatric Cardiology at the Jim Pattison Children’s Hospital in Saskatoon, Saskatchewan. A paediatric cardiologist prescreened patients for study eligibility and reviewed the home-based exercise programme to ensure safety (43% [21 of 49] of participants identified met inclusion criteria). Exclusion criteria for children with CHD included cardiac surgery within the last 6 months, inability to perform moderate-to-vigorous activity, and inability to follow verbal commands related to the experimental procedures. Fourteen children with CHD completed both pre- and post-programme measures with the final analysed sample including 7-10 (n varied for outcomes) children with CHD because of data quality/signal loss (see Fig. 1 for details). The reasons for withdrawal from the study are included in Figure 1. Nine typically developing children (CTL) between the ages of 9 and 16, recruited through word of mouth and posters, completed a one-time assessment of all measures (Fig. 2). The CTL group did not participate in the exercise intervention or post-testing session, as CTL data were intended as a reference only. Inclusion criteria for the CTL group included the absence of cardiovascular and respiratory disease, and ability to perform moderate-to-vigorous activity and follow verbal commands related to the experimental procedures. Figure 2 details study enrolment for CTL. Consent was obtained from all parents and/or legal guardians and assent was obtained from all children.
Figure 1.
Flow diagram of the study design for participants with CHD. “Insufficient peak test” indicates that participants did not meet the criteria for a “good effort” test as per an RER >0.90. CHD, congenital heart disease; HR, heart rate; RER, respiratory exchange ratio; TOI; tissue oxygenation index.
Figure 2.
Flow diagram of the study design for CTL participants. CTL, control; HR, heart rate.
Pre- and post-programme measurements
Participants completed a peak O2 test to volitional fatigue on an electromagnetically braked cycle ergometer (Ergoline 800S; SensorMedics Corp, Yorba Linda, CA). Before each test, flow and volume were calibrated using a 3-L syringe. Gas analysers were calibrated using gases of known gas concentrations of O2 (16%) and carbon dioxide (4%). Participants were instructed to refrain from heavy exercise, caffeine, and large meals before the test. Participants sat quietly on the cycle ergometer for 3 minutes to obtain nonexercising baseline data. A modified Oslo protocol, designed for peak exercise testing children,30,31 was used to ensure that participants could complete the exercise test properly by achieving at least the first 2 stages of the test. The reproducibility of this test has been validated previously.31 The test began at 25 W for 2 minutes, followed by 25 W increments every 2 minutes. Participants were provided with standard verbal checkups at regular intervals by the investigator. Exercise was terminated when participants indicated that they wished to stop or if they failed to sustain a pedal rate of 65 revolutions/min. Results of the test were accepted if the respiratory exchange ratio was greater than 0.90. Breath-by-breath gas exchange and heart rate parameters were measured as the highest 30-s values within the last 1 minute of exercise (SensorMedics Vmax 229; VIASYS Healthcare Respiratory Technologies, Yorba Linda, CA). Beat-by-beat heart rate was monitored with a 3-lead ECG (VIASYS Healthcare Respiratory Technologies).
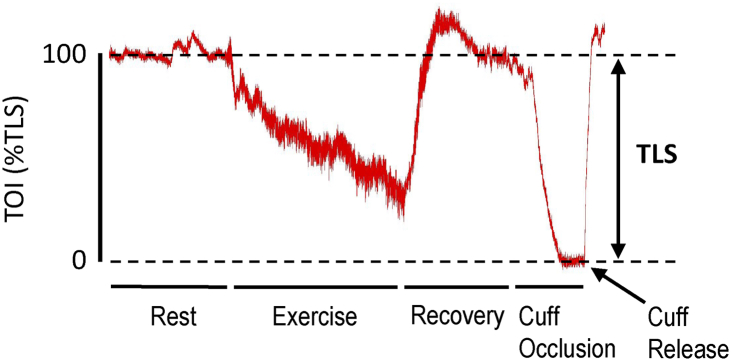
Muscle oxygenation (determined by TOI) was measured using continuous wave NIRS, with 2 nonstick diodes placed on the skin surface on the right vastus lateralis (NIRO-200NX; Hamamatsu Photonics K.K., Hamamatsu, Shizuoka, Japan). Placement was determined as midway between the femoral head and the lateral epicondyle of the femur. The proximal diode emits light into the tissue at 3 wavelengths (735, 810, and 850 nm), and the distal diode measures the returning wavelengths not absorbed by haemoglobin. Light emitted from the diode penetrates the skin, subcutaneous fat, and underlying muscle and is either absorbed by haemoglobin or myoglobin or scattered within the tissue.32 Adipose tissue thickness does not alter the NIRS signal.33 The interdiode surface distance was 3 cm for all participants, and the depth of penetration of the near-infrared light was approximately equal to half the distance between the light source and the diode.20,32 Right thigh skinfold and girth were used to validate the depth of near-infrared light penetration. A thick black cloth was placed over the diodes to block ambient light that may interfere with the NIRS signal. A tensor bandage was secured over the diodes and the cloth to hold them in place during exercise. To ensure that NIRS diodes were placed in the same location before and after testing, the investigator referred to anatomic landmarks and anthropometric measurements taken at the diode site during preliminary testing to reduce pre- vs post-measurement variability. Once the peak O2 test recovery period (4 minutes) was complete, a blood pressure cuff was inflated on the thigh above the NIRS diodes to suprasystolic pressure (220 mm Hg) for 3 minutes to establish the total labile signal (TLS; the difference between the nonexercising baseline and nadir TOI). Determining the TLS enabled calibration of the physiological TOI range, and changes in TOI before and after training were expressed as a percentage of the TLS (Fig. 3). Peak TOI (%TLS) was measured as the highest 30-s value within the last 1 minute of exercise.
