Abstract
Kinetic parameters for nitrate reduction in intact sediment cores were investigated by using the acetylene blockage method at five sites along the Swale-Ouse river system in northeastern England, including a highly polluted tributary, R. Wiske. The denitrification rate in sediment containing added nitrate exhibited a Michaelis-Menten-type curve. The concentration of nitrate for half-maximal activity (Kmap) by denitrifying bacteria increased on passing downstream from 13.1 to 90.4 μM in the main river, but it was highest (640 μM) in the Wiske. The apparent maximal rate (Vmaxap) ranged between 35.8 and 324 μmol of N m−2 h−1 in the Swale-Ouse (increasing upstream to downstream), but it was highest in the Wiske (1,194 μmol N m−2 h−1). A study of nitrous oxide (N2O) production at the same time showed that rates ranged from below the detection limit (0.05 μmol of N2O-N m−2 h−1) at the headwater site to 27 μmol of N2O-N m−2 h−1 at the downstream site. In the Wiske the rate was up to 570 μmol of N2O-N m−2 h−1, accounting for up to 80% of total N gas production.
The massive use of nitrogen-based fertilizer has inevitably increased the concentrations of nitrate in many rivers (14, 15). This has led to the acceleration of the nitrogen cycle, especially those processes that use nitrate as the substrate, such as denitrification, the biological reduction of nitrogen oxides (nitrate and nitrite) to N-containing gases (N2 and N2O) in environments with relatively low levels of oxygen (9). Interest in denitrifying bacteria has also been stimulated by concern over the recognition of the role of N2O in the destruction of stratospheric ozone (5) and in the radiative heat budget of the atmosphere (19).
Although rivers are usually the first aquatic system to receive the excess of nitrate, fewer studies have been undertaken on them than on soils or estuaries. Only one (2, 27) or a few sites down the river (4) have been sampled, and it is therefore difficult to generalize from the results. According to the river continuum concept of Vannote et al. (28), the physical and chemical variables of water and sediments should present a gradient from the headwaters to the estuary, which should elicit a series of responses within the denitrifying bacterial population. For instance, in the Swale-Ouse river system in northeastern England we have observed (18) a consistent increase (up to 30 times) in the denitrification rate of sediment cores on passing downstream.
There have apparently been no studies to determine the apparent affinity (Kmap) and maximal capacity (Vmaxap) for nitrate utilization of denitrifying bacteria down a whole river; however, this is important for clarifying the relationship between nitrate availability in the environment and nitrate use by denitrifying bacteria. Previous attempts to measure the kinetics of nitrate utilization in soils and sediments have been hampered by a number of problems. The earliest studies employed slurry techniques (7, 12, 17), where the structure of the sediment is destroyed and hence the in situ oxygen and nitrate gradients. The values of Kmap and Vmaxap obtained are only meaningful under conditions of no limitation and cannot be incorporated into predictive models. Other researchers have used intact sediment cores to study the kinetics of nitrate reduction through measuring the decrease in nitrate concentration in the overlying water (1). The long incubation period, possible errors in measuring nitrate, and the unknown fate of the depleted nitrate make uncertain the significance of the reported kinetic parameters.
The aim of the present study was to investigate the effect of nitrate concentration on the denitrification rate and N2O production in intact sediment cores taken from a river continuum.
MATERIALS AND METHODS
Study area.
The study was performed on the Swale-Ouse river system. A detailed description of the geography of the area has been provided by Jarvie et al. (8), the vegetation has been surveyed by Holmes and Whitton (6), and biological studies have been reviewed by Whitton and Lucas (29). The Swale rises on the Northern Pennines, running 117 km to its confluence with the Ure, where it becomes the Ouse (Fig. 1). The river becomes tidal (but still freshwater) at km 145.0. (Main river distances are measured downstream from the junction of two upland tributaries; the location of a site on a tributary is given by a negative value indicating the distance upstream, together with the point at which it enters the main river.)
FIG. 1.
