Abstract
Sudden cardiac arrest in the young is a rare event with a range of potential causes including cardiomyopathies, ion channelopathies, and autonomic nervous system dysfunction. Investigations into the cause involve a multidisciplinary team, including cardiologists, geneticists, and psychologists. In addition to a detailed medical history, family history and circumstances surrounding the event are important in determining the cause. Clinical investigations including an electrocardiogram are fundamental in diagnosis and should be interpreted cautiously because some children may have atypical presentations and an evolving phenotype. The potential for misdiagnosis exists that could lead to incorrect long-term management strategies. If an inherited condition is suspected, genetic testing of the patient and cascade screening of family members is recommended with genetic counselling and psychological support. Medical management is left to the treating physician acknowledging that a clear diagnosis cannot be made in approximately half of cases. Secondary prevention implantable defibrillators are widely deployed but can be associated with complications in young patients. A plan for safe return to activity is recommended along with a proper transition of care into adulthood. Broad screening of the general population for arrhythmia syndromes is not recommended; preventative measures include screening paediatric patients for risk factors by their primary care physician. Several milestone events or activities that take place in youth could be used as opportunities to promote safety. Further work into risk stratification of this paediatric population through patient registries and greater awareness of cardiopulmonary resuscitation and automated external defibrillator use in saving lives is warranted.
Résumé
L’arrêt cardiaque subit chez les enfants est un événement rare dont les causes possibles comprennent la cardiomyopathie, la maladie des canaux ioniques ou une dysfonction du système nerveux autonome. Pour cerner la cause exacte, on fait appel à une équipe multidisciplinaire composée notamment de cardiologues, de généticiens et de psychologues. En plus de recueillir les antécédents médicaux complets du patient, il est également important de s’enquérir des antécédents familiaux ainsi que des circonstances entourant l’événement. Les examens cliniques comme un électrocardiogramme sont par ailleurs essentiels pour établir le diagnostic, mais doivent être interprétés avec prudence, car chez certains enfants, le tableau peut être atypique et le phénotype peut évoluer. Une erreur de diagnostic pourrait fausser la stratégie de prise en charge à long terme. Si l’on soupçonne une cause héréditaire, un dépistage génétique est recommandé pour le patient et pour chacun des membres de sa famille de même qu’une consultation génétique et un soutien psychologique. Il revient au médecin traitant de déterminer la conduite à suivre et de ne pas perdre de vue que, dans environ la moitié des cas, il n’est pas possible de poser un diagnostic avec certitude. Les défibrillateurs implantables sont largement employés en prophylaxie secondaire, mais s’accompagnent d’un risque de complications chez les jeunes patients. Un plan de retour prudent à l’activité physique est recommandé, et le jeune patient devra être suivi jusqu’à l’âge adulte. Le dépistage systématique des symptômes de l’arythmie dans la population générale n’est pas recommandé. En revanche, le médecin de première ligne peut, à titre préventif, évaluer les facteurs de risque chez les patients pédiatriques. Plusieurs événements marquants ou étapes clés dans la vie de l’enfant pourraient être l’occasion de promouvoir les mesures de sécurité à cet égard. Enfin, il est nécessaire d’effectuer d’autres études sur la stratification des risques dans la population pédiatrique à l’aide des registres de patients et de promouvoir les manœuvres de réanimation cardiorespiratoire ainsi que le recours aux défibrillateurs externes automatisés pour sauver des vies.
Sudden cardiac arrest (SCA) is rare in children with estimates between 1 and 3 cases/100,000 children.1 Most cases occur in apparently previously well children. Prompt recognition of the situation by witnesses with immediate administration of cardiopulmonary resuscitation (CPR) and access to an automated external defibrillator (AED) can be lifesaving. Details of a witnessed event are critical in guiding therapy and diagnostic testing. After initial stabilization and resuscitation, further management is guided by neurologic, physical recovery and identification of treatable underlying conditions. Potential causes are numerous and each condition is rare, necessitating the management by specialized multidisciplinary teams (Fig. 1). Despite systematic and thorough evaluations, approximately 50% of SCA cases remain unexplained.2 In this review, we focus on the most frequently encountered genetic and congenital causes of SCA in the young, with the additional inclusion of some newly recognized conditions and potential autonomic nervous system (ANS) contributors. This review does not address acquired causes including SCA secondary to underlying infection (eg, myocarditis), illness (eg, malignancy), trauma, accidents, and drug abuse. Where relevant, we include atypical features relevant to the paediatric presentation or that may contribute to a delay in diagnosis. Practical recommendations for management, follow-up of patients and their families are presented.
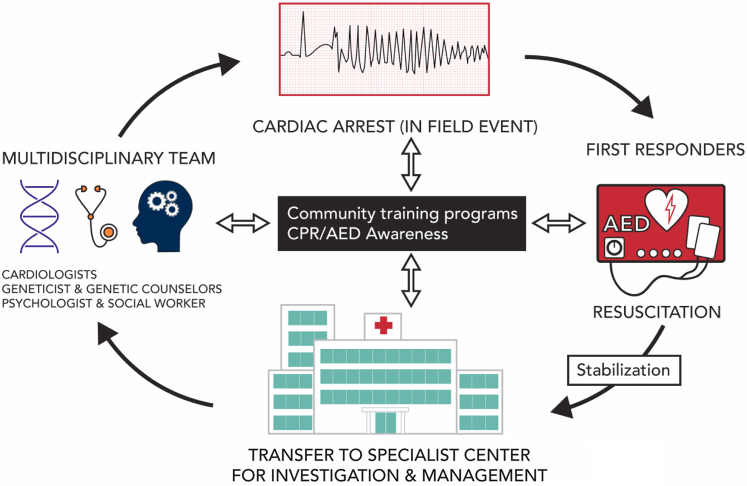
Figure 1.
Overview of events that occur after a sudden cardiac arrest (SCA). Subsequent to an infield event, first responders and/or bystander attend the scene with an AED and/or CPR in an effort to resuscitate the victim. After stabilization, the patient is transferred to a special center for investigation and management. Investigation of the patient with SCA should involve a multidisciplinary approach by a team of specialists that include cardiologists, genetic counsellors, and psychologists. An important part of the workup is the electrocardiogram recording, especially those acquired at the time of the event. Promoting safe communities with overall greater CPR and AED awareness will impact not only the infield event but also outcomes of SCA survivors. AED, automated external defibrillator; CPR, cardiopulmonary resuscitation.
What Is Young?
Sudden infant death syndrome (SIDS) occurs in infants typically aged 2-3 months with an incidence of 0.2-0.5 per 1000 live births in the majority of countries.3 Several of the conditions, especially cardiomyopathies and Brugada syndrome (BrS), do not present in their typical form until beyond the paediatric age group, usually defined as younger than 18 years of age. Most studies have limited their participants to those experiencing cardiac arrest and/or death before ages 35-40 years. Therefore, although this review does focus on paediatric aspects, much of the information is pertinent for any patient with SCA younger than 35-40 years.
Causes of Sudden Unexpected Death in the Young
The most common cause of sudden unexpected death (SUD) in those aged 1-35 years is arrhythmic syndromes.4 These conditions are largely heritable and include cardiomyopathies and ion channelopathies, characterized by variable expressivity and incomplete penetrance among family members. Paediatric patients are often asymptomatic or misdiagnosed with benign conditions such as vasovagal syncope; the first presenting symptom can be an SCA and/or SUD. These conditions can be detected incidentally or through cascade family screening. Other causes of SCA and/or SUD in the young include noncardiac causes such as an abnormal functioning ANS (Fig. 2).
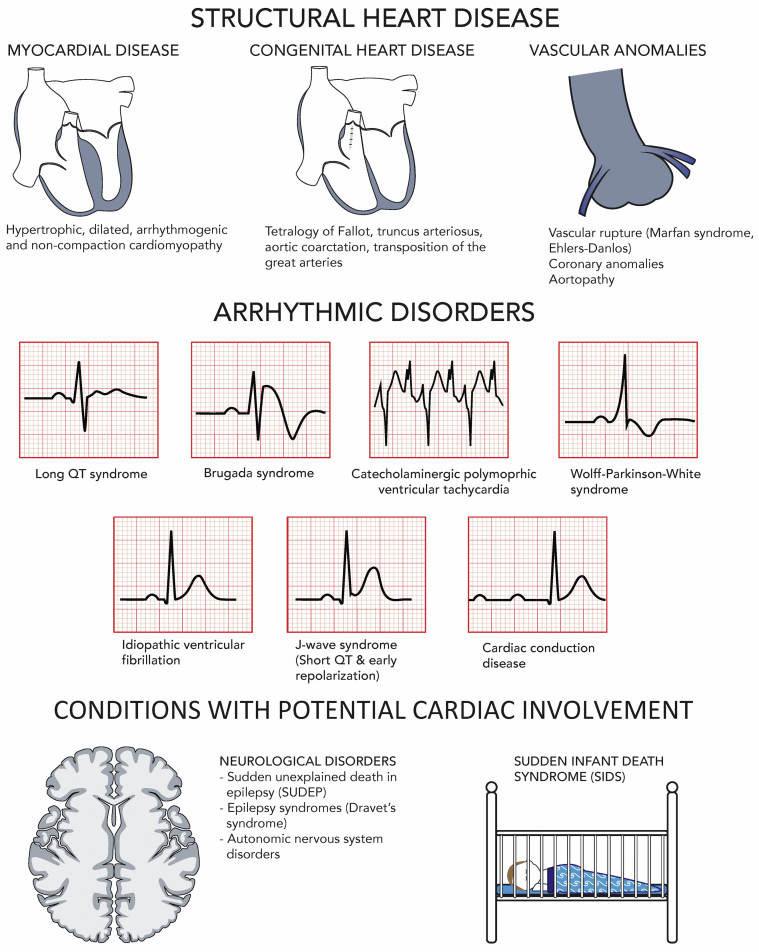
Figure 2.
