Abstract
Background
Dextro-transposition of the great arteries is a congenital heart defect with eventually lethal life-threatening consequences of hypoxic low cardiac output. When a balloon atrial septostomy (BAS) is needed, it is performed shortly after birth to create an interatrial shunt and improve systemic blood oxygenation and haemodynamic conditions. In 2019 and 2020, the withdrawal of some balloon atrioseptostomy catheters from the market led to increased use of catheters with different materials, shapes, and sizes. The main objective of this study was to investigate whether the size of the Miller and Fogarty balloon (Edwards Lifesciences) in its 2 variations, the 4.0 cc and the 1.8 cc, had a different impact on the systemic oxygen saturation, on the atrial septal defect (ASD) size, or on the type and frequency of procedure-related complications.
Methods
We conducted a retrospective study on 134 consecutive patients diagnosed with dextrotransposition of the great arteries between 2002 and 2018 who underwent BAS in a tertiary paediatric hospital in Canada.
Results
BAS resulted in a significant increase in oxygen saturation of 18.91% ± 12.95% points (P < 0.0001) and a significant increase in the resulting ASD by 3.92 ± 1.58 mm (P < 0.0001). There was no significant difference in resulting oxygen saturation (P = 0.8370) or the final ASD size (P = 0.2193) based on the balloon size. Severe or life-threatening complications were rare (1%) with no subsequent patient demise.
Conclusions
This is the first study to show that the small balloon is as efficient as the large balloon catheter including in premature patients. This raises the question whether different balloon sizes are necessary.
Résumé
Contexte
La dextro-transposition des gros vaisseaux (dTGV) est une cardiopathie congénitale dont les conséquences peuvent être mortelles en raison du bas débit cardiaque et de l’état hypoxique. Lorsqu’une septostomie auriculaire par ballonnet est nécessaire, l’intervention est réalisée après la naissance pour créer une communication interauriculaire (CIA); cette ouverture améliore l’oxygénation de la circulation sanguine systémique ainsi que les conditions hémodynamiques. En 2019 et en 2020, le retrait du marché de certains cathéters utilisés lors des septostomies auriculaires par ballonnet a entraîné une hausse de l’usage de nouveaux cathéters offerts en différentes formes et tailles. L’objectif principal de cette étude était de déterminer si la différence de taille des ballonnets de Miller et de Fogarty (Edwards Lifesciences), respectivement de 4,0 cc et de 1,8 cc, a un effet sur la saturation en oxygène de la circulation sanguine systémique, sur la taille de la CIA ou sur le type et la fréquence des complications liées à l’intervention.
Méthodologie
Nous avons mené une étude rétrospective comptant 134 patients consécutifs qui ont présenté une dTGV entre 2002 et 2018 et qui ont subi une septostomie auriculaire par ballonnet dans un hôpital pédiatrique tertiaire canadien.
Résultats
Les septostomies auriculaires par ballonnet ont donné lieu à une hausse significative de 18,91 ± 12,95 points de pourcentage (p < 0,0001) de la saturation en oxygène et à une hausse significative de 3,92 ± 1,58 mm (p < 0,0001) de la CIA qui a résulté de l’intervention. La taille du ballonnet n’a pas entraîné de différence significative en ce qui concerne la saturation en oxygène qui a résulté de l’intervention (p = 0,8370) ou la taille finale de la CIA (p = 0,2193). Les complications graves ou mettant la vie du patient en danger ont été rares (1 %) et aucun patient n’est décédé suite à l'intervention.
Conclusion
Il s’agit de la première étude qui démontre que le petit ballonnet est aussi efficace que le gros ballonnet, y compris chez les enfants prématurés. Cette conclusion soulève la question à savoir si différentes tailles de ballonnets sont nécessaires.