Location of the sampling sites in the Swale-Ouse and R. Wiske.
The Swale-Ouse shows a consistent trend of changes in many physical and chemical features on passing downstream, while the whole river system is subject to marked variations in discharge. An important tributary is the Wiske, a lowland and highly organically polluted stream, that receives treated sewage effluent from a town of 10,700 inhabitants.
The oxygen concentration in water at sites on the main river was always over 93% saturation (based on monthly data taken between 0900 to 1200 h), but this value fell to 64% in the Wiske.
Sediment and water collection.
Samples were taken from upstream (km −2.5 [0.0] and km 10.9), midstream (km 49.9), and downstream (km 107.9 and km 145.0, i.e., freshwater tidal) sites (Fig. 1). In addition, sediment was collected from the Wiske (km −1.7 [86.1]). Samples were collected between 18 and 24 April 1997, with repeat sampling at two sites, upstream (km 10.9) and the Wiske, on 18 June and 4 August 1997.
Intact sediment cores were taken in Plexiglas cylinders (3.5-cm inner diameter, 25-cm height, with a vertical series of silicone rubber inserts to allow injection at different positions) from a defined 2-m2 area at positions where sediment was accumulating. Each tube was sealed with a rubber bung, and the core was removed carefully. The bottom of the core was sealed with an additional rubber bung. Special care was taken to preserve the sediment structure. The pH of the water was determined with a glass combination electrode (Russell CW76) and a Wissenschaftliche Technische Werkstätten meter. Water samples were taken at the same time and filtered through 0.45-μm (pore size) membrane filters. Nitrite and nitrate were determined according to the method of Stainton et al. (26).
Depletion of in situ nitrate.
In order to establish the required nitrate concentration during experimental studies, nitrate and nitrite were removed from the sediment by using natural denitrification activity, as adapted from the method of Murray et al. (12), with river water being substituted by a medium lacking N or P. This medium was modified from that used by Chu (3) and contained (per liter) 4.28 mg of KCl, 54.75 mg of CaCl2 · 6H2O, 25 mg of MgSO4 · 7H2O, 15.85 mg of NaHCO3, 2.44 mg of FeCl2 · 6H2O, 3.33 mg of Na2EDTA, and 0.25 ml of AC microelement stock (11). The medium was buffered with HEPES (0.6 g liter−1) to the natural pH. All samples were incubated in the dark for 24 h at 15°C.
Assay for denitrification activity and N2O production.
The acetylene (C2H2) block method (24) was used to determine the denitrification rate. This method is based on assessing the rate of N2O accumulation in cores to which C2H2 has been added to block the bacterial reduction of N2O to N2. Net N2O production was also determined in intact sediment cores incubated without C2H2. After incubation to remove environmental nitrate and nitrite, the supernatant water was drained off and replaced with the freshwater medium with the required nitrate concentration supplied as KNO3. Control experiments with KCl showed that K+ had no influence on the results. Nitrate concentrations used in the assays were modified to reflect the expected denitrification rate and ranged from 0 to 140 μM for the upstream sites, 357 μM for downstream sites, and 11,420 μM for the Wiske. Two triplicate sets of cores were used for each concentration assayed at each site. The first set was incubated without C2H2 to measure net N2O production, while the second was incubated with C2H2 to determine the denitrification rate (N2O plus N2 production). For the latter, C2H2 saturated medium with the required nitrate concentration was added in the overlying water to obtain 10% C2H2 (final concentration). C2H2 saturated medium was prepared by flushing for 20 to 30 min with pure C2H2 (previously passed for 30 min through 0.1 N phosphoric acid to remove ammonium contamination). Then 1.0 ml of this solution was injected (where required) into the sediment through the holes in the cylinder at each centimeter depth for the top 3 cm. Cores were filled with the test medium, sealed without gas space, and incubated at 15°C in the dark for between 3 and 5 h. Immediately before the assay, another three cores from each site were used to determine the initial N2O concentration. In addition, another two sets of three cores were used to determine denitrification and N2O production by using river water from each site. For this analysis, cores were incubated at the same time and under similar conditions to those described above but with C2H2 saturated river water (denitrification rate) or natural river water (N2O production).