Figure 3.
Representative data illustrating total labile signal (TLS) calibration. Tissue oxygenation index (TOI) is scaled from the nonexercising baseline value (100% TOI) to the value during ischemia from femoral cuff inflation above systolic pressure (0% TOI).
Home-based exercise programme
Children with CHD completed a home-based exercise programme after the completion of preliminary measures. The programme was 12 weeks long, with three 30- to 45-minute sessions completed per week (36 sessions total). The primary focus of the exercise sessions was to improve strength and aerobic capacity. Each exercise session included a strength component (exercises targeting the lower body), aerobic component (brisk walking, running, stair climbing), and flexibility component (warm-up stretches). Strength exercises comprised activities that use the participant’s own body weight (no equipment was required). The 12-week exercise programme was divided into 3 different phases of 4 weeks. In each phase, the intensity of the strength and aerobic exercises was increased for number and/or duration completed, and new exercises were added. Participants were provided detailed instructions for completing each session, including pictures and informative videos of specific movements (see Supplemental Appendix S1). Participants were instructed to complete each session at or above a rating of perceived exertion (RPE) of 4-6 (moderate-intensity exercise). The RPE scale was explained in detail, and take-home information was given to participants to ensure that they understood the RPE scale properly while exercising (see Supplemental Appendix S1). Logbooks were given to each participant to record the completion of each session, the RPE level attained during the session, as well as any other physical activity they participated in each week. We did not calibrate RPE by having children exercise at the high and low ends of the RPE scale, and we acknowledge that this may contribute to participants exercising at lower intensity than intended. Participants were also asked to record if they missed any sessions along with the reason why they were not able to complete it (eg, illness). Weekly follow-ups were conducted by phone, email, and/or in-person to aid in adherence and to facilitate questions regarding the programme. All participants were given the opportunity to have the researcher come to their home and complete an exercise session alongside them at least 1-2 times per phase, to ensure proper technique and completion of the exercise sessions. Participants who resided further than 100 km away from the testing site did not receive an in-person exercise session.
Biweekly in-person sessions
To facilitate study adherence, monitor the exercise technique and provide feedback in real time in-person, and demonstrate home-based exercise sessions, participants with CHD attended 6 biweekly in-person sessions (3-4 hours in length) at the University of Saskatchewan Physical Activity Complex. Sessions had a multidisciplinary approach beyond the scope of the current study, including mental wellness and physical literacy sessions along with physical activity promotion. For the current study, only the physical activity portion of these sessions was considered when interpreting results. Physical activities included swimming, rock climbing, yoga, gymnastics, and sports such as basketball and volleyball. In addition, each participant completed 20 minutes of aerobic exercise on a cycle ergometer at an RPE intensity of 4-6 (scale was visually presented to them). Participants then completed 15 minutes of resistance training led by the investigator, including similar exercises to the home-based exercise programme. Similar to the home-based programme, exercise was primarily lower-body resistance exercise. The upcoming 2 weeks of the home-based programme was reviewed with participants, and new exercises or progressions were demonstrated in-person.
Data analysis
The primary outcome was TOI measured by NIRS on the right vastus lateralis. The secondary outcomes were exercise tolerance measured by peak O2 and heart rate. We report TOI as a percent value scaled to the TLS based on the highest 5-s average at baseline (100%) and the lowest 5-s value at the nadir during circulatory occlusion of the right thigh (0%). Peak TOI, O2, and heart rate were analysed as the average value over 30 s over the last 1 minute of exercise. Pre- vs post-training changes in the TOI %TLS (total labile signal) and O2 reserve (peak – nonexercising baseline), and peak heart rate were determined using Cohen’s d effect size analysis (small effect ≥ 0.20; medium effect ≥ 0.50; large effect ≥ 0.80). Statistical analyses were conducted using 2-tailed t-tests with the Holm-Šídák correction for multiple comparisons. χ2 assessed between-group sex differences. Data were analysed with GraphPad Prism (Version 9.1.0, San Diego, CA) and are reported as mean ± standard deviation, where P < 0.05 was considered statistically significant.
Results
Demographics
By design, there was no difference in age and sex between CHD and CTL (Table 1). Children with CHD had greater mass compared with CTL (Table 1). Participant diagnosis, surgical intervention, time since last cardiac-related surgical intervention, and cardiac status at the time of study are listed in Table 2.