Causes of SUD in the young.
Cardiomyopathies
Cardiomyopathies are associated with an anatomically normal heart with abnormal cardiac myocyte structure or function. Myopathic changes result in areas of ischemia or microinfarcts and fibrosis that act as substrates for re-entry and abnormal automaticity triggering arrhythmias.5 Cardiomyopathies often result from genetic alterations in proteins found in the sarcomere, desmosomes, or cytoskeleton. Each has distinct morphological features identifiable with imaging, initially echocardiography and in some cases magnetic resonance imaging. Hypertrophic cardiomyopathy (HCM) accounts for most cases of sudden cardiac death (SCD) in the young6 and less commonly dilated cardiomyopathy (DCM), arrhythmogenic cardiomyopathy (ACM), and left ventricular noncompaction (LVNC). Cases of SCA have occurred in those harbouring a genetic risk in the absence of an overt phenotype.7
Hypertrophic cardiomyopathy
HCM, a common cause of SCD in young athletes, is characterized by an abnormally thickened ventricular myocardium that manifests during midlife, though can present in infancy and throughout childhood.8 Most cases are due to causative variants in genes encoding the sarcomere. Inheritance is usually autosomal dominant with variable penetrance. SCA in HCM is thought to occur as a result of ventricular tachycardia (VT) and/or ventricular fibrillation (VF) but may also result from atrial fibrillation (AF) or rapid atrial tachycardia with increasing myocardial ischemia, diastolic dysfunction, systemic hypotension, decreased stroke volume, and secondary ventricular arrhythmias.9 A model for SCD risk prediction in paediatric HCM has been reported.10
Dilated cardiomyopathy
DCM is characterized by an enlarged, dilated left and/or right ventricle with or without decreased systolic function.11 Causes of DCM in children are diverse with approximately 70% of cases categorized as idiopathic. Children with severe cases of DCM often present with heart failure. Older age at diagnosis, positive family history, and severity of left ventricular dysfunction are linked to increased risk of SCD.12 Genetic causes of DCM include causative variants in genes encoding the sarcomere, desmosome, cytoskeleton, and mitochondria.13 Truncating variants in TTN account for up to 25% of idiopathic DCM, but onset typically occurs in the fifth and sixth decades of life.
Arrhythmogenic cardiomyopathy
ACM is characterized by an enlarged, dilated right and/or left ventricle with or without decreased systolic function and associated with frequent arrhythmias due to fibrofatty degeneration of the right and/or left ventricular myocardium.14 The right ventricle is predominantly affected; a study of ACM mutation positive patients revealed that 96% have an abnormality in the right ventricle and 52% in the left ventricle.15 Most cases are autosomal dominant and due to causative variants in genes encoding the desmosome. During early stages of the disease, ACM consists of a subclinical phase where structural abnormalities are concealed and patients exhibit no symptoms, though patients may experience SCA and/or SUD. This concealed phase, linked to variants in the PKP2 gene,16 may resemble ion channelopathies due to the presence of ventricular arrhythmias and the absence of structural changes on imaging. Task force criteria are not applicable to children, and this diagnosis requires a high degree of suspicion. Phenotyping the parents is an important step if one suspects ACM in a child. ACM typically presents between the second and fourth decade of life, although in paediatric patients, it presents more often as SCA and/or SCD than in adults.14,17
Left ventricular noncompaction
LVNC is a heterogeneous cardiomyopathy thought to result from intrauterine arrest of the compaction process, frequently affecting the left ventricular apex and characterized by a prominent left ventricular mesh of trabeculated and/or noncompacted hypertrophied endocardium, deep intertrabecular recesses, and compacted and/or thin epicardial layer.18 Patients are asymptomatic or experience thromboembolic events, congestive heart failure, and life-threatening arrhythmias. LVNC may occur in isolation or with other cardiomyopathy phenotypes, arrhythmias, congenital heart diseases (CHDs) (including LV and/or right ventricle outflow tract abnormalities, Ebstein’s anomaly, tetralogy of Fallot, and double outlet right ventricle), neuromuscular diseases, and genetic syndromes. LVNC may be sporadic or familial, inherited in an autosomal dominant or X-linked recessive manner. Causative variants include genes encoding the sarcomere, Z-disc, cytoskeleton, and mitochondria. Although historically considered a cardiomyopathy, there should be caution in making this diagnosis as LV trabeculation is now widely considered a physiological trait.
Ion channelopathies
Ion channelopathies are typically associated with normal cardiac anatomy and result from genetic alterations in membrane ion channels or intracellular proteins that affect ion transport. Electrocardiogram (ECG) abnormalities at baseline or under particular stimuli may assist in differentiating ion channelopathies among living SCA survivors.
Long QT syndrome
Long QT syndrome (LQTS) is characterized by a prolonged corrected QT interval (QTc) with abnormalities in T-wave morphology on an ECG with risk of malignant arrhythmias.19 Caution is needed when measuring the QT interval in children due to the inaccuracies of various correction formulae and difficulties with measurement.20 Therefore, it is advised that the ECG be repeated to overcome these confounders and revisit an LQTS diagnosis as the child matures (eg, when the child is school aged, can complete an exercise stress test [EST] typically around ages 8-10). The LQTS phenotype ranges from asymptomatic to syncope and SCD caused by torsade de pointes. SIDS has been attributed to LQTS in approximately 10% of cases.21 LQTS can be categorized into 3 main subtypes (LQT1-3) due to causative variants in 3 major susceptibility genes KCNQ1, KCNH2, and SCN5A or acquired from drugs, CNS processes, or electrolyte and/or metabolic imbalance as is seen in TANGO2 disease.22 Severe forms of LQTS presenting in infancy or early childhood as life-threatening arrhythmias are due to variants in genes encoding calmodulin and triadin.23 Males are at greater risk of cardiac events up to age 15 (puberty phase), and the risk increases in females during adulthood.24 Because of the risk of life-threatening arrhythmic events, guidelines recommend that patients with a clinical LQTS diagnosis regardless of phenotype be treated with β-blockers.25
Catecholaminergic polymorphic ventricular tachycardia
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is characterized by bidirectional and polymorphic VT (PVT) during emotional stress or exercise, normal resting ECG, and no structural abnormalities.26 Symptoms associated with CPVT include palpitations, (near-)syncope, and cardiac arrest and/or death. The phenotype is usually unmasked during an EST with the presence of premature ventricular complexes progressing to bigeminy and ventricular tachyarrhythmias27 as well as atrial tachyarrhythmias in some cases.28,29 CPVT is inherited in an autosomal dominant form with variants in RYR2, which encode the cardiac ryanodine receptor, accounting for the majority of cases and less commonly, CASQ2.30
Brugada syndrome
BrS is characterized by a coved-type elevated ST segment in the right precordial leads on an ECG.31 Variants in the SCN5A gene account for 20%-25% of BrS patients with the majority remaining gene elusive and likely to have a strong polygenic contribution.32 BrS manifests primarily in males during adulthood although can present in the young and usually occurs during rest and/or sleep, times of high vagal tone, and fever.25 Young patients may be asymptomatic or can present with a spectrum of abnormalities including sinus node dysfunction, atrial arrhythmias, first-degree atrioventricular block, intraventricular conduction delay, potentially lethal arrhythmias, and SCD secondary to PVT and/or VF33; the original eponymous series did include several young children.31
Short QT syndrome
Short QT syndrome (SQTS) is an extremely rare condition, inherited in an autosomal dominant pattern and high penetrance.34 It is characterized by an abnormal shortening of the QTc with prominent and peaked T waves and no evident structural heart disease. Causative variants in 3 genes encoding potassium channels (KCNH2, KCNQ1, and KCNJ2) are implicated with the most prevalent subtype associated with gain-of-function variants in KCNH2 (SQTS1). SCA, palpitations, syncope, and AF can occur under varying circumstances such as a loud noise, at rest, normal activity, or exercise and often occurs in the first year of life. Application of the Gollob score (a score diagnostic for SQTS developed in the adult SQTS population based on 4 criteria: QT interval, clinical history, family history, and genotype) was challenging to apply in paediatric SQTS patients due to the high rate of inappropriate shocks in this population.
