Abstract
Background:
The association between belonging to a disadvantaged socio-economic status or social class and health outcomes has been consistently documented during recent decades. However, a meta-analysis quantifying the association between belonging to a lower social class and the risk of dementia has yet to be performed. In the present work, we sought to summarise the results of prospective, longitudinal studies on this topic.
Methods:
We conducted a systematic review and meta-analysis of prospective, longitudinal studies measuring the association between indicators of social class and the risk of all-cause/Alzheimer’s dementia. The search was conducted in four databases (Medline, Embase, Web of Science and PsychInfo). Inclusion criteria for this systematic review and meta-analysis were: (a) longitudinal prospective study, (b) aged ⩾60 years at baseline, (c) issued from the general population, (d) no dementia at baseline and (e) mention of social class as exposure. Exclusion criteria were: (a) study of rare dementia types (e.g. frontotemporal dementia), (b) abstract-only papers and (c) articles without full text available. The Newcastle–Ottawa scale was used to assess the risk of bias in individual studies. We calculated the overall pooled relative risk of dementia for different social class indicators, both crude and adjusted for sex, age and the year of the cohort start.
Results:
Out of 4548 screened abstracts, 15 were included in the final analysis (76,561 participants, mean follow-up 6.7 years (2.4–25 years), mean age at baseline 75.1 years (70.6–82.1 years), mean percentage of women 58%). Social class was operationalised as levels of education, occupational class, income level, neighbourhood disadvantage and wealth. Education (relative risk (RR)=2.48; confidence interval (CI) 1.71–3.59) and occupational class (RR=2.09; CI 1.18–3.69) but not income (RR=1.28; CI 0.81–2.04) were significantly associated with the risk of dementia in the adjusted model. Some of the limitations of this study are the inclusion of studies predominantly conducted in high-income countries and the exclusion of social mobility in our analysis.
Conclusions:
We conclude that there is a significant association between belonging to a social class and the risk of dementia, with education and occupation being the most relevant indicators of social class regarding this risk. Studying the relationship between belonging to a disadvantaged social class and dementia risk might be a fruitful path to diminishing the incidence of dementia over time. However, a narrow operationalisation of social class that only includes education, occupation and income may reduce the potential for such studies to inform social policies.
Keywords: Age, aging, Alzheimer’s disease, dementia, social class, social status, social inequalities, social determinants of health, systematic review, meta-analysis, education, occupation, neighbourhood, income, wealth
Introduction
Early studies on social class and dementia
The association between belonging to a disadvantaged social class and health outcomes has been consistently documented during recent decades, and dementia is no exception. The study by Stern et al. [1], published in 1994, was one of the first to link dementia risk to ‘our social class hierarchies’, with occupational class and years of education as indicators. Evans et al. [2] then reported the association between socio-economic status and dementia – measured with education, occupation and income. Their work and the following studies [3–6] revealed that the relationship between social class and dementia risk may be more complex than originally posited [7]. First, there is no consensus on the best social class indicators [7]. Second, studies report a large between-studies variability in the magnitude of association [1,8]. Finally, multiple interactions between social class and other factors (e.g. APOE genotype) can be found to influence dementia risk [2,8,9]. Despite a presence and interest in studying the influence of social class on various health outcomes, a meta-analysis quantifying the association between belonging to a disadvantaged social class and the risk of dementia has yet to be performed. In the present work, we sought to summarise the results of prospective, longitudinal studies on the subject to gain insight into the mechanisms of this relationship.
