Abstract
As the demographics of congenital heart disease (CHD) have shifted, there are now more adults living with CHD than children in North America. This presents unprecedented challenges as patients with CHD acquire noncardiac comorbidities and seek care for a variety of reasons, including noncardiac surgery and emergency department (ED) visits. CHD shifts from a one organ problem to a multisystem disease and requires a team of specialists to maintain high-quality longitudinal care. In this review, we summarize the challenges patients with CHD and their providers face as they age. We review the demographics of CHD and health care utilization. We examine the rates of noncardiac comorbidities and the current quality of care received by adult patients with CHD.
Résumé
Les caractéristiques démographiques des patients atteints d’une cardiopathie congénitale ont changé : à l’heure actuelle, en Amérique du Nord, il y a plus d’adultes qui présentent une cardiopathie congénitale que d’enfants. Cela représente un défi sans précédent lorsque ces patients ont des troubles concomitants non cardiaques et doivent subir une chirurgie non cardiaque, par exemple, ou se rendre aux urgences pour toutes sortes de raisons. On ne parle alors plus de l’atteinte d’un seul organe, mais bien d’une maladie multisystémique. Une équipe pluridisciplinaire sera nécessaire pour maintenir des soins longitudinaux de haute qualité.
Dans notre article, nous faisons le point sur les défis que les personnes atteintes d’une cardiopathie congénitale et les dispensateurs de soins doivent affronter à mesure que le patient avance en âge. Nous passons en revue les données démographiques sur les cardiopathies congénitales ainsi que celles sur l’utilisation des services de santé. Nous examinons la fréquence des comorbidités non cardiaques, tout comme la qualité des soins que reçoivent actuellement les patients atteints d’une cardiopathie congénitale.
Before modern surgical techniques were developed, 78% of children with hypoplastic left heart syndrome died within their first month of life.1 Nearly 50% of the children born with tricuspid atresia, tetralogy of Fallot, and complete atrioventricular septal defects would not reach their first birthday.1 More than 60% of children born with transposition of the great arteries died before 9 months of age.1 In the last 50 years, there has been a demographic shift in high-income countries, with the majority of those born with congenital heart disease (CHD) living to adulthood and many achieving older age.2 This success presents new challenges as there are now more adults living with CHD than children.3 Although a normal life expectancy is not possible for all, many will live well into adulthood, providing time to acquire adult comorbidities.4
In this narrative review, we summarize the challenges adults with CHD (ACHD) and their providers face in this new era. To incorporate the patient voice in the review, we have included focus group quotations from patients living with moderate-to-complex CHD. These were collected after approval by our institutional research ethics board.
Chronologic Age vs Biologic Age
In Western society, we measure age chronologically (literal number of years alive). We use this as a proxy for life expectancy and to predict expected overall functioning.5 For those with ACHD, their chronologic age likely does not capture the entirety of their circumstances. As longitudinal cohort studies are published in the most complex populations, we find that many patients with ACHD will not reach their chronologically expected life expectancies.6 In addition, young people do not fit the cultural paradigm of heart disease, making the journeys of those with ACHD socially complex.
“I know that more education could get me further and I would enjoy it, but do I want to make a financial investment in my career? It (CHD) holds you back. What if I put time into a career and I’m going to drop dead? Maybe stable and mediocre is OK.” Quote from a patient with aortic coarctation in her 30s.
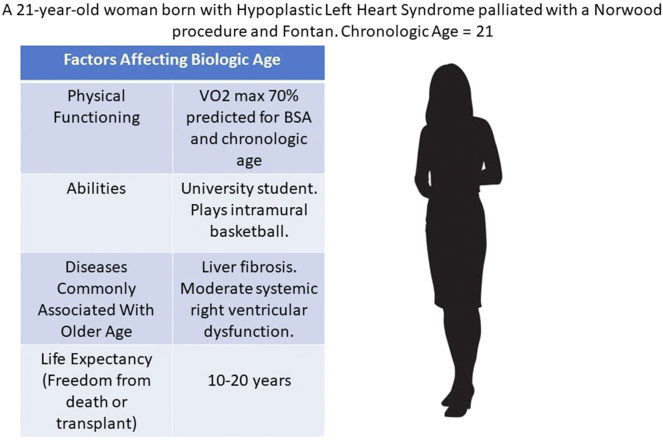
For those with CHD, biologic age provides a more comprehensive assessment. This is a rethinking of chronological age including physical functioning, ability, diseases commonly associated with older age, and life expectancy.5 For a patient with complex CHD, this paradigm may be more useful overall but presents a cognitive challenge for both patients and providers. For example, a woman with hypoplastic left heart syndrome palliated with a Norwood procedure may be chronologically in her 20s, but liver disease, decreased exercise tolerance, systemic ventricular dysfunction, and a reduced life expectancy make her biologically much older (Fig. 1).6
Figure 1.
The disconnect between chronological age (what we expect) and biologic age (true functioning) in young adults living with congenital heart disease.6
Growing Old With Congenital Heart Disease
Cardiac comorbidities in the aging congenital heart disease population
As cohort data for older adults with CHD are published, patterns and trends emerge (Table 1). Heart failure is the most common complication experienced by adults with CHD as they age. Heart failure in ACHD is most often borne of a slow, gradual decline in underlying physiologic lesions (valvular disease, systemic ventricular dysfunction, and shunting) despite childhood corrective surgeries.4 A more rapid progression to heart failure may occur in those with infective endocarditis or atrial tachyarrhythmias.4 No matter the chronology, multiple hospitalizations for heart failure are correlated with increased mortality.7 More than 50% of Québec ACHD patients over the age of 40 admitted to hospital with heart failure were readmitted within 1 year.7 Adding to the complexity, heart failure in the ACHD population presents differently than that in older adults with acquired heart disease, perhaps leading to delayed recognition.6 Frank pulmonary and pedal oedema are very late signs of circulation failure in ACHD, whereas sarcopenia and cachexia, decline in exercise tolerance, ascites with liver failure, and worsening cyanosis can be early signs missed by general practitioners.4,8 Also, traditional medical therapy for heart failure is often ineffective or untested in ACHD populations.8
Table 1.