Table 1.
Participant demographics
| CHD before training (n = 10) | CHD after training (n = 10) | CTL (n = 9) | |
|---|---|---|---|
| Age (y) | 13 ± 1 | 13 ± 1 | 12 ± 3 |
| Sex (female:male) | 4:6 | – | 5:4 |
| Height (cm) | 160 ± 10 | 161 ± 10 | 149 ± 13 |
| Weight (kg) | 54 ± 11∗ | 54 ± 12∗ | 41 ± 12 |
| BMI (kg/m2) | 21 ± 4 | 21 ± 4 | 18 ± 3 |
| Reside (U:R) | 7:3 | – | 9:0 |
Values are mean ± standard deviation.
Comparisons were made using multiple 2-tailed t-tests with the Holm-Šídák correction for multiple comparisons.
BMI, body mass index; CHD, congenital heart disease; CTL, control; R, rural; U, urban.
Significantly different vs CTL (P = 0.048).
Table 2.
CHD participant characteristics
| Age (y) and sex | Diagnosis | Surgical intervention | Years since last intervention | Cardiac status at the time of the study∗ |
|---|---|---|---|---|
| 12, male | PV stenosis | Balloon valvuloplasty | 12 | Moderate pulmonary insufficiency, trivial pulmonary stenosis, mild RV dilation, and normal BiV function |
| 13, male | d-TGA | Balloon atrial septostomy, arterial switch, RVOT patch, and balloon branch pass | 10 | Mild main pulmonary artery gradient, mild aortic insufficiency, normal chamber sizes, and normal BiV function |
| 12, male | TOF | VSD patch, RVMB resection, and pulmonary valvuloplasty | 12 | Trivial RV outflow tract gradient, mild pulmonary insufficiency, no VSD, normal RV dimensions, and normal BiV function |
| 12, female | PV stenosis, ASD | Balloon valvuloplasty, ASD closure, and TV repair | 8 | Trivial pulmonary stenosis, mild pulmonary insufficiency, mild to moderate tricuspid regurgitation, normal chamber sizes, and normal BiV function |
| 11, male† | cc-TGA | ASD closure and pacemaker | 1 | Small ASD with left to right shunting, moderate supravalvar pulmonary stenosis, trivial mitral regurgitation, mild tricuspid regurgitation, mild pulmonary insufficiency, normal BiV function, and normal pacemaker function |
| 13, female | TOF | Right Blalock-Taussig shunt and TOF repair | 13 | Moderate RV dilation, trivial tricuspid regurgitation, severe pulmonary insufficiency, normal BiV function, and trivial pulmonary stenosis |
| 16, female | TOF | TOF repair and pacemaker | 16 | Mild RV dilation, mild pulmonary insufficiency, no pulmonary stenosis, normal BiV function, and stable pacemaker function |
| 15, female | d-TGA, VSD | Arterial switch, VSD patch, CoA repair, and ASD suture | 15 | Moderate pulmonary stenosis, mild aortic insufficiency, no recoarctation, and normal BiV function |
| 13, male | CoA, VSD, PDA, BAV, left SVC to dilated coronary sinus | End-to-end CoA repair, VSD closure, PA banding and debanding, and RVMB resection | 13 | No aortic stenosis or insufficiency, mild ascending aorta and aortic root dilation, no aortic arch gradient, and normal BiV function |
| 13, male | CoA, BAV | End-to-end coarctation repair | 10 | No aortic stenosis or insufficiency, no dilation to the ascending aorta, normal BiV function, and no recoarctation of the aorta |
ASD, atrial septal defect; AVSD, atrioventricular septal defect; BAV, bicuspid aortic valve; BiV, biventricular; cc-TGA, congenitally corrected transposition of the great arteries; CoA, coarctation of the aorta; DILV, double inlet left ventricle; d-TGA, dextro-transposition of the great arteries; PDA, patent ductus arteriosus; PV, pulmonary valve; RVMB, right ventricular muscle band; RVOT, right ventricular outflow tract; SVC, superior vena cava; TOF, tetralogy of Fallot; TV, tricuspid valve; VSD, ventricular septal defect.
All patients were NYHA functional class I.
Cardiac-related medication: acetylsalicylic acid.