At the end of incubation, the cores were vigorously shaken, and 5 ml of the resulting slurry was transferred to a 12.5-ml gas container, which included 0.1 ml of 40% formaldehyde, and immediately frozen until N2O analysis. After the gas container was shaken for 2 min to equilibrate gas in the headspace and sediments, 2 ml of the headspace was removed for N2O measurement. This was done by using a gas chromatograph equipped with 63Ni electron capture detector (Perkin-Elmer 2000; detector temperature 350°C; flow of 20 ml min−1). The N2O concentration was determined by using a standard curve generated with purified N2O (BOC, Ltd.). The N2O concentration in the headspace was used to back-calculate the amount of N2O in the sediment by using Henry’s law. N2O production (expressed as micromoles of N2O-N per square meter per hour) and denitrification rate (N2O plus N2, expressed as micromoles of N per square meter per hour) were calculated after subtracting the initial N2O in the core and taking into account the incubation period and the area of the core.
The kinetic parameters for denitrification and N2O production were computed from the Lineweaver-Burk transformation (1/V versus 1/S) of the Michaelis-Menten equation, where V is the denitrification rate or N2O production, and S is the concentration of nitrate. Because the denitrifying enzyme activity was assayed in a complex system, the kinetic parameters were termed as apparent concentration of NO3− for half-maximal activity (Kmap) and apparent maximal activity (Vmaxap).
RESULTS
Environmental data.
The data for sediments obtained at the six sites are shown in Table 1. With respect to sediment particle size, there was an increase in the silt-plus-clay fractions and a decrease in the sand fraction on passing downstream. In general, total carbon and nitrogen in the sediment increased on passing downstream.
TABLE 1.
Sediment characteristics of the top centimeter for sampling sites on the Swale-Ouse and R. Wiske
| River and site | Distance (km) | Carbon and nitrogen contenta
|
Composition (%)b
|
||||
|---|---|---|---|---|---|---|---|
| % C | % N | C/N | Silt + clay | Fine sand | Sand | ||
| Swale | |||||||
| Ravenseat | −2.5, 0.0c | 0.17 | 0.009 | 19.5 | 8.3 | 36.1 | 43.5 |
| Ivelet Bridge | 10.9 | 0.43 | 0.037 | 14.8 | 11.2 | 50.6 | 20.0 |
| Catterick Bridge | 49.9 | 0.56 | 0.034 | 17.4 | 23.4 | 41.3 | 13.6 |
| Thornton Manor | 107.9 | 0.26 | 0.017 | 17.6 | 31.7 | 35.0 | 1.6 |
| Naburn Weir | 145 | 0.51 | 0.034 | 16.8 | 38.1 | 11.2 | 0.6 |
| Wiske (Wiske) | −1.7, 86.1c | 0.40 | 0.031 | 12.5 | 10.0 | 58.7 | 14.1 |
Monthly average values (excluding February) for 1997.
Average obtained from January, April, July, and October 1997.
The first value is the distance upstream from the main river. The second value is the distance down the main river where the tributary enters.
Nitrate depletion.
The denitrification rate and N2O production in the cores after incubation for 24 h to remove sediment nitrate and nitrite are shown in Table 2. Denitrification was still detectable, though rates were low: the rates were 5 μmol of N m−2 h−1 in the headwater site and 31.4 μmol of N m−2 h−1 in the Wiske. These values represent, respectively, 16 and 3% of the maximum rates found during assays with the saturation level of nitrate (see below). N2O production was very low in the Wiske (2.7 μmol of N2O m−2 h−1) and lower or negative (net consumption of N2O) at the other sites (Table 2).
TABLE 2.
