Abstract
Background
Several medication choices are available for acute and prophylactic treatment of refractory supraventricular tachycardia (SVT) in infants. There are almost no controlled trials, and medication choices are not necessarily evidence based. Our objective was to report the effectiveness of management strategies for infant SVT.
Methods
A registry of infants admitted to hospital with re-entrant SVT and no haemodynamically significant heart disease were prospectively followed at 11 international tertiary care centres. In addition, a systematic review of studies on infant re-entrant SVT in MEDLINE and EMBASE was conducted. Data on demographics, symptoms, acute and maintenance treatments, and outcomes were collected.
Results
A total of 2534 infants were included: n = 108 from the registry (median age, 9 days [0-324 days], 70.8% male) and n = 2426 from the literature review (median age, 14 days; 62.3% male). Propranolol was the most prevalent acute (61.4%) and maintenance treatment (53.8%) in the Registry, whereas digoxin was used sparingly (4.0% and 3.8%, respectively). Propranolol and digoxin were used frequently in the literature acutely (31% and 33.2%) and for maintenance (17.8% and 10.1%) (P < 0.001). No differences in acute or prophylactic effectiveness between medications were observed. Recurrence was higher in the Registry (25.0%) vs literature (13.4%) (P < 0.001), and 22 (0.9%) deaths were reported in the literature vs none in the Registry.
Conclusion
This was the largest cohort of infants with SVT analysed to date. Digoxin monotherapy use was rare amongst contemporary paediatric cardiologists. There was limited evidence to support one medication over another. Overall, recurrence and mortality rates on antiarrhythmic treatment were low.
Résumé
Contexte
De nombreux choix de médicaments existent pour le traitement aigu et prophylactique de la tachycardie supraventriculaire (TSV) réfractaire chez les nourrissons. Or, il n’y a presque pas d’essais contrôlés à ce sujet, et les choix de médicaments ne sont pas nécessairement fondés sur des données probantes. Notre objectif était de faire état de l’efficacité des stratégies de prise en charge de la TSV chez les nourrissons.
Méthodologie
Un registre des nourrissons admis à l’hôpital pour une TSV par réentrée, sans cardiopathie d’importance hémodynamique, a été tenu de façon prospective dans 11 centres de soins tertiaires à l’échelle mondiale. De plus, une revue systématique des études sur la TSV par réentrée chez le nourrisson a été effectuée dans MEDLINE et EMBASE. Des données sur les caractéristiques démographiques, les symptômes, les traitements aigus et d’entretien, et les résultats ont été recueillis.
Résultats
Un total de 2 534 nourrissons ont été inclus : n = 108 du registre (âge médian de 9 jours [0-324 jours], 70,8 % de sexe masculin) et n = 2 426 de la revue de la littérature (âge médian de 14 jours; 62,3 % de sexe masculin). Le propranolol était le traitement de soins aigus (61,4 %) et d’entretien (53,8 %) le plus fréquent dans le registre, alors que la digoxine a été utilisée occasionnellement (respectivement dans 4,0 % et 3,8 % des cas). Dans la littérature, le propranolol et la digoxine étaient fréquemment utilisés en soins aigus (31 % et 33,2 %) et en traitement d’entretien (17,8 % et 10,1 %) (p < 0,001). Aucune différence n’a été observée entre les médicaments au chapitre de l’efficacité du traitement de soins aigus ou du traitement prophylactique. Le taux de récurrence était plus élevé dans le registre (25,0 %) que dans la littérature (13,4 %) (p < 0,001), et 22 (0,9 %) décès ont été signalés dans la littérature, mais aucun dans le registre.
Conclusion
Il s’agit de la plus grande cohorte de nourrissons atteints de TSV analysée à ce jour. De nos jours, les cardiologues pédiatriques prescrivent rarement la digoxine en monothérapie. Peu de données probantes favorisent l’utilisation d’un médicament par rapport à l’autre. Dans l’ensemble, les taux de récurrence et de mortalité sous traitement antiarythmique étaient faibles.
Supraventricular tachycardia (SVT) is the most common chronic paediatric arrhythmia, estimated to occur in 1 in 250 to 1 in 1000 children.1, 2, 3 The most common mechanisms of SVT in paediatric patients are atrioventricular re-entrant tachycardia (AVRT) and atrioventricular nodal re-entrant tachycardia (AVNRT), with AVRT being the most prevalent in infants under 1 year of age.4 Children most commonly present with SVT in infancy, and the majority present in the first 4 months of life.5, 6, 7, 8 Within 48 hours of presentation, over 50% of infants develop heart failure, which may lead to cardiovascular collapse or mortality, and although in some cases the acute SVT episode may terminate spontaneously, pharmacological intervention is often required.5,6,9, 10, 11, 12, 13
Current treatments to abort an SVT episode acutely include vagal manoeuvres and adenosine, whereas antiarrhythmic medications are used acutely when those measures fail as well as for preventative or maintenance therapy.14 Although vagal manoeuvres and adenosine are standard first-line treatments, the choice of which medication to add acutely or as chronic maintenance varies widely.15, 16, 17, 18 Management decisions are currently based on experiential factors such as physician preference and institutional practice as opposed to controlled trials. There have been few prospective studies examining the utility of antiarrhythmic therapies for acute SVT termination and prevention of refractory SVT in infants.19, 20, 21, 22
As SVT continues to be a common problem globally, for which management options are numerous, with no clear “best option,” we sought to gather new data and collate the evidence available. The objectives of this multicentre prospective registry and systematic literature review were to determine the most effective antiarrhythmic treatments for acute management of SVT in infants, as well as which antiarrhythmic agents were most effective in prevention of later recurrences.
Methods
From October 2015 to December 2020, infants <1 year of age admitted to hospital for SVT were enrolled at 11 international tertiary care centres into a prospective observational registry (“Registry”). Consent was obtained from the patient’s parent or legal guardian. Patients were included if they had a diagnosis of a short RP interval (RP << PR; RP interval measured from onset of the R wave to the onset of next P wave, PR interval measured from onset of the P wave to onset of subsequent R wave) tachycardia consistent with AVRT or AVNRT and who subsequently received pharmacological treatment for SVT. Infants with junctional ectopic tachycardia, ectopic atrial tachycardia, or permanent junctional reciprocating tachycardia were excluded, as these arrhythmias require more complex treatment approaches.23 Infants with atrial flutter were also excluded; this does not usually require maintenance therapy.15 The presumptive diagnosis of re-entrant arrhythmias (AVRT and AVNRT) was based on published criteria.4,24 Patients were also excluded if they had haemodynamically significant structural heart disease. Participants were followed for 1 year, and information regarding patient demographics, medical history, acute and chronic SVT management, and outcomes during the first year after initial presentation were collected and managed using the REDCap electronic data capture tool.25,26 Outcomes of interest included initial adequate SVT control (defined as no further sustained SVT after intervention) and continued SVT control (no further episodes requiring medication change, readmission to hospital, or presentation to emergency department), time to medication withdrawal during follow-up, rate of SVT recurrence, and adverse events, defined as death, cardiac arrest, proarrhythmia, bradycardia, hypotension, medication intolerance, or need for mechanical support (ventilator or extracorporeal membrane oxygenation). The protocol received ethical approval at each participating site. The diagnosis and treatment decisions were all made at the participating sites based on their clinical decision making. There were no management strategies dictated by participation in the Registry.