Wolff-Parkinson-White syndrome
Although not related to ion channels, Wolff-Parkinson-White syndrome (WPW) is an important cause of SCA and/or SUD in the young and is frequently encountered in clinical practice. One in 1000 individuals have a WPW pattern on ECG. WPW arises from abnormal cardiac conduction due to an accessory pathway between the atrium and ventricle that bypass the AV node. Most cases of WPW are sporadic though there are associations with cardiomyopathy and structural heart disease. Variants in PRKAG2, which leads to alterations in AMP-activated protein kinase, and other deleterious variants in genes associated with AF have been described in some cases.35 Typically, WPW is associated with non–life-threatening supraventricular tachycardia; however, AF, atrial flutter, or atrial tachycardia can develop in children and lead to VF.36 Loss of accessory pathway conduction in noninvasive testing was thought to identify patients at low risk of a cardiac event; however, it is difficult to identify those at risk of SCA.37 Unlike other primarily electrical conditions, catheter-based ablation is curative.38
Idiopathic ventricular fibrillation
Idiopathic ventricular fibrillation (IVF) is a rare cause of SCA characterized by VF of unknown origin.39 IVF is a distinct condition because a subgroup of IVF patients have been shown to have a primary electrical disease termed short-coupled VF.40 The term IVF has been inconsistently used in the literature to describe survivors of cardiac arrest with no explanation despite clinical testing.41 Therefore, a standardized algorithm consisting of clinical tests with high yield such as cardiac magnetic resonance imaging (CMRI), EST, and sodium-channel blocker challenge has been developed to facilitate a uniform diagnosis of IVF in these patients.41
Early repolarization syndrome
Although a common ECG finding in the general population, especially healthy adolescents, an early repolarization (ER) pattern in the inferior and/or lateral leads is associated with increased risk of VF and SCD in older invidividuals.42 Patients are typically diagnosed with early repolarization syndrome (ERS) when resuscitated from a documented episode of IVF and/or PVT or SCD victims with a previous ECG and negative autopsy.25 ERS is likely polygenetic and influenced by nongenetic factors.27 An ER pattern in the paediatric population does not appear to be a marker of potentially fatal arrhythmias, and the finding of ERS in a paediatric patient after SCA should not be considered an underlying diagnosis.43
Congenital complete heart block
Congenital complete heart block is a complete absence of conduction between the atria and ventricles due to either immune mediated injury in utero, genetic variants in multiple genes, or as a result of CHD.44 It is accompanied by bradycardia and can be associated with ventricular dysfunction. Repolarization can be abnormal and approximately 20% can develop cardiac dysfunction. Pacing indications are based on risk factors for sudden death, including symptoms, very slow ventricular escape rates, and ventricular dysfunction. Patients typically require a pacemaker with careful consideration of leads, their management, and mode of pacing in the young.45
Congenital heart disease
Because of increases in prenatal screening and corrective surgery, survival into adulthood has increased dramatically for young patients with CHD.46 The most common types of CHD diagnoses implicated in SCA and/or SUD in the young vary based on demographics and geographic locations and include coarctation of aorta, transposition of the great arteries, and univentricular heart.47,48 Although most patients are diagnosed with CHD before death, unrecognized CHD continues to account for cases of SCD in the young.47,48 If left untreated, CHD can lead to cardiac hypertrophy, fibrosis, cardiac failure with thin-walled ventricle, and increased risk of lethal cardiac arrhythmias.46 In surgically treated CHDs, the intervention itself may cause further damage and act as a focus for cardiac arrhythmias and SCD.
Aortopathy
Individuals with a dilated aorta are at increased risk of aortic aneurysm, dissection, and rupture under conditions of high wall stress such as during heavy exercise especially isometric exercise.49 Several inherited disorders predispose to aortic dilation and rupture including Marfan and Ehler-Danlos syndromes. Other CHDs such as bicuspid aortic valve, a common condition in 1%-2% of the population, appears to be heritable and is frequently associated with a dilated ascending aorta. Further, patients with tetralogy of Fallot, truncus arteriosus, and transposition of the great arteries have been shown to develop dilatation of the neoaortic root. A dilated aorta is associated with decreased elasticity, increased stiffness of the aortic wall, negatively influencing ventricular function due to increased afterload, and ventricular hypertrophy. Medical therapies aimed at reducing blood pressure with or without a reduction in heart rate including β-blockers and angiotensin-converting enzyme inhibitors may be beneficial; patients considered at high risk of aortic dissection often require surgical management.
Congenital coronary artery anomalies
Congenital coronary anomalies are malformations that are rare (<1% of population) and found either in isolation or coexist with complex CHDs.50 They are typically diagnosed in the young using echocardiography although computed tomography and magnetic resonance imaging are generally recognized as the gold standard. These are often incidental findings in otherwise healthy children, but can be identified in the evaluation of SCA and/or SUD. The mechanism leading to sudden death is thought to involve ischemia, fibrosis, and malignant arrhythmias. Sudden death particularly in young athletes is often the first presenting symptom. In a case series of postmortem young athletes, it was demonstrated that these patients had experienced exertional syncope or chest pain particularly in the setting of anomalous left main coronary artery origin before death.51 Patients with coronary artery anomalies are at risk of cardiac arrest, with the anomalous origin of the right coronary artery from the left aortic sinus of Valsalva having a more benign clinical course vs left coronary artery anomalies from the right aortic sinus of Valsalva that often require surgical correction.
Emerging Causes of Unexplained SCA and/or SCD
Novel cardiac arrhythmia syndromes
Several novel cardiac arrhythmia syndromes have been documented in recent years. Triadin knockout syndrome is reportedly caused by homozygous TRDN variants leading to risk of arrhythmias in the young.52 It is a recessively inherited cardiac arrhythmia syndrome manifesting with extensive T-wave inversion in precordial leads V1-V4 with consistent or transient QT prolongation, exercise-induced syncope or cardiac arrest in early childhood (less than or equal to 5 years of age), and possible noncardiac involvement with mild-to-moderate skeletal muscle weakness. An autosomal dominant cardiac syndrome termed familial ST-segment depression syndrome with ECG changes including ST segment depression, AF, and ventricular arrhythmias has been identified.53 Calcium release deficiency syndrome has been linked to loss of function RYR2 variants and early after depolarizations in contrast to gain of function variants and delayed after depolarizations typically associated with CPVT.54 Patients with calcium release deficiency syndrome demonstrate no arrhythmias using conventional EST and are at risk of SCD.
Autonomic nervous system
Perturbations in the parasympathetic and/or sympathetic systems of the ANS or autonomic conflict, defined as concomitant sympathetic and parasympathetic stimulation, have been implicated in cardiac arrhythmias, SCD, SUD in epilepsy, and SIDS.5,55, 56, 57 It is well known that the ANS influences inherited arrhythmia syndromes. For example, sympathectomy has been used to treat LQTS and CPVT. BrS manifests under conditions of high vagal tone. ANS testing in an adolescent patient whose pacemaker recorded PVT at the time of a syncopal event revealed impaired vagal and sympathetic baroreflex function that likely contributed to the arrhythmia.58 In susceptible individuals, these episodes of PVT and/or VF could progress to an SCA.
The Workup
Investigation of paediatric SCA should be targeted towards the etiologies described above,59 acknowledging that a clear diagnosis cannot be made in a significant proportion. Misdiagnoses based on limited or inconclusive data contribute to severe adverse patient outcomes, including recurrent life-threatening events. The management of patients after SCA has recently been addressed in detail in a joint publication by the Asia Pacific Heart Rhythm Society and Heart Rhythm Society,4 and as such we focus on features specific to paediatric patients (Fig. 3).
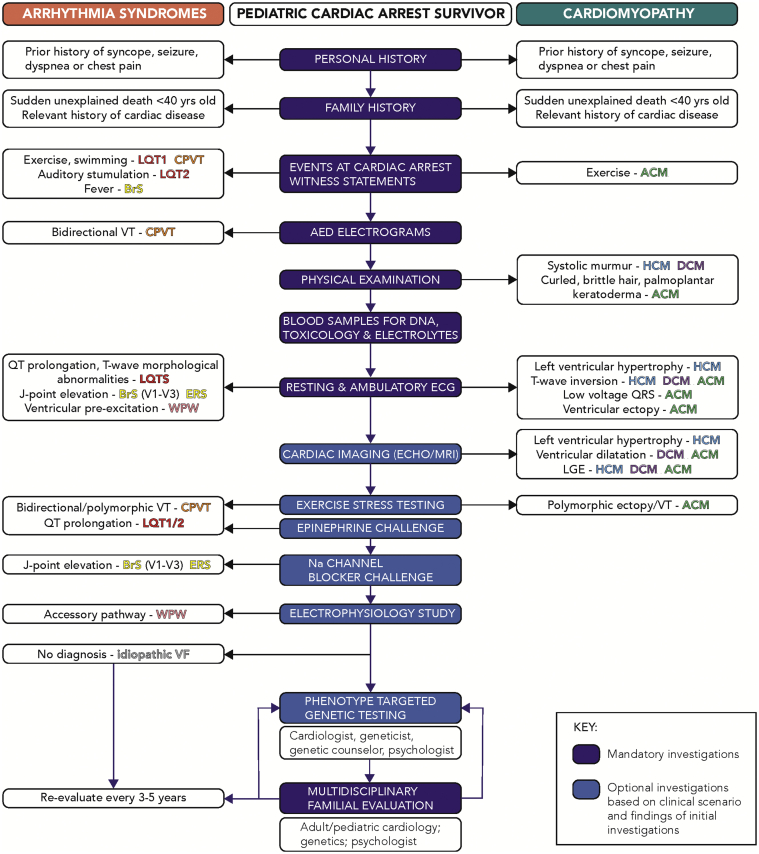
Figure 3.
The workup of a paediatric cardiac arrest survivor; ACM, arrhythmogenic cardiomyopathy; AED, automated external defibrillator; BrS, Brugada syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ECG, electrocardiogram; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; LGE, late gadolinium enhancement; LQTS, long QT syndrome; MRI, magnetic resonance imaging; QRS, Q wave, R wave, S wave; VF, ventricular fibrillation; VT, ventricular tachycardia; WPW, Wolff-Parkinson-White syndrome.