Net N2O production and denitrification rate with no nitrate after 24 h at 15°C with nitrogen-free freshwater medium
| River and site | Distance (km) | N2O production (μmol of N2O-N m−2 h−1 ± SD) | Denitrification (μmol of N m−2 h−1 ± SD) |
|---|---|---|---|
| Swale | |||
| Ravenseat | −2.5, 0.0 | 0.74 ± 0.62 | 5.0 ± 4.0 |
| Ivelet Bridge (April) | 10.9 | −1.42 ± 0.93 | 5.5 ± 0.6 |
| Ivelet Bridge (June) | −2.34 ± 0.71 | 3.5 ± 0.7 | |
| Ivelet Bridge (August) | |||
| Catterick Bridge | 49.9 | 0.25 ± 0.61 | 13.4 ± 1.6 |
| Thornton Manor | 107.9 | 1.23 ± 0.56 | 12.2 ± 3.4 |
| Naburn Weir | 145 | 0.83 ± 0.86 | 9.2 ± 0.36 |
| Wiske | |||
| Wiske (April) | −1.7, 86.1 | 2.72 ± 2.52 | 23.1 ± 9.4 |
| Wiske (June) | −1.57 ± 0.13 | 31.4 ± 3.8 | |
| Wiske (August) | −9.70 ± 2.35 | 6.6 ± 5.8 |
Kinetics for denitrification and N2O production.
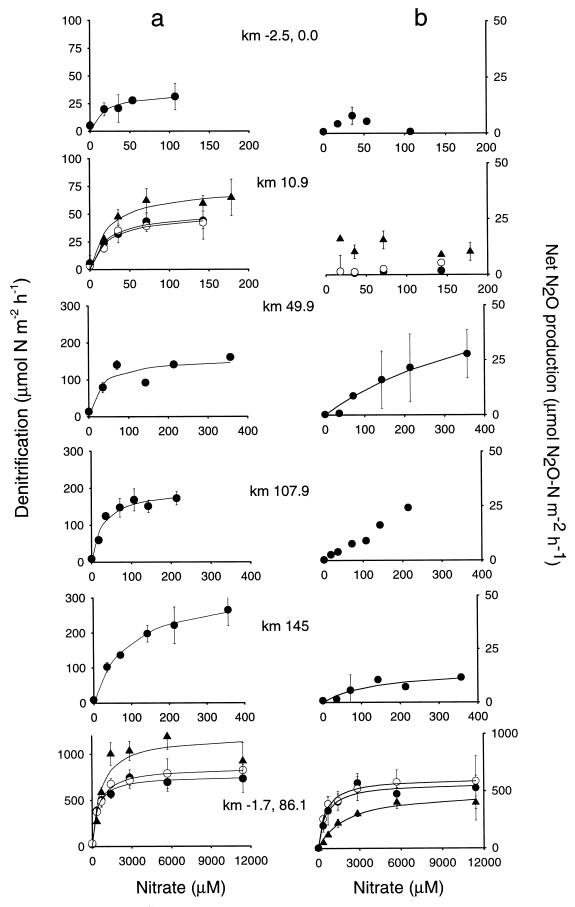
Nitrate addition in the overlying water resulted in an increase in the denitrification rate (Fig. 2a). The response of denitrification to nitrate at all sites could be fitted successfully (minimum regression coefficient of 0.9) to a Michaelis-Menten-type curve (Fig. 2a and Table 3). During the April survey (Table 3), Vmaxap increased on passing down the river (from 35.8 to 324 μmol of N m−2 h−1), but it was highest on the Wiske (758 μmol of N m−2 h−1). The value for Vmaxap in the Wiske in August was still higher (1,194 μmol of N m−2 h−1). Kmap constants increased in April on passing down the main river from 13.1 to 90.4 μM nitrate (Table 3). The overall highest value for Kmap (640 μM) was for the Wiske in August. Where repeat measurements were made (km 10.9 and Wiske), values for Vmaxap and Kmap were similar in April and June, but they were about one-third higher in August (Table 3).
FIG. 2.
