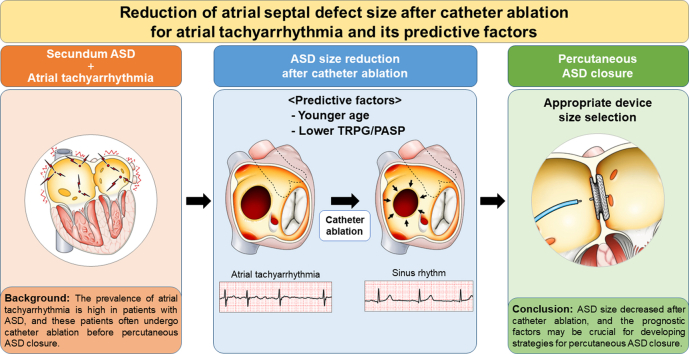
Abstract
The prevalence of atrial tachyarrhythmia is high in patients with atrial septal defect (ASD), and catheter ablation (CA) is often performed before percutaneous ASD closure. We aimed to clarify the effect of CA on the ASD size. We analysed 16 patients with secundum ASD who had a history of CA for atrial tachyarrhythmia and underwent ASD size evaluation before and after CA. The size of ASD significantly decreased after CA. Younger age and lower tricuspid regurgitation pressure gradients and pulmonary arterial systolic pressures were associated with size reduction. These factors are crucial for making strategies of percutaneous ASD closure.
Graphical abstract
Résumé
La prévalence de la tachyarythmie auriculaire est élevée chez les patients qui présentent une communication interauriculaire (CIA), et l’on pratique souvent une ablation par cathéter avant la fermeture percutanée de la CIA. Notre objectif consistait à éclaircir l’effet de l’ablation par cathéter sur la taille de la CIA. Pour ce faire, nous avons analysé 16 patients présentant une CIA de type ostium secundum, ayant déjà subi une ablation par cathéter et chez qui la taille de la CIA a été évaluée avant et après l’intervention. La taille de la CIA a diminué de manière significative après l’ablation par cathéter. Les facteurs associés à la réduction de la taille de la communication comprennent un âge plus jeune, des gradients de pression plus faibles pour l’insuffisance tricuspidienne et une pression artérielle systolique moins élevée. Il est crucial de tenir compte de ces facteurs lors de l’établissement d’une stratégie pour la fermeture percutanée d’une CIA.
Percutaneous closure of a secundum atrial septal defect (ASD) has been widely accepted as an alternative to surgical repair.1,2 Precise evaluation of ASD morphology and size by transesophageal echocardiography (TEE) before its closure is crucial for successful device deployment.
The prevalence of atrial tachyarrhythmia (ATA) (eg, atrial fibrillation [AF] and atrial flutter) is high in patients with ASD.3 Nowadays, such patients often undergo catheter ablation (CA) before percutaneous ASD closure owing to development of transcatheter treatments. Although it has been reported that left atrial (LA) size decreases after CA,4,5 the effect of CA on the ASD size is unknown. Therefore, we aimed to clarify the effect of CA for ATA on the secundum ASD size and identify prognostic factors for the ASD size reduction after CA.
Methods
In this single-center, retrospective study, we analyzed data from adult patients with secundum ASD (≥18 years of age) who had a history of CA for ATA, who underwent percutaneous ASD closure between January 2015 and March 2021 in our hospital, and who underwent ASD size evaluation by TEE before and after CA. Among the 245 consecutive patients with ASD who underwent percutaneous ASD closure, 37 patients had a history of CA for ATA and 16 patients were evaluated by TEE before and after CA. The information on each patient’s ATA and CA procedures is summarized in Supplemental Table S1. One patient experienced recurrence of AF after final CA. In this patient, paroxysmal AF was documented by portable electrocardiograph only once during the 6 months after CA and the ASD size was relatively small (maximum diameter after CA: 10.2 mm). Thus, it was considered that CA could be performed again even after ASD device deployment. Therefore, the patient underwent percutaneous ASD closure. None of the 16 patients had multiple ASDs. The maximum and minimum diameters of ASDs were measured by 2-dimensional (2D) or 3-dimensional (3D) TEE. The detailed TEE evaluation of ASD has been described previously.2 Twelve patients underwent 2D TEE and 3D TEE before and after CA, and 4 underwent only 2D TEE before and/or after CA. The ASD area was estimated using diameter measurements as follows, assuming that the defect was elliptical: ASD area (cm2) = maximum diameter (cm) × minimum diameter (cm) × π/4.