Effect of exercise training on TOI at peak exercise
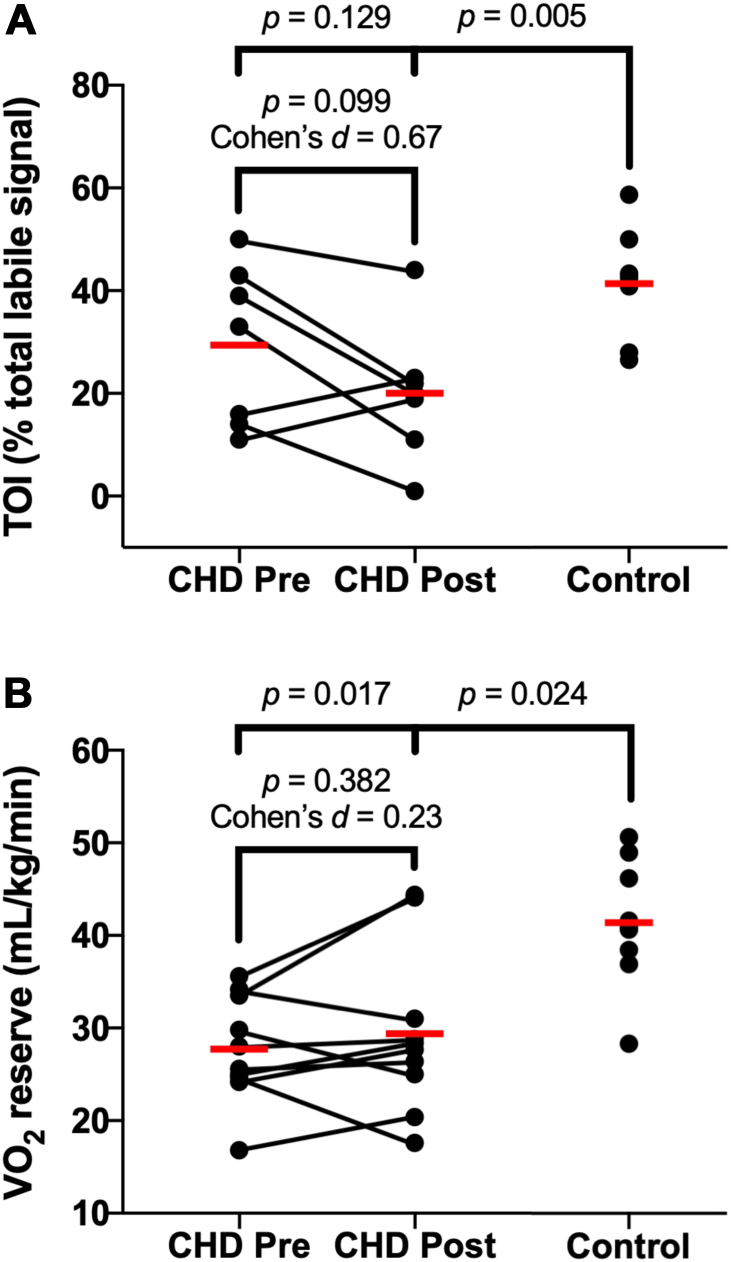
There was a medium effect of training on TOI at peak exercise in CHD (pre: 30 ± 16 %TLS vs post: 20 ± 13 %TLS; Cohen’s d = 0.67, P = 0.099; Fig. 4A). Before training, TOI at peak exercise was not different between CHD (30 ± 16 %TLS) and CTL (41 ± 11 %TLS; P = 0.129; Fig. 4A). After training, children with CHD had lower TOI at peak exercise compared with CTL (CHD-post: 20 ± 13 %TLS vs 41 ± 11 %TLS; P = 0.005; Fig. 4A).
Figure 4.
(A) TOI and (B) O2 reserve in children with CHD compared with CTL at peak exercise. Data points are individual data. The red line is the group mean. Comparisons were made using multiple 2-tailed t-tests with the Holm-Šídák correction for multiple comparisons. Cohen’s d effect size analysis was completed for pre- to post-testing in CHD, where a small effect is ≥0.20, a medium effect is ≥0.50, and a large effect is ≥0.80. CHD, congenital heart disease; CTL, control; Pre, pre-exercise training; Post, post-exercise training; TOI, tissue oxygenation index.
Effect of exercise training on O2 reserve
There was a small effect of training on O2 reserve in CHD (pre: 27.2 ± 5.7 mL/kg/min vs post: 29.4 ± 8.8 mL/kg/min; Cohen’s d = 0.23, P = 0.382; Fig. 4B). The improvement in O2 reserve approximates a half (0.6) metabolic equivalent (MET) increase in exercise capacity. O2 reserve remained lower between CHD and CTL before and after training (Fig. 4B).
Effect of exercise training on heart rate at peak exercise
There was a small effect of training on lowering heart rate at peak exercise (pre: 175 ± 23 beats/min vs post: 169 ± 21 beats/min; Cohen’s d = 0.27, P = 0.18) in CHD. Heart rate at peak exercise tended to be lower in CHD compared with CTL pre- and post-training (pre: 175 ± 23 beats/min and post: 169 ± 21 beats/min vs CTL: 189 ± 11 beats/min; all P > 0.05).
Exercise compliance
Nine of 10 participants submitted their exercise compliance logbook (1 participant did not submit the exercise compliance logbook). In these participants, 26 ± 11 of 36 (71%) home-based exercise sessions were completed (range: 4-36 sessions). Seven of 9 participants completed >60% of sessions (30 ± 6 of 36 sessions; range: 22-36). In 8 of 10 participants who recorded their exercise intensity, exercise was performed at an RPE of 4-6 or greater 81% ± 27% of the time. Nine of 10 participants attended 5 ± 1 of 6 (89%) biweekly in-person exercise sessions (range: 4-6 sessions).