Disease-specific outcomes for CHD > 40 years of age
| CHD diagnosis | Cardiac comorbidities | Noncardiac comorbidities | Predictors of hospitalization and/or mortality | Reference |
|---|---|---|---|---|
| Complete AVSD (n = 43, >50% with T21) | Pacemaker (14%) NYHA>II (35%) Pulm HTN (30%) |
GI/hepatobiliary (28%) Renal (23%) |
During median follow-up of 1.7 y, there were 10 unplanned hospitalizations and 4 deaths. Predictor: >NYHA class | J Clin Med 2021;10:3665 |
| Single ventricle physiology (n = 49) | NYHA>II (>78%) Arrhythmia with DCCV (41%) |
Thyroid dysfunction (55%) Renal disease (31%) Stroke (27%) Venous thrombosis (18%) |
During median follow-up of 4.9 y, there were 10 deaths and 72% were admitted for HF. Predictors: cirrhosis, renal dysfunction | J Clin Med 2021;10:3665 |
| PAH-ACHD (n = 65, 71% shunt lesions) | Arrhythmias (31%) Thromboembolism (14%) |
Not reported | During median follow-up of 4.2 y, 16 (25%) of patients died. Predictors: >BNP, >Cr | J Clin Med 2020;9:4071 |
AVSD, atrioventricular septal defect; BNP, brain natriuretic peptide; CHD, congenital heart disease; Cr, creatinine; DCCV, direct current cardioversion; GI, gastrointestinal; HF, heart failure; HTN, hypertension; NYHA, New York Heart Association; PAH-AHCD, pulmonary arterial hypertension in adult congenital heart disease.
In addition to heart failure, arrhythmias requiring cardioversion and/or device management are especially common in adults with CHD over the age of 40.9 Scar from prior surgeries, artificial material in the atria (ie, Mustard or Senning operations), and atrial volume overload from worsening valvular disease or failing ventricles are all pathophysiologic culprits.9 Atrial arrhythmias can either precipitate overt heart failure in patients with ACHD or be the harbinger of worsening prognosis. In patients with a right atrial to pulmonary artery Fontan circulation, for example, atrial arrhythmias should prompt a robust discussion of Fontan conversion, cardiac transplantation, or palliative care.4
It is estimated that >50% of patients with ACHD will experience an atrial arrhythmia before they reach 65 years of age. In contrast, the lifetime risk of the development of atrial fibrillation in the general population is less than 25%.10 Traditional thromboembolic risk scores for atrial arrhythmia, many of them incorporating chronologic age, are not applicable to ACHD.11 Contrary to the general population, younger patients with higher complexity of CHD may be at higher risk of thromboembolic stroke than their older counterparts.11 There is also no clear evidence for a thromboprophylaxis strategy (antiplatelets vs anticoagulants), and bleeding risk in patients with CHD is not negligible. This leads to treatment quandaries for the increasing number of middle-aged patients with CHD and their physicians.
Patients with CHD are also testing the layering of acquired vascular risk factors onto native disease. Adults with CHD are known to have higher rates of diabetes and hypertension (HTN) than the general population.12 Because of cognitive biases held by non-CHD practitioners about young patients, many of those with CHD and HTN remain undertreated.13 Pregnancy considerations in women of childbearing age may also limit pharmacologic options for treatment.14 Also, the overweight and obesity epidemic affecting young adults in North America has not spared those with CHD. The consequences of obesity may be more grave, though, in those with underlying cardiac disease.15 The rate of metabolic syndrome in American adults with CHD is higher than in the general population.16 Although dementia was a rare complication in the largest senior CHD cohort to date, with only approximately 1% prevalence, it was a significant predictor of mortality and may be the downstream consequence of a combination of congenital and acquired vascular disease.9,17
Noncardiac comorbidities in the aging CHD population
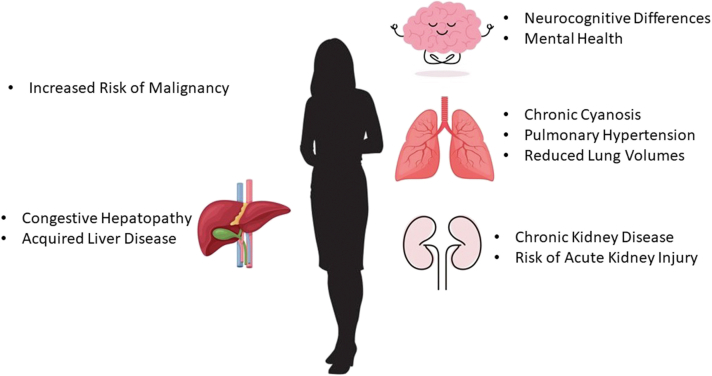
As patients with CHD age, many are surprised to find that multisystem disease may limit their life expectancy more than their isolated cardiac anomalies. Patients with Fontan circulations dying from hepatocellular carcinoma (HCC) and patients with Eisenmenger syndrome dying from malignancy are borne of decreased upfront cardiovascular mortality in CHD. Multisystem disease in the aging patient with CHD brings unprecedented challenges (Fig. 2).