Because of our familiarity with diverse treatment protocols and low event rates, a systematic review of the literature on infant SVT was also conducted. A comprehensive search strategy (Supplemental Methods) was developed to capture all relevant studies from Ovid MEDLINE and EMBASE databases from 1964 until January 2021. A paediatric search filter developed at the University of Alberta was adapted and used to narrow results to the paediatric population.27 Backward and forward citation chaining was conducted using ISI Web of Science and Google Scholar. Search results were deduplicated and independently screened by 2 investigators (NW and AL) to identify relevant studies for inclusion in the review. Studies reporting patients <1 year of age with AVRT or AVNRT and no haemodynamically significant structural heart disease were included. Similar to the registry, infants with junctional ectopic tachycardia, ectopic atrial tachycardia, or permanent junctional reciprocating tachycardia were excluded. In cases of multiple studies reporting on the same cohort, the most recent study was included. The corresponding authors of studies from the same institution were contacted to ensure that no duplication of patients occurred. Reviews, editorials, case reports, conference proceedings, and non–English language papers were excluded. Data on patient demographics, SVT diagnosis, acute and prophylactic management, postdischarge recurrences, and outcomes at follow-up were extracted from included studies.
Statistical analysis
Data were summarized using counts and percentages for categorical variables, whereas continuous variables were summarized using means and standard deviations. A χ2 test was used to compare the effectiveness of monotherapy with combination therapy in controlling the rate of SVT. The effectiveness of different monotherapies in controlling SVT was also compared using a χ2 test. To examine time to consistent sinus rhythm and time to breakthrough events at first follow-up, Kaplan-Meier survival curves were estimated with 95% confidence intervals (CI), stratified by monotherapy and combination therapy. Possible differences in curves were assessed using a log-rank test. χ2 testing was used to compare medication usage rates in the Registry with the literature. Comparison of recurrence rates in the Registry with the literature was conducted by computing risk ratios. For pooling drug use proportions and recurrence rates across studies, we used a random-effects meta-analysis model based on generalized linear mixed-effects models with logit link functions. Results are displayed with forest plots, and analyses were performed using the meta package in R. All analysis was performed using R statistical software.28
Results
Prospective registry
A total of 108 infants were enrolled into the Registry throughout the study period. Patient characteristics are shown in Table 1. The mean age at presentation was 20.1 days (range, 0-324 days, standard deviation [SD] = 44.21 days), and 70.8% of infants were male. Fifty-four (50.5%) infants had AVRT, 3 (2.8%) had AVNRT, and 51 (46.7%) had undifferentiated re-entrant SVT (either AVNRT or AVRT). Pre-excitation on baseline electrocardiogram was observed in 35 (32.4%) infants, of whom 27 (77.1%) were diagnosed with AVRT and 8 (22.9%) had undifferentiated re-entrant SVT. Most patients presented because of an incidental finding of elevated heart rate (46.2%) or the presence of symptoms (30.2%), and 14.8% had evidence of cardiovascular compromise during the initial episode with other reasons for cardiovascular compromise not listed being poor feeding, decreased heart function, and features of hydropic fetalis.
Table 1.
Registry patient characteristics
| Demographics | n = 108 |
|---|---|
| Age at presentation (d), mean (SD) | 20.12 (44.21) |
| Male (%) | 70.8 |
| Weight (kg), mean (SD) | 3.54 (1.34) |
| Height (cm), mean (SD) | 49.86 (6.86) |
| Ethnicity, n (%) | |
| Asian | 16 (15.7) |
| Black | 1 (1.0) |
| Caucasian | 73 (71.6) |
| Hispanic | 3 (2.9) |
| South Asian | 2 (2.0) |
| Southeast Asian | 4 (3.9) |
| Other | 3 (2.9) |
| Diagnosis, n (%) | |
| AVRT | 54 (50.0) |
| AVNRT | 3 (2.8) |
| Undifferentiated | 51 (47.2) |
| Pre-excitation | 35 (32.4) |
| AVRT | 27 (77.1) |
| Undifferentiated | 8 (22.9) |
| Primary reason for presentation, n (%) | |
| Incidental finding of elevated heart rate | 54 (50.0) |
| Known in utero SVT | 18 (16.7) |
| Symptomatic | 36 (33.3) |
| Symptoms at presentation (n = 36), n (%) | |
| Decreased urine output | 4 (11.1) |
| Decreased peripheral perfusion | 9 (25.0) |
| Lethargy, irritability | 18 (50.0) |
| Respiratory symptoms (tachypnea, increased work of breathing) | 18 (50.0) |
| Poor feeding | 19 (52.8) |
| Emesis | 2 (5.6) |
| Abnormal heart rate | 3 (8.3) |
| Evidence of cardiovascular compromise during initial episode | 16 (14.8) |
| Decreased urine output | 2 (12.5) |
| Decreased peripheral perfusion | 8 (50.0 |
| Decreased neurological perfusion (lethargy, inappropriate responses to stimulation) | 5 (31.3) |
| Hypotension | 5 (31.3) |
| Increased work of breathing | 6 (37.5) |
| Tachypnea | 6 (37.5) |
| Other | 4 (25.0) |
AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; SD, standard deviation; SVT, supraventricular tachycardia.