Clinical history and examination
Investigations should begin with a detailed personal medical history, focusing on symptoms immediately before the event, and nature of events surrounding the cardiac arrest. One-fifth of patients experience syncope or seizures before cardiac arrest,60 although for many this is the sentinel event.61,62 Cerebral anoxia during cardiac arrhythmias leading to seizure activity or convulsive syncope have been mistaken for epilepsy, and the results of any prior neurological investigations should be determined, which are often normal.63,64 Notably, a small proportion of patients with KCNH2 variants have a true overlap syndrome between epilepsy and LQTS65 and RYR2 variants with epilepsy.66 Whether an SCA has a cardiac or neurological etiology can be challenging to determine; risk factors for cardiac arrest or SUD in epilepsy include refractory epilepsy often associated with conditions such as Dravet syndrome, neurological disability, or autism.67,68 A neurology consult is often helpful if there is doubt.
Specific triggers may be temporally associated with cardiac arrest and should be sought during the history. Arrhythmia in both LQT1 and CPVT is triggered by adrenergic stimulation, such as exercise and emotion, although a minority of events may occur at rest.26 Swimming is a specific trigger in LQTS1 and CPVT; however, autonomic conflict may play a role because coactivation of the sympathetic and parasympathetic systems during cold water immersion has been described as proarrhythmic.69 Auditory stimuli are associated with LQT2. One of the challenges in the history is that almost every child has been active and performed the same activity countless times before the SCA. This speaks to the unknown factors that cause SCA, especially in genetic conditions present since birth. We hypothesize that adolescence is a time of particular risk due to hormonal and neural factors, the same that contribute to the spike in reflex syncope at this age. Certain medications may exacerbate ion channel dysfunction leading to enhanced ECG changes and arrhythmia in either LQTS (www.crediblemeds.org) or BrS (www.brugadadrugs.org); fever is an important trigger for events in paediatric BrS and events may be mistaken as febrile seizures.70,71 A febrile illness with respiratory or gastrointestinal symptoms may be indicative of viral myocarditis.
Noncardiac features may also be helpful in determining possible cardiac etiologies. A history or clinical evidence of subtle skeletal myopathy may be indicative of desmin- or lamin-mediated cardiomyopathies; intermittent episodes of periodic paralysis, short stature, and classical facial features may be evident in Andersen-Tawil syndrome due to variants in KCNJ2.72 Recurrent episodic chest pain secondary to myocardial inflammation and cardiocutaneous features (tightly curled hair, palmoplantar hyperkeratosis) are important features of desmoplakin ACM.73
Family history
In a child with a potentially inherited disorder, a 3-generation family history is key in the investigation. This is ideally performed by a genetic counsellor or nurse with specific training and expertise because evidence supports increased detection of critical family details as compared with when history taking is performed by physicians.74 Any cardiac investigations in family members with a relevant history or symptoms should be acquired, as well as autopsy details of any prior SCD cases within the family, including any genetic findings from autopsy, if available. Immediately after the cardiac arrest may be an opportune time to acquire all relevant information and to speak with multiple family members because a diagnosis is more likely.75 Construction of a detailed pedigree is also key in evaluating genetic findings and segregation across different family members with clinical findings and the family itself as the most powerful tool for subsequent validation of any identified variants.
Clinical investigations
The approach to clinical investigations in patients after SCA includes several baseline investigations, provocative tests, genetic testing recommendations, and cascade testing of family members.4 In any potentially arrhythmic cardiac arrest, ECG recordings are key, especially those acquired at the time of the event. ECG traces from the AED acquired during the resuscitation may provide diagnostic information leading to the early instigation of appropriate management strategies. Bidirectional tachycardia in the setting of an exertional cardiac arrest is almost pathognomonic of CPVT and may be captured as patients with this adrenergic-triggered disorder of calcium handling in cardiac myocytes often require multiple episodes of defibrillation.76
The 12-lead ECG is fundamental to the investigation in young patients, and the ECG (resting, exercise, or during drug provocation) has a high diagnostic yield in familial investigations after SCD.75 Importantly, ECG abnormalities may result from the cardiac arrest, and so early findings should be interpreted with caution, especially minor degrees of QT prolongation and T-wave morphological abnormalities. However, classical cardiac conduction, depolarization, and repolarization abnormalities associated with different cardiac genetic disorders may be evident providing valuable insights to the underlying diagnosis. Ventricular premature beats persisting after the cardiac arrest may reflect underlying myocardial instability and reperfusion or a key component of an ACM phenotype. There are several normal variants in the young that should not raise concern, including T-wave inversion in the right precordial leads; first degree AV block and ER.77
Once the patient is ambulatory, the EST may reveal the classical features of CPVT, which are typically absent when the patient is nonambulatory and many sedative agents used after cardiac arrest have profound antiarrhythmic effects by reducing adrenergic drive. A burst EST has been shown to elicit new and more complex arrhythmias in CPVT patients vs standard EST.78 Most patients suffering cardiac arrest secondary to LQTS will typically have overt QT prolongation and T-wave morphological abnormalities, but demonstration of normal repolarization reserve on a postarrest EST is an important negative finding. In patients too young to undergo diagnostic treadmill testing, provocative testing with epinephrine challenge may be considered for the diagnosis of LQTS and CPVT. Ambulatory monitoring or telemetry should be performed as part of the initial workup and follow-up if no diagnosis is made.
Echocardiography will identify many structural features of cardiac disease such as left ventricular hypertrophy, mitral valve anomalies, and outflow tract obstruction in HCM or left ventricular dysfunction in DCM and/or ACM. Echocardiography has many advantages, especially ease of use at bedside, but again findings (especially ventricular function) need to be interpreted considering the overall situation and temporal relationship to the cardiac arrest.
CMRI may add further information, primarily the identification of delayed gadolinium enhancement indicative of myocardial inflammation, oedema, or fibrosis, and provides quantitative analysis of right ventricular size and function for assessment of ACM. Left ventricular delayed enhancement in a subepicardial or mid-myocardial pattern may be suggestive of viral myocarditis or an early ACM, and further clinical and genetic evaluation is important to differentiate these diagnoses with fundamentally different implications for both the patient and wider family.79,80 CMRI in younger children often requires sedation or anaesthesia; however, the risk of any major adverse events is low.81
ECG changes in BrS are highly dynamic and may change from diagnostic to normal within a short period of time especially if initially exacerbated by a dynamic environmental trigger such as fever,70,82 or by pharmacological provocation. Importantly, a BrS ECG phenotype may be seen early after defibrillation and may represent a phenocopy rather than evidence of the condition.83 Myocardial sodium blockade using a class 1 antiarrhythmic such as ajmaline, flecainide, or procainamide can unmask the latent ECG changes,84 although the prevalence of false-positive findings is high.85 Catecholamine infusions can also be helpful in unmasking a CPVT phenotype in whom other investigations may be normal.86
An electrophysiology study may be considered in cases where no other disorder has been identified4 including cases where bundle branch re-entrant VT, pre-excited AF, or rapid supraventricular tachycardia degenerated into VF. Right ventricular voltage mapping may be considered for detection of subclinical ACM.4
Myocardial biopsy may provide additional diagnostic information. A rare cause of cardiac arrest that requires a histologic diagnosis is histiocytoid cardiomyopathy, classically seen in females of non-Caucasian heritage aged less than 2 years.87 This diagnosis is important as spontaneous regression appears to occur after the age of 2-3 years, which has major implications for longer-term management and decision-making.
Genetic testing
Genetic testing is increasingly recognized as a tool in personalized medicine.88 It is a process of identifying the underlying variant causing disease in a family. Because most families have their own unique variant, determining with confidence which variant is causative of disease is a time-consuming process. Standardized approaches for variant interpretation include the 5-tier system from the American College of Medical Genetics.89,90 In circumstances where a paediatric survivor of a cardiac arrest has a relative who passed away suddenly, genetic findings from the deceased relative could shed light on a possible familial causative variant. If a causative (likely pathogenic or pathogenic) variant is identified, then asymptomatic family members can be tested for the variant. Those shown to carry an autosomal dominant variant can be followed clinically and their own first-degree relatives will now be considered to have a 1 in 2 risk of disease and relatives of those with an autosomal recessive variant would have a 1 in 4 risk of disease. Those who do not carry the variant can be released from all future clinical surveillance and cannot pass this on to their children. Being able to release relatives from unnecessary screening and worry is why genetic testing is a cost-effective addition to family management compared with clinical screening alone.91,92
In recent years, there has been increasing stringency of the level of evidence required to consider a variant as causative, with reclassification of variants sometimes necessary. As new evidence emerges, or as variant interpretation criteria are refined, the interpretation of a variant may shift.91 As the phenotype evolves, it is important to revisit the phenotype and genetic testing should include genes where there is a robust gene-disease association; genetic tests with more comprehensive genomic coverage may lead to more diagnostic yield but may also lead to increased discovery of variants of uncertain significance.4 Broader genetic assessment may be considered in select circumstances, for example, assessment for de novo variation or when a heritable phenotype is being mapped within a family. The yield of genetic testing depends on the phenotype93 and can be as high as 80% in typical LQTS, 50% in ACM, and lower in atypical presentations.94 The need for open and clear communication with the family is essential in such settings and demonstrates the value of robust pre- and post-test genetic counselling, as well as strong rapport with the family.