The first identification of dextrotransposition of the great arteries (d-TGA) is credited to Matthew Baillie in 1797, who described the morphology of the disease, “a congenital heart defect in which neonates are born with severe cyanosis secondary to a switching of the main pulmonary artery and the aorta.”1 The incidence of d-TGA in the province of Québec, Canada, is similar to the international incidence (1 per 5062), representing 5%-7% of all congenital heart diseases.2 The main problem in this condition is hypoxemia, which can lead to acidemia and low cardiac output. The urgent need to improve the systemic oxygenation is necessary to allow the lowering of the pulmonary vascular resistance and to increase the mixing of the oxygenated and deoxygenated blood across an atrial septal defect (ASD) shunt. The ductus arteriosus (DA) also plays a major role by acting as a left-to-right shunt and increasing pulmonary reoxygenation and venous return to the left atrium. For this, prostaglandin E1 (PGE1) is used to prevent the DA from closing, and in cases where the ASD is not large enough to improve oxygenation, one can enlarge it percutaneously through a balloon atrial septostomy (BAS).3,4 The BAS is ideally performed within the first 24 hours after birth, in general, but may be urgently required within minutes after birth in critical situations.5
There are complications attributed to BAS such as stroke, arrhythmias (supraventricular tachycardia and atrial fibrillation), valve or vessel damage, cardiac perforation, failure of balloon deflation, and balloon fragment embolization.3,6, 7, 8, 9, 10, 11, 12 As a result, interventionalists attempt to carefully select patients who would benefit most from this procedure by outweighing the risks and the timing of the procedure.
Atrioseptostomy balloons come in different sizes from various manufacturers. The choice of the size of the balloon should theoretically depend on factors related to the thickness of the septum secundum and the baby’s body habitus (weight, height, premature birth, and in utero growth pattern),13 but there is a premise suggesting that an ASD cannot be created with a shunt greater than the dimensions of the fossa ovalis.14 In other words, BAS cannot create a communication larger than the fossa ovalis because it only dislocates the septum primum membrane and does not tear the septum secundum. Even though there were different brands with different sizes of balloons available on the market, Miller and Fogarty 4 cc (19 mm) and 1.8 cc (15 mm) balloons (Edwards Lifesciences, Irvine, CA), Rashkind 2 cc balloon (9.5 mm) (Medtronic, Minneapolis, MN), and Z6 1 cc (9.5 mm) and 2 cc (13.5 mm) balloons (NuMed, Inc, Hopkinton, NY), it seems that the brand of the balloon does not have an impact on the achieved ASD size.10,15,16
More recently, fewer companies have continued to produce the BAS catheters. On April 26, 2019, Edwards Lifesciences recalled the Miller and Fogarty atrioseptostomy dilation catheters (Edwards Lifesciences, Irvine, CA) "due to balloon deflation after deployment, and reports of fragmentation or detachment" issues.12 Medtronic did the same on November 3, 2020.11 NuMed (Cornwall, ON, Canada) redesigned its Z-5 balloons after users’ concerns, with some subtle changes (short distal tip for improved balloon rewrapping, and the catheter body is radiopaque to facilitate reliable positioning of the catheter), without modifying the sizes of the balloon, and continuing manufacturing 2 balloon sizes. Meanwhile, the NuMed Z-6 9.5 mm and 13.5 mm balloons are available, increasing the need to reassess the material manufacturing. Answering the question of whether the size of the balloon matters for the success of the BAS procedures or not becomes a timely matter.
The main objective of this study was to investigate whether the size of the Miller and Fogarty balloon (Edwards Lifesciences) in its 2 variations, the 4.0 cc (inflation diameter: 19 mm) and the 1.8 cc (inflation diameter: 15 mm), had a different impact on the systemic oxygen saturation, on the ASD size, or on the type and frequency of procedure-related complications.
Materials and Methods
Sampling method and criteria
This retrospective cohort study was performed at the tertiary perinatal centre with paediatric cardiology and fetal diagnostic service CHU Sainte-Justine Children’s hospital (Université de Montréal, Montréal, Canada). The retrospective study covers the period between 2002 and 2018 inclusively. The study was approved by the hospital’s ethics board committee. Parental consent was waived according to applicable laws on retrospective clinical studies in the province of Québec. Initially, we identified a total of 178 patients diagnosed with d-TGA. Patients were excluded if they did not undergo a BAS procedure and if they had associated complex congenital heart diseases such as Taussig-Bing syndrome, tricuspid atresia, or double inlet left ventricle. The decision to proceed performing a BAS carries some subjective elements based on the attending cardiologist’s approach and depends on factors such as the initial oxygen saturations at birth, size of the interatrial communication, and likelihood of earlier atrial switch operation (ASO). Most of the cases that did not require BAS had other left-to-right shunts that helped mixing in the oxygenation (ie, ventricular septal defect or a large ASD) before the ASO, and performing a BAS might not be necessary because the initial oxygen saturations can be sufficiently adequate to support the baby until the ASO is performed. Regarding the ASO, it is a common practice in our institution not to operate in the immediate days after birth. This relates to various aspects of institutional logistics considerations. However, 47 of 116 (40%) and 44 of 116 (38%) cases were operated between 3 and 10 days of life and between 10 and 16 days of life, respectively. Otherwise, some reasons for delayed ASO include prematurity, other underlying anatomic conditions (eg, coarctation of the aorta or other associated cardiac malformations), or comorbidities that led to contradictions for early surgery (eg, necrotizing enterocolitis, appendicitis, various infections, and major post-BAS complications).