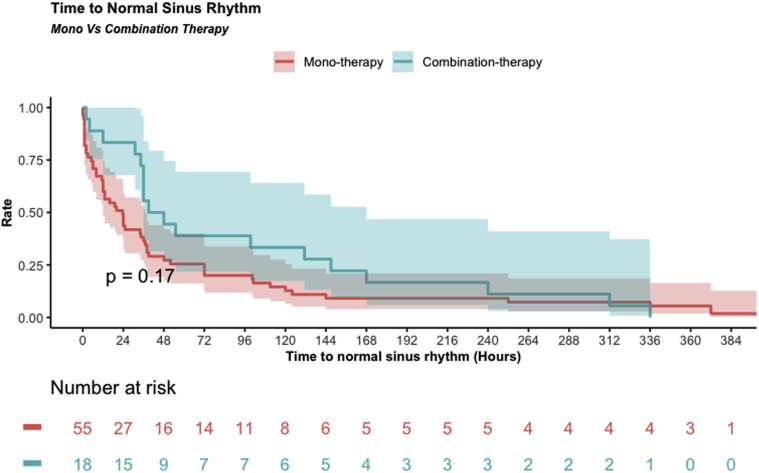
A total of 101 patients (93.5%) received antiarrhythmic therapy in addition to adenosine or vagal manoeuvres to treat the acute SVT episode or prevent recurrences after termination (Table 2). The most common first-choice acute antiarrhythmic was propranolol (62 of 101, 61.4%). Adequate SVT control was achieved in 56 of 80 (70%) patients receiving monotherapy, including 45 of 62 (72.6%) patients on propranolol. Of those who received combination therapy (n = 21), 12 (57.1%) achieved adequate control. χ2 testing found no significant difference in the rate of SVT control between all treatments (P = 0.07), nor when comparing monotherapy with combination therapy (P = 0.39). Of the 33 patients in whom SVT control was not achieved on the first medication, 32 (97%) underwent a medication change and 1 (3%) did not have a change prescribed. SVT control was achieved after 1 change in treatment regimen in 10 of 16 (62.5%) patients and 9 of 16 (56.3%) patients on combination therapy (Table 3). Nine patients (69.2%) were subsequently controlled after 2 changes in treatment, and 4 patients (30.8%) required 3 changes. Figure 1 shows a Kaplan-Meier curve of time from initiation of treatment to consistent normal sinus rhythm. The median time to normal sinus rhythm was 24 hours for patients on acute monotherapy and 48 hours for patients on combination therapy, but the log-rank test found no significant difference in time to adequate SVT control (P = 0.17).
Table 2.
Acute antiarrhythmic therapy—first regimen
| Monotherapy (n = 80) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication used | Route (IV) | IV starting dosage (mg/d) | IV maximum dosage (mg/d) | Route (PO) | PO starting dosage (mg/d) | PO maximum dosage (mg/d) | Other | Other starting dosage (mg/d) | Other starting dosage (mg/d) | Adequate SVT control | |
| Digoxin | 5 (6.3%) | 2 (40.0%) | 0.025 (0.02-0.03) | 0.025 (0.02-0.03 | 3 (75.0%) | 0.019 (0.012-0.024 | 0.023 (0.012-0.024) | n/a | n/a | n/a | 3/5 (60.0%) |
| Esmolol | 7 (8.8%) | 7 (100%) | 144 (72-627.84) | 216 (72-627.84) | n/a | n/a | n/a | n/a | n/a | n/a | 2/7 (28.6%) |
| Flecainide | 3 (3.8%) | n/a | n/a | n/a | 3 (100%) | Not reported | Not reported | n/a | n/a | n/a | 3/3 (100%) |
| Propranolol | 62 (77.5%) | 3 (4.8%) | 1.0 (0.5-1.5) | 1.5 (1.0-2.0) | 58 (93.5%) | 3.96 (0.5-20) | 8 (0.9-24.96) | 1 (1.6%) | 0.96 | 0.96 | 45/62 (72.6%) |
| Sotalol | 3 (3.8%) | n/a | n/a | n/a | 3 (100%) | 21 (5-27.75) | 21 (9-27.75) | n/a | n/a | n/a | 3/3 (100%) |
| Adequate SVT control | 56 (70.0%) | ||||||||||
| Adverse events | 9 (11.3%) | ||||||||||
| Combination therapy (n = 21) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First medication | Medication route | Starting dosage (mg/d) | Maximum dosage (mg/d) | Second medication | Medication route | Starting dosage (mg/d) | Maximum dosage (mg/d) | Third medication | Medication route | Starting dosage (mg/d) | Maximum dosage (mg/d) |
| Digoxin | IV | 0.015 | 0.015 | Propranolol | PO | 2.2 | 2.2 | n/a | n/a | n/a | n/a |
| Propranolol | IV | 0.9 | 2.1 | Digoxin | IV | 0.03 | 0.03 | n/a | n/a | n/a | n/a |
| Amiodarone | IV | 10.08 | 14.4 | Esmolol | IV | 648 | 1944 | Flecainide | PO | 10 | 15 |
| Propranolol | IV | 0.9 | 1.5 | Flecainide | PO | 16 | 16 | n/a | n/a | n/a | n/a |
| Digoxin | IV | Not reported | Not reported | Propranolol | PO | 9 | 9 | n/a | n/a | n/a | n/a |
| Propranolol | PO | 16 | 24 | Flecainide | PO | Not reported | 20 | n/a | n/a | n/a | n/a |
| Amiodarone | IV | 14.05 | 21.0816 | Propranolol | PO | 10 | 12 | n/a | n/a | n/a | n/a |
| Amiodarone | IV | 7.2 | 21.6 | Esmolol | IV | 72 | 151.2 | Propafenone | PO | 60 | 60 |
| Propafenone | PO | Not reported | Not reported | Amiodarone | IV | 24.048 | 24.048 | n/a | n/a | n/a | n/a |
| Propranolol | PO | 4 | 12 | Sotalol | Unspecified | Not reported | Not reported | n/a | n/a | n/a | n/a |
| Propranolol | PO | 8 | 16 | Propafenone | PO | 45 | 90 | n/a | n/a | n/a | n/a |
| Propranolol | PO | Not reported | Not reported | Sotalol | PO | Not reported | Not reported | n/a | n/a | n/a | n/a |
| Propranolol | PO | 2.4 | 6.4 | Esmolol | IV | 3.8448 | 3.8448 | n/a | n/a | n/a | n/a |
| Esmolol | IV | 72 | 504 | Propranolol | PO | 2.89 | 2.89 | n/a | n/a | n/a | n/a |
| Flecainide | PO | 12 | 12 | Propranolol | PO | 6 | 9.6 | n/a | n/a | n/a | n/a |
| Esmolol | IV | 108 | 144 | Propranolol | IV | Not reported | Not reported | n/a | n/a | n/a | n/a |
| Esmolol | IV | 446.4 | 1116 | Propranolol | IV | 1.55 | 2.3 | n/a | n/a | n/a | n/a |
| Propranolol | PO | 4.5 | 9 | Esmolol | IV | 64.8 | 64.8 | n/a | n/a | n/a | n/a |
| Propranolol | PO | 1.83 | 3.65 | Esmolol | IV | 144 | 648 | n/a | n/a | n/a | n/a |
| Propranolol | PO | 12.8 | 19.2 | Esmolol | IV | 72 | 576 | n/a | n/a | n/a | n/a |
| Procainamide | IV | Not reported | Not reported | Propranolol | PO | 12 | 20 | n/a | n/a | n/a | n/a |
| Adequate SVT control | 12 (57.1%) | ||||||||||
| Adverse events | 0 (0.0%) | ||||||||||
Patients on combination therapy in whom adequate SVT control was not achieved are bolded. Dosages are reported as medians and ranges.
