Abstract
We report the case of an adult who had a cardiac arrest in the setting of pulmonary hypertension and a previously repaired intermediate atrioventricular septal defect, with left main coronary trunk stenosis due to dilatation of the main pulmonary artery. In patients with pulmonary hypertension exhibiting anginal symptoms, it is advisable to perform chest contrast computed tomography to confirm the pulmonary artery diameter and the presence of coronary artery compression. In addition, our case highlights the importance of early collaboration among specialists during the transition from adolescence to adulthood.
Résumé
Nous décrivons le cas d’un adulte ayant subi un arrêt cardiaque alors qu’il présentait une hypertension pulmonaire et qu’il avait déjà subi la réparation d’une communication septale auriculoventriculaire intermédiaire, avec sténose de l’artère coronaire gauche principale causée par la dilatation de l’artère pulmonaire principale. Chez les patients atteints d’hypertension pulmonaire qui présentent des symptômes angineux, il est recommandé d’effectuer une tomodensitométrie thoracique avec produit de contraste pour confirmer le diamètre de l’artère pulmonaire et la présence d’une compression de l’artère coronaire. Notre cas souligne également l’importance d’établir sans tarder une collaboration entre spécialistes lors de la transition entre l’adolescence et l’âge adulte.
Case Presentation
An adult patient previously presented with asymptomatic left axis deviation and right bundle branch block at age 7, which was identified on an electrocardiogram at the compulsory regular medical check-ups in Japanese elementary schools. He was diagnosed with intermediate atrioventricular septal defect, spontaneously closed ventricular septal defect, and pulmonary arterial hypertension (PAH) (Table 1). The patient promptly underwent intracardiac repair, but severe PAH remained after the surgery.
Table 1.
Cardiac catheterization results
| Age (y) | 7 | 12 | 19 | 20 | 22 |
| PA pressure (mm Hg) | 85/47 (63) | 70/27 (45) | 80/43 (58) | 85/39 (57) | 78/35 (55) |
| PA wedge pressure (mm Hg) | 9 | (5) | (5) | (11) | (12) |
| LV (mm Hg) | 98/EDP11 | 93/EDP5 | 76/EDP5 | 105/EDP10 | 95/EDP13 |
| LVEF (%) | – | 56 | – | 53 | – |
| RV (mm Hg) | 87/EDP11 | 65/EDP5 | 81/EDP8 | 79/EDP9 | 83/EDP10 |
| RVEF (%) | – | 51 | – | 46 | – |
| RpI (Woods units m2) | 9.4 | 10.8 | 17.9 | 14 | 10.6 |
| C.I. (L/min m2) | 5.5 | 4.8 | 3.8 | 3.5 | 4.1 |
C.I., cardiac index; EDP, end-diastolic pressure; LV, left ventricle; LVEF, left ventricular ejection fraction; PA, pulmonary artery; RpI, pulmonary vascular resistance index; RV, right ventricle; RVEF, right ventricular ejection fraction.
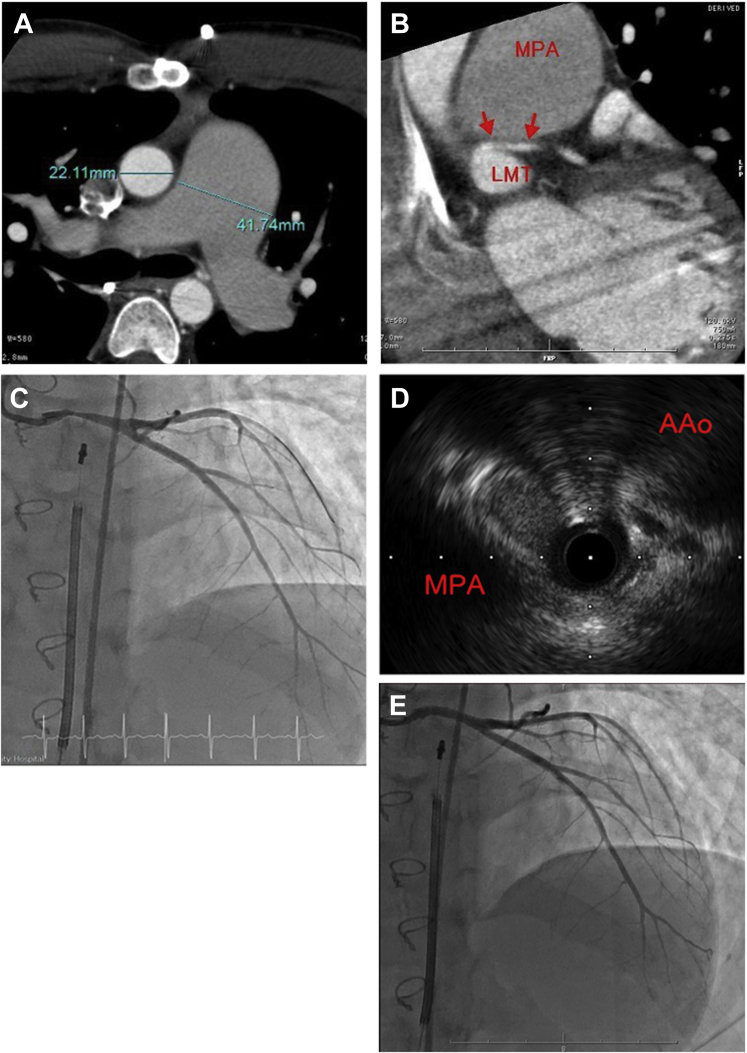
Around the age of 12, he began to have occasional chest pain after exercise. Coronary angiography did not reveal any abnormalities. At the age of 19, sildenafil and bosentan were administered at the maximum dose, namely 60 mg/d and 125 mg/d, respectively. At the age of 22, he lost consciousness after exercise and bystander cardiopulmonary resuscitation was started. Ventricular fibrillization was confirmed using electrocardiogram on the arrival of the emergency team. The patient returned to sinus rhythm with 150 J cardiac defibrillation but had a prolonged disturbance of consciousness. He was transported to hospital, placed on a ventilator, and treated with hypothermia at 33°C in the intensive care unit. He was extubated on the third day of hospitalization, when he regained consciousness and showed no neurological sequelae. Subsequent chest contrast computed tomography (CT) and cardiac catheterization (Fig. 1) revealed compression of the left main coronary trunk (LMT) by a markedly enlarged pulmonary artery and severe pulmonary hypertension (PH). After interdisciplinary consultation (paediatrics, and the adult cardiology and adult respiratory departments), a subcutaneous cardioverter-defibrillator was implanted and treatment with oral selexipag was started at 0.4 mg/d. Subsequently, stenting of the LMT was performed. An electrophysiology study detected no arrhythmogenic substrate. After the examination, the patient was referred to adult cardiology and adult respiratory departments for medical follow-up. After stent placement, his chest pain on exertion resolved.