Figure 2.
Not just the heart; adult congenital heart disease has become a multisystem disease as patients reach older age.
Liver disease in ACHD
In the last 2 decades, awareness of Fontan-associated liver disease has increased significantly. It is now recognized as a leading cause of morbidity and mortality in the adult Fontan population. Screening paradigms for cirrhosis and HCC have been developed, and frameworks for heart-liver transplantation have been instituted.18 But patients with Fontan circulations are not the only patients with ACHD at risk of significant hepatic morbidity. Hepatologists are now integral to the ACHD management team.
Right heart failure leading to congestive hepatopathy, cirrhosis, and HCC in non-Fontan repaired CHD has been reported.19 Hepatic dysfunction is associated with reduced long-term mortality in patients with Ebstein anomaly.20 Patients with ACHD may also belong to the cohort of those who acquired hepatitis during childhood surgeries before the screening of blood products. Lack of appropriate hepatitis C screening in this population can lead to delayed diagnosis and treatment.21 In addition, >80% of patients with ACHD drink alcohol and >25% binge drink.22,23 Although these rates may be similar to those in chronological age-matched peers, the potential consequences of alcohol consumption in young adults with underlying liver disease are more dire. Similarly, although the prevalence of overweight and obesity in young adults with CHD is consistent with that of their peers, the long-term hepatic consequences (ie, nonalcoholic fatty liver disease) are likely more severe.
Outside of the Fontan population, there are no clear guidelines for counselling and screening for liver disease in young adult survivors of CHD. This makes multidisciplinary involvement paramount. Some experts advocate for the routine assessment of thrombocytopenia and other hepatic biomarkers in the ACHD population, with a low index for triggering HCC surveillance.19
Malignancy and ACHD
The lifetime risk of cancer in those with CHD is 23% higher than age- and sex-matched controls.24 Malignancies of the skin, hematopoietic, and lymphoid cancers are the most common.24 Cancer is associated with some CHD syndromes (ie, trisomy 21 and leukaemia), and pheochromocytoma has specifically been associated with cyanotic CHD due to chronic hypoxia.25
The elevated risk of malignancy in those with ACHD persists, though, even after the exclusion of genetic syndromes and organ transplantation.26 The proposed biological mechanisms include loss of function genetic variants in patients with CHD that code regulatory proteins.27 As malignancy risk is higher in those with congenital heart surgery performed at less than 1 year of age, with presumed thymectomy, it has also been postulated that alteration of T-cell profiles may play a role.24
The lifetime risk of ionizing radiation exposure in children with CHD also must be examined. Previously, when life expectancies of children with CHD were short, the risk of radiation was negligible. Now, as patients with CHD are living to older age, it must be carefully contemplated. Although imaging is necessary for the long-term monitoring of patients with ACHD, thoughtful selection of imaging modality (magnetic resonance imaging or cardiac computed tomography), an understanding of cumulative radiations dosing, and optimization principles (patient positioning, scan range, and timing protocols) are all important considerations.28 Alarmingly, routine screening for malignancy (ie, breast, colon, and cervical) is suboptimal in women with CHD, perhaps indicating a sense of futility by general practitioners who are underestimating life expectancy in this population.29 Although procedures requiring ionizing radiation have certainly contributed to the improvements in CHD life expectancy overall, guidelines for proper stewardship have been developed to prevent unnecessary downstream cancer risk and reduce lifetime cumulative dose.28
Pulmonary compromise in ACHD
Each congenital cardiac anatomy and surgical correction has secondary effects on lung function. The heterogeneous ACHD population requires comanagement with respirologists. Pulmonary HTN from Eisenmenger syndrome or late after shunt closure carries significant morbidity and mortality. Differential blood flow to the lungs and chronic thromboembolic disease also present important challenges in the CHD population.30
Even without significant pulmonary HTN or thromboembolic disease, restrictive ventilatory disorder associated with reduced forced vital capacity is very common in adults with CHD.31 Reduced lung volumes are independently associated with reduced survival in patients with CHD.32 Inspiratory muscle dysfunction and diaphragmatic weakness can contribute to overall exercise intolerance.31 Although deficits may not be present during day-to-day activities, limitations at higher levels of exertion may lead those with ACHD to avoid exercise, leading to a negative spiral of inactivity. There is also a higher prevalence of sleep-disordered breathing in adult patients with CHD. Daytime symptoms of sleep-disordered breathing can contribute to exercise intolerance in this population.33 In addition to the effects on physical activity, restrictive lung defects may portend worse surgical risk in the ACHD population. The number of prior chest wall incisions was independently associated with mortality and prolonged ventilation >7 days in adults with CHD undergoing cardiac surgery.34
Renal disease in ACHD
Renal dysfunction, defined by reduced estimated glomerular filtration rate (eGFR), is present in at least 50% of patients with ACHD.35 In one large series, an eGFR <60 mL/min/1.73 m2 was present in 9% of patients with ACHD.35 In patients with a Fontan circulation and a normal eGFR, significant albuminuria was present in 33%, suggesting a degree of mild chronic kidney disease (CKD) not detectable with standard screening.36 Despite the prevalence of CKD in survivors of paediatric CHD surgeries, renal surveillance in this population is often suboptimal.37
The causes of renal dysfunction are multiple, including the chronic renal hypoperfusion and increased neurohormonal activity observed in other heart failure populations.35 ACHD presents unique renal challenges, though. Chronic hypoxia contributes to renal compromise due to the increase in blood viscosity associated with secondary erythrocytosis.38 Renal dysfunction has also been reported in concert with infective endocarditis, suggestive of cardioembolic renal infarction. When present in the setting of prosthetic material endocarditis in patients with ACHD, renal dysfunction was associated with a 7-fold increase in multiorgan failure and/or death.39
Independent of etiology, renal dysfunction in ACHD portends worse outcomes. A moderate-to-severe reduction in eGFR is associated with a 3-fold higher risk of mortality.35 In young adults with CHD admitted to an intensive care unit, acute kidney injury was associated with longer hospital stays and increased mortality at 5 years.40 Early screening for and management of CKD should be instituted for all patients living with ACHD.