Responses of denitrification rate (a) and N2O production (b) to nitrate additions at the sampling sites in April 1997 (•). For the Ivelet Bridge (km 10.9) and Wiske sites the assays were repeated in June (○) and August (▴) 1997. The bars denote the standard deviations of three replicates. Note the different scales for denitrification rate, N2O production, and nitrate concentration.
TABLE 3.
Kinetic parameters for denitrification rate and N2O production estimated according to the Lineweaver-Burk transformation of the Michaelis-Menten equation
| River and site | Distance (km) | Denitrification
|
N2O production
|
||||
|---|---|---|---|---|---|---|---|
| Vmaxap (μmol of N m−2 h−1) | Kmap (μM) | r2a | Vmaxap (μmol of N2O m−2 h−1) | Km (μM) | r2 | ||
| Swale | −2.5, 0.0 | ||||||
| Ravenseat | 10.9 | 35.8 | 13 | 0.95 | |||
| Ivelet Bridge (April) | 52 | 21 | 0.98 | ||||
| Ivelet Bridge (June) | 51 | 23 | 0.98 | ||||
| Ivelet Bridge (August) | 74 | 23 | 0.98 | ||||
| Catterick Bridge | 49.9 | 144 | 18 | 0.90 | 70.2 | 517 | 0.98 |
| Thornton Manor | 107.9 | 197 | 29 | 0.98 | |||
| Naburn Weir | 145 | 324 | 90 | 0.99 | 15.5 | 138 | 0.93 |
| Wiske | |||||||
| Wiske (April) | −1.7, 86.1 | 758 | 351 | 0.99 | 570 | 543 | 0.97 |
| Wiske (June) | 860 | 460 | 0.99 | 626 | 532 | 0.98 | |
| Wiske (August) | 1,194 | 640 | 0.98 | 496 | 1,952 | 0.98 | |
Coefficient of determination. See Results.
The production of N2O in the cores without C2H2 could only be fitted to a Michaelis-Menten curve for three sites (km 49.9, km 145.0, and Wiske [Fig. 2b]). At the two upstream sites (km −2.5 [0.0] and km 10.9), N2O production was less than 15 μmol of N2O-N m−2 h−1 and without any clear trend with increasing nitrate concentration. The maximum values for N2O production were 70.2, 15.5, and 570 μmol of N2O-N m−2 h−1 in April for km 49.9, km 145.0, and the Wiske, respectively, and 626 and 496 μmol of N2O-N m−2 h−1 in June and August for the Wiske (Table 3). The Kmap values were 517, 138, and 542 μM in April for the same sites and 532 and 1,952 μM in June and August for the Wiske (Table 3). The Kmap value for N2O production for these sites was higher than the Kmap value for the denitrification rate, while the Vmaxap values were lower.
The proportion of N2O to total N gases (N2O + N2) ranged between 2.4 and 38% and between 2 and 58% at the headwater and km 10.9 sites, respectively (Table 4), but there was no clear trend with increasing nitrate concentration. The values increased on addition of nitrate to the km 49.9 and km 107.9 samples but not to the km 145.0 sample. In the Wiske the values exceeded 50% at all nitrate concentrations in April and June (Table 4).
TABLE 4.
Percentage of N2O related to total N gases evolved (N2O + N2) for the nitrate concentration assayed
| Nitrate concn (μM) | % N2O in samples from:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ravenseat | Ivelet Bridge
|
Catterick Bridge | Thornton Manor | Naburn Weir | R. Wiske
|
|||||
| April | June | August | April | June | August | |||||
| 17.8 | 21 | 6 | 7.5 | 58 | 4.3 | |||||
| 37.7 | 38 | 2 | 3.4 | 21 | 1.0 | 3.0 | <0.0 | |||
| 53.6 | 19 | 3 | 6.4 | 25 | ||||||
| 71.4 | 6.0 | 5.1 | 6.0 | |||||||
| 107.1 | 2.3 | 5.3 | ||||||||
| 142.8 | 4 | 12 | 15 | 17 | 10 | 6.4 | ||||
| 214.3 | 15 | 14 | 3.2 | |||||||
| 357.1 | 17 | 11 | 50 | 68 | 18 | |||||
| 714.3 | 65 | 80 | 20 | |||||||
| 1,428 | 71 | 62 | 22 | |||||||
| 2,857 | 76 | 73 | 29 | |||||||
| 5,714 | 68 | 73 | 33 | |||||||
| 11,429 | 72 | 75 | 43 | |||||||
When river water (with its natural nitrate concentration) was used instead of medium, the denitrification rate was linearly related (r2 = 0.89; slope = 0.92) to the rate predicted from the Michaelis-Menten curve for the nitrate concentration in the river.