Data collection
The demographic data collected from clinical records included sex, gestational age, birth weight, date of birth, cardiac diagnosis including the association of complex cardiac malformations, BAS date and time relative to the time of birth, vascular access route for the procedure, and the length in days between birth and the arterial switch operation. The clinical data variables collected include saturation before and after BAS, and number of days on PGE1 after BAS. The outcome variables included the change in the saturation after the BAS, the change in the ASD size after the BAS, and complications related to the procedure. The pertinent anatomic data included the dimensions of the ASD before and after BAS, measured in the subcostal view. Saturation is continuously monitored as per our clinical management standards. The preductal oxygen saturations before and after BAS measured under FiO2 21%-25% were captured 15 minutes before and 3 hours after the BAS, respectively. These time frames are taken to maintain consistency and for comparison purposes. The 3-hour postintervention saturation measure was elected to ensure that all possible weaning (eg, O2 and mechanical ventilation parameters) was done at a stable state. The oxygen saturation values were measured by Masimo SET pulse oximetry with Signal Extraction Technology (SET, Irvine, CA). In our practice, pulse oximetry is always monitored on the right upper limb and on a lower limb to monitor for cerebral oxygen supply and to estimate the degree of total mixing reaching the lower segment. Saturation recorded on the upper limb (the lowest in this anatomy) was the one considered for this study; it reflects the degree of oxygenation leaving the right (systemic) ventricle and reaching the brain. The anatomic data were measured using transthoracic echocardiography, with a Vivid E95-GE health care ultrasound machine or equivalent.
Septostomy balloons
The septostomy balloons used were the Miller and Fogarty balloon atrioseptostomy catheters (Edwards Lifesciences). The Miller & Fogarty balloon atrioseptostomy catheter has either a maximum balloon inflation volume of 4.0 cc and a maximal diameter of 19 mm, or of 1.8 cc and a maximum diameter of 15 mm. According to local practice, the smaller balloon was used as a first choice for low-birth weight and premature babies and for cases with a small fossa ovalis; otherwise, the larger was used in general as a first attempt. Typically, if the smaller balloon did not achieve satisfactory detachment of the septum primum and a nonrestrictive interatrial shunt resulted and/or improved saturation achieved, the largest balloon was attempted. Also, in the case where a larger balloon was used but did not cross the septum/fossa ovalis because of a small size fossa, the smaller balloon was attempted subsequently. The latter situation was more occasional than the first scenario. There are situations where a 4 cc balloon did not cross the fossa ovalis at full inflation but was able to cross at submaximal inflation (3 cc instead) without resorting to the smaller balloon. For each patient, the balloon is inflated to full capacity (4 cc) and occasionally with an extra 1 cc to perform the septostomy.
Statistical analysis
Patients were divided into 2 groups based on the balloon size used during the BAS procedure: 4.0 cc group and 1.8 cc group. Comparisons between separate groups were performed using independent sample t-tests. Welch’s independent t-test was performed when sample sizes and variances were unequal between the groups. Within-group comparisons were performed using dependent sample t-tests. For categorical data, Fisher’s exact test was used. Log rank statistic for the survival curves was used to compare freedom from PGE1 and from switch operation between patients who underwent 1.8 cc vs 4.0 cc BAS. Time to arterial switch operation was also compared between cases who underwent BAS vs those who did not require BAS. Basic statistical analyses were performed on the GraphPad Prism 9 software (Dotmatics, San Diego, CA). Actuarial survival analysis and graphs were performed in SigmaPlot 13.0 software (SYSTAT Software, Point Richmond, CA). A P value of <0.05 was considered as significant.