Mechanisms linking social class and dementia
Belonging to a given social class has been found to change a person’s cognitive health outcomes in advanced age in multiple ways. First, individuals in more advantaged social classes have better access to education [10–12], creative and cognitively demanding occupations [10,13,14] and cognitively complex leisure activities such as going to the theatre, opera and museums [15,16]. Exposure to lifelong cognitive stimulation of this sort enables the development of so-called cognitive/brain reserve, delaying the clinical manifestation of existing dementia-type brain changes [17–20]. Second, these individuals endure less chronic background stress associated with, for example, insecure housing, work and bill payments [21,22]. These lower levels of stress contribute to better cardiovascular [23] and cognitive health [24], both of which also contribute to a lower risk of dementia [25,26]. Third, people in more advantageous social classes have more confidence in institutions [27] and consequently are more prone to rely on formal support to fulfil their health needs [28,29]. As such, they have a higher probability of receiving timely medical and social care [28,30] – another factor associated with better health outcomes. Finally, people of higher social classes are exposed to a lesser extent to environmental factors linked to dementia risk, such as air and noise pollution, limited greenness and poor walkability of the place [31,32]. Inversely, disadvantaged groups and individuals belonging to lower social classes are more exposed to risk factors and environmental conditions associated with much higher health risks. This phenomenon, called ‘double jeopardy’ [33], has been previously explored in the field of cognitive health of older people [34].
Challenges in studying the association between social class and dementia
There are some notable difficulties in studying the association between social class and dementia. For one, each society has its own understanding of what are considered valuable resources (e.g. capital/means of production [35,36], spiritual [37] or cultural [38,39] leadership, respect/recognition [40] or decisional power [41]), the possession of which determines one’s affiliation to a higher social class [42,43]. Therefore, social inequalities in dementia may appear along different axes of social stratification (e.g. wealth, cultural capital, occupational prestige) in different societies. Thus, comparing associations between belonging to social class and dementia risk across countries must take these possible differences into account. Moreover, some individual characteristics such as race and gender should be considered because they could modify the association between social class indicators and dementia risk [44,45] observed in the general population.
As we are interested in how social stratification affects the cognitive health of older people, we preferred the term ‘social class’ over ‘socio-economic status’. While the difference between the two terms is a subject of ongoing discussion [46], it is generally acknowledged that the former is a broader concept and includes notions of privilege and lifestyle which may be as influential on the risk of dementia as socio-economic position.
The necessity of the review and objectives
A systematic review with meta-analysis targeting the association between belonging to a disadvantaged social class and dementia risk can clarify the mechanisms at play by answering the following questions: (1) What is the magnitude of this association overall? (2) Which social class indicators are associated with greater dementia risk? (3) To what extent is the association moderated by individual participant characteristics (gender, race/ethnicity) and the context (time period, country)? We limited our analysis to prospective, longitudinal studies, as they provide the highest levels of evidence for prognostic studies [47].
Methods
The registration number of this review on the PROSPERO International prospective register of systematic reviews (https://www.crd.york.ac.uk/prospero) is CRD42020166244. The registration record was automatically published exactly as submitted due to the COVID-19 pandemic in 2020. The divergence with the protocol concerned inclusion criteria, as we did not include studies on cognitive functioning, minimal cognitive impairment and cognitive decline. This decision was made to keep the focus of the current work reasonably narrow. The diversity in operationalisation of cognitive decline and cognitive impairment, as well as the diversity in cognitive functions considered, would require a presentation of results far above the limits of the journal publication.
The search strategy was developed by researchers (Y.B. and G.M.) with the help of a university librarian specialising in public health. The search period included all available publications until 7 October 2021 (the day of the last search in all the databases), with no limit for the oldest publication. The search strategy used both MeSH terms and keywords and was constructed using four key concepts: social class, dementia, longitudinal studies and aging (see Supplemental Material for the PubMed search terms). We included journal articles and governmental reports, while dissertations, book chapters, and conference papers were excluded. No contact with study authors was undertaken. We searched in PubMed, Embase, PsychInfo and Web of Science databases. Covidence and Endnote reference management software was used for independent abstract screening and full-text management. Articles in English and French were retained.