Discussion
We observed that home-based resistance training had a medium effect of reducing TOI at peak exercise in children with CHD. The change in TOI conferred a small effect (increase of approximately 0.6 METs) in O2 reserve. We also observed a small (effect) reduction in the heart rate at peak exercise. Together, these findings indicate that peripheral factors that are known to contribute to exercise tolerance may possibly be ameliorated with a chronic and primarily resistance exercise intervention in children with CHD.
Effects of exercise training on TOI in children with CHD
We found that the reduction in TOI at peak exercise was augmented after strength-based exercise training in children with CHD. TOI reflects the balance between O2 delivery and utilization;20 during exercise, TOI transiently decreases (and the arterial-venous O2 content difference increases) as O2 is used at the muscle to meet increased metabolic demands in healthy individuals.34 In children with CHD, this response has been shown to be abnormal compared with healthy age- and sex-matched peers due to reduced O2 delivery and uptake at the working muscle.21,23,35,36 Aerobic exercise interventions have been used to improve the impaired exercise TOI that contributes to exercise intolerance in CHD.14,22 Moalla et al.22 associated improvements in muscle oxygenation after an aerobic interval training programme to both an increase in cardiac output and arterial-venous O2 content difference and capillary density. In the present study, we demonstrated a further reduction in TOI at peak exercise. Costes et al.37 reported significant reductions in muscle oxygenation during submaximal exercise in young healthy males after an aerobic exercise intervention, with no change in O2. In that study, muscle biopsy of the vastus lateralis indicated that an augmented reduction in TOI post-training was secondary to increased capillarization and oxidative enzyme capacity at the muscle, thus enhancing the matching of capillary flow to metabolic demand.37 Indeed, an enhancement of capillary flow without a concomitant increase in O2 delivery further decreases TOI after training37 as the arterial-venous O2 content difference would increase if O2 delivery did not undergo a similar increase. Similar findings results have also been reported in competitive cyclists during a submaximal endurance test after an interval training programme.38 More recently, a study in patients with chronic obstructive pulmonary disease and healthy sedentary participants showed training-induced increases in skeletal muscle aerobic capacity secondary to enhancement of the muscle capillary network and mitochondrial respiratory capacity without a change in muscle blood volume.39 Although difficult to compare our findings, these prior reports reveal possible mechanisms that may explain the reduction in TOI we observed post-training in children with CHD.
Exercise training has been shown to improve blood-muscle O2 transfer and subsequently increasing arterial-venous O2 content difference during exercise in patients with heart failure.40,41 Similarly, our exercise intervention may have elicited increases in capillary number and density, slowing blood flow at the working muscle and allowing for an increase in O2 offloaded from the blood to the muscle (see Fig. 5 for illustration of this phenomenon). With an increase in mitochondrial density the muscle can use the increased O2 available and the arterial-venous O2 content difference increase (reflected as a reduction in/lower TOI). An increase in O2 extraction and utilization is especially likely to occur without a subsequent increase in O2 delivery that would normal facilitate a higher TOI. Indeed, we found that heart rate decreased somewhat (small effect) after exercise training.
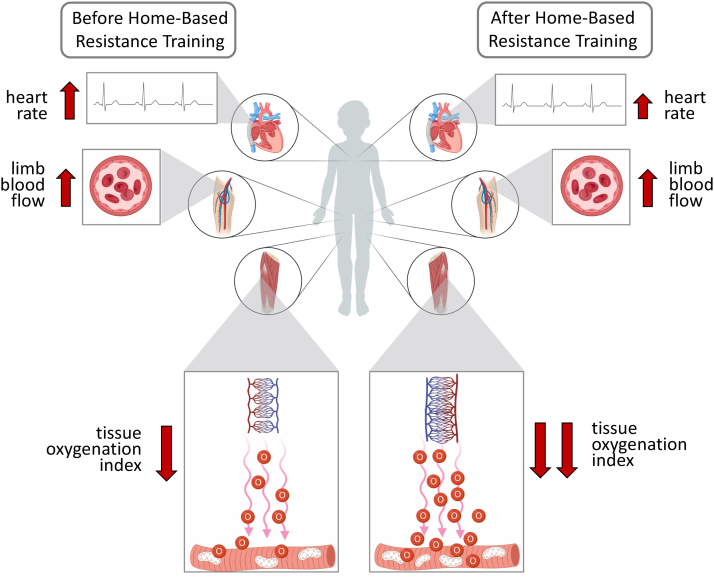
Figure 5.