Results
A total of 178 patients diagnosed with d-TGA were identified. Eighteen cases were excluded from analysis because they did not require BAS, and another 26 cases were excluded for associated complex malformations. The final series consisted of 134 patients. The umbilical venous access was used in 57% and femoral access in 43%. An internal jugular venous access was used in 1 case with the interrupted inferior vena cava. For the femoral access, a 7 F size catheter was used in 75% of the patients and a 6 F size was used for the other 25%. There were no statistical differences between the choice of the balloon size (4.0 cc vs 1.8 cc) in terms of gestational age (P = 0.71) or birth weight (P = 0.11). There were no significant differences in other clinical variables either (Table 1).
Table 1.
Demographic and clinical data of patients who underwent the BAS procedure
| Demographic and clinical variables | Group 4.0 cc balloon (N = 88) | Group 1.8 cc balloon (N = 46) | P value |
|---|---|---|---|
| Male/female | 62/26 (2.4) | 28/18 (1.5) | 0.3329 |
| Gestational age (wk) | 38.77 ± 1.37 | 38.67 ± 1.70 | 0.7074 |
| Birth weight (kg) | 3.42 ± 0.44 | 3.27 ± 0.52 | 0.1061 |
| Average number of days between birth and switch procedure | 13.38 ± 10.64 | 12.61 ± 7.46 | 0.6361 |
| Venous access ratio (umbilical/femoral) | 45/37 (1.2) | 29/17 (1.7) | 0.4563 |
| Number of days PGE administered | 5.31 ± 5.96 | 4.23 ± 5.28 | 0.3084 |
| Systemic oxygen saturation (%) | |||
| Pre-BAS | 65.11 ± 15.18 | 66.12 ± 14.01 | 0.7157 |
| Post-BAS | 84.49 ± 7.72 | 84.77 ± 6.46 | 0.8370 |
| Atrial septal defect size (mm) | |||
| Pre-BAS | 2.92 ± 1.27 | 2.66 ± 1.20 | 0.2951 |
| Post-BAS | 6.88 ± 1.74 | 6.53 ± 1.42 | 0.2193 |
Data are mean ± standard deviation for quantitative continuous variables and proportions for nominal data.
BAS, balloon atrial septostomy; PGE, prostaglandin E.
There were a total of 5 premature infants (gestational age <37 weeks) in this study. The average weight of these patients was 2.40 ± 0.71 kg for those who had BAS with the 4.0 cc balloon (n = 2) and 2.40 ± 0.41 kg for those who had BAS with the 1.8 cc balloon (n = 3) (P = 0.99). The PGE1 was administered in 124 patients (92.5%) for an average of 4.89 ± 5.65 days, with no significant difference in the number of days of PGE1 infusion, the balloon size (P = 0.31), or the type of venous access (P = 0.45). Seventeen (14%) of the patients who required PGE1 had it administered until the day of the switch procedure.
Because the duration of PGE usage may be influenced by time until surgery, we investigated the influence of balloon on clinically acceptable mixing as requiring continuation of PGE or not by the time of surgery. Accordingly, there was no significant difference according to balloon size distribution. Of the 17 patients maintained on PGE, 7 (41%) were in the 1.8 cc group and 10 (59%) in the 4.0 cc group, and this was compared with 36 (36%) and 63 (64%), respectively, of the 99 cases who were weaned off PGE (P = 0.788). In the same line of the potentially impactful outcome, for cases who required continuous PGE perfusion until ASO, balloon size did not affect the time interval from BAS to ASO (8.43 ± 4.16 [median: 8 days] for the 1.8 cc group vs 12.10 ± 3.35 [median: 13 days] for the 4.0 cc group [P = 0.0691]).
We did a similar analysis for the 99 cases who were weaned off PGE in advance of the day of surgery. Discontinuation of PGE infusion was recorded within 2.97 ± 4.46 days after BAS in the 1.8 cc group (median: 1 day) and 3.57 ± 3.34 days in the 4.0 cc group (median: 2 days), with no statistically significant difference (P = 0.160). The time interval between BAS and ASO between these 2 groups was also comparable (10.22 ± 7.69 days [median: 8.5 days] in the 1.8 cc group and 9.84 ± 11.00 days [median: 6 days] in the 4.0 cc group [P = 0.332]). In addition, there was no significant difference in age at which ASO was performed (13.19 ± 7.92 days vs 13.41 ± 11.09 days, respectively [P = 0.607]).