We included prospective longitudinal studies including participants aged ⩾60 years without dementia at baseline issued from the general population. To be included, studies had to report the risk of dementia (all-cause dementia or Alzheimer’s dementia) as assessed by the authors of included studies with an individual’s social class indicator. Social class as an exposure or confounder had to be explicitly stated by the authors, and indicators of social class had to be clearly defined. If a concept (e.g. education) was considered as an indicator of social class in one article but was not so in another, we only included the first article in the review, even if both reported an association between the concept and dementia (e.g. if an article defined social class through occupation, and education was used as a cofounder, we extracted data on occupation only, even if other studies defined social class using education). We excluded studies conducted in non-general populations (e.g. professional communities or clinical populations), studies with no indicators clearly attributed to social class (e.g. a group of variables was considered as indicators of social class and lifestyle without distinction between two) and, for feasibility reasons, studies reporting the association between social class and cognitive health outcomes other than all-cause dementia and Alzheimer’s dementia (e.g. cognitive functioning, cognitive decline, dementia mortality, vascular dementia only). In addition, we did not include studies looking at the association between social mobility/childhood socioeconomic status and dementia risk to keep the scope of the current review reasonably narrow. For this same reason, we did not include studies with combined indexes of social class; nor did we consider the interaction between indicators and other variables in the analysis.
Article selection was conducted by Y.B. and A.K. The screening of abstracts was done independently under the supervision of G.M.; cases of disagreement were resolved by G.M. The selection of full texts was made by Y.B. under the supervision of G.M.
Data extraction was done by Y.B. and X.M. Y.B. conducted the initial extraction, and X.M. verified its quality. We extracted general information pertaining to each publication, including year, author, journal, setting (country, city), recruitment period, participation rate (recruited/included in the analysis), mean length of follow-up and type of cognitive evaluation (e.g. screening tests, clinical interview) both at baseline and at follow-up. We extracted the data on the indicators of social class (e.g. education, income, occupation). For each indicator, we extracted the number of individuals classified as belonging to the ‘advantaged’ and ‘disadvantaged’ classes, as well as the number of dementia cases in each class. If the exposure was presented in more than two categories (e.g. primary education, high school and university degree), we extracted the data from the extremities of the exposure classification (e.g. primary education vs. university degree). When no information on the number of participants belonging to each exposure/disease group was available, we extracted the association’s effect size (risk ratio, hazard ratio, incidence rate ratio) from the unadjusted model presenting the association between social class and dementia risk. If the unadjusted model was not reported, we extracted the effect size from the adjusted one. If results were reported for more than one non-overlapping group of participants (e.g. black and white racial groups), we treated them as distinct populations.
Missing data
We applied pairwise deletion to deal with missing data for all the variables except the year of the cohort’s start. We imputed it as the year of the study publication minus years of follow-up minus two years.
Data analysis
We first reviewed the indicators used in the operationalisation of social class. The objective of this part of the work was twofold. First, we sought to prevent bias from the categorisation of continuous variables when using cut points (e.g. the categories of high and low education might have been dichotomised at cut points of three years or 13 years). If this occurred, we excluded the variable from the analysis. Second, we wanted to ensure we did not miss narrower categories within indicators of social class which could contribute to our understanding of the association between the social standing and dementia risk.
Meta-analysis and meta-regression
To understand the extent to which social class is associated with the risk of dementia, we calculated its pooled relative risk both in the fixed-effect model and in the random-effect model using a restricted maximum-likelihood estimator. To understand which indicators of social class might be the most impactful on the risk of dementia, we undertook the same analysis in the subgroups of indicators of social class. Then, in a random-effect model, created for each indicator of social class separately, we adjusted the association between indicators of social class and dementia risk for the percentage of women, the average age at baseline and the year of the start of the cohort. Finally, we suggested that if there was a difference in the effect of social class indicators between countries, there should be a correlation between true effect size in studies conducted within the same country. Thus, controlling for the countries where the studies were conducted would improve the fit of the meta-regression model. We finally compare the model fit of two meta-regression models with and without applying the fixed effect on the country of the studies’ origin and the first year of recruitment in a likelihood ratio test. Its non-significance would mean an insignificant effect of the country/period context on the relationships between the social class indicators and dementia risk.