DISCUSSION
Nitrate depletion and freshwater medium.
The successful application of kinetic studies to the sediments required removal of nitrate and nitrite, and this was largely achieved. This suggests that any nitrate produced via nitrification of the ammonium in the sediment was also consumed by natural denitrification during this period. The highest denitrification persisting after 24 h of incubation in the absence of nitrate was at the headwater site (Ravenseat).
Influence of nitrate addition on denitrification and N2O production.
The denitrification rate in intact sediment cores responded strongly to nitrate addition and followed a Michaelis-Menten-type curve, as found by other researchers for freshwater (1) and estuarine (10, 16, 17) sediments. Repeat measurements on different dates at particular sites showed only moderate differences.
The Vmaxap values for denitrification rate were mostly similar to the maximum rates reported by Pattinson et al. (18) for field conditions in a 17-month survey of intact sediment cores from the same sites. However, comparisons with other studies are complicated by the fact that these have been made at a range of temperatures. Ideally, results would be presented for both the field temperature and a standard laboratory temperature. In the case of the Swale-Ouse, the values are comparable because the temperature at the time of the maximum value was always within 5°C of the standard temperature of 15°C.
Other published kinetic data apparently refer only to experiments with slurries, so caution is needed in comparing our data with previous data. However, the values for Vmaxap at the headwater site and at the main river (35.8 to 324 μmol of N m−2 h−1) are well within the range of denitrification rates measured at near-ambient conditions for rivers (0 to 345 μmol of N m−2 h−1) and for coastal marine sediments (0 to 888 μmol of N m−2 h−1) (23), although denitrification was always detectable in our studies. The uppermost value on the Wiske (1,194 μmol of N m−2 h−1 in August) exceeded this range. The Kmap values may be compared with the lower (8 μM) and upper (344 μM) values found in previous studies (10, 12, 16, 17) with the slurry technique.
Another problem in assessing results is the fact that denitrification in the sediment has been related to the nitrate concentration in the overlying water. Although transport of nitrate into the sediment is likely to be rapid, the lower and variable nitrate concentration in the sediment means that the Kmap value has inevitably been overestimated. However, we advocate the use of cores to obtain realistic measurements of kinetic parameters to be incorporated into integrating models.
Net N2O production occurred at all sites, but only at some sites was there an increase with increasing nitrate concentration. Compared to other studies, the proportions of N2O related to total N gases (N2 + N2O) for the highest nitrate concentrations (Table 4) were high, reaching 80% in the Wiske in June. The published values for N2O in aquatic sediments have been obtained from pore water profiles (25) and direct N2O flux measurements from cores (13, 21); net N2O flux is generally less than 2 μmol of N2O m−2 h−1 and less than 5% of N2 production (23). As N2O production is an intermediate product in at least three processes in the nitrogen cycle, each influenced by a range of environmental factors, it is difficult to explain these high values. However, eutrophication is one factor reported to lead to increased N2O production, as shown in Narragansett Bay (20), where N2O fluxes increased around 10-fold from the relatively unpolluted lower- and mid-bay sediments to the eutrophic upper-bay sediments. A study (22) on a marine mesocosm showed that N2O flux from sediment and the N2O/N2 ratio increased markedly in relation to nitrate input.
Ecological significance.