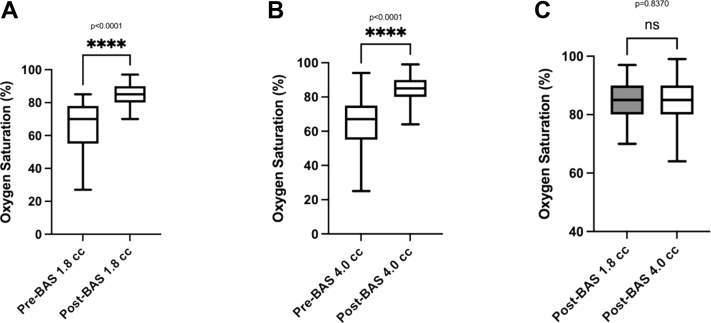
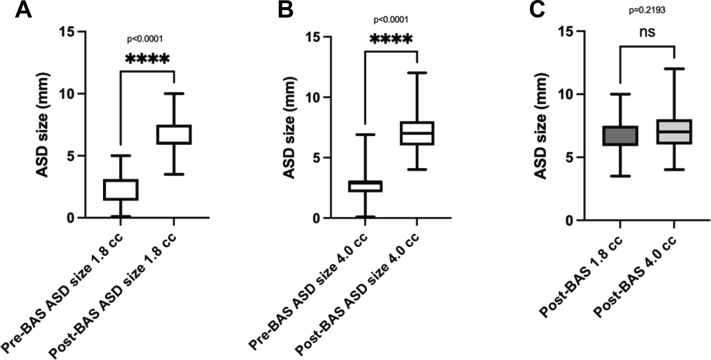
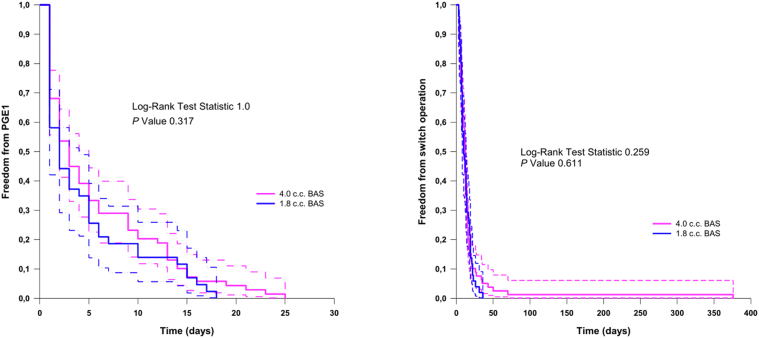
The BAS resulted in a significant increase in oxygen saturation of 18.91% ± 12.95% points from baseline (P < 0.0001) (Fig. 1, A and B). There was no significant difference based on the balloon size in the final oxygen saturation after the procedure (Fig. 1C). The average increase in ASD size was 3.92 ± 1.58 mm (Fig. 2, A and B). However, there was no significant difference in the final size of the ASD when comparing both balloon sizes (P = 0.22) (Fig. 2c). Stratified analyses of the haemoglobin oxygen saturation outcome showed lower values when the umbilical route was possible compared with femoral access cases (83.33% ± 7.21% vs 86.04% ± 6.91%; P = 0.036) with no related clinical significance. However, freedom from PGE1 infusion was comparable between the 1.8 cc balloon BAS cases and the 4.0 cc balloon (P = 0.317), as well as the interval to the switch operation (P = 0.61) (Fig. 3). Finally, a comparison between cases who underwent BAS and a concurrent series of patients who did not require BAS (n = 18) showed no statistically significant difference in the duration of PGE1 infusion (P = 0.154) (data not shown).
Figure 1.
Oxygen saturation before and after the BAS procedure using the (A) 1.8 cc balloon, (B) 4.0 cc balloon, and (C) comparison between both sizes. BAS, balloon atrial septostomy.
Figure 2.
ASD size before and after the BAS procedure using the (A) 1.8 cc balloon, (B) 4.0 cc balloon, and (C) comparison between both sizes. ASD, atrial septal defect; BAS, balloon atrial septostomy.
Figure 3.
Kaplan-Meier estimates demonstrating no statistically significant difference between the 1.8 cc balloon and the 4.0 cc balloon groups in terms of freedom from PGE1 (left panel) or from switch operation (right panel). BAS, balloon atrial septostomy; PGE1, prostaglandin E1.