Publication bias was assessed by the funnel plot and the trim and trill test which recalculates the pooled effect size including the values of hypothetical studies needed to make the funnel plot symmetrical.
The risk of bias in individual studies was assessed with the Newcastle–Ottawa scale [48]. The scale ranks the observational studies included in systematic reviews and meta-analyses based on the risk of selection bias, comparability and outcome assessment. Each criterion is assessed using a star ranking, with a maximum of nine stars. A higher number of stars is associated with a lower risk of bias.
All analyses were made with the Metafor [49] package in R (The R Foundation for Statistical Computing, Vienna, Austria) statistical software.
Results
Article selection
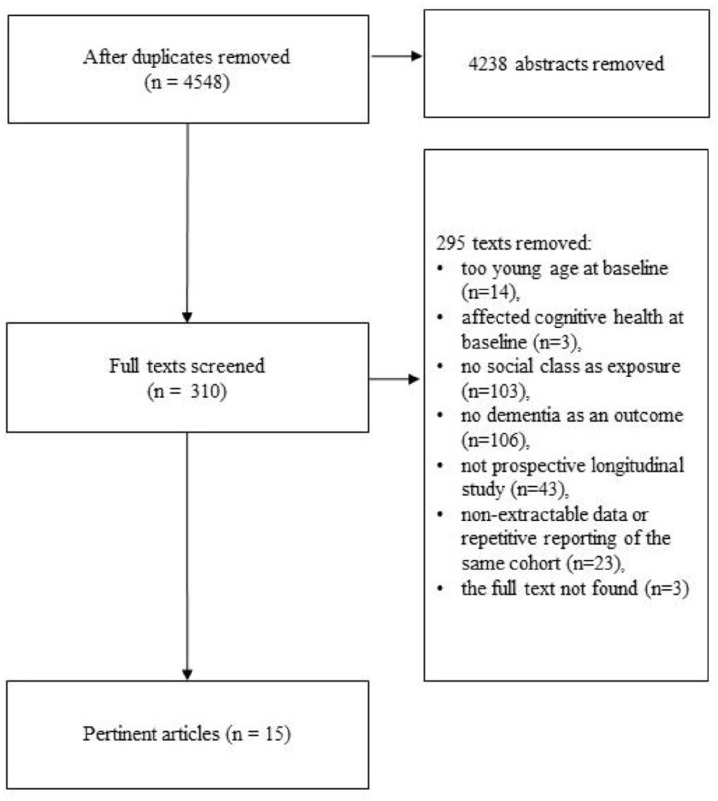
The PRISMA [50] flow chart of study selection is presented in Figure 1. Out of 4548 abstracts screened, 310 full-text documents were retained. Out of them, 295 articles were excluded for the following reasons: age <60 years old (n=14), not only including cognitively healthy people on baseline (n=3), no social class as exposure (n=103), no dementia as an outcome (n=106), not a prospective longitudinal study (n=43), non-extractable effect size or repetitive reporting of the same cohort (n=23) and the full text was not found (n=3). A total of 15 articles containing information on 18 non-overlapping samples were analysed. Descriptions of the selected studies [1,2,4,8,9,44,45,51–58] with principal outcomes are presented in Table I.
Figure 1.
PRISMA flow chart of study selection.
Table I.