Depiction of the main study findings. The illustration indicates physiological responses during exercise before (left) and after (right) home-based resistance training. There was a small effect of training on lowering heart rate at peak exercise after training. It is also hypothesized that there was also no increase in the limb blood flow (and “bulk” oxygen delivery) during exercise from before to after training. There was a medium effect of exercise training on decreasing TOI (tissue oxygenation index) at peak exercise that is hypothesized to occur secondary to an increase in skeletal muscle capillarity and/or muscle metabolism. The increase in capillary density would subsequently slow the flux of red blood cells across the muscle bed, thus facilitating greater time for potential diffusion of oxygen to the mitochondria. Greater metabolic activity and/or number of mitochondria after training facilitates greater oxygen extraction from blood and effectively increases the arterial-venous oxygen content difference and is noted by a lower TOI value. By way of the Fick equation where O2 = cardiac output × arterial-venous oxygen content difference, the small effect of exercise training on increasing O2 reserve approximately 0.6 metabolic equivalents was likely driven by an increase in the arterial-venous oxygen content difference (lower TOI) and not an increase in cardiac output (heart rate or stroke volume) as heart rate was somewhat lower after training and is a known major determinant of cardiac output. TOI, tissue oxygenation index.
O2 response to exercise training in children with CHD
There was a small effect of training on O2 reserve (1.6 mL/kg/min or 0.6 METs) in children with CHD. Previous work has not yet led to a consensus for the intervention required to elicit significant increases in O2 in children with CHD. The data indicate that aerobic interval training elicits increases in peak O2 of 3-4 mL/kg/min;1,22,29 however, no change in peak O2 after similar training has also been reported.28 Cordina et al.42 implemented a resistance training programme that resulted in a significant increase of absolute peak O2 of 0.5 L/min, and Rhodes et al.24 found an increase in peak O2 of approximately 4.0 mL/kg/min after a combined aerobic and strength training intervention. Similarly, our study included primarily resistance-based exercise supplemented with aerobic training; however, it did elicit only modest changes in O2. It is possible that maturational differences and varying lesion types in our study group elicited varying physiological adaptation to our exercise training programme. Indeed, our group consisted of a heterogeneous sample of CHD phenotypes, including both simple and complex lesions. Various central hemodynamic factors can be implicated as a result of lesion structure and morphology such as cardiac output, heart rate, left ventricular ejection fraction, and atrial and ventricular pressure.43 As a result, lesion type and severity are a major determinant of O2 for individuals with CHD5 and thus could impact exercise efficacy.
Exercise intensity may be an important factor in our results. Intensity is widely regarded as the most important determinant of an exercise prescription’s effectiveness for increasing O2.44 Unlike previous studies, our intervention only used an RPE scale to monitor intensity, as our programme was designed to be feasible and easy for children to follow. Rhodes et al.24 used heart rate at the ventilatory threshold as a target exercise intensity for participants. They were also able to monitor their participants more closely, with supervised exercise sessions 2 times per week for 12 weeks, compared with our intervention where supervised exercise only occurred every 2 weeks. Therefore, our method of self-reported intensity may not have had a strong enough stimulus to elicit greater changes in O2.
Effectiveness of the home-based exercise programme
Our novel strength-based home-exercise intervention demonstrated that a programme designed for research methodology as well as real-world application may confer physiological adaptation in children with CHD. Previous studies have primarily implemented aerobic-based exercise interventions,1,13,22,29 likely due to ease of programme implementation and the ability of these programmes to produce significant improvements in exercise tolerance. Although both are important, it is difficult to generalize these results and translate them into feasible clinical rehabilitation programmes. By including primarily lower body strength exercises related to functional movement patterns, and supplementing these exercises with aerobic and play components, our study addressed key aspects of daily activities for children with CHD. Developing our intervention with a focus on real-world feasibility and activities of daily living strengthened the impact our results may have on future studies and may help address a gap in approaches to exercise rehabilitation for children with CHD.
Limitations
This study was limited to participants who lived in close proximity to the study location (Saskatoon, Saskatchewan, Canada). All participants were recruited from a single institution and were classified as NYHA I, and therefore are not representative of all children with CHD. Our small heterogeneous sample is also a limitation. Ideally, an in-depth study of specific based on lesion type or severity would allow for more precision exercise training recommendations. The addition of a CHD control group and a training group consisting of healthy age- and sex-matched children may have strengthened our results. A more stringent inclusion criterion for compliance may provide a better indication of adaptations to our exercise intervention.
Previous reports using NIRS to evaluate TOI have not included TLS, making it difficult to ascertain whether the magnitude of change in TOI (either increase or decrease) is related to the relative baseline TOI level for each participant. It is important to note that the TLS of muscle oxygenation is an indicant of changes in training-related TOI augmentation secondary to microvascular adaptations and not baseline TOI values. In the present study, calibration of the physiological TOI range via the TLS allowed reliable comparisons and generalizations regarding peripheral responses to exercise training. Therefore, our approach to calibrating TOI further bolsters our conclusions that peripheral skeletal muscle/microvascular factors are associated with exercise intolerance in children with CHD.
Conclusions
Our data indicate in a heterogeneous group of children with CHD that a home-based exercise intervention including strength, aerobic, and play activities decreased TOI at peak exercise. The exercise training–induced reduction in TOI led to a small (effect) increase in O2 reserve. Given that heart rate was moderately reduced after exercise, it is likely that training did not increase the exercise cardiac output. Based on the small change in exercise reserve we observed, home-based resistance training may confer benefit for activities of daily living in children with CHD.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards except for registration in a database. The study was approved by the Biomedical Research Ethics Board of the University of Saskatchewan (Bio #15-148).