Procedure-related complications occurred in 6% (8 of 134). Complications were mild in 5 including 2 cases of transitory AV-block, 1 case of thrombosis of the right femoral vein responding to short heparin therapy, 1 case of transitory ventricular tachycardia, and 1 case with an inconsequential accidental puncture of the left and right femoral arteries. The first severe complication included a case of cerebrovascular stroke with transitory convulsion but without long-term neurologic sequelae. This case was not due to technical complications such as prolonged procedure time or balloon rupture. The other complication is a case of ischemia of the right lower limb that led to a transtibial amputation due to multiple aggravating factors including needle puncture of the femoral artery (no introducer sheath inserted), delayed access to the patient, and delayed resuscitation vascular access that resulted in low cardiac output and acidosis in a significantly cyanotic baby. In another case with severe desaturation an accidental cutting of the umbilical venous catheter occurred while attempting to replace it with the BAS catheter. The latter technical complication required abdominal surgery for the extraction of the catheter by juxta-umbilical skin incision. No deaths occurred because of the procedure, and no significant technical complications related to the catheter balloons were recorded either.
Discussion
The rationale behind performing the BAS procedure is to improve haemodynamic conditions before the arterial switch operation so that this surgery is performed on a stable patient. As expected, we found a significant increase in the systemic oxygen saturation and the ASD size after the procedure with a tangible benefit for the patient. However, this improvement in oxygen saturation did not correlate with balloon dimension. Sehgal et al.17 conducted a retrospective study on 25 neonates and found no correlation between the size of the ASD and the improvement in oxygenation after the procedure according to the following variables: FiO2, PaO2, PaO2/FiO2, oxygen saturations/FiO2, preductal oxygen saturations, and oxygen index. Two other independent studies, one by Matter et al.16 and the other by Cherif et al.,18 also found that the brand of the balloon or the size of the ASD after the BAS did not have an impact on the improvement in systemic oxygen saturation after the procedure.
The lack of significant differences in clinical variables in our study between the 4.0 cc and 1.8 cc groups (the number of days on PGE1, the change in the saturation, the change in the size of the ASD, and complications related to the procedure) seriously questions whether it is necessary to have different sizes for BAS in production for the needs of babies with d-TGA. A smaller balloon requires a smaller introducer’s profile; hence vascular injury and potential thrombosis may be reduced. This is particularly impactful for the prematurely born and the low-birth-weight neonate. It seems logical to use smaller atrioseptostomy balloons on premature babies because of their body habitus compared with full-term babies. Simpson et al.19 in their experience of cardiac catheterization of low-birth-weight infants report that in 11 of 16 cases of patients who underwent BAS, the balloon size of 1.2-1.8 mL was used. However, in our study, we did not find any significant difference in the balloon size used for premature or full-term babies.
The umbilical access is the first choice in the study centre, and the femoral access is only used when the septostomy is not feasible with the former. The stratified outcome variables (improvement of systemic oxygen saturation) were more favourable for the femoral route according to our observations with higher systemic oxygen saturation. However, conceptually speaking, this may not be attributed to the route of access.
The use of PGE1 in patients with d-TGA maintains the patency of the DA and decreases pulmonary vascular resistance, which increases venous return to the left atrium and thus blood mixing at the atrial level.7,20,21 In our study, PGE1 was maintained in 92.5% of the infants after the BAS procedure for an average of 4.89 ± 5.65 days and until the day of surgery in 14%. Despite an adequate ASD size after BAS, rebound hypoxia was the attributable cause for protracted use of PGE1.22,23 According to a series of 45 neonates, the risk was 3-fold higher in infants in whom PGE1 discontinuation was early (16 of 25; 64%) compared with those who had a late PGE1 discontinuation (4 of 20; 20%) after the BAS procedure (P < 0.006) due to rebound hypoxia.7
Severe or life-threatening complications represent the main obstacle to adopting any particular procedure. In our series, 2% of the patients suffered nonlethal serious complications. There are conflicting findings related to post-BAS complications, specifically the risk of brain injury. In a large series of 17,392 neonates, Hamzah et al.24 reported stroke events in 1.1% compared with 0.6% in those who did not undergo BAS (P < 0.0001). On the opposite, in a meta-analysis of 10,108 patients including 22.4% who underwent BAS, Polito et al.25 did not find increased odds of perioperative brain injury. In a multicentric prospective longitudinal investigation on brain injury in 64 infants with cyanotic congenital heart disease, Beca et al.26 found similar rates in infants with TGA compared with other cardiopathies, whether they had undergone BAS or not. In our series, 1 patient sustained a brain injury after BAS, although no serial neurology imaging was systematically performed. However, as Petit et al.27 suggest with their series of 26 neonates, the preoperative brain injury could also be associated with hypoxemia and the time delay between birth and performing the switch operation.