Description of included studies. Principal outcomes are given with 95% confidence interval.
| Author, publication year | Cohort, start, country | Follow-up, years | Participants, a n sub-cohort | Age, b years | Education, years | Manual workers, % | Women, % | Reference | Exposure c and principal outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Bickel et al., 1994 | 1986, Germany | 7.8 | 314 | 74 | 34% | 64% | Crude annual incidence rate: O: professional: 7.1; occupied solely with housework or helping in family business: 10.1 | ||
| Cadar et al., 2018 | 2002, UK | 7 | 1808, born 1900–1925 | 81 | 8.3 | 58% | More privileged | Adjusted, E: HR=0.72 (0.49–1.07); N: HR=1.11 (0.68–1.81); W: HR=1.35 (0.85–2.14) | |
| 4412, born 1926–1943 | 71 | 9.2 | 53% | Adjusted, E: HR=1.21 (0.81–1.79); N: HR=1.50 (0.91–2.49); W: HR=1.68 (1.05–2.86) | |||||
| Chen et al., 2011 | 2001, China | 7.5 | 1526 | 72 | 9.5 | 24% | 43% | More privileged | Adjusted, E: OR=2.12 (1.03–4.38); I: OR=1.13 (0.56–2.24); O: OR=1.33 (0.60–2.99) |
| Contador et al., 2015 | 1994, Spain, | 3.8 | 2711 | 73 | 6.9 | 85% | 57% | More privileged | Adjusted, N: RR=4.36 (2.35–8.09) |
| De Deyn et al., 2011 | 2006, Belgium | 3 | 825 | 78 | 9.3 | 63% | Crude annual proportion of cases: N: high mean education: 5.9%; low mean education level: 10.1% | ||
| Evans et al., 1997 | 1982, USA | 4.3 | 642 | 74 | 9.1 | 56% | Less privileged | Adjusted, E: OR=0.83 (0.75–0.92); I: OR=0.80 (0.63–1.02); O: OR=0.96 (0.93–0.99) | |
| Hasselgren et al., 2019 | 2000, Sweden | 10 | 580 | 71 | 9.1 | 44% | 61% | Less privileged | Adjusted, E: HR=0.72 (0.35–1.49); O: HR=0.91 (0.49–1.71) |
| Karp et al., 2004 | 1987, Sweden | 3 | 931 | 81 | 8.2 | 35% | 76% | More privileged | Adjusted, O: RR=1.1 (0.7–1.7) |
| Letellier et al., 2018 | 1999, France | 12 | 7016 | 74 | 18 | 62% | Less privileged | Adjusted, E: HR=0.57 (0.49–0.67); I: HR=0.72 (0.62–0.84) | |
| More privileged | Adjusted, O: HR=1.64 (1.39–1.94); LA: HR=1.03 (0.91–1.17); N: HR=1.15 (0.93–1.42) | ||||||||
| Ouvrard et al., 2020 | 1988, France | 25 | 3431 | 75 | 57% | Less privileged | Adjusted, N: HR=1.10 (0.89–1.36) | ||
| Paykel et al., 1994 | 1988, UK | 2.4 | 483 | 82 | 59% | 66% | Crude annual incidence rate, O: higher occupational class 3.6 (2.5–5.2); lower occupational class: 4.4 (2.9–6.8) | ||
| Samuel et al., 2020 | 2012, USA | 5 | 3785 | 76 | 12.6 | 58% | 56% | Less privileged | Adjusted, E: hOR=0.73 (0.65–0.83); I: hOR=0.84 (0.74–0.95); O: hOR=1.10 (0.97–1.24) |
| Stern et al., 2020 | 1988, USA | 3 | 593 | 74 | 9.6 | 55% | 73% | More privileged | Adjusted, E: HR=2.02 (1.33–3.06); O: HR=2.25 (1.32–3.84) |
| Takasugi et al., 2019 | 2010, Japan | 6 | 24,175, males | 74 | 9.9 | 18% | 0% | More privileged | Adjusted, E: HR=1.34 (1.04–1.73); I: HR=0.97 (0.83–1.13); O: HR=1.25 (0.99–1.58) |
| 27,888, females | 74 | 9.0 | 39% | 100% | Adjusted, E: HR=1.21 (1.00–1.45); I: HR=0.83 (0.72–0.96); O: HR=0.95 (0.80–1.13) | ||||
| Yaffe et al., 2013 | 1997, USA | 6 | 1019, black | 73 | 12.6 | 47% | More privileged | Adjusted, E: HR=1.75 (1.26–2.43); I: HR=1.45 (1.07–1.96), | |
| 1438, white | 74 | 10.8 | 55% | Adjusted, E: HR=0.99 (0.66–1.47); I: HR=1.49 (0.80–2.77) |
Participants at follow-up.