IV, intravenous route; n/a, not available; PO, oral route; SVT, supraventricular tachycardia.
Table 3.
Acute antiarrhythmic therapy—second regimen
| Monotherapy (n = 16) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medication used | Route (IV) | IV starting dosage (mg/d) | IV maximum dosage (mg/d) | Route (PO) | PO starting dosage (mg/d) | PO maximum dosage (mg/d) | Adequate SVT control | |
| Digoxin | 5 (31.2%) | 1 (20.0%) | Not reported | Not reported | 4 (80.0%) | 0.031 (0.014-0.068) | 0.031 (0.014-0.068) | 4 (80.0%) |
| Esmolol | 3 (18.8%) | 3 (100%) | 72 (72-446.4) | 360 (108-669.6) | n/a | n/a | n/a | 1 (33.3%) |
| Flecainide | 2 (12.5%) | 1 (50.0%) | Not reported | Not reported | 1 (50.0%) | 24 | 24 | 1 (50.0%) |
| Propranolol | 4 (25.0%) | 1 (25.0%) | 1.14 | 1.5 | 3 (75.0%) | 2.628 (1.14-9) | 6.9 (1.5-14.68) | 2 (50.0%) |
| Sotalol | 1 (6.3%) | n/a | n/a | n/a | 1 (100%) | 15 | 15 | 1 (100%) |
| Procainamide | 1 (6.3%) | 1 (100%) | 28.8 | 28.8 | n/a | n/a | n/a | 1 (100%) |
| Adequate SVT control | 10 (62.5%) | |||||||
| Adverse events | 0 (0.0%) | |||||||
| Combination therapy (n = 16) | |||||||
|---|---|---|---|---|---|---|---|
| First medication | Medication route | starting dosage (mg/d) | Maximum dosage (mg/d) | Second medication | Medication route | starting dosage (mg/d) | Maximum dosage (mg/d) |
| Esmolol | IV | 144 | 504 | Amiodarone | IV | 7.2 | 21.6 |
| Propranolol | PO | Not reported | Not reported | Digoxin | Unspecified | Not reported | Not reported |
| Propranolol | PO | 12 | 12 | Digoxin | PO | 0.03 | 0.03 |
| Flecainide | PO | 10 | 10 | Propranolol | PO | 6.08 | 6.08 |
| Sotalol | PO | Not reported | Not reported | Flecainide | PO | Not reported | Not reported |
| Sotalol | PO | 27 | 27 | Digoxin | PO | 0.03 | 0.03 |
| Esmolol | IV | 72 | 504 | Sotalol | PO | 24 | 30 |
| Propranolol | PO | 7.5 | 11.28 | Flecainide | PO | 18 | 18 |
| Propranolol | PO | 8.31 | 8.31 | Flecainide | PO | 11.4 | 11.4 |
| Propranolol | PO | 1.5 | 1.5 | Digoxin | PO | 0.012 | 0.012 |
| Esmolol | IV | 144 | 144 | Sotalol | PO | 3 | 3 |
| Esmolol | IV | 72 | 72 | Propranolol | PO | 4.3 | 8.6 |
| Esmolol | IV | 446.4 | 446.4 | Flecainide | PO | 9.6 | 9.6 |
| Sotalol | PO | 2.85 | 3.8 | Sotalol | PO | Not reported | Not reported |
| Esmolol | IV | 648 | 648 | Sotalol | PO | 7.3 | 30 |
| Propranolol | PO | 12.8 | 19.2 | Procainamide | IV | 28.8 | 43.2 |
| Adequate SVT control | 9 (56.3%) | ||||||
| Adverse events | 3 (18.8%) | ||||||
Second treatment regimen attempted for patients in whom adequate SVT control was not achieved with the first regimen. Patients on combination therapy in whom adequate SVT control was not achieved are bolded. Dosages are reported as medians and ranges.
IV, intravenous route; n/a, not available; PO, oral route; SVT, supraventricular tachycardia.
Figure 1.
Kaplan-Meier curve of time to normal sinus rhythm for Registry patients on acute monotherapy and combination therapy.
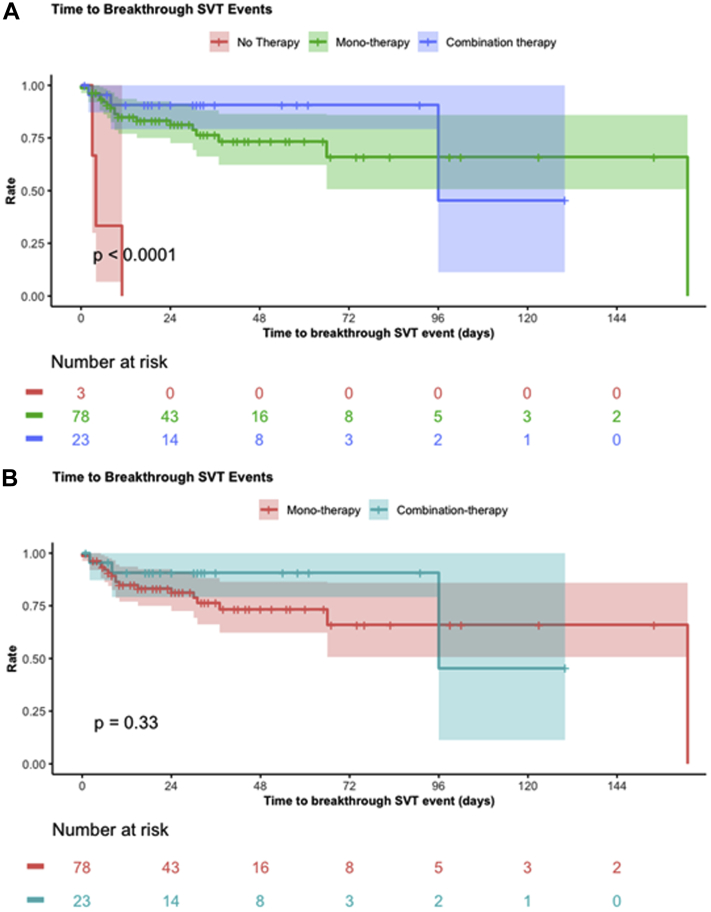
Follow-up information was available for 104 patients with a median follow-up length of 321 days (range, 24-960 days). Propranolol was the most prescribed medication for maintenance after discharge, used in 56 (53.8%) patients (Table 4). Combination therapy was used in 23 (22.1%) patients. Three (2.9%) patients did not receive prophylactic therapy at discharge. SVT recurrence requiring presentation to the emergency department, readmission to hospital, or medication change during follow-up occurred in 26 of 104 (25.0%) patients, with no significant difference in SVT recurrence between different chronic antiarrhythmics (P = 0.07) or between monotherapy and combination therapy (P = 0.12). All 3 (100%) patients who did not receive chronic prophylactic therapy at discharge experienced breakthrough SVT by the time of first follow-up. No significant difference in time to breakthrough events after discharge was observed between patients treated with monotherapy and combination therapy (P = 0.33) (Fig. 2A); however, patients who were not treated after discharge had earlier recurrences (P < 0.0001) (Fig. 2B). All 3 patients on no treatment had a recurrent episode before their first follow-up visit and were readmitted to hospital after presenting to emergency department. Two patients were started on propranolol and 1 was started on flecainide. Digoxin was used during acute or chronic therapy in 5 of 35 (14.3%) patients with pre-excitation. No adverse events were observed in any of these patients, and the 2 patients who received chronic digoxin did not experience SVT recurrence while on medication.