Mental health in ACHD
In recent decades, it has become clear that the rate of mental health disorders in those with ACHD is higher than that of the general population.41 Some is attributable to underlying syndromic diseases (ie, 22q11.2 microdeletion), but much is not. There are also important intersections between the challenges of living with a chronic disease, quality of life, and psychosocial circumstances.42 Traumatic childhood experiences due to early health care exposure and neurocognitive differences are highly prevalent.43
In one series, 35% of patients with ACHD had anxiety and 13% had depression.44 Both anxiety and depression, which may not be identified during routine cardiac clinical care, correlated with nonadherence to care. Men with ACHD had higher rates of denial of illness impact than women counterparts, which was also associated with greater nonadherence to care.44 Depression among patients with ACHD has also been correlated with increased substance abuse (excessive alcohol use and cigarette smoking). This suggests that untreated mental health disease can directly affect physical health and longevity for those with ACHD.45
Patients with ACHD also have high rates of self-reported post-traumatic stress disorder, either globally or related to the trauma of early surgery (11%-21%).41 Unfortunately, <50% were under appropriate psychological care for their symptoms, suggesting that many are suffering in silence without the knowledge of their cardiologists.41
Robust neurocognitive screening and psychological care programmes have been recommended for ACHD programmes but are rarely implemented because of financial constraints on the health care system.46 This is a clear area for improvement in the care of patients with ACHD as we recognize that CHD has implications on all aspects of health and not just the heart.
Hospital-Based Care of Patients With ACHD
As patients with CHD age, they should have longitudinal care by their ACHD care team. They will also have contact with the health care system for a host of other reasons such as contraception and pregnancy care, noncardiac comorbidities, noncardiac surgery, and ED visits. As there has been a rapid demographic shift in CHD care over the last 3 decades, many current adult medical practitioners have received no formalized training in managing ACHD.47 These factors lead to difficult interactions for both physicians and patients.
“They (non-CHD physicians) either ask all these questions about my heart when I’m there for a sore throat or they don’t want to touch me. There’s no in-between.” Quote from a Fontan patient in her 30s.
For paediatric cardiologists and cardiac surgeons of the past, a patient with complex CHD living to a sufficient age to die from acquired adult disease would represent both a success and a challenge. In this section, we summarize the challenges for practitioners and the quality of non-CHD care received by patients with ACHD to date.
It is important to highlight the difficulty in using administrative databases for tracking the system-level care of CHD. International Classification of Diseases–Ninth Revision codes are notoriously vague in classifying CHD, with many patients being coded with “unspecified congenital heart disease” out of necessity due to lack of specificity. Also, coding patients based on their original anatomy negates the impact of different surgical techniques on long-term morbidity and mortality.48 Much of the literature we review below suffers from these limitations.
Emergency room visits by patients with ACHD
In temporal data from the United States, there has been a significant increase in the number of ED visits by patients with ACHD over the past 2 decades.49 These increases were across the board, independent of severity of CHD and region. The prevalence of comorbidities such as HTN, diabetes, CKD, obesity, and smoking among those presenting to ED with CHD also increased substantially. Almost two-thirds of those presenting to the ED required admission to hospital, with many of those with complex CHD requiring transfer to a larger centre from their local ED. Chest pain and respiratory disorders were the most common presenting concerns.49 The high proportion of patients with ACHD admitted to hospital may be attributable to a low-risk tolerance among ED physicians and a hesitance to discharge patients with complex disease with which they have low familiarity.
The dearth of trained ACHD physicians nationwide to provide quality outpatient care may also be contributing to the burden of ED visits.50 The distance from the ACHD centre has been correlated with an increase in ED visits, indicating that those living in more rural settings may seek ED care more frequently due to lack of access.51 In addition, those with ACHD living in more economically deprived areas of the United States had 74% higher risk of visiting an ED than those living in regions associated with higher socioeconomic status.52
Despite the significant increase in patients with ACHD presenting to EDs, 75% of North American general emergency medicine residency training directors rated ACHD education for their trainees as either “low priority” or “unnecessary.”53 This reveals a clear mismatch between the need for ACHD continuing education and non-CHD provider perceptions of that need.
Outpatient cardiac care provided by non-ACHD trained specialists leads to more death, permanent disability, and inadequate management among patients with moderate-to-complex CHD.54 It also leads to significantly higher cost to the health care system, including ED visits and hospitalizations.55 Nonattendance, for a variety of reasons, at specialized ACHD clinics leads to a 3-fold increase in ED visits.56 Although quality ACHD outpatient care is both clinically superior and more cost-effective, it is not available to many living with ACHD as their numbers swell.50 This will contribute to the higher burden on nonspecialized providers.