Age at baseline.
Exposure: O: occupation; E: education; N: neighbourhood; W: wealth; I: income; LA: living alone.
HR: hazard ratio; RR: relative risk; OR: odds ratio.
Description of study populations
Studies retained for analysis contained information on 85,406 participants. The mean follow-up was 7.2 years (2.4–25 years), the mean age of participants at baseline was 74.2 years (70.6–82.1 years, n=18), the mean percentage of women was 57% (0–100%, n=10), the mean number of education years was 9.6 (6.9–12.6, n=11) and the mean percentage of manual workers was 43% (18–85%, n=9). Four studies [2,44,52,57] reported ethnic/racial composition of participants. The percentage of underrepresented minorities varied from 0% to 100% of samples. In three studies [1,51,55], the year of the cohort’s start was imputed as described in the Missing Data subsection of the Methods section. Most of the selected studies tested more than one indicator of social class: education was tested in 12 samples, income was tested in nine, neighbourhood in six, occupation in 10, wealth in two and living alone in one. Considering the small number of samples measuring the association between wealth/living alone and the risk of dementia, these samples were excluded from a part of analysis.
Diagnosis of dementia was established with: (a) a clinical interview by two or more trained professionals with a medical degree (e.g. psychiatrically trained physician, neurologist) [4,51], (b) a combination of self-reported diagnosis, cognitive screening tests and medical history analysis with caregiver’s information [44,52,57], (c) a combination of cognitive screening and a following in-person clinical investigation with or without analysis of medical history and an interview with a caregiver [1,2,8,9,53–56) or (d) administrative data [45].
Qualitative description of the social class indicators
Education was considered as an indicator of social status by the majority of authors [1,2,8,44,45,52,53,57,58]. Most of them reported the relative risk of dementia associated with each additional level of education (e.g. primary vs. secondary, primary vs. some college), with rare exceptions. Stern et al. [1] dichotomised education as less than eight years or eight years or more of schooling. Evans et al. [2] measured education as the total number of years of schooling. Yaffe et al. [44] also used literacy level, defined as reading capacity corresponding to >9th versus ⩽9th grade.
Income [2,44,45,53,57,58] and wealth [52] were also widely used as indicators of social class. Some authors proposed a classification of income based on a fixed amount of earnings per year (e.g. Evans et al. [2]: >US$10,000 per year vs. <US$5000 per year in 1982). This form of classification is convenient to use during the study period but can be difficult to interpret in today’s money, given inflation and wage conversion to fit current incomes. Thus, Samuel et al. [57] proposed the classification of income as a percentage of the poverty rate (>500%, ⩽500%), which could be more appropriate for communication with a wider audience. From this point of view, the relative lack of income in terms of ‘financial problems’ [53], ‘financial strain’ [57] or ‘financial inadequacy’ [44] could also be an easy-to-interpret indicator of social class.
Occupation was also widely considered [1,2,4,8,45,52,54,56–58]. In the majority of studies, professional and technical jobs were considered as indicators of a higher social status, and a lower status was attributed to participants without occupation, manual workers and peasants. Some studies used the official state classifications of occupations, while Evans et al. [2] used occupational prestige scores. Two distinct occupational categories were found in workplace hierarchies. The first was self-employed [4,8,51] workers who showed a dementia risk comparable with those of professional or academic workers. The second category was housewives [1,2,4,8,51]. The social status of the housewives is difficult to establish [4,8], as their education level might be higher than that of working women [4]; however, they do not have the prestige or income associated with participation in the labour market. Thus, in selected studies, they were either excluded from analysis [8] or considered as a distinctive occupational category [1,2,4,51]. In the latter case, the dementia risk associated with housekeeping was compatible with those of non-skilled manual occupations [1,2,51].