The maximum diameters and areas of ASDs before and after CA were compared, and we defined the ASD size-reduction group as follows: (1) >1 mm decrease in maximum diameter and (2) 15% decrease in ASD area after CA. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Keio University School of Medicine (approval number: 20160249). All patients provided written informed consent before the procedure. Clinical data were collected before CA, including age, sex, type of ATA (paroxysmal or persistent/chronic), blood levels of brain natriuretic peptide, and transthoracic echocardiography (TTE) analyses, and were compared between the groups.
Normally distributed data were expressed as mean ± standard deviation, nonparametric data were presented as median (interquartile range), and categorical data were shown as absolute values (percent). Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS Statistics, Version 25.0 (IBM Corp, Armonk, NY).
Results
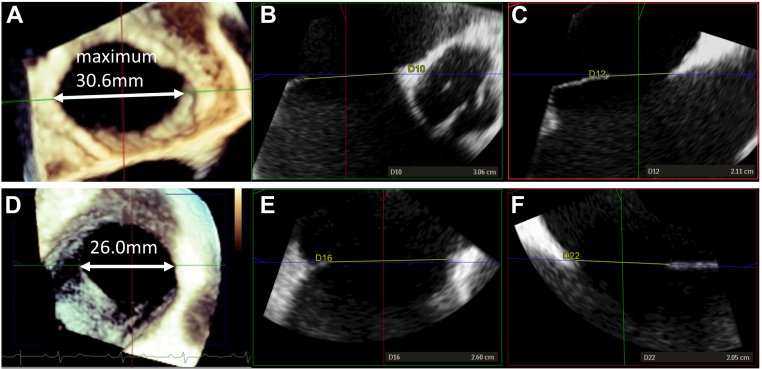
TEE was performed at 227 ± 156 days after CA. Compared with the measurements before CA, the maximum and minimum diameters and areas of ASDs decreased after CA (maximum diameter: 19.2 ± 8.0 to 17.9 ± 8.0 mm, P = 0.001; minimum diameter: 14.8 ± 6.6 to 13.6 ± 6.6 mm, P < 0.001; area: 2.6 ± 2.3 to 2.3 ± 2.3 cm2, P < 0.001). Figure 1 shows the representative images of TEE. Patients were divided into 2 groups according to the ASD size: an unchanged group (n = 9) and a size-reduction group (n = 7). None of the patients showed an increase in the ASD size. Patients in the size-reduction group were younger and had lower tricuspid regurgitation pressure gradients (TRPG) and pulmonary arterial systolic pressures (PASP) than those in the unchanged group (age: 57.9 ± 5.7 vs 67.7 ± 9.6 years, P = 0.031; TRPG: 24.3 ± 7.9 vs 36.2 ± 9.6 mm Hg, P = 0.034; PASP: 30.2 ± 7.1 vs 41.7 ± 10.3 mm Hg, P = 0.034) (Table 1). None of the patients in the size-reduction group had more than mild tricuspid regurgitation (TR). The ASD size reduction was not associated with the type of ATA, left atrium size, mitral regurgitation more than mild, left ventricular ejection fraction (modified Simpson method), ASD size before CA, or the use of diuretics.
Figure 1.
Representative images of transesophageal echocardiography (TEE). (A) Three-dimensional (3D) TEE, (B) maximum diameter, and (C) minimum diameter of an atrial septal defect (ASD) before catheter ablation (CA). (D) 3D TEE, (E) maximum diameter, and (F) minimum diameter of an ASD after CA.
Table 1.