Table 4.
Discharge medications (n = 104)
| Medication | Count | Discharge dosage (mg/d) | Count (combinations categorized) | Breakthrough SVT |
|---|---|---|---|---|
| Amiodarone | 5 (3.8%) | 17 (10-22) | 2 (1.9%) | 2/2 (100%) |
| Digoxin | 14 (10.5%) | 0.03 (0.015-0.07) | 4 (3.8%) | 1/4 (25.0%) |
| Flecainide | 24 (18.0%) | 18 (9-40) | 11 (10.6%) | 3/11 (27.3%) |
| Propranolol | 74 (55.6%) | 9 (0.9-24.96) | 56 (53.8%) | 13/56 (23.2%) |
| Sotalol | 11 (8.3%) | 22.5 (3.6-45) | 5 (4.8%) | 2/5 (40.0%) |
| Propafenone | 2 (1.5%) | 75 (60-90) | n/a | n/a |
| None | 3 (2.3%) | n/a | 3 (2.9%) | 3/3 (100%) |
| Combination | n/a | n/a | 23 (22.1%) | 2/23 (8.7%) |
Medications prescribed at discharge and the rate of breakthrough SVT events throughout follow-up for each discharge medication.
n/a, not available; SVT, supraventricular tachycardia.
Figure 2.
Kaplan-Meier curves of time to breakthrough SVT events assessed at the first follow-up visit. (A) Comparison of monotherapy, combination therapy, and no therapy. (B) Comparison of maintenance monotherapy and combination therapy. SVT, supraventricular tachycardia.
Two (1.9%) infants at the same institution underwent ablation within the first year after initial presentation. The first patient was 81 days and weighed 5.1 kg at ablation and had continued recurrent SVT before the ablation despite 3 different combination therapies. The second patient was 512 days and weighed 10.5 kg at ablation and had suspected allergy to digoxin and flecainide. Both patients had successful ablations without any complications, and antiarrhythmic medications were discontinued during follow-up. There were no deaths in this cohort.
Systematic review
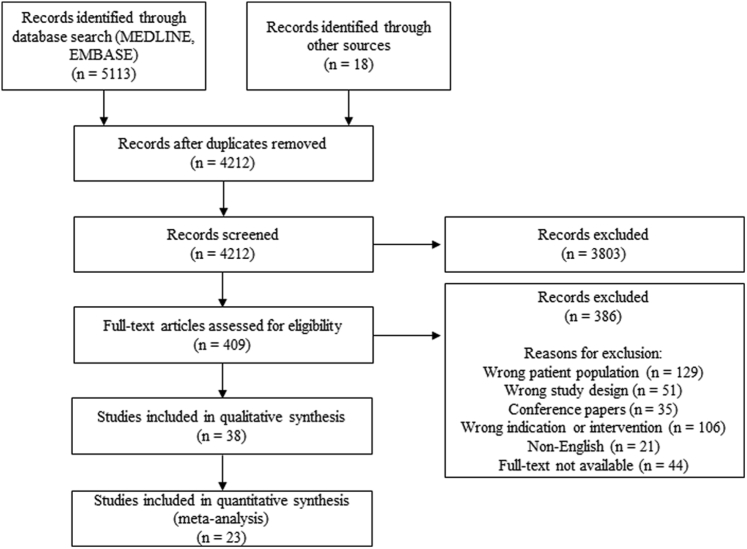
The literature search returned 5113 results from Ovid MEDLINE and EMBASE, with an additional 18 studies identified through citation chaining and other sources (Fig. 3). A total of 4212 studies were screened for eligibility, and full-text assessment was completed for 409 articles, resulting in 23 studies published between 1983 and 2021 included in analysis. Retrospective studies were most common amongst included studies (n = 16), with 6 prospective studies and 1 randomized trial. A summary of the included studies and findings is provided in Supplemental Table S1.