Genetic counselling
Genetic counselling is a process aimed at supporting patients and family members to understand and adapt to the medical, psychosocial, and familial implications of heritable diseases.95,96 Genetic counselling is not merely education but also includes psychological support, and for this reason, appropriately qualified health professionals will ideally perform this, including genetic counsellors and genetic nurses. Genetic counsellors have become a large allied health workforce in many countries worldwide, and their role in the cardiology clinic is well described.97 Cardiac genetic counsellors are often key members of the multidisciplinary team.98
Genetic counsellors play a critical role in the process of genetic testing, including both pre- and post-test genetic counselling. Careful decisions around the type of genetic testing performed and conservative interpretation of genetic variants are critical. Likewise, given the often low yield of genetic testing, maintaining realistic expectations about the chance a causative variant will be identified is also important. In addition, discussions include inheritance risks, education and awareness, variant classification, developing a 3-generation family history, and psychosocial support.4 Psychosocial aspects of genetic counselling include psychological support, empathic listening, crisis intervention skills, knowledge of family dynamics, coping models, processes of grief, and adjustment to a new diagnoses, all of which align with core competencies of genetic counselling accreditation.99
Care of the Patient and Family
Clinical management
In as many as 50% of cases, a reversible cause can be identified and successfully treated, such as anomalous course of the left coronary artery that can be surgically corrected, or ventricular pre-excitation secondary to an accessory pathway. It is always important to demonstrate a clinical picture consistent with cardiac arrest (eg, in the setting of ventricular pre-excitation rapid antegrade conduction via the accessory pathway during AF or programmed atrial stimulation), to ensure that such findings are not incidental bystanders and the true cause has been overlooked.
In patients with an identified cause of SCA, β-blockers are commonly prescribed27 and often prescribed in unexplained SCA also. Implantable cardioverter-defibrillators (ICDs) are also recommended for most patients after cardiac arrest, although in the young this provides unique challenges both in terms of implantation and longer-term management given the high rate of complications.100,101 Evolving evidence supports medical management of LQT1.102 Increasing experience suggests that combination therapy with the β-blocker nadolol and flecainide, and the early use of cardiac sympathetic denervation, is emerging as a preferable strategy to early ICD implantation in CPVT.
Specific lifestyle and pharmacological management are ultimately guided by the precise diagnosis, although increasingly patients with ICDs are safely returning to sports with specific levels of exertion dictated by the underlying diagnosis.101 Lack of compliance and poor effectiveness of current treatments have been attributed to cardiac adverse events.103 In families where there is no familial SUD syndrome, the risk of cardiac events in first degree relatives is low.104
Psychological support
For those who experience a resuscitated SCA, this is often a traumatic event for both the patient and family. The addition of complex genetic testing findings has potential to cause even greater burden. Effectively conveying genetic information can be a challenge and the need for integration with a clinical psychologist has become evident.4,105 Further, up to 95% of family members report psychological difficulties highlighting the need for support.106 In children, we know that a diagnosis of an inherited arrhythmia syndrome can lead to general and cardiac focused anxiety,107 and for the parents the uncertainty and risk of SCA for their children can cause distress.108 Importantly, although lifesaving, adjustment to life with an ICD can be problematic,109 and consistent risk factors for poor coping after an ICD are young age at implantation and multiple ICD shocks.110 Therefore, incorporating clinical psychological support in the multidisciplinary team is critical and for frontline clinic staff such as genetic counsellors, nurses, and cardiologists to be mindful of the potential for poor coping.
Physical activity after SCA
Many SCA events occur during exercise111 and adrenergic stimulation is a recognized trigger for arrhythmias in patients with certain channelopathies (eg, LQTS and CPVT) and cardiomyopathies. Therefore, the return to physical activity needs to be carefully managed with the SCA survivor. Reports of safety of exercise in LQTS and CPVT have been published. Importantly, in young athletes with ICDs, sport participation was not associated with significant adverse events, although appropriate and inappropriate shocks did occur.101,112 There has been a shift from dogmatic recommendations to a shared decision model, in which the priority is to work collaboratively with the patients to determine what their desired level of participation is and to ensure that they are fully informed.113 This includes ensuring that safety parameters are available (family member with knowledge of CPR, AED located in the vicinity of the activity). Most young patients surviving SCA can return to full participation with a few caveats (Table 1). It is the health care provider’s responsibility to ensure that treatment is optimized for the phenotype. This includes revisiting the phenotype over time. Approximately 20% of diagnosis reclassification was reported in the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry, a registry of unexplained cardiac arrest patients and family members.114 Programming the ICD to avoid inappropriate shocks is essential; verifying that there is not T-wave oversensing on EST can be helpful. The patient must participate in “Safe Sports” behaviour.115 Participation after diagnosis with ACM requires a more nuanced discussion. Exercise intensity and dosage can accelerate the disease progression in some patients with ACM.116
Table 1.
Patient and provider responsibilities for returning to sports
| Provider |
|
| Patient |
|
| Full participation not recommended |
|
Follow-up
Implementation of current guidelines can be challenging and varies widely.117 The management of the underlying condition and frequency of follow-up visits is left to the treating cardiologist. In patients with no apparent clinical diagnosis at the time of presentation, long-term follow-up should focus on the emergence of specific cardiomyopathies, where the arrhythmic phenotype precedes the classical structural, electrocardiographic, and histologic features.16 Repeated cardiological evaluation (ECG, EST, and echocardiogram) and advanced investigations (ranging from drug provocation to magnetic resonance imaging) in SCA survivors and first-degree relatives whose first evaluation was normal or inconclusive are recommended every 3-5 years (more frequently if there is more than 1 sudden death in the family) until age 45.4
Transition to adult care
A paediatric SCA victim will likely require monitoring and care into adulthood. Unfortunately, only 30% of adult CHD patients who transitioned from paediatric care received the recommended cardiac care.118 Components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational and/or vocational needs of the adolescent as they transition. The transition process should begin before adolescence, be located close to the patient in order to mitigate any potential travel barriers, flexible, and tailored to the developmental and psychosocial status of the patient.119,120 Unsuccessful transition has been shown to lead to increased hospital visits, adverse events, and even death. It is important to identify adult cardiac providers with the requisite knowledge and expertise in genomics as so many of the conditions have an underlying genetic basis and family follow-up is crucial.
Vocations
Because of the risks of sudden incapacitation, medical regulations exist that vary by country and region regarding high-hazard professions such as commercial driving, firefighting, police or military service, and professional driving.121, 122, 123, 124, 125 The maximal allowable risk for acute incapacitation varies and is dependent on the profession and/or employer. For example, the maximum acceptable risk for acute incapacitation for commercial pilots is 1%, whereas for private automobile drivers it is 22%.121,126 Similarly, in some countries, patients with ICDs are completely prohibited from professional driving, whereas in other areas, there is more variation.127 Specific recommendations should take into consideration such things as the patient’s specific medical condition, distance travelled, and driving behaviour. Advances in technology such as driverless vehicles could play a role in the amount of acceptable risk due to these medical conditions.
Risk Prediction and Prevention
Prevention of SUD in the young has focused on various cardiovascular screening strategies.128 Although ECG screening is routinely performed in certain countries and populations such as in athletes, broad screening for arrhythmia syndromes is not recommended because of potential false positives, inaccurate diagnoses, high costs, and lack of willingness to pursue further diagnostic testing in some. Use of ECG screening is particularly challenging in children and adolescents due to alterations in depolarization and repolarization patterns with aging and growth.129 Emphasis has focused on screening family members of affected individuals, creating awareness of the prodrome, and educating primary care providers of the risk factors for SCA and/or SCD.129,130 In addition, the updated policy statement from the American Academy of Pediatrics provides information for the primary care physician on screening children prone to SCA and SCD to aid in the prevention of SCA.130 Risk stratification strategies have been primarily adult based and those specific to the young have been limited to specific disease populations, retrospective, small cohort studies and should be applied cautiously.128 Multicentre registry studies with thorough phenotyping are required to better determine risk factors of SCA and accurately direct medical management and ICD therapy.131
Making Communities Safe
The community response to an SUD or SCA can be very powerful. Families and organizations impacted have created foundations, support groups, and action plans (eg, https://parentheartwatch.org). Organizations such as the Hearts in Rhythm Organization (https://hiro.heartsinrhythm.ca/) and Sudden Arrhythmia Death Syndromes Foundation (https://www.sads.ca; https://www.sads.org) have created awareness programs, funded research, and provided education for families affected by SCA and/or SUD and for their care providers. Prompt bystander CPR and access to defibrillation are lifesaving in SCA; however, the annual rate of CPR and AED training is low.132 Because of various barriers, implementation has not been easy and the public health impact of this rare but tragic event is not large. Several milestone events or activities that take place in early childhood and/or adolescence including high school graduation, obtaining a driver’s license, vaccinations, and social media can be used as opportunities to promote safety in youth. There are pockets of intensified risk that have been targeted for AED placement, including some creative ones (eg, Tokyo marathon, drone approach, and cellphone apps). Several campaigns and events that promote CPR and AED training (eg, CPR month in Canada, National CPR, and AED Awareness week in the USA) are ongoing.