The Vmaxap value showed a marked increase coincident with increasing nitrate concentration on passing downriver, and it seems almost certain that this is a key factor. However, other factors must be considered when assessing the results shown in Fig. 2. These factors include the number of denitrifying bacteria, their genetic and physiological properties, and the optimal conditions of the process (e.g., the concentrations of suitable carbon substrates, phosphate, and oxygen). Downstream, the sediment particles were finer, presumably decreasing oxygen penetration, and the carbon content of the upper 2 cm of sediment was higher (Table 1), all features likely to favor denitrification. Although the changes in oxygen concentration were not investigated, the high O2 saturation usually present in the river water and the relatively low incubation period minimized the disturbance of sediment in our experimental setup.
The Kmap values increased on passing downstream and were maximum in the Wiske. This trend agrees with the observed increase in nitrate concentration on passing downstream. In environments with high affinity (low Kmap) and high nitrate concentration or in those with low affinity (high Kmap) and low nitrate concentration, nitrate reduction by denitrifying bacteria would be inefficient. The Kmap values calculated in this study for each site were compared with the nitrate data available for 1995 to 1996 from other sources (Environment Agency and the LOIS data base). For the upland sites, the Kmap values were higher than the nitrate concentration in the river, and therefore the response of denitrification to the nitrate concentration is likely to be linear for most of the year. However, the Kmap values for all sites on the main river and the Wiske were lower than the nitrate concentration for most of the year, and therefore nitrate reduction by denitrifying bacteria is probably nitrate saturated, especially during winter and spring. This suggests that other variables, such as temperature, organic carbon, and oxygen, must be considered when studying the seasonal changes in denitrification (18). As denitrifying bacteria are taxonomically heterogeneous (10), it is likely that these factors also exert a selective pressure on groups that are dominant at particular times of the year.
ACKNOWLEDGMENTS
This work was performed as part of the Land Ocean Interaction Study programme (NERC grant GST/01/A770), which included a studentship for S.N.P. R.G. acknowledges a postdoctoral grant from the Ministerio de Educación y Cultura, Subprograma General en el Extranjero.
We are most grateful to R. V. Smith, Department of Agricultural and Environmental Science, the Queens University of Belfast, Belfast, Northern Ireland, for helpful discussion.
Footnotes
This is Land Ocean Interaction Study programme publication no. 414.
REFERENCES
- 1.Andersen J M. Rates of denitrification on undisturbed sediment from six lakes as a function of nitrate concentration, oxygen and temperature. Arch Hydrobiol. 1977;80:147–159. [Google Scholar]
- 2.Christensen P J, Sørensen J. Denitrification in sediment of lowland streams: regional and seasonal variation in Gelaek and Rabis Baek, Denmark. FEMS Microbiol Ecol. 1988;53:335–344. [Google Scholar]
- 3.Chu S P. The influence of the mineral composition of the media on the growth of planktonic algae. I. Methods and culture media. J Ecol. 1942;30:284–325. [Google Scholar]
- 4.Cooke J G, White R E. Spatial distribution of denitrifying activity in a stream draining an agricultural catchment. Freshwater Biol. 1987;18:509–519. [Google Scholar]
- 5.Crutzen P J. Atmospheric chemical processes of the oxides of nitrogen, including nitrous oxide. In: Delwiche C C, editor. Denitrification, nitrification, and atmospheric nitrous oxide. New York, N.Y: John Wiley & Sons, Inc.; 1981. pp. 17–44. [Google Scholar]
- 6.Holmes N T H, Whitton B A. Macrophytic vegetation of the River Swale, Yorkshire. Freshwater Biol. 1977;7:545–558. [Google Scholar]