For unfamiliar ED providers, there are now guidelines available to provide a framework for the ED care of patients with ACHD and their common presentations.57 The publication of prior ACHD guidelines has been shown to improve quality of care overall, but the impact of these guidelines on ED providers has not yet been assessed. Current ED management of patients with ACHD has been found lacking, though. Although patients with ACHD are more likely to suffer from arrhythmia and heart failure than age-matched non-CHD cohorts, and less likely to have acute coronary syndromes (ACS) and atherosclerotic disease, they are more likely to be inappropriately investigated for ACS after presentation with noncardiac chest pain. This leads to both wasted cost to the health care system and increased risk to patients undergoing unnecessary procedures (ie, cardiac catheterization).58 Traditional risk stratification scores had good predictive values in patients with ACHD but were adhered to less, indicating an inappropriately higher index of suspicion for ACS among nonspecialized providers.58
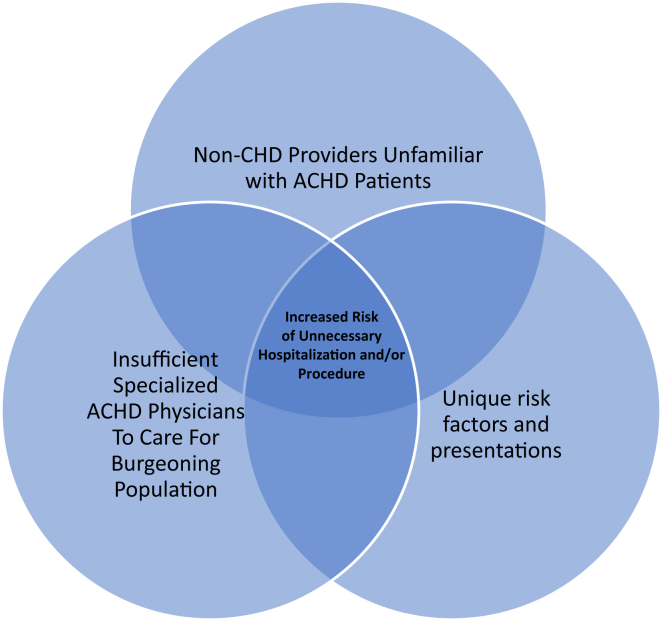
As more patients with ACHD present to EDs for care, their unique characteristics lead to a triad of risk (Fig. 3). Continuing medical education for non-CHD providers is required to combat this risk as the number of patients with ACHD continues to increase.
Figure 3.
Triad of risk in patients with adult congenital heart disease (ACHD) presenting to the emergency department.
Noncardiac surgery in patients with ACHD
As patients with CHD age, in addition to requiring further cardiac surgery at specialized centres, many also require noncardiac surgery during their lifetimes. The average anaesthesiologist and surgeon may have little to no familiarity with the complex underlying haemodynamics of CHD survivors.34 Although ideal, transfer to a tertiary care centre with ACHD providers will not always be feasible due to availability or the urgency of the needed surgery.34 Concomitant liver dysfunction, renal dysfunction, coagulopathies, and abnormal lung function present additional challenges and may be an unwelcome surprise to non-CHD practitioners.34
Much of the literature regarding noncardiac surgery in patients with CHD describes outcomes in teenagers and adolescents. In children with repaired transposition of the great arteries (atrio-ventricular concordance and ventricular-arterial discordance), the risk of significant perioperative complications (bleeding, failed extubation, and dysrhythmia) after noncardiac surgery was 8%.59 In one series, 31% of children with Fontan circulation undergoing noncardiac surgery with general anaesthesia had significant perioperative complications. Lower ejection fraction of the single ventricle was associated with worse outcomes.60 In another series, 50% of patients with a Fontan circulation required noncardiac surgery over 17-year follow-up. Although there was only 1 perioperative death, 15% required transfusions due to bleeding complications, which may affect their long-term heart transplant prospects.61 In a large case-control study, children with moderate-to-complex CHD had at least 2-fold increased mortality and risk of reintubation compared with children without CHD undergoing similar procedures.62 Even at a large tertiary care paediatric hospital, the risk of major adverse events after noncardiac surgery in patients with a single ventricle was 11%.63
As more patients with CHD survive to adulthood and acquire adult comorbidities, the likelihood that they will require noncardiac surgery increases and their perioperative risk becomes more complex. This is exacerbated by the heterogeneous nature of the adult CHD population that makes generalization regarding risk stratification difficult.64 Although recommendations suggest that patients with moderate-to-complex CHD should have surgery in regional centres of expertise, U.S. data suggest that 74% of outpatient noncardiac surgeries occur at nonspecialized centres.65 From 2002 to 2009, the number of patients with ACHD presenting for noncardiac surgery in North America increased significantly.66 This necessitates continuing medical education for all anaesthesiologists and surgeons, not just those operating in highly specialized ACHD centres.
Compared with a matched cohort, those with ACHD experienced higher rates of both perioperative morbidity and mortality.66 This highlights the medical vulnerability of the ACHD population and the need for standardized mitigation strategies.54 Nonelective surgery status was a predictor of increased mortality, suggesting that lack of time for providers to familiarize themselves with complex anatomy was detrimental.66 Although most patients with CHD die from sudden cardiac death or heart failure, 16% of all CHD patient deaths can be attributed to perioperative complications and 1% of all deaths were a result of noncardiac surgeries.67 This presents a target for improvement as we aim to lengthen the life expectancy of the average CHD survivor.
Conclusions
In summary, patients with CHD are living longer in unprecedented numbers. In addition to their native cardiac disease, they are developing acquired cardiac diseases with as-yet undefined intersections. Also, as patients with ACHD live into older age, their disease changes from a one-system problem to a multisystem disorder. Involvement of a wide umbrella of specialists is now paramount. Because of a dearth of ACHD trained cardiologists, specialists and general practitioners will need continuing medical education to keep up with the needs of this growing population and maintain quality of care. The rapidly changing demographics of the CHD population present both a challenge and an opportunity.
Ethics Statement
This review adheres to our institutional ethical guidelines and focus group quotations were obtained after approval from our local research ethics board.