Figure 3.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
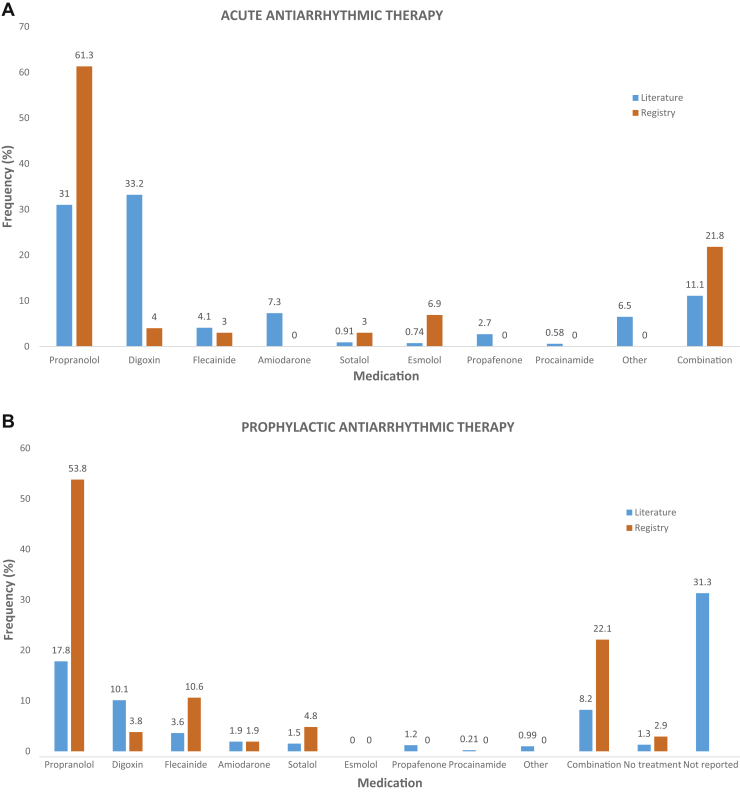
The 23 studies provided information on 2426 patients. The patients were predominantly male (mean, 63.14%, SD = 10.07), and the mean age at admission was 29.33 days (SD = 38.46). SVT diagnosis was unspecified re-entrant SVT in 84.3% of patients and AVRT in 15.7%. Pre-excitation was present in 8.4%, which was significantly lower than in the Registry (32.4%, P < 0.001), suggesting that previous studies may not have recognized or reported pre-excitation. Digoxin (31.0%) and propranolol (33.2%) were the most commonly used acute antiarrhythmic medications, whereas combination therapy was used in 11.1% (Fig. 4A), representing a significant difference in acute antiarrhythmic usage rates when compared with the Registry (P < 0.001). For prophylactic maintenance therapy, propranolol (17.8%) and digoxin (10.1%) usage were most commonly reported in the literature (Fig. 4B), whereas propranolol was the most common in the Registry (54.8%) (P < 0.001). Chronic combination therapy was used more frequently in the Registry than in the literature (22.1% vs 8.2%, P < 0.001).
Figure 4.
Comparison of (A) acute and (B) prophylactic antiarrhythmic usage rates in the Registry and literature.
There was an overall recurrence rate of 13.4% (n = 255) in the literature population, representing a risk ratio of 2.38 (95% CI: 1.67-3.38, P < 0.001) when compared with the 25.0% recurrence rate found in the Registry. Ablation was required in 20 (0.8%) patients in the included studies, and there were 22 (0.9%) deaths.
Meta-analysis
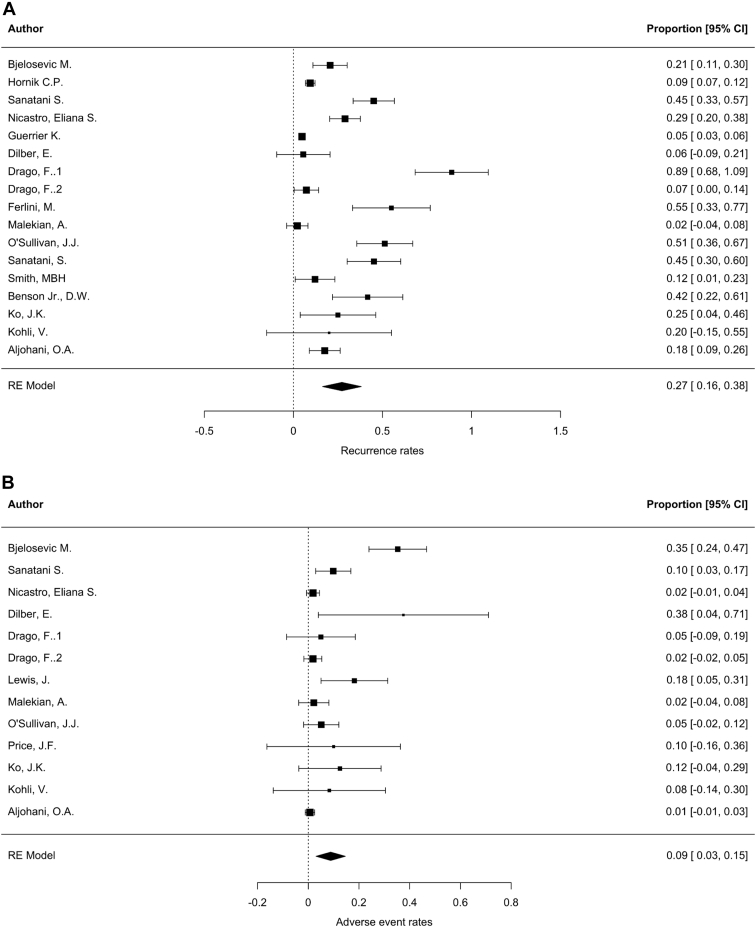
Random-effects meta-analysis of studies that reported data on SVT recurrence or adverse events resulted in an estimate of 27% recurrence rate (95% CI: 16%-38%) and 9% adverse event rate (95% CI: 3%-15%) (Fig. 5).
Figure 5.
Forest plots representing the results of random-effects (RE) meta-analysis of relevant studies identified through the systematic review. (A) Estimation of recurrence rate; (B) estimation of adverse event rate. CI, confidence interval.
Discussion
Pharmacological management of SVT in infants is based primarily on physician experience and retrospective studies. Because the typically small number of cases of infant SVT evaluated at any given institution and the range of antiarrhythmic therapies available, randomized controlled trials with sufficient power to detect statistically significant differences in treatment efficacy are difficult in this patient population. To address this limitation, a systematic review was used to collect all the published data on infant SVT and generate a large study population for analysis in conjunction with our multicentre prospective registry. This contributed to the largest cohort of infants with SVT and structurally normal hearts analysed to date.
Because of the retrospective nature and differences in study methodologies used, we had limited evidence to support one medication over another acutely or as maintenance therapy. No differences were seen in terms of successful acute termination, time to adequate control, recurrence rate, or time to breakthrough events. Antiarrhythmic therapy was highly effective overall, as the initial maintenance regimen in the Registry cohort was successful in achieving adequate SVT control in most patients, and the majority of regimens attempted had at least 50% effectiveness. Statistically comparing the efficacy of specific medications with high confidence was challenging in this cohort due to the high proportion of patients who received propranolol and low numbers of patients receiving other monotherapies.