Comparisons between the unchanged and size-reduction groups of patients with atrial septal defects (ASDs)
| ASD unchanged size group (n = 9) | ASD size-reduction group (n = 7) | P values | |
|---|---|---|---|
| Age (y) | 67.7 ± 9.6 | 57.9 ± 5.7 | 0.031 |
| Sex: male, n (%) | 4 (44.4) | 4 (57.1) | 1.000 |
| BNP (pg/mL) | 246 [77-310] | 161 [87-205] | 0.606 |
| PAF, n (%) | 5 (55.6) | 3 (42.9) | 1.000 |
| Diuretics, n (%) | 4 (44.4) | 2 (28.6) | 0.633 |
| CA to TEE after CA (d) | 246 ± 180 | 202 ± 126 | 0.594 |
| TEE evaluation | |||
| Maximum diameter before CA (mm) | 19.9 ± 8.7 | 18.3 ± 7.6 | 0.708 |
| Minimum diameter before CA (mm) | 15.3 ± 7.4 | 14.1 ± 6.0 | 0.716 |
| ASD area before CA (cm2) | 2.8 ± 2.8 | 2.3 ± 1.7 | 0.681 |
| Maximum diameter after CA (mm) | 19.2 ± 8.9 | 16.2 ± 6.8 | 0.740 |
| Minimum diameter after CA (mm) | 14.5 ± 7.0 | 12.3 ± 6.1 | 0.666 |
| ASD area after CA (cm2) | 2.6 ± 3.0 | 1.8 ± 1.4 | 0.675 |
| TTE evaluation | |||
| Qp/Qs before CA | 2.3 [1.5-2.6] | 2.4 [1.9-2.7] | 0.456 |
| LA diameter (mm) | 4.0 ± 0.5 | 4.4 ± 0.4 | 0.144 |
| MR > mild | 2 (22.2) | 0 (0.0) | 0.475 |
| TR > mild | 6 (66.7) | 0 (0.0) | 0.011 |
| TRPG (mm Hg) | 36.2 ± 9.6 | 24.3 ± 7.9 | 0.026 |
| PASP (mm Hg) | 41.7 ± 10.3 | 30.2 ± 7.1 | 0.034 |
| LVEF (modified Simpson method) (%) | 61.7 ± 7.6 | 62.8 ± 5.8 | 0.759 |
Data are presented as mean ± standard deviation, median [with interquartile ranges], or absolute values (percent).
BNP, brain natriuretic peptide; CA, catheter ablation; LA, left atrium; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PAF, paroxysmal atrial fibrillation; PASP, pulmonary artery systolic pressure; Qp/Qs, ratio of pulmonary perfusion to systemic perfusion; TEE, transesophageal echocardiography; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient; TTE, transthoracic echocardiography.
Discussion
The main findings of this study are as follows: (1) the size of the ASD significantly decreased after CA, and (2) younger age and lower TRPG and PASP before CA were associated with a reduction in the ASD size. To the best of our knowledge, this is the first study that evaluated the size of the ASD before and after CA.
A detailed evaluation of ASD morphology and size before percutaneous closure is crucial to avoid serious complications such as cardiac erosion. The size of ASD occlusion devices (eg, Amplatzer septal occluder) increases in 1 mm increments, and the ASD size before device closure should be measured in millimetres. Therefore, a 1 mm change of the ASD size is important, and reassessment of the ASD size by TEE after CA is essential for successful and safe transcatheter closure. Patients with large ASDs (>38 mm) are usually referred for surgical closure because the largest ASD occlusion device currently available in Japan is 38 mm. However, if a patient with ATA has a planned CA, the ASD size is expected to decrease and become suitable for percutaneous closure after the CA, especially in young patients.
The precise mechanism of the ASD size reduction remains uncertain in the present study. It has been reported that LA volume decreases, and atrial systolic function is reduced after CA for AF, because of reverse remodelling and shrinkage from ablation-induced fibrosis.4, 5, 6, 7 The decrease in the LA size and the reduction of a left-to-right shunt because of decreased LA systolic function may partly explain this mechanism. Furthermore, a decrease in the right atrial (RA) size may affect the results. Patients with moderate or severe TR were not included in the ASD size-reduction group in our study. It has been reported that longstanding AF leads to RA enlargement, tricuspid valve annular dilatation, and subsequent TR.8,9 In AF patients with less than mild TR, successful CA led to right heart reverse remodelling, but the effect of CA on AF in patients with moderate or severe TR has remained unknown.10 It was suggested that in patients with ASD, AF, and moderate or severe TR, right heart remodelling became irreversible, which otherwise resulted in the ASD size reduction after CA.