Acknowledgments
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Samánek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol. 1992;13:152–158. doi: 10.1007/BF00793947. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P., Ionescu-Ittu R., Mackie A.S., et al. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157. doi: 10.1016/j.jacc.2010.03.085. [DOI] [PubMed] [Google Scholar]
- 3.Marelli A.J., Ionescu-Ittu R., Mackie A.S., et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 4.Crossland D.S., Van De Bruaene A., Silversides C.K., Hickey E.J., Roche S.L. Heart failure in adult congenital heart disease: from advanced therapies to end-of-life care. Can J Cardiol. 2019;35:1723–1739. doi: 10.1016/j.cjca.2019.07.626. [DOI] [PubMed] [Google Scholar]
- 5.Artz A.S. Biologic vs physiologic age in the transplant candidate. Hematology Am Soc Hematol Educ Program. 2016;2016:99–105. doi: 10.1182/asheducation-2016.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis M., Zannino D., du Plessis K., et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71:1009–1017. doi: 10.1016/j.jacc.2017.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Wang F., Liu A., Brophy J.M., et al. Determinants of survival in older adults with congenital heart disease newly hospitalized for heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006490. [DOI] [PubMed] [Google Scholar]
- 8.Zentner D., Celermajer D.S., Gentles T., et al. Management of people with a Fontan circulation: a Cardiac Society of Australia and New Zealand Position statement. Heart Lung Circ. 2020;29:5–39. doi: 10.1016/j.hlc.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Reich K., Moledina A., Kwan E., Keir M. Congenital heart disease (CHD) in seniors: a retrospective study defining a brand new cohort. Can Geriatr J. 2020;23:270–276. doi: 10.5770/cgj.23.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D.M., Wang T.J., Leip E.P., et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 11.Khairy P., Aboulhosn J., Broberg C.S., et al. Thromboprophylaxis for atrial arrhythmias in congenital heart disease: a multicenter study. Int J Cardiol. 2016;223:729–735. doi: 10.1016/j.ijcard.2016.08.223. [DOI] [PubMed] [Google Scholar]
- 12.Moons P., Van Deyk K., Dedroog D., Troost E., Budts W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612–616. doi: 10.1097/01.hjr.0000197472.81694.2b. [DOI] [PubMed] [Google Scholar]
- 13.de Bono J.P., Freeman L.J. Long term follow up of patients with repaired aortic coarctations. Heart. 2005;91:537–538. doi: 10.1136/hrt.2004.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windram J., Grewal J., Bottega N., et al. Canadian Cardiovascular Society: clinical practice update on cardiovascular management of the pregnant patient. Can J Cardiol. 2021;37:1886–1901. doi: 10.1016/j.cjca.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Pasquali S.K., Marino B.S., Pudusseri A., et al. Risk factors and comorbidities associated with obesity in children and adolescents after the arterial switch operation and Ross procedure. Am Heart J. 2009;158:473–479. doi: 10.1016/j.ahj.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Deen J.F., Krieger E.V., Slee A.E., et al. Metabolic syndrome in adults with congenital heart disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.114.001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afilalo J., Therrien J., Pilote L., Ionescu-Ittu R., Martucci G., Marelli A.J. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. doi: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 18.Emamaullee J., Zaidi A.N., Schiano T., et al. Fontan-associated liver disease: screening, management, and transplant considerations. Circulation. 2020;142:591–604. doi: 10.1161/CIRCULATIONAHA.120.045597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustyn A., Peng L., Singal A.G., Yopp A.C. Surveillance for hepatocellular carcinoma secondary to cardiogenic cirrhosis in patients with congenital heart disease. Clin Res Cardiol. 2015;104:446–449. doi: 10.1007/s00392-015-0809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egbe A.C., Miranda W.R., Dearani J., Kamath P.S., Connolly H.M. Prognostic role of Hepatorenal Function Indexes in patients with Ebstein anomaly. J Am Coll Cardiol. 2020;76:2968–2976. doi: 10.1016/j.jacc.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox D.A., Ginde S., Tweddell J.S., Earing M.G. Outcomes of a hepatitis C screening protocol in at-risk adults with prior cardiac surgery. World J Pediatr Congenit Heart Surg. 2014;5:503–506. doi: 10.1177/2150135114547587. [DOI] [PubMed] [Google Scholar]
- 22.Overgaard D., Schrader A.M., Lisby K.H., et al. Substance use, dental hygiene, and physical activity in adult patients with single ventricle physiology. Congenit Heart Dis. 2014;9:75–82. doi: 10.1111/chd.12086. [DOI] [PubMed] [Google Scholar]