Conclusion
SCA in a young otherwise healthy individual is rare. Investigations into the cause of the SCA involve a multidisciplinary approach and may not result in a diagnosis despite extensive clinical testing. Care should be tailored to the patient, involve the family, and include psychological support. Further work into risk prediction and greater awareness of the benefits of CPR and AED training is warranted.
Acknowledgements
We would like to thank Dr Prince Joseph Kannankeril and Dr James Clifford Perry for their review and editorial comments. We would also like to thank the patients and families who support the research and learning that continue to advance our understanding of this important and challenging field.
Ethics Statement
Ethical approval was not obtained for this review since it did not involve human participants or clinical data.
Funding Sources
No funding was received for this study.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Couper K., Putt O., Field R., et al. Incidence of sudden cardiac death in the young: a systematic review. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krahn A.D., Healey J.S., Chauhan V., et al. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 3.Duncan J.R., Byard R.W. In: SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future. Duncan J.R., Byard R.W., editors. University of Adelaide Press; Adelaide: 2018. Sudden infant death syndrome: an overview. [PubMed] [Google Scholar]
- 4.Stiles M.K., Wilde A.A.M., Abrams D.J., et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm. 2021;18:e1–e50. doi: 10.1016/j.hrthm.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda T., Fitzgerald K.K., Temple J. Sudden cardiac death in children and young adults without structural heart disease: a comprehensive review. Rev Cardiovasc Med. 2020;21:205–216. doi: 10.31083/j.rcm.2020.02.55. [DOI] [PubMed] [Google Scholar]
- 6.Meyer L., Stubbs B., Fahrenbruch C., et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age. Circulation. 2012;126:1363–1372. doi: 10.1161/CIRCULATIONAHA.111.076810. [DOI] [PubMed] [Google Scholar]
- 7.Christiaans I., Lekanne dit Deprez R.H., van Langen I.M., Wilde A.A. Ventricular fibrillation in MYH7-related hypertrophic cardiomyopathy before onset of ventricular hypertrophy. Heart Rhythm. 2009;6:1366–1369. doi: 10.1016/j.hrthm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Ellims A.H. Hypertrophic cardiomyopathy in the adolescent. Aust Fam Physician. 2017;46:553–557. [PubMed] [Google Scholar]
- 9.O'Mahony C., Elliott P., McKenna W. Sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2013;6:443–451. doi: 10.1161/CIRCEP.111.962043. [DOI] [PubMed] [Google Scholar]
- 10.Miron A., Lafreniere-Roula M., Steve Fan C.-P., et al. A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation. 2020;142:217–229. doi: 10.1161/CIRCULATIONAHA.120.047235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Towbin J.A., Lowe A.M., Colan S.D., et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 12.Bharucha T., Lee K.J., Daubeney P.E.F., et al. Sudden death in childhood cardiomyopathy. J Am Coll Cardiol. 2015;65:2302–2310. doi: 10.1016/j.jacc.2015.03.552. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum A.N., Agre K.E., Pereira N.L. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat Rev Cardiol. 2020;17:286–297. doi: 10.1038/s41569-019-0284-0. [DOI] [PubMed] [Google Scholar]
- 14.Corrado D., Basso C., Judge D.P. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121:784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- 15.Te Riele A.S., James C.A., Philips B., et al. Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol. 2013;24:1311–1320. doi: 10.1111/jce.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingles J., Bagnall R.D., Yeates L., et al. Concealed arrhythmogenic right ventricular cardiomyopathy in sudden unexplained cardiac death events. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.002355. [DOI] [PubMed] [Google Scholar]
- 17.Te Riele A., James C.A., Sawant A.C., et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy in the pediatric population: clinical characterization and comparison with adult-onset disease. JACC Clin Electrophysiol. 2015;1:551–560. doi: 10.1016/j.jacep.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Towbin J.A., Jefferies J.L. Cardiomyopathies due to left ventricular noncompaction, mitochondrial and storage diseases, and inborn errors of metabolism. Circ Res. 2017;121:838–854. doi: 10.1161/CIRCRESAHA.117.310987. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz P.J., Crotti L., Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gow R.M., Ewald B., Lai L., Gardin L., Lougheed J. The measurement of the QT and QTc on the neonatal and infant electrocardiogram: a comprehensive reliability assessment. Ann Noninvasive Electrocardiol. 2009;14:165–175. doi: 10.1111/j.1542-474X.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnestad M., Crotti L., Rognum T.O., et al. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 22.Berat C.M., Montealegre S., Wiedemann A., et al. Clinical and biological characterization of 20 patients with TANGO2 deficiency indicates novel triggers of metabolic crises and no primary energetic defect. J Inherit Metab Dis. 2021;44:415–425. doi: 10.1002/jimd.12314. [DOI] [PubMed] [Google Scholar]
- 23.Adler A., Novelli V., Amin A.S., et al. An international, multicentered, evidence-based reappraisal of genes reported to cause congenital long QT syndrome. Circulation. 2020;141:418–428. doi: 10.1161/CIRCULATIONAHA.119.043132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locati E.H., Zareba W., Moss A.J., et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the international LQTS registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 25.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Roston T.M., Yuchi Z., Kannankeril P.J., et al. The clinical and genetic spectrum of catecholaminergic polymorphic ventricular tachycardia: findings from an international multicentre registry. Europace. 2018;20:541–547. doi: 10.1093/europace/euw389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priori S.G., Blomström-Lundqvist C., Mazzanti A., et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 28.Leenhardt A., Lucet V., Denjoy I., et al. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 29.Sy R.W., Gollob M.H., Klein G.J., et al. Arrhythmia characterization and long-term outcomes in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2011;8:864–871. doi: 10.1016/j.hrthm.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 30.Ng K., Titus E.W., Lieve K.V., et al. An International multicenter evaluation of inheritance patterns, arrhythmic risks, and underlying mechanisms of CASQ2-catecholaminergic polymorphic ventricular tachycardia. Circulation. 2020;142:932–947. doi: 10.1161/CIRCULATIONAHA.120.045723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 32.Brugada R., Campuzano O., Sarquella-Brugada G., Brugada J., Brugada P. Brugada syndrome. Methodist Debakey Cardiovasc J. 2014;10:25–28. doi: 10.14797/mdcj-10-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez Corcia M.C., Sieira J., Sarkozy A., et al. Brugada syndrome in the young: an assessment of risk factors predicting future events. Europace. 2017;19:1864–1873. doi: 10.1093/europace/euw206. [DOI] [PubMed] [Google Scholar]
- 34.Campuzano O., Sarquella-Brugada G., Cesar S., et al. Recent advances in short QT syndrome. Front Cardiovasc Med. 2018;5:149. doi: 10.3389/fcvm.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coban-Akdemir Z.H., Charng W.L., Azamian M., et al. Wolff-Parkinson-White syndrome: de novo variants and evidence for mutational burden in genes associated with atrial fibrillation. Am J Med Genet A. 2020;182:1387–1399. doi: 10.1002/ajmg.a.61571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etheridge S.P., Escudero C.A., Blaufox A.D., et al. Life-threatening event risk in children with Wolff-Parkinson-White syndrome: a multicenter international study. JACC Clin Electrophysiol. 2018;4:433–444. doi: 10.1016/j.jacep.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Escudero C.A., Ceresnak S.R., Collins K.K., et al. Loss of ventricular preexcitation during noninvasive testing does not exclude high-risk accessory pathways: a multicenter study of WPW in children. Heart Rhythm. 2020;17:1729–1737. doi: 10.1016/j.hrthm.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Pappone C., Vicedomini G., Manguso F., et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130:811–819. doi: 10.1161/CIRCULATIONAHA.114.011154. [DOI] [PubMed] [Google Scholar]
- 39.Visser M., van der Heijden J.F., Doevendans P.A., et al. Idiopathic ventricular fibrillation: the struggle for definition, diagnosis, and follow-up. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003817. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg C., Davies B., Mellor G., et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: a report from the CASPER registry. Eur Heart J. 2021;42:2827–2838. doi: 10.1093/eurheartj/ehab275. [DOI] [PubMed] [Google Scholar]
- 41.Alqarawi W., Dewidar O., Tadros R., et al. Defining idiopathic ventricular fibrillation: a systematic review of diagnostic testing yield in apparently unexplained cardiac arrest. Heart Rhythm. 2021;18:1178–1185. doi: 10.1016/j.hrthm.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Tikkanen J.T., Anttonen O., Junttila M.J., et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 43.Safa R., Thomas R., Karpawich P.P. Electrocardiographic early repolarization characteristics and clinical presentations in the young: a benign finding or worrisome marker for arrhythmias. Congenit Heart Dis. 2017;12:99–104. doi: 10.1111/chd.12410. [DOI] [PubMed] [Google Scholar]