Neighbourhood as an indicator of social class was considered as an aggregated variable, calculated using indicators of social classes from its residents (e.g. occupation [9,54], education [9,55], income [9,52]). In several studies, some neighbourhood-specific characteristics were also considered, such as an unemployment rate [9], health deprivation and disability, barriers to housing and services, living environment deprivation and crime [52]. In the study by Letellier et al. [58], the neighbourhood deprivation score used was calculated from a range of economic and social indicators of deprivation.
Other axes of social stratification
The variables discussed in this section were not considered as social class indicators but were tested as probable modifiers in the relationships between social class and dementia. Yaffe et al. [44] showed that the effect of race, namely, higher incidence of dementia in black people versus white people, disappeared if adjusted to social class indicators. Hasselgren et al. [8] and Tacasugi et al. [45] both reported different patterns of association between social class and dementia across genders.
Meta-analysis and meta-regression
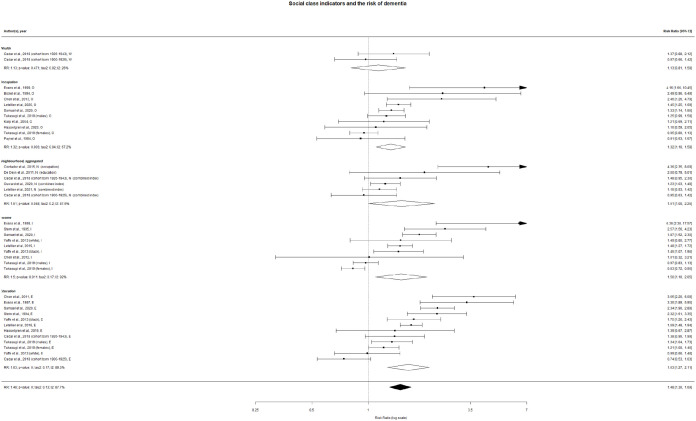
The pooled crude relative risk (RR) of dementia associated with belonging to lower social class was 1.33 (confidence interval (CI) 1.27–1.38; I2=84%) in a fixed-effect model and 1.48 (CI 1.30–1.69; I2=88%) in a random-effect model (Figure 2). Education, income and occupations were all significantly associated with dementia risk in both fixed-effect and random-effect crude models.
Figure 2.
Individual and pooled risk ratios of the association between the social status and dementia risk (forest plot), random effect. Letters after the authors’ names mean the indicators of social class (W: wealth; O: occupation; N: neighbourhood; E: education; I: income). In parentheses, for all the indicators except neighbourhood: the sub-cohorts; for the neighbourhood: the characteristic of the population by which the social class was attributed to the neighbourhood.
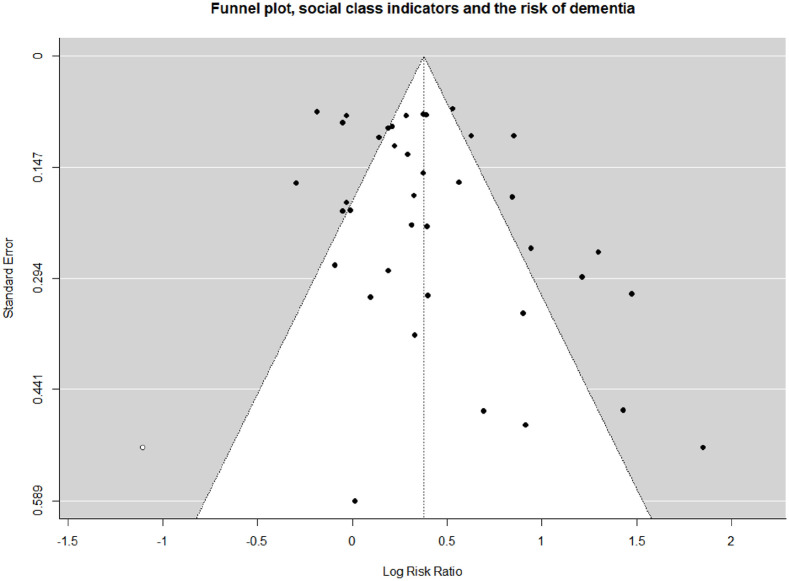
The funnel plot presented in Figure 3 suggests the presence of a publication bias. The trim-and-fill test finds one missing study from the left side of the pooled relative risk. The recalculated pooled relative risk was 1.46 (CI 1.28–1.66) in the random-effect model.