- 23.Reid G.J., Webb G.D., McCrindle B.W., Irvine M.J., Siu S.C. Health behaviors among adolescents and young adults with congenital heart disease. Congenit Heart Dis. 2008;3:16–25. doi: 10.1111/j.1747-0803.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- 24.Karazisi C., Dellborg M., Mellgren K., et al. Risk of cancer in young and older patients with congenital heart disease and the excess risk of cancer by syndromes, organ transplantation and cardiac surgery: Swedish health registry study (1930-2017) Lancet Reg Health Eur. 2022;18:100407. doi: 10.1016/j.lanepe.2022.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adunuri N., Mrkobrada M. Not so incidental: pheochromocytoma in an adult with unrepaired cyanotic heart disease. Eur J Case Rep Intern Med. 2018;5:000959. doi: 10.12890/2018_000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mejia E.J., Rossano J.W. Congenital heart disease and the risk of cancer: the importance of understanding associated comorbidities. Lancet Reg Health Eur. 2022;18:100415. doi: 10.1016/j.lanepe.2022.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton S.U., Shimamura A., Newburger P.E., et al. Association of damaging variants in genes with increased cancer risk among patients with congenital heart disease. JAMA Cardiol. 2021;6:457–462. doi: 10.1001/jamacardio.2020.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francone M., Gimelli A., Budde R.P.J., et al. Radiation safety for cardiovascular computed tomography imaging in paediatric cardiology: a joint expert consensus document of the EACVI, ESCR, AEPC, and ESPR. Eur Heart J Cardiovasc Imaging. 2022;23:e279–e289. doi: 10.1093/ehjci/jeac048. [DOI] [PubMed] [Google Scholar]
- 29.Christman M.P., Castro-Zarraga M., Defaria Yeh D., Liberthson R.R., Bhatt A.B. Adequacy of cancer screening in adult women with congenital heart disease. ISRN Cardiol. 2013;2013 doi: 10.1155/2013/827696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neunhaeuserer D., Battista F., Mazzucato B., et al. Exercise capacity and cardiorespiratory fitness in children with congenital heart diseases: a proposal for an adapted NYHA classification. Int J Environ Res Public Health. 2022;19:5907. doi: 10.3390/ijerph19105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiesshoefer J., Orwat S., Henke C., et al. Inspiratory muscle dysfunction and restrictive lung function impairment in congenital heart disease: association with immune inflammatory response and exercise intolerance. Int J Cardiol. 2020;318:45–51. doi: 10.1016/j.ijcard.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 32.Laohachai K., Ayer J. Impairments in pulmonary function in Fontan patients: their causes and consequences. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.825841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada G., Takeuchi D., Inai K., Shinohara T., Nakanishi T. Prevalence and risk factors of sleep apnea in adult patients with congenital heart disease. Cardiol Young. 2019;29:576–582. doi: 10.1017/S1047951119000179. [DOI] [PubMed] [Google Scholar]
- 34.Lei Lei E., Heggie J. Adult congenital heart disease and anesthesia: an educational review. Paediatr Anaesth. 2021;31:123–131. doi: 10.1111/pan.13982. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos K., Diller G.P., Koltsida E., et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320–2328. doi: 10.1161/CIRCULATIONAHA.107.734921. [DOI] [PubMed] [Google Scholar]
- 36.Lee D., Levin A., Kiess M., et al. Chronic kidney damage in the adult Fontan population. Int J Cardiol. 2018;257:62–66. doi: 10.1016/j.ijcard.2017.11.118. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Lopez S., Huynh L., Benisty K., et al. Paucity of renal follow-up by school age after neonatal cardiac surgery. Cardiol Young. 2020;30:822–828. doi: 10.1017/S1047951120001067. [DOI] [PubMed] [Google Scholar]
- 38.Spence M.S., Balaratnam M.S., Gatzoulis M.A. Clinical update: cyanotic adult congenital heart disease. Lancet. 2007;370:1530–1532. doi: 10.1016/S0140-6736(07)61647-X. [DOI] [PubMed] [Google Scholar]
- 39.Arvanitaki A., Ibrahim W., Shore D., et al. Epidemiology and management of Staphylococcus aureus infective endocarditis in adult patients with congenital heart disease: a single tertiary center experience. Int J Cardiol. 2022;360:23–28. doi: 10.1016/j.ijcard.2022.04.078. [DOI] [PubMed] [Google Scholar]
- 40.Fuhrman D.Y., Nguyen L., Joyce E.L., Priyanka P., Kellum J.A. Outcomes of adults with congenital heart disease that experience acute kidney injury in the intensive care unit. Cardiol Young. 2021;31:274–278. doi: 10.1017/S1047951120003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng L.X., Khan A.M., Drajpuch D., et al. Prevalence and correlates of post-traumatic stress disorder in adults with congenital heart disease. Am J Cardiol. 2016;117:853–857. doi: 10.1016/j.amjcard.2015.11.065. [DOI] [PubMed] [Google Scholar]
- 42.Bang J.S., Jo S., Kim G.B., et al. The mental health and quality of life of adult patients with congenital heart disease. Int J Cardiol. 2013;170:49–53. doi: 10.1016/j.ijcard.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Keir M., Bailey B., Lee A., et al. Narrative analysis of adults with complex congenital heart disease: childhood experiences and their lifelong reverberations. Congenit Heart Dis. 2018;13:740–747. doi: 10.1111/chd.12647. [DOI] [PubMed] [Google Scholar]
- 44.White K.S., Pardue C., Ludbrook P., et al. Cardiac denial and psychological predictors of cardiac care adherence in adults with congenital heart disease. Behav Modif. 2016;40:29–50. doi: 10.1177/0145445515613329. [DOI] [PubMed] [Google Scholar]
- 45.Khan M., Monaghan M., Klein N., Ruiz G., John A.S. Associations among depression symptoms with alcohol and smoking tobacco use in adult patients with congenital heart disease. Congenit Heart Dis. 2015;10:E243–E249. doi: 10.1111/chd.12282. [DOI] [PubMed] [Google Scholar]