Incorporating the results of the review, we found a difference in antiarrhythmic usage rates, particularly digoxin, between the literature and Registry. Digoxin use in the literature was reported more often than propranolol before the year 2000 (Supplemental Fig. S1) and was found to be the most popular antiarrhythmic for infants without pre-excitation in a 2006 North American survey.17 The limited use of digoxin by contemporary paediatric cardiologists in our Registry may suggest a trend away from digoxin with increasing propranolol use, even in patients without pre-excitation, which is consistent with recent database studies that demonstrate similar trends over time.18,29 The 23.2% recurrence rate for Registry patients discharged on propranolol is consistent with studies identified in the review.20,29,30 Previous reports on digoxin, however, were conflicting. Benson et al., O’Sullivan et al., and Sanatani et al. found limited prophylactic effectiveness, with at least 55% recurrence rate on digoxin, whereas recent studies comparing digoxin and propranolol have found either no difference in recurrence or a higher rate of recurrence when receiving propranolol.20,29,31, 32, 33 Thus, it appears that the trend towards propranolol use over digoxin is not based on literature-presented evidence. Despite the difference in usage rates, combining the Registry data into the literature would not significantly alter the data obtained from the review.
Medications other than propranolol or digoxin as initial second-line agents were used less commonly in both the Registry and literature. Bjeloševič et al.34 demonstrate an overall low recurrence risk among infants receiving flecainide, propafenone, sotalol, or combination therapy for prophylaxis. Flecainide monotherapy, occasionally with the addition of propranolol in refractory cases, was found to be effective in preventing recurrences in 3 additional studies included in the review.19,32,35
Overall, mortality and recurrence rates were low in the Registry and literature, suggesting that the typical treatment duration of 6-12 months for infants with SVT may be overly conservative. Acceptably low recurrence risk may be achieved with shorter treatment durations. A previous randomized controlled trial found that most patients on propranolol or digoxin were arrhythmia-free at 4 months of age, and Aljohani et al. have shown that discontinuing therapy at 4-6 months of age led to similar recurrence rates as longer treatment durations.20,36 Given our findings, there is no need for further prospective studies and randomized trials to compare treatment efficacy between medications and determine optimal treatment length. In addition, it is unlikely that any group would undertake a randomized controlled trial, as because of the small differences between treatments and low event rate, the numbers needed to demonstrate a significant difference may not be feasible.37
Limitations
As mentioned, there were a high proportion of patients receiving propranolol and small sample sizes of patients receiving other antiarrhythmic medications. This limited the ability to make direct comparisons between different antiarrhythmic agents in terms of effectiveness or survival time. The majority of patients enrolled in the Registry were Caucasian, which is not representative of the general population. Moreover, it is possible that SVT recurrences are underestimated in clinical studies due to under-reporting.38 In addition, antiarrhythmic medication availability is not uniform around the world that influences anti-arrhythmic medication use by institutions. In terms of the systematic review, studies in which a proportion of patients did not meet inclusion criteria (eg, patients with other types of arrhythmia and/or congenital heart disease, or patients older than 1 year of age), and in which data specific to relevant patients could not be isolated, were excluded from statistical analysis. As a result, the review was not able to capture data for all literature-reported patients who meet the inclusion criteria. An additional limitation was the possibility of the same patient being reported in multiple registries.
Conclusion
Overall, recurrence risk in the Registry and literature was low for patients on antiarrhythmic therapy, and mortality was low. Current data suggest that propranolol and digoxin are both effective initial second-line agents for SVT management in the infant population, but do not support any antiarrhythmic medication over another, despite the current move away from digoxin. Recurrences can occur in the first year of life, but ultimately 1 or more antiarrhythmic medications can effectively suppress SVT. The results of the present registry and review highlight the need for more clinical trials and prospective studies to more effectively compare treatment efficacy across the range of antiarrhythmic medications and provide further evidence to guide future management.
Acknowledgements
We would like to thank the participants for their cooperation and contribution to this study.
Ethics Statement
Research was conducted in accordance to the ethical guidelines of the Declaration of Helsinki. Ethical approval for this study was obtained at the coordinating site from the University of British Columbia Children's and Women's Health Centre of British Columbia Research Ethics Board (H14-03380) and at each of the participating sites.
Funding Sources
None.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2021.09.001.
Supplementary Material
References
- 1.Ros S.P., Fisher E.A., Bell T.J. Adenosine in the emergency management of supraventricular tachycardia. Pediatr Emerg Care. 1991;7:222–223. doi: 10.1097/00006565-199108000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Reyes G., Stanton R., Galvis A.G. Adenosine in the treatment of paroxysmal supraventricular tachycardia in children. Ann Emerg Med. 1992;21:1499–1501. doi: 10.1016/s0196-0644(05)80069-1. [DOI] [PubMed] [Google Scholar]
- 3.Anand R.G., Rosenthal G.L., Van Hare G.F., Snyder C.S. Is the mechanism of supraventricular tachycardia in pediatrics influenced by age, gender or ethnicity? Congenit Heart Dis. 2009;4:464–468. doi: 10.1111/j.1747-0803.2009.00336.x. [DOI] [PubMed] [Google Scholar]
- 4.Ko J.K., Deal B.J., Strasburger J.F., Benson D.W., Jr. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 5.Doniger S.J., Sharieff G.Q. Pediatric dysrhythmias. Pediatr Clin North Am. 2006;53:85–105. doi: 10.1016/j.pcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Till J.A., Shinebourne E.A. Supraventricular tachycardia: diagnosis and current acute management. Arch Dis Child. 1991;66:647–652. doi: 10.1136/adc.66.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfammatter J.P., Stocker F.P. Results of a restrictive use of antiarrhythmic drugs in the chronic treatment of atrioventricular reentrant tachycardias in infancy and childhood. Am J Cardiol. 1998;82:72–75. doi: 10.1016/s0002-9149(98)00232-x. [DOI] [PubMed] [Google Scholar]