This study has several limitations. First, right heart catheterization was not routinely performed before the CA, and pulmonary hypertension was not evaluated precisely. TRPG and PASP evaluated by TTE were associated with the ASD size reduction, but the influence of TR on the measurement could be ignored. Second, the number of patients was small, which might have reduced the study’s statistical power. Moreover, other factors related to the reduction of the ASD size after CA might exist. Third, we estimated the ASD area assuming that the defect was elliptical, and the precise area was not measured. However, we believe that the estimation was reasonable because the occluder size was selected based on the maximum and minimum diameters in clinical practice. Fourth, TTE was not evaluated routinely after CA before ASD closure. The change in RA and LA size or cardiac function, which might have been impaired due to tachycardia-induced cardiomyopathy before CA, could be helpful to speculate the mechanism of the ASD size reduction. Fifth, although the size of the ASDs significantly decreased after CA, the average reduction of the maximum diameter was only 1.3 mm, and it is possible that this reduction was due to a measurement error. Further structured prospective studies, which should include haemodynamic data, cardiac magnetic resonance imaging, and other parameters, are required to validate our findings and elucidate the underlying mechanisms. In addition, we believe that an important part of these future studies would be LA and RA volume assessment.
Conclusion
ASD size decreased after CA, and the prognostic factors may be crucial for developing strategies for percutaneous ASD closure.
Acknowledgments
Ethics Statement
This research adhered to the relevant ethical guidelines.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at 10.1016/j.cjcpc.2022.08.001.
Supplementary Material
References
- 1.Villablanca P.A., Briston D.A., Rodés-Cabau J., et al. Treatment options for the closure of secundum atrial septal defects: a systematic review and meta-analysis. Int J Cardiol. 2017;241:149–155. doi: 10.1016/j.ijcard.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 2.Kitakata H., Itabashi Y., Kanazawa H., et al. Appropriate device selection for transcatheter atrial septal defect closure using three-dimensional transesophageal echocardiography. Int J Cardiovasc Imaging. 2021;37:1159–1168. doi: 10.1007/s10554-020-02095-x. [DOI] [PubMed] [Google Scholar]
- 3.Murphy J.G., Gersh B.J., McGoon M.D., et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med. 1990;323:1645–1650. doi: 10.1056/NEJM199012133232401. [DOI] [PubMed] [Google Scholar]
- 4.Thomas L., Boyd A., Thomas S.P., Schiller N.B., Ross D.L. Atrial structural remodelling and restoration of atrial contraction after linear ablation for atrial fibrillation. Eur Heart J. 2003;24:1942–1951. doi: 10.1016/j.ehj.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Beukema W.P., Elvan A., Sie H.T., Misier A.R., Wellens H.J. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005;112:2089–2095. doi: 10.1161/CIRCULATIONAHA.104.484766. [DOI] [PubMed] [Google Scholar]
- 6.Wylie J.V., Jr., Peters D.C., Essebag V., Manning W.J., Josephson M.E., Hauser T.H. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:656–662. doi: 10.1016/j.hrthm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Hof I.E., Velthuis B.K., Chaldoupi S.M., et al. Pulmonary vein antrum isolation leads to a significant decrease of left atrial size. Europace. 2011;13:371–375. doi: 10.1093/europace/euq464. [DOI] [PubMed] [Google Scholar]
- 8.Sanfilippo A.J., Abascal V.M., Sheehan M., et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990;82:792–797. doi: 10.1161/01.cir.82.3.792. [DOI] [PubMed] [Google Scholar]
- 9.Utsunomiya H., Itabashi Y., Mihara H., et al. Functional tricuspid regurgitation caused by chronic atrial fibrillation: a real-time 3-dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.004897. [DOI] [PubMed] [Google Scholar]
- 10.Itakura K., Hidaka T., Nakano Y., et al. Successful catheter ablation of persistent atrial fibrillation is associated with improvement in functional tricuspid regurgitation and right heart reverse remodeling. Heart Vessels. 2020;35:842–851. doi: 10.1007/s00380-019-01546-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.