Figure 1.
Chest contrast computed tomography and cardiac catheterization immediately after cardiac arrest. (A) Dilated main pulmonary artery (right) and ascending aorta (left). (B) Left main coronary artery is compressed between the main pulmonary artery and the sinus of Valsalva. (C) Contrast-enhanced image of the left main coronary artery. (D) Proximal portion of the left main coronary artery with 90% stenosis. (E) Left coronary angiography after stenting. AAo, ascending aorta; LMT, left main coronary trunk; MPA, main pulmonary artery.
In addition, whole-exome sequencing was performed. Although no pathogenic variants were found in genes responsible for PAH, RNF213 (NM_001256071.3) c.14429 G>A p.Arg4810Lys was identified. Other abnormal vascular lesions, including Moyamoya disease, were not detected.
Discussion
This PAH case was classified as PAH associated with congenital heart disease (CHD-PAH).1,2 Although the timing of the spontaneous ventricular septal defect closure was unknown, the high pulmonary blood flow likely induced severe PH before the congenital heart disease was detected. It is possible that primum atrial septal defect also played some role in the progression of PAH, due to increased pulmonary blood flow. Meanwhile, RNF213 p.Arg4810Lys variant was detected in this patient. It is known as a risk allele for PAH, and a poor prognosis was reported in carriers of the variant with idiopathic PAH.3 The significance of this variant in patients with CHD-PAH is unknown but should be investigated in the future.
In 1997, Patrat et al.4 reported a PH patient with angina pectoris due to coronary artery compression induced by pulmonary artery enlargement. Since then, several similar cases have been reported. To the best of our knowledge, our case is the first report of cardiac arrest due to LMT compression by dilated pulmonary artery caused by PH. However, it is highly possible that similar cases occurred in patients suffering from sudden deaths caused by PH.
Galiè et al5 reported that among 765 patients with PAH, 16% had anginal symptoms and approximately 40% had LMT stenosis of 50% or more. The best predictor of >50% LMT stenosis was PA diameter ≥40 mm on chest contrast CT. The pulmonary artery diameter of our patient met this criterion.5
In patients with PH exhibiting anginal symptoms, performing chest contrast CT is advisable to confirm the pulmonary artery diameter and the presence of coronary artery compression. Identifying such an issue in a timely manner could potentially allow early intervention and/or prevent adverse events. The importance of the timely and full transition of patients with CHD-PAH from paediatric to adult congenital heart teams is also highlighted.
Novel Teaching Points.
-
•
In patients with pulmonary hypertension exhibiting anginal symptoms, it is advisable to perform chest contrast computed tomography to confirm the pulmonary artery diameter and the presence of coronary artery compression.
-
•
Our case highlights the importance of early collaboration among specialists during the transition from adolescence to adulthood.
Ethics Statement
Written informed consent to participate in this study was provided by the patient. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frost A., Badesch D., Gibbs J.S.R., et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53:1801904. doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraide T., Kataoka M., Suzuki H., et al. Poor outcomes in carriers of the RNF213 variant (p.Arg4810Lys) with pulmonary arterial hypertension. J Heart Lung Transplant. 2020;39:103–112. doi: 10.1016/j.healun.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Patrat J.F., Jondeau G., Dubourg O., et al. Left main coronary artery compression during primary pulmonary hypertension. Chest. 1997;112:842–843. doi: 10.1378/chest.112.3.842. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N., Saia F., Palazzini M., et al. Left main coronary artery compression in patients with pulmonary arterial hypertension and angina. J Am Coll Cardiol. 2017;69:2808–2817. doi: 10.1016/j.jacc.2017.03.597. [DOI] [PubMed] [Google Scholar]