Emphasizing the low probability of complications will help decision-making. Some groups have questioned the benefit of BAS, whereas others continue to favour BAS considering no difference in the rate of mortality between those who undergo a BAS and those who do not.24 Needless to say, the noninferiority consideration does not take into account the risk factors in those who “required” BAS for reasons of low cardiac output and significant hypoxia.
The present study has inherent limitations besides its retrospective design. First, this is a single-centre study, which limits the generalizability of the conclusions as opposed to multicentred collaborative studies or prospective protocol-based studies. Secondly, the echocardiographic measurements of the ASD were performed by different experienced cardiologists, well versed and experienced in echocardiography, without a specific protocol that summarizes where and how the measurements should be performed. The 3 hours of cutoff observation in our methodology cannot exclude possible supplemental oxygen requirement in some cases. Admittedly, a number of cases could have required higher oxygen supplementation at or beyond 3 hours after BAS.
Conclusions
This is the first study in patients with d-TGA to study the influence of the BAS balloon size on the change in oxygen saturation after the BAS as the primary outcome. Ultimately, the balloon size used for the atrial septostomy seems irrelevant to the degree of improvement in oxygen saturation or to the ASD size and provides similar palliation, while awaiting the arterial switch operation. Further consideration by manufacturers and prospective studies are warranted to confirm or reject our findings.
Ethics Statement
This retrospective study was conducted in compliance with established ethical standards. The study was approved by the CHU Sainte-Justine’s ethics board committee. Parental consent was waived according to applicable laws on retrospective clinical studies in the province of Québec.
Acknowledgments
Funding Sources
Partial funding for the conduct of this study were secured from BoBeau Coeur (BoBeauCoeur.org) funds for paediatric cardiology applied clinical research (student grant to JW).
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Marathe S.P., Talwar S. Surgery for transposition of great arteries: a historical perspective. Ann Pediatr Cardiol. 2015;8:122–128. doi: 10.4103/0974-2069.157025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renaud C, Raboisson M, Dallaire F, Drolet C, Rohlicek C. Incidence of d-transposition of the great arteries in Québec, where do we stand? Can J Cardiol 201531:S28-S29.
- 3.Mukherjee D., Lindsay M., Zhang Y., et al. Analysis of 8681 neonates with transposition of the great arteries: outcomes with and without Rashkind balloon atrial septostomy. Cardiol Young. 2010;20:373–380. doi: 10.1017/S1047951110000296. [DOI] [PubMed] [Google Scholar]
- 4.Baker E.J., Allan L.D., Tynan M.J., et al. Balloon atrial septostomy in the neonatal intensive care unit. Br Heart J. 1984;51:377–378. doi: 10.1136/hrt.51.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaujois L., Boucoiran I., Preuss C., et al. Relationship between interatrial communication, ductus arteriosus, and pulmonary flow patterns in fetuses with transposition of the great arteries: prediction of neonatal desaturation. Cardiol Young. 2017;27:1280–1288. doi: 10.1017/S1047951117000087. [DOI] [PubMed] [Google Scholar]
- 6.Cinteza E., Carminati M. Balloon atrial septostomy—almost half a century after. Maedica (Bucur) 2013;8:280–284. [PMC free article] [PubMed] [Google Scholar]
- 7.Finan E., Mak W., Bismilla Z., McNamara P.J. Early discontinuation of intravenous prostaglandin E 1 after balloon atrial septostomy is associated with an increased risk of rebound hypoxemia. J Perinatol. 2008;28:341–346. doi: 10.1038/jp.2008.11. [DOI] [PubMed] [Google Scholar]
- 8.McQuillen P.S., Hamrick S.E., Perez M.J., et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 9.Hijazi Z.M., Abu Ata I., Kuhn M.A., et al. Balloon atrial septostomy using a new low-profile balloon catheter: initial clinical results. Cathet Cardiovasc Diagn. 1997;40:187–190. doi: 10.1002/(sici)1097-0304(199702)40:2<187::aid-ccd17>3.0.co;2-o. [discussion: 191] [DOI] [PubMed] [Google Scholar]