- 44.Baruteau A.E., Pass R.H., Thambo J.B., et al. Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr. 2016;175:1235–1248. doi: 10.1007/s00431-016-2748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton R.M. Editorial commentary: live better electrically? Optimizing the timing and application of pacing in congenital heart block. Trends Cardiovasc Med. 2020;30:287–288. doi: 10.1016/j.tcm.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Sheppard M.N. Sudden death in congenital heart disease: the role of the autopsy in determining the actual cause. J Cardiovasc Dev Dis. 2020;7:58. doi: 10.3390/jcdd7040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynge T.H., Jeppesen A.G., Winkel B.G., et al. Nationwide study of sudden cardiac death in people with congenital heart defects aged 0 to 35 years. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005757. [DOI] [PubMed] [Google Scholar]
- 48.Serinelli S., Arunkumar P., White S. Undiagnosed congenital heart defects as a cause of sudden, unexpected death in children. J Forensic Sci. 2018;63:1750–1755. doi: 10.1111/1556-4029.13779. [DOI] [PubMed] [Google Scholar]
- 49.Papagiannis J. Sudden death due to aortic pathology. Cardiol Young. 2017;27:S36–42. doi: 10.1017/S1047951116002213. [DOI] [PubMed] [Google Scholar]
- 50.Rizzo S., De Gaspari M., Frescura C., et al. Sudden death and coronary artery anomalies. Front Cardiovasc Med. 2021;8:636589. doi: 10.3389/fcvm.2021.636589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basso C., Maron B.J., Corrado D., Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 52.Altmann H.M., Tester D.J., Will M.L., et al. Homozygous/compound heterozygous triadin mutations associated with autosomal-recessive long-QT syndrome and pediatric sudden cardiac arrest: elucidation of the triadin knockout syndrome. Circulation. 2015;131:2051–2060. doi: 10.1161/CIRCULATIONAHA.115.015397. [DOI] [PubMed] [Google Scholar]
- 53.Bundgaard H., Jons C., Lodder E.M., et al. A novel familial cardiac arrhythmia syndrome with widespread ST-segment depression. N Engl J Med. 2018;379:1780–1781. doi: 10.1056/NEJMc1807668. [DOI] [PubMed] [Google Scholar]
- 54.Sun B., Yao J., Ni M., et al. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.aba7287. [DOI] [PubMed] [Google Scholar]
- 55.Franciosi S., Perry F.K.G., Roston T.M., et al. The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton Neurosci. 2017;205:1–11. doi: 10.1016/j.autneu.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Lieve K.V.V., Dusi V., van der Werf C., et al. Heart rate recovery after exercise is associated with arrhythmic events in patients with catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007471. [DOI] [PubMed] [Google Scholar]
- 57.Chang Liu M., Tester M.A., Franciosi S., et al. Potential role of life stress in unexplained sudden cardiac arrest. CJC Open. 2021;3:285–291. doi: 10.1016/j.cjco.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tester M.A., Hockin B.C.D., David T., et al. Polymorphic ventricular tachycardia associated with an episode of reflex syncope: Is this the needle in the haystack? HeartRhythm Case Rep. 2018;4:510–513. doi: 10.1016/j.hrcr.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rucinski C., Winbo A., Marcondes L., et al. A population-based registry of patients with inherited cardiac conditions and resuscitated cardiac arrest. J Am Coll Cardiol. 2020;75:2698–2707. doi: 10.1016/j.jacc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Lahrouchi N., Raju H., Lodder E.M., et al. Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol. 2017;69:2134–2145. doi: 10.1016/j.jacc.2017.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hylind R.J., Chandler S.F., Beausejour Ladouceur V., et al. Clinical and genetic findings in children presenting with ventricular fibrillation as the first manifestation of cardiovascular disease. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunningham T., Roston T.M., Franciosi S., et al. Initially unexplained cardiac arrest in children and adolescents: a national experience from the Canadian Pediatric Heart Rhythm Network. Heart Rhythm. 2020;17:975–981. doi: 10.1016/j.hrthm.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 63.MacCormick J.M., McAlister H., Crawford J., et al. Misdiagnosis of long QT syndrome as epilepsy at first presentation. Ann Emerg Med. 2009;54:26–32. doi: 10.1016/j.annemergmed.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 64.Hayashi M., Denjoy I., Extramiana F., et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 65.Johnson J.N., Hofman N., Haglund C.M., et al. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–231. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yap S.M., Smyth S. Ryanodine receptor 2 (RYR2) mutation: a potentially novel neurocardiac calcium channelopathy manifesting as primary generalised epilepsy. Seizure. 2019;67:11–14. doi: 10.1016/j.seizure.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Chahal C.A.A., Salloum M.N., Alahdab F., et al. Systematic review of the genetics of sudden unexpected death in epilepsy: potential overlap with sudden cardiac death and arrhythmia-related genes. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craig D.P., Choi Y.Y., Hughes E., et al. Paediatric sudden unexpected death in epilepsy: a parental report cohort. Acta Neurol Scand. 2021;143:509–513. doi: 10.1111/ane.13378. [DOI] [PubMed] [Google Scholar]
- 69.Shattock M.J., Tipton M.J. Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590:3219–3230. doi: 10.1113/jphysiol.2012.229864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peltenburg P.J., Blom N.A., Vink A.S., et al. In children and adolescents from Brugada syndrome-families, only SCN5A mutation carriers develop a type-1 ECG pattern induced by fever. Circulation. 2020;142:89–91. doi: 10.1161/CIRCULATIONAHA.120.045720. [DOI] [PubMed] [Google Scholar]
- 71.Skinner J.R., Chung S.K., Nel C.A., et al. Brugada syndrome masquerading as febrile seizures. Pediatrics. 2007;119:e1206–e1211. doi: 10.1542/peds.2006-2628. [DOI] [PubMed] [Google Scholar]
- 72.Tsuboi M., Antzelevitch C. Cellular basis for electrocardiographic and arrhythmic manifestations of Andersen-Tawil syndrome (LQT7) Heart Rhythm. 2006;3:328–335. doi: 10.1016/j.hrthm.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hylind R., Beausejour-Ladouceur V., Plovanich M.E., et al. Cardiocutaneous features of autosomal dominant desmoplakin-associated arrhythmogenic cardiomyopathy. Circ Genom Precis Med. 2020;13 doi: 10.1161/CIRCGEN.120.003081. [DOI] [PubMed] [Google Scholar]
- 74.Waddell-Smith K.E., Donoghue T., Oates S., et al. Inpatient detection of cardiac-inherited disease: the impact of improving family history taking. Open Heart. 2016;3 doi: 10.1136/openhrt-2015-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan H.L., Hofman N., van Langen I.M., van der Wal A.C., Wilde A.A. Sudden unexplained death: heritability and diagnostic yield of cardiological and genetic examination in surviving relatives. Circulation. 2005;112:207–213. doi: 10.1161/CIRCULATIONAHA.104.522581. [DOI] [PubMed] [Google Scholar]
- 76.Bellamy D., Nuthall G., Dalziel S., Skinner J.R. Catecholaminergic polymorphic ventricular tachycardia: the cardiac arrest where epinephrine is contraindicated. Pediatr Crit Care Med. 2019;20:262–268. doi: 10.1097/PCC.0000000000001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma S., Drezner J.A., Baggish A., et al. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057–1075. doi: 10.1016/j.jacc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Roston T.M., Kallas D., Davies B., et al. Burst exercise testing can unmask arrhythmias in patients with incompletely penetrant catecholaminergic polymorphic ventricular tachycardia. JACC Clin Electrophysiol. 2021;7:437–441. doi: 10.1016/j.jacep.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 79.DeWitt E.S., Chandler S.F., Beausejour-Ladouceur V., et al. Phenotypic variability and outcome of arrhythmogenic cardiomyopathy in pediatric and adolescent patients. Prog Pediatr Cardiol. 2017;46:31. [Google Scholar]
- 80.Martins D., Ovaert C., Khraiche D., et al. Myocardial inflammation detected by cardiac MRI in arrhythmogenic right ventricular cardiomyopathy: a paediatric case series. Int J Cardiol. 2018;271:81–86. doi: 10.1016/j.ijcard.2018.05.116. [DOI] [PubMed] [Google Scholar]
- 81.Odegard K.C., DiNardo J.A., Tsai-Goodman B., et al. Anaesthesia considerations for cardiac MRI in infants and small children. Paediatr Anaesth. 2004;14:471–476. doi: 10.1111/j.1460-9592.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 82.Page S.P., Archbold A., Abrams D.J. Chest pain and ST elevation. BMJ. 2012;344:e4323. doi: 10.1136/bmj.e4323. [DOI] [PubMed] [Google Scholar]
- 83.Wilde A.A., Antzelevitch C., Borggrefe M., et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 84.Antzelevitch C., Brugada P., Borggrefe M., et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 85.Hasdemir C., Payzin S., Kocabas U., et al. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2015;12:1584–1594. doi: 10.1016/j.hrthm.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Krahn A.D., Healey J.S., Chauhan V.S., et al. Epinephrine infusion in the evaluation of unexplained cardiac arrest and familial sudden death: from the cardiac arrest survivors with preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol. 2012;5:933–940. doi: 10.1161/CIRCEP.112.973230. [DOI] [PubMed] [Google Scholar]
- 87.Xie H., Chen X., Chen N., Zhou Q. Sudden death in a male infant due to histiocytoid cardiomyopathy: an autopsy case and review of the literature. Am J Forensic Med Pathol. 2017;38:32–34. doi: 10.1097/PAF.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 88.Parker L.E., Landstrom A.P. The clinical utility of pediatric cardiomyopathy genetic testing: from diagnosis to a precision medicine-based approach to care. Prog in Pediatr Cardiol. 2021;62:101413. doi: 10.1016/j.ppedcard.2021.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingles J., Macciocca I., Morales A., Thomson K. Genetic testing in inherited heart diseases. Heart Lung Circ. 2020;29:505–511. doi: 10.1016/j.hlc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 90.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ingles J., McGaughran J., Scuffham P.A., Atherton J., Semsarian C. A cost-effectiveness model of genetic testing for the evaluation of families with hypertrophic cardiomyopathy. Heart. 2012;98:625–630. doi: 10.1136/heartjnl-2011-300368. [DOI] [PubMed] [Google Scholar]