- 46.Keir M., Ebert P., Kovacs A.H., et al. Neurocognition in adult congenital heart disease: how to monitor and prevent progressive decline. Can J Cardiol. 2019;35:1675–1685. doi: 10.1016/j.cjca.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Gurvitz M., Lui G.K., Marelli A. Adult congenital heart disease-preparing for the changing work force demand. Cardiol Clin. 2020;38:283–294. doi: 10.1016/j.ccl.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Riehle-Colarusso T.J., Bergersen L., Broberg C.S., et al. Databases for congenital heart defect public health studies across the lifespan. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal S., Sud K., Khera S., et al. Trends in the burden of adult congenital heart disease in US emergency departments. Clin Cardiol. 2016;39:391–398. doi: 10.1002/clc.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen L.T., Maul T.M., Hindes M., et al. Current and future status of adult congenital training in North America. Am J Cardiol. 2015;115:1151–1153. doi: 10.1016/j.amjcard.2015.01.552. [DOI] [PubMed] [Google Scholar]
- 51.Khan A.M., McGrath L.B., Ramsey K., et al. Distance to care, rural dwelling status, and patterns of care utilization in adult congenital heart disease. Pediatr Cardiol. 2022;43:532–540. doi: 10.1007/s00246-021-02750-7. [DOI] [PubMed] [Google Scholar]
- 52.Tillman A.R., Colborn K.L., Scott K.A., et al. Associations between socioeconomic context and congenital heart disease related outcomes in adolescents and adults. Am J Cardiol. 2021;139:105–115. doi: 10.1016/j.amjcard.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 53.Cross K.P., Santucci K.A. Transitional medicine: will emergency medicine physicians be ready for the growing population of adults with congenital heart disease? Pediatr Emerg Care. 2006;22:775–781. doi: 10.1097/01.pec.0000245178.13418.4f. [DOI] [PubMed] [Google Scholar]
- 54.Cordina R., Nasir Ahmad S., Kotchetkova I., et al. Management errors in adults with congenital heart disease: prevalence, sources, and consequences. Eur Heart J. 2018;39:982–989. doi: 10.1093/eurheartj/ehx685. [DOI] [PubMed] [Google Scholar]
- 55.Willems R., Ombelet F., Goossens E., et al. Different levels of care for follow-up of adults with congenital heart disease: a cost analysis scrutinizing the impact on medical costs, hospitalizations, and emergency department visits. Eur J Health Econ. 2021;22:951–960. doi: 10.1007/s10198-021-01300-5. [DOI] [PubMed] [Google Scholar]
- 56.Awh K., Venuti M.A., Gleason L.P., et al. Clinic nonattendance is associated with increased emergency department visits in adults with congenital heart disease. Congenit Heart Dis. 2019;14:726–734. doi: 10.1111/chd.12784. [DOI] [PubMed] [Google Scholar]
- 57.Chessa M., Brida M., Gatzoulis M.A., et al. Emergency department management of patients with adult congenital heart disease: a consensus paper from the ESC Working Group on Adult Congenital Heart Disease, the European Society for Emergency Medicine (EUSEM), the European Association for Cardio-Thoracic Surgery (EACTS), and the Association for Acute Cardiovascular Care (ACVC) Eur Heart J. 2021;42:2527–2535. doi: 10.1093/eurheartj/ehab272. [DOI] [PubMed] [Google Scholar]
- 58.Gales J., Krasuski R.A., Awerbach J.D. Emergency department evaluation of chest pain among adult congenital heart disease patients. Am Heart J. 2020;222:191–198. doi: 10.1016/j.ahj.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Christensen R.E., Reynolds P.I., Bukowski B.K., Malviya S. Anaesthetic management and outcomes in patients with surgically corrected D-transposition of the great arteries undergoing non-cardiac surgery. Br J Anaesth. 2010;104:12–15. doi: 10.1093/bja/aep332. [DOI] [PubMed] [Google Scholar]
- 60.Rabbitts J.A., Groenewald C.B., Mauermann W.J., et al. Outcomes of general anesthesia for noncardiac surgery in a series of patients with Fontan palliation. Paediatr Anaesth. 2013;23:180–187. doi: 10.1111/pan.12020. [DOI] [PubMed] [Google Scholar]
- 61.Palumbo T., Sluysmans T., Rubay J.E., Poncelet A.J., Momeni M. Long-term outcome and anaesthetic management for non-cardiac surgery after Fontan palliation: a single-centre retrospective analysis. Cardiol Young. 2015;25:1148–1154. doi: 10.1017/S1047951114001814. [DOI] [PubMed] [Google Scholar]
- 62.Faraoni D., Zurakowski D., Vo D., et al. Post-operative outcomes in children with and without congenital heart disease undergoing noncardiac surgery. J Am Coll Cardiol. 2016;67:793–801. doi: 10.1016/j.jacc.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 63.Brown M.L., DiNardo J.A., Odegard K.C. Patients with single ventricle physiology undergoing noncardiac surgery are at high risk for adverse events. Paediatr Anaesth. 2015;25:846–851. doi: 10.1111/pan.12685. [DOI] [PubMed] [Google Scholar]
- 64.Gerardin J.F., Earing M.G. Preoperative evaluation of adult congenital heart disease patients for non-cardiac surgery. Curr Cardiol Rep. 2018;20:76. doi: 10.1007/s11886-018-1016-5. [DOI] [PubMed] [Google Scholar]
- 65.Maxwell B.G., Maxwell T.G., Wong J.K. Decentralization of care for adults with congenital heart disease in the United States: a geographic analysis of outpatient surgery. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maxwell B.G., Wong J.K., Kin C., Lobato R.L. Perioperative outcomes of major noncardiac surgery in adults with congenital heart disease. Anesthesiology. 2013;119:762–769. doi: 10.1097/ALN.0b013e3182a56de3. [DOI] [PubMed] [Google Scholar]
- 67.Engelings C.C., Helm P.C., Abdul-Khaliq H., et al. Cause of death in adults with congenital heart disease—an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol. 2016;211:31–36. doi: 10.1016/j.ijcard.2016.02.133. [DOI] [PubMed] [Google Scholar]