- 10.Boehm W., Emmel M., Sreeram N. Balloon atrial septostomy: history and technique. Images Paediatr Cardiol. 2006;8:8–14. [PMC free article] [PubMed] [Google Scholar]
- 11.Medtronic recalls Rashkind balloon septostomy catheters for quality issues; 2020. 2020. https://www.fda.gov/medical-devices/medical-device-recalls/medtronic-recalls-rashkind-balloon-septostomy-catheters-quality-issues Available at:
- 12.FDA Class 1 device recall Miller balloon atrioseptostomy catheter; 2019. 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?ID=171709
- 13.Korns M.E., Garabedian H.A., Lauer R.M. Anatomic limitations of balloon atrial septostomy. Hum Pathol. 1972;3:345–349. doi: 10.1016/s0046-8177(72)80035-2. [DOI] [PubMed] [Google Scholar]
- 14.Kishve P., Motwani R. Morphometric study of fossa ovale in human cadaveric hearts: embryological and clinical relevance. Anat Cell Biol. 2021;54:42–50. doi: 10.5115/acb.20.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hijazi Z.M., Geggel R.L., Aronovitz M.J., et al. A new low profile balloon atrial septostomy catheter: initial animal and clinical experience. J Invasive Cardiol. 1994;6:209–212. [PubMed] [Google Scholar]
- 16.Matter M., Almarsafawy H., Hafez M., Attia G., Abou Elkhier M.M. Balloon atrial septostomy: the oldest cardiac interventional procedure in Mansoura. Egypt Heart J. 2011;63:125–129. [Google Scholar]
- 17.Sehgal K., Sehgal K., Varma S. Size of atrial septal defect after balloon atrial septostomy does not correlate with immediate improvement in oxygenation. World J Pediatr Surg. 2021;4 doi: 10.1136/wjps-2020-000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherif A., Mourali S., Farhati A., Ezzar T., Mechmeche R.L. 'effet immédiat de l'atrioseptostomie de Rashkind sur la saturation systémique dans la transposition des gros vaisseaux [The immediate effect of Rashkind's atrioseptostomy on systemic saturation in transposition of the great arteries] Tunis Med. 2004;82:1107–1110. [in French] [PubMed] [Google Scholar]
- 19.Simpson J.M., Moore P., Teitel D.F. Cardiac catheterization of low birth weight infants. Am J Cardiol. 2001;87:1372–1377. doi: 10.1016/s0002-9149(01)01555-7. [DOI] [PubMed] [Google Scholar]
- 20.Olley P.M., Coceani F., Bodach E. E-type prostaglandins: a new emergency therapy for certain cyanotic congenital heart malformations. Circulation. 1976;53:728–731. doi: 10.1161/01.cir.53.4.728. [DOI] [PubMed] [Google Scholar]
- 21.Lang P., Freed M.D., Bierman F.Z., Norwood W.I., Jr., Nadas A.S. Use of prostaglandin E1 in infants with d-transposition of the great arteries and intact ventricular septum. Am J Cardiol. 1979;44:76–81. doi: 10.1016/0002-9149(79)90253-4. [DOI] [PubMed] [Google Scholar]
- 22.Benson L.N., Olley P.M., Patel R.G., Coceani F., Rowe R.D. Role of prostaglandin E1 infusion in the management of transposition of the great arteries. Am J Cardiol. 1979;44:691–696. doi: 10.1016/0002-9149(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 23.Driscoll D.J., Kugler J.D., Nihill M.R., McNamara D.G. The use of prostaglandin E1 in a critically ill infant with transposition of the great arteries. J Pediatr. 1979;95:259–261. [PubMed] [Google Scholar]
- 24.Hamzah M., Othman H.F., Peluso A.M., Sammour I., Aly H. Prevalence and outcomes of balloon atrial septostomy in neonates with transposition of great arteries. Pediatr Crit Care Med. 2020;21:324–331. doi: 10.1097/PCC.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 25.Polito A., Ricci Z., Fragasso T., Cogo P.E. Balloon atrial septostomy and pre-operative brain injury in neonates with transposition of the great arteries: a systematic review and a meta-analysis. Cardiol Young. 2012;22:1–7. doi: 10.1017/S1047951111001909. [DOI] [PubMed] [Google Scholar]
- 26.Beca J., Gunn J., Coleman L., et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807–1811. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 27.Petit C.J., Rome J.J., Wernovsky G., et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–716. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]