- 7.Hordijk C A, Snieder M, Van Engelen J J M, Cappenberg T E. Estimation of bacterial nitrate reduction rates at in situ concentrations in freshwater sediments. Appl Environ Microbiol. 1987;53:217–223. doi: 10.1128/aem.53.2.217-223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvie H P, Neal C, Robson A J. The geography of the Humber catchment. Sci Total Environ. 1997;194/195:87–99. [Google Scholar]
- 9.Knowles R. Denitrification. Microbiol Rev. 1982;46:43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike I, Hattori A, Goering J J. Controlled ecosystem pollution experiment: effect of mercury on enclosed water columns. 6. Denitrification by marine bacteria. Mar Sci Commun. 1978;4:1–12. [Google Scholar]
- 11.Kratz W A, Myers J. Nutrition and growth of several blue-green algae. Am J Bot. 1955;42:282–287. [Google Scholar]
- 12.Murray R E, Parsons L L, Smith M S. Kinetics of nitrate utilization by mixed populations of denitrifying bacteria. Appl Environ Microbiol. 1989;55:717–721. doi: 10.1128/aem.55.3.717-721.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishio T, Koike I, Hatorri A. Estimates of denitrification and nitrification in coastal and estuarine sediments. Appl Environ Microbiol. 1983;45:440–450. doi: 10.1128/aem.45.2.444-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OECD. Environmental data compendium, 1985. Paris, France: OECD; 1985. [Google Scholar]
- 15.OECD. Environmental data compendium, 1987. Paris, France: OECD; 1987. [Google Scholar]
- 16.Oremland R S, Umberger C, Culbertson C W, Smith R L. Denitrification in San Francisco Bay intertidal sediments. Appl Environ Microbiol. 1984;47:1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oren A, Blackburn T H. Estimation of sediment denitrification rates at in situ nitrate concentrations. Appl Environ Microbiol. 1979;37:174–176. doi: 10.1128/aem.37.1.174-176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattinson S N, García-Ruiz R, Whitton B A. Spatial and seasonal variation in denitrification in the Swale-Ouse system, a river continuum. Sci Total Environ. 1998;200:289–306. [Google Scholar]
- 19.Rohde H. A comparison of the contribution of various gases to the greenhouse effect. Science. 1990;248:1217. doi: 10.1126/science.248.4960.1217. [DOI] [PubMed] [Google Scholar]
- 20.Seitzinger S P, Pilson M E Q, Nixon S W. Nitrous oxide production in nearshore marine sediments. Science. 1983;222:1244–1246. doi: 10.1126/science.222.4629.1244. [DOI] [PubMed] [Google Scholar]
- 21.Seitzinger S P, Nixon S W, Pilson M E Q. Denitrification and nitrous oxide production in a coastal marine ecosystem. Limnol Oceanogr. 1984;29:73–83. [Google Scholar]
- 22.Seitzinger S P, Nixon S W. Eutrophication and the rate of denitrification and N2O production in coastal marine sediments. Limnol Oceanogr. 1985;30:1332–1339. [Google Scholar]
- 23.Seitzinger S P. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr. 1988;33:702–724. [Google Scholar]
- 24.Sørensen J. Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Appl Environ Microbiol. 1978;36:139–143. doi: 10.1128/aem.36.1.139-143.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørensen J. Occurrence of nitric and nitrous oxides in a coastal marine sediment. Appl Environ Microbiol. 1978;36:809–813. doi: 10.1128/aem.36.6.809-813.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stainton M P, Capel M J, Armstrong F A J. The chemical analysis of freshwater. Miscellaneous special publication no. 25. 2nd ed. Winnipeg, Canada: Fisheries and Environment Canada, Fisheries and Marine Service; 1977. [Google Scholar]
- 27.Torre M, Rebillard J P, Ayphassorho H, Labroue L, Helmer C. Etude expérimentale de la dénitrification in situ en eaux courantes: application à la rivière Charente. Ann Limnol. 1992;28:263–271. [Google Scholar]
- 28.Vannote R L, Minshall G W, Cummins K W, Sedell J R, Cushing C E. The river continuum concept. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 29.Whitton B A, Lucas M. Biology of the Humber rivers. Sci Total Environ. 1997;194/195:247–262. doi: 10.1016/s0048-9697(97)88162-4. [DOI] [PubMed] [Google Scholar]