Funding Sources
The Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation funded this study.
Disclosures
The authors have no conflicts of interest to disclose.
Author Contributions
D. S. Lahti, C. Pockett, N. G. Boyes, S. J. Butcher, K. D. Wright, M. C. Erlandson, C. R. Tomczak were contributed to the study conception. All authors contributed to the study design. Material preparation, data collection and analysis were performed by D. S. Lahti, C. Pockett, N. G. Boyes, and C. R. Tomczak. The first draft of the manuscript was written by D. S. Lahti and C. R. Tomczak. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The support of participating families is greatly appreciated.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2022.08.002
Supplementary data
References
- 1.Amiard V., Jullien H., Nassif D., et al. Effects of home-based training at dyspnea threshold in children surgically repaired for congenital heart disease. Congenit Heart Dis. 2008;3:191–199. doi: 10.1111/j.1747-0803.2008.00191.x. [DOI] [PubMed] [Google Scholar]
- 2.Fredriksen P.M., Kahrs N., Blaasvaer S., et al. Effect of physical training in children and adolescents with congenital heart disease. Cardiol Young. 2000;10:107–114. doi: 10.1017/s1047951100006557. [DOI] [PubMed] [Google Scholar]
- 3.Tikkanen A.U., Oyaga A.R., Riaño O.A., et al. Paediatric cardiac rehabilitation in congenital heart disease: a systematic review. Cardiol Young. 2012;22:241–250. doi: 10.1017/S1047951111002010. [DOI] [PubMed] [Google Scholar]
- 4.Roche S.L., Silversides C.K. Hypertension, obesity, and coronary artery disease in the survivors of congenital heart disease. Can J Cardiol. 2013;29:841–848. doi: 10.1016/j.cjca.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Kempny A., Dimopoulos K., Uebing A., et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life single centre experience and review of published data. Eur Heart J. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 6.Myers J., Prakash M., Froelicher V., et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 7.Diller G.P., Dimopoulos K., Okonko D., et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 8.Thaulow E., Fredriksen P.M. Exercise and training in adults with congenital heart disease. Int J Cardiol. 2004;97:35–38. doi: 10.1016/j.ijcard.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Passino C., Aimo A., Mirizzi G., Emdin M. Exercise intolerance in heart failure with preserved ejection fraction: a reappraisal of central mechanisms? Int J Cardiol. 2018;254:248–249. doi: 10.1016/j.ijcard.2017.11.114. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos K., Diller G.P., Piepoli M.F., Gatzoulis M.A. Exercise intolerance in adults with congenital heart disease. Cardiol Clin. 2006;24:641–660. doi: 10.1016/j.ccl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner H., Bonhoeffer P., De Groot N.M.S., et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 12.Brassard P., Ferland A., Marquis K., et al. Impact of diabetes, chronic heart failure, congenital heart disease and chronic obstructive pulmonary disease on acute and chronic exercise responses. Can J Cardiol. 2007;23:89–96. doi: 10.1016/s0828-282x(07)71018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minamisawa S., Nakazawa M., Momma K., et al. Effect of aerobic training on exercise performance in patients after the Fontan operation. Am J Cardiol. 2001;88:695–698. doi: 10.1016/s0002-9149(01)01822-7. [DOI] [PubMed] [Google Scholar]
- 14.Moalla W., Maingourd Y., Gauthier R., et al. Effect of exercise training on respiratory muscle oxygenation in children with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:604–611. doi: 10.1097/01.hjr.0000201515.59085.69. [DOI] [PubMed] [Google Scholar]
- 15.Opocher F., Varnier M., Sanders S.P., et al. Effects of aerobic exercise training in children after the Fontan operation. Am J Cardiol. 2005;95:150–152. doi: 10.1016/j.amjcard.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes J., Curran T.J., Camil L., et al. Sustained effects of cardiac rehabilitation in children with serious congenital heart disease. Pediatrics. 2006;118:586–593. doi: 10.1542/peds.2006-0264. [DOI] [PubMed] [Google Scholar]
- 17.Warburton D.E.R., Taylor A., Bredin S.S.D., et al. Central haemodynamics and peripheral muscle function during exercise in patients with chronic heart failure. Appl Physiol Nutr Metab. 2007;32:318–331. doi: 10.1139/h06-085. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari M., Muthalib M., Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans Math Phys Eng Sci. 2005;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 19.Leclair E., Borel B., Baquet G., et al. Reproducibility of measurement of muscle deoxygenation in children during exercise. Pediatr Exerc Sci. 2010;22:183–194. doi: 10.1123/pes.22.2.183. [DOI] [PubMed] [Google Scholar]
- 20.Hamaoka T., McCully K.K., Quaresima V., et al. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12 doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 21.Moalla W., Dupont G., Costes F., et al. Performance and muscle oxygenation during isometric exercise and recovery in children with congenital heart diseases. Int J Sports Med. 2006;27:864–869. doi: 10.1055/s-2006-923787. [DOI] [PubMed] [Google Scholar]