- 92.Catchpool M., Ramchand J., Martyn M., et al. A cost-effectiveness model of genetic testing and periodical clinical screening for the evaluation of families with dilated cardiomyopathy. Genet Med. 2019;21:2815–2822. doi: 10.1038/s41436-019-0582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofman N., Tan H.L., Alders M., et al. Yield of molecular and clinical testing for arrhythmia syndromes: report of 15 years’ experience. Circulation. 2013;128:1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091. [DOI] [PubMed] [Google Scholar]
- 94.Harris S.L., Lubitz S.A. Clinical and genetic evaluation after sudden cardiac arrest. J Cardiovasc Electrophysiol. 2020;31:570–578. doi: 10.1111/jce.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biesecker B.B. Goals of genetic counseling. Clin Genet. 2001;60:323–330. doi: 10.1034/j.1399-0004.2001.600501.x. [DOI] [PubMed] [Google Scholar]
- 96.National Society of Genetic Counselors’ Definition Task Force. Resta R., Biesecker B.B., et al. A new definition of genetic counseling: National Society of Genetic Counselors' Task Force report. J Genet Couns. 2006;15:77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- 97.Ingles J., Yeates L., Semsarian C. The emerging role of the cardiac genetic counselor. Heart Rhythm. 2011;8:1958–1962. doi: 10.1016/j.hrthm.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 98.Caleshu C., Kasparian N.A., Edwards K.S., et al. Interdisciplinary psychosocial care for families with inherited cardiovascular diseases. Trends Cardiovasc Med. 2016;26:647–653. doi: 10.1016/j.tcm.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 99.Austin J., Semaka A., Hadjipavlou G. Conceptualizing genetic counseling as psychotherapy in the era of genomic medicine. J Genet Couns. 2014;23:903–909. doi: 10.1007/s10897-014-9728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Werf C., Lieve K.V., Bos J.M., et al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J. 2019;40:2953–2961. doi: 10.1093/eurheartj/ehz309. [DOI] [PubMed] [Google Scholar]
- 101.Saarel E.V., Law I., Berul C.I., et al. Safety of sports for young patients with implantable cardioverter-defibrillators: long-term results of the multinational ICD sports registry. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.118.006305. [DOI] [PubMed] [Google Scholar]
- 102.Vincent G.M., Schwartz P.J., Denjoy I., et al. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe H., van der Werf C., Roses-Noguer F., et al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10:542–547. doi: 10.1016/j.hrthm.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Werf C., Stiekema L., Tan H.L., et al. Low rate of cardiac events in first-degree relatives of diagnosis-negative young sudden unexplained death syndrome victims during follow-up. Heart Rhythm. 2014;11:1728–1732. doi: 10.1016/j.hrthm.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 105.Ingles J., Spinks C., Yeates L., et al. Posttraumatic stress and prolonged grief after the sudden cardiac death of a young relative. JAMA Intern Med. 2016;176:402–405. doi: 10.1001/jamainternmed.2015.7808. [DOI] [PubMed] [Google Scholar]
- 106.Mion M., Case R., Smith K., et al. Follow-up care after out-of-hospital cardiac arrest: a pilot study of survivors and families’ experiences and recommendations. Resusc Plus. 2021;7:100154. doi: 10.1016/j.resplu.2021.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Last A.N., English J., Pote H., et al. Anxiety in children attending a specialist inherited cardiac arrhythmia clinic: a questionnaire study. BMJ Paediatr Open. 2018;2 doi: 10.1136/bmjpo-2018-000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farnsworth M.M., Fosyth D., Haglund C., Ackerman M.J. When I go in to wake them … I wonder: parental perceptions about congenital long QT syndrome. J Am Acad Nurse Pract. 2006;18:284–290. doi: 10.1111/j.1745-7599.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 109.DeMaso D.R., Lauretti A., Spieth L., et al. Psychosocial factors and quality of life in children and adolescents with implantable cardioverter-defibrillators. Am J Cardiol. 2004;93:582–587. doi: 10.1016/j.amjcard.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 110.Sears S.F., Jr., Conti J.B. Quality of life and psychological functioning of icd patients. Heart. 2002;87:488–493. doi: 10.1136/heart.87.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodman J.M., Burr J.F., Banks L., Thomas S.G. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can J Cardiol. 2016;32:523–532. doi: 10.1016/j.cjca.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 112.Lampert R., Olshansky B., Heidbuchel H., et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation. 2013;127:2021–2030. doi: 10.1161/CIRCULATIONAHA.112.000447. [DOI] [PubMed] [Google Scholar]
- 113.Tobert K.E., Bos J.M., Garmany R., Ackerman M.J. Return-to-play for athletes with long QT syndrome or genetic heart diseases predisposing to sudden death. J Am Coll Cardiol. 2021;78:594–604. doi: 10.1016/j.jacc.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 114.Vittoria Matassini M., Krahn A.D., Gardner M., et al. Evolution of clinical diagnosis in patients presenting with unexplained cardiac arrest or syncope due to polymorphic ventricular tachycardia. Heart Rhythm. 2014;11:274–281. doi: 10.1016/j.hrthm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 115.Cheung C.C., Laksman Z.W., Mellor G., Sanatani S., Krahn A.D. Exercise and inherited arrhythmias. Can J Cardiol. 2016;32:452–458. doi: 10.1016/j.cjca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 116.Lie O.H., Dejgaard L.A., Saberniak J., et al. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–753. doi: 10.1016/j.jacep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 117.Behr E.R., Scrocco C., Wilde A.A.M., et al. Investigation on Sudden Unexpected Death in the Young (SUDY) in Europe: results of the European Heart Rhythm Association Survey. Europace. 2022;24:331–339. doi: 10.1093/europace/euab176. [DOI] [PubMed] [Google Scholar]
- 118.Banerjee R., Patel M.S. Adults with congenital heart disease: the critical transition from pediatric to adult care. J Clin Outcomes Manag. 2018;25:467–478. [Google Scholar]
- 119.Knauth A., Verstappen A., Reiss J., Webb G.D. Transition and transfer from pediatric to adult care of the young adult with complex congenital heart disease. Cardiol Clin. 2006;24:619–629, vi. doi: 10.1016/j.ccl.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 120.LeComte K., Sinclair B., Cockell S., et al. Ensuring a successful transition and transfer from pediatric to adult care in patients with congenital heart disease. BCMJ. 2016;58:389–395. [Google Scholar]
- 121.Simpson C., Dorian P., Gupta A., et al. Assessment of the cardiac patient for fitness to drive: drive subgroup executive summary. Can J Cardiol. 2004;20:1314–1320. [PubMed] [Google Scholar]
- 122.Epstein A.E., Miles W.M., Benditt D.G., et al. Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation. 1996;94:1147–1166. doi: 10.1161/01.cir.94.5.1147. [DOI] [PubMed] [Google Scholar]
- 123.Epstein A.E., Baessler C.A., Curtis A.B., et al. Addendum to “Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations: a medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology”: public safety issues in patients with implantable defibrillators: a scientific statement from the American Heart Association and the Heart Rhythm Society. Circulation. 2007;115:1170–1176. doi: 10.1161/CIRCULATIONAHA.106.180203. [DOI] [PubMed] [Google Scholar]
- 124.Petch M.C. Driving and heart disease. Eur Heart J. 1998;19:1165–1177. doi: 10.1053/euhj.1998.1120. [DOI] [PubMed] [Google Scholar]
- 125.Brignole M., Alboni P., Benditt D.G., et al. Guidelines on management (diagnosis and treatment) of syncope-update 2004. Executive summary. Eur Heart J. 2004;25:2054–2072. doi: 10.1016/j.ehj.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 126.Nicol E.D., Rienks R., Gray G., et al. An introduction to aviation cardiology. Heart. 2019;105:s3–8. doi: 10.1136/heartjnl-2018-313019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Margulescu A.D., Anderson M.H. A review of driving restrictions in patients at risk of syncope and cardiac arrhythmias associated with sudden incapacity: differing global approaches to regulation and risk. Arrhythm Electrophysiol Rev. 2019;8:90–98. doi: 10.15420/aer.2019.13.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ackerman M., Atkins D.L., Triedman J.K. Sudden cardiac death in the young. Circulation. 2016;133:1006–1026. doi: 10.1161/CIRCULATIONAHA.115.020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aro A.L., Chugh S.S. Prevention of sudden cardiac death in children and young adults. Prog Pediatr Cardiol. 2017;45:37–42. doi: 10.1016/j.ppedcard.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Erickson C.C., Salerno J.C., Berger S., et al. Sudden death in the young: information for the primary care provider. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052044. [DOI] [PubMed] [Google Scholar]
- 131.Behr E., Ensam B. New approaches to predicting the risk of sudden death. Clin Med (Lond) 2016;16:283. doi: 10.7861/clinmedicine.16-3-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anderson M.L., Cox M., Al-Khatib S.M., et al. Rates of cardiopulmonary resuscitation training in the United States. JAMA Intern Med. 2014;174:194–201. doi: 10.1001/jamainternmed.2013.11320. [DOI] [PMC free article] [PubMed] [Google Scholar]