- 8.Bauersfeld U., Pfammatter J.P., Jaeggi E. Treatment of supraventricular tachycardias in the new millennium—drugs or radiofrequency catheter ablation? Eur J Pediatr. 2001;160:1–9. doi: 10.1007/pl00008409. [DOI] [PubMed] [Google Scholar]
- 9.Perry J.C. In: The Science and Practice of Pediatric Cardiology. Garson A. Jr., Bricher J.T., Fisher D.J., Neish S.N., editors. Lea & Febiger; Philadelphia: 1997. Supraventricular tachycardia; pp. 2059–2101. [Google Scholar]
- 10.Garson A., Jr., Gillette P.C., McNamara D.G. Supraventricular tachycardia in children: clinical features, response to treatment, and long-term follow-up in 217 patients. J Pediatr. 1981;98:875–882. doi: 10.1016/s0022-3476(81)80578-1. [DOI] [PubMed] [Google Scholar]
- 11.Nadas A.S., Daeschner C.W., Roth A., Blumenthal S.L. Paroxysmal tachycardia in infants and children: study of 41 cases. Pediatrics. 1952;9:167–181. [PubMed] [Google Scholar]
- 12.Deal B.J., Keane J.F., Gillette P.C., Garson A., Jr. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol. 1985;5:130–135. doi: 10.1016/s0735-1097(85)80095-4. [DOI] [PubMed] [Google Scholar]
- 13.Benson D.W., Jr., Dunnigan A., Benditt D.G., Pritzker M.R., Thompson T.R. Transesophageal study of infant supraventricular tachycardia: electrophysiologic characteristics. Am J Cardiol. 1983;52:1002–1006. doi: 10.1016/0002-9149(83)90520-9. [DOI] [PubMed] [Google Scholar]
- 14.Kugler J.D., Danford D.A. Management of infants, children, and adolescents with paroxysmal supraventricular tachycardia. J Pediatr. 1996;129:324–338. doi: 10.1016/s0022-3476(96)70063-x. [DOI] [PubMed] [Google Scholar]
- 15.Brugada J., Blom N., Sarquella-Brugada G., et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337–1382. doi: 10.1093/europace/eut082. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J., Arora G., Tudorascu D.L., et al. Acute management of refractory and unstable pediatric supraventricular tachycardia. J Pediatr. 2017;181:177–182. doi: 10.1016/j.jpeds.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 17.Wong K., Potts J., Etheridge S., Sanatani S. Medications used to manage supraventricular tachycardia in the infant a North American survey. Pediatr Cardiol. 2006;27:199–203. doi: 10.1007/s00246-005-1126-x. [DOI] [PubMed] [Google Scholar]
- 18.Chu P.Y., Hill K.D., Clark R.H., Smith P.B., Hornik C.P. Treatment of supraventricular tachycardia in infants: analysis of a large multicenter database. Early Hum Dev. 2015;91:345–350. doi: 10.1016/j.earlhumdev.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlini M., Colli A.M., Bonanomi C., et al. Flecainide as first-line treatment for supraventricular tachycardia in newborns. J Cardiovasc Med. 2009;10:372–375. doi: 10.2459/JCM.0b013e328329154d. [DOI] [PubMed] [Google Scholar]
- 20.Sanatani S., Potts J.E., Reed J.H., et al. The study of antiarrhythmic medications in infancy (SAMIS): a multi-center, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984–991. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 21.Kohli V. Oral flecainide is effective in management of refractory tachycardia in infants. Indian Heart J. 2013;65:168–171. doi: 10.1016/j.ihj.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malekian A., Khalilian M.R., Dehdashtian M., Aramesh M.R., Heydaripoor K. Evaluation and management of neonatal supraventricular tachycardia. J Compr Ped. 2016;7 [Google Scholar]
- 23.Tavera M.C., Bassareo P.P., Neroni P., et al. Supraventricular tachycardia in neonates: antiarrhythmic drug choice dilemma. J Matern Fetal Neonatal Med. 2011;24:541–544. doi: 10.3109/14767058.2010.509915. [DOI] [PubMed] [Google Scholar]
- 24.Colucci R.A., Silver M.J., Shubrook J. Common types of supraventricular tachycardia: diagnosis and management. Am Fam Physician. 2010;82:942–952. [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tjosvold L., Campbell S., Dorgan M. Filter to retrieve pediatric articles in the OVIDMedline database. John W. Scott Health Sciences Library, University of Alberta. https://guides.library.ualberta.ca/c.php?g=342568&p=5096194 2020. Available at: Accessed April 23, 2021.
- 28.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing. [Google Scholar]
- 29.Hornik C.P., Chu P.Y., Li J.S., et al. Comparative effectiveness of digoxin and propranolol for supraventricular tachycardia in infants. Pediatr Crit Care Med. 2014;15:839–845. doi: 10.1097/PCC.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicastro E.S., Majdalani M.G., Abello M.S., et al. Experience using propranolol for the management of supraventricular tachycardia in patients younger than 1 year. Arch Argent Pediatr. 2020;118:273–276. doi: 10.5546/aap.2020.eng.273. [DOI] [PubMed] [Google Scholar]
- 31.Benson D.W., Dunnigan A., Benditt D.G., et al. Prediction of digoxin treatment failure in infants with supraventricular tachycardia: role of transesophageal pacing. Pediatrics. 1985;75:288–293. [PubMed] [Google Scholar]
- 32.O’Sullivan J.J., Gardiner H.M., Wren C. Digoxin or flecainide for prophylaxis of supraventricular tachycardia in infants? J Am Coll Cardiol. 1995;26:991–994. doi: 10.1016/0735-1097(95)00291-9. [DOI] [PubMed] [Google Scholar]
- 33.Sanatani S., Hamilton R.M., Gross G.J. Predictors of refractory tachycardia in infants with supraventricular tachycardia. Pediatr Cardiol. 2002;23:508–512. doi: 10.1007/s00246-002-1514-4. [DOI] [PubMed] [Google Scholar]
- 34.Bjeloševič M., Illíková V., Tomko J., et al. Supraventricular tachyarrhythmias during the intrauterine, neonatal, and infant period: a 10-year population-based study. Pacing Clin Electrophysiol. 2020;43:680–686. doi: 10.1111/pace.13964. [DOI] [PubMed] [Google Scholar]
- 35.Drago F., Silvetti M.S., De Santis A., et al. Paroxysmal reciprocating supraventricular tachycardia in infants: electrophysiologically guided medical treatment and long-term evolution of the re-entry circuit. Europace. 2008;10:629–635. doi: 10.1093/europace/eun069. [DOI] [PubMed] [Google Scholar]
- 36.Aljohani O.A., Herrick N.L., Borquez A.A., et al. Antiarrhythmic treatment duration and tachycardia recurrence in infants with supraventricular tachycardia. Pediatr Cardiol. 2021;42:716–720. doi: 10.1007/s00246-020-02534-5. [DOI] [PubMed] [Google Scholar]
- 37.Perry J.C. Supraventricular tachycardia treatment efficacy in infants: on further review. Circ Arrhythm Electriophysiol. 2012;5:882–883. doi: 10.1161/CIRCEP.112.977454. [DOI] [PubMed] [Google Scholar]
- 38.Anjewierden S., Humpherys J., LaPage M.J., Asaki S.Y., Aziz P.F. Detection of tachyarrhythmias in a large cohort of infants using direct-to-consumer heart rate monitoring. J Pediatr. 2021;232 doi: 10.1016/j.jpeds.2020.12.080. 147-53.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.