- 22.Moalla W., Elloumi M., Chamari K., et al. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl Physiol Nutr Metab. 2012;37:621–630. doi: 10.1139/h2012-036. [DOI] [PubMed] [Google Scholar]
- 23.Vandekerckhove K., Coomans I., Moerman A., et al. Differences in cerebral and muscle oxygenation patterns during exercise in children with univentricular heart after Fontan operation compared to healthy peers. Int J Cardiol. 2019;290:86–92. doi: 10.1016/j.ijcard.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes J., Curran T.J., Camil L., et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 25.Tomassoni T.L. Role of exercise in the management of cardiovascular disease in children and youth. Med Sci Sports Exerc. 1996;28:406–413. doi: 10.1097/00005768-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Casillas J.M., Gremeaux V., Damak S., et al. Exercise training for patients with cardiovascular disease. Ann Readapt Med Phys. 2007;50:403–418. doi: 10.1016/j.annrmp.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Bhasipol A., Sanjaroensuttikul N., Pornsuriyasak P., et al. Efficiency of the home cardiac rehabilitation program for adults with complex congenital heart disease. Congenit Heart Dis. 2018;13:1–7. doi: 10.1111/chd.12659. [DOI] [PubMed] [Google Scholar]
- 28.Brassard P., Poirier P., Martin J., et al. Impact of exercise training on muscle function and ergoreflex in Fontan patients: a pilot study. Int J Cardiol. 2006;107:85–94. doi: 10.1016/j.ijcard.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg B., Fripp R.R., Lister G., et al. Effect of physical training on exercise performance of children following surgical repair of congenital heart disease. Pediatrics. 1981;68:691–699. [PubMed] [Google Scholar]
- 30.Brøgger R.J., Mathisen G., Pettersen S.A. Effect of high intensity activity on children’s aerobic power. J Phys Educ Sport. 2013;13:511–516. [Google Scholar]
- 31.Fredriksen P.M., Ingjer F., Nystad W., Thaulow E. Aerobic endurance testing of children and adolescents—a comparison of two treadmill protocols. Scand J Med Sci Sport. 1998;8:203–207. doi: 10.1111/j.1600-0838.1998.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari M., Mottola L., Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 33.Quaresima V., Komiyama T., Ferrari M. Differences in oxygen re-saturation of thigh and calf muscles after two treadmill stress tests. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:67–73. doi: 10.1016/s1095-6433(01)00531-1. [DOI] [PubMed] [Google Scholar]
- 34.Belardinelli R., Barstow T.J., Porszasz J., Wasserman K. Changes in skeletal muscle oxygenation during incremental exercise measured with near infrared spectroscopy. Eur J Appl Physiol Occup Physiol. 1995;70:487–492. doi: 10.1007/BF00634377. [DOI] [PubMed] [Google Scholar]
- 35.Moalla W., Dupont G., Temfemo A., et al. Assessment of exercise capacity and respiratory muscle oxygenation in healthy children and children with congenital heart diseases. Appl Physiol Nutr Metab. 2007;33:434–440. doi: 10.1139/H07-196. [DOI] [PubMed] [Google Scholar]
- 36.Ross F.J., Arakaki L.S.L., Ciesielski W.A., et al. Assessment of muscle oxygenation in children with congenital heart disease. 2019;29:850–857. doi: 10.1111/pan.13668. [DOI] [PubMed] [Google Scholar]
- 37.Costes F., Prieur F., Féasson L., et al. Influence of training on NIRS muscle oxygen saturation during submaximal exercise. Med Sci Sports Exerc. 2001;33:1484–1489. doi: 10.1097/00005768-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Neary J.P., McKenzie D.C., Bhambhani Y.N. Effects of short-term endurance training on muscle deoxygenation trends using NIRS. Med Sci Sports Exerc. 2002;34:1725–1732. doi: 10.1097/00005768-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Barberan-Garcia A., Munoz P.A., Gimeno-Santos E., et al. Training-induced changes on quadriceps muscle oxygenation measured by near-infrared spectroscopy in healthy subjects and in chronic obstructive pulmonary disease patients. Clin Physiol Funct Imaging. 2019;39:284–290. doi: 10.1111/cpf.12572. [DOI] [PubMed] [Google Scholar]
- 40.Poole D.C., Hirai D.M., Copp S.W., Musch T.I. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:1050–1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirai D.M., Musch T.I., Poole D.C. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol. 2015;309:1419–1439. doi: 10.1152/ajpheart.00469.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordina R.L., O’Meagher S., Karmali A., et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Bassareo P.P., Saba L., Solla P., et al. Factors influencing adaptation and performance at physical exercise in complex congenital heart diseases after surgical repair. Biomed Res Int. 2014;2014 doi: 10.1155/2014/862372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swain D.P. Moderate or vigorous intensity exercise: which is better for improving aerobic fitness? Prev Cardiol. 2005;8:55–58. doi: 10.1111/j.1520-037x.2005.02791.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.