Figure 3.
Probability of publication bias (funnel plot). A filled study is market by a white dot.
Table II reports the results of the multivariate models, where the association between each social class indicator and dementia risk was adjusted for participants’ age and sex and the year of the cohort start. It must be noted that a sufficient number of observations was found for education, occupation and income, but not for wealth and neighbourhood. We found a significant association between the risk of dementia and education (RR=2.52; CI 1.81–3.51) and occupation (RR=2.37; CI 1.58–3.56), while this association was not significant for income (RR=1.13; CI 0.76–1.70). Gender was the only robustly significant cofounder, and only for the association between occupation and risk of dementia (RR=0.7; CI 0.52–0.93; ref.=males).
Table II.
Meta-regression results: effect of cofounders (year of the cohort’s start, age at baseline, percentage of women) on the association between the belonging to the ‘lower’ social class and dementia risk in selected indicators of social class.
| Social class indicator | Crude regression, effect of social class indicators on the risk of dementia, RR | Adjusted regression | |||
|---|---|---|---|---|---|
| Effect of social class indicators, RR | Effect of covariates, RR | ||||
| Year of recruitment | Age | Sex (ref.=males) | |||
| Occupation | 1.32 (1.10-1.58)** | 2.37 (1.58–3.56)*** | 0.99 (0.98–1.00)* | 0.98 (0.94–1.03) | 0.7 (0.52–0.93)* |
| Income | 1.50 (1.10–2.05)* | 1.13 (0.76–1.70) | 0.95 (0.94–0.96)*** | 1.4 (1.27–1.54)*** | 0.93 (0.75–1.15) |
| Education | 1.63 (1.27–2.11)*** | 2.52 (1.81–3.51) | 0.99 (0.98–1.00) | 0.96 (0.93–1.00)* | 0.85 (0.62–1.15) |
| Neighbourhood | 1.51 (1.00-2.26)* | – | |||
| Wealth | 1.13 (0.81–1.58) | – | |||
Note: Please note that due to weak sample size, adjusted regression was impossible for neighbourhood and wealth indicators of social class. For all indicators, the reference group is more privileged social class. ***p <0.0001, **p < 0.001, *p < 0.05.
Testing the race/ethnicity variable as a modifier of the association also did not yield statistically significant results (p=0.2). Also, we did find a better model fit when applying a fixed effect to the countries where the studies were conducted or to the time period for any indicator of social class.
The risk of bias in individual studies is presented in Table III. Two studies were classified as having ‘excellent’ methodological quality (eight stars out of a possible nine), 10 were classified as ‘good’ (seven stars) and four as ‘acceptable’ (five or six stars). The highest-rated criteria were ‘representativeness of the exposed cohort’ and ‘selection of the non-exposed cohorts’. The worst-rated criterion was ‘ascertainment of exposure’ because the indicators of individual social status were generally self-reported.
Table III.
Risk of bias in individual studies.
| Author, year | Selection | Comparability | Outcome |
|---|---|---|---|
| Bickel et al., 1994 | *** | ** | *** |
| Cadar et al., 2018 | *** | ** | ** |
| Chen et al., 2011 | *** | ** | ** |
| Contador et al., 2015 | *** | ** | ** |
| De Deyn et al., 2011 | *** | ** | ** |
| Evans et al., 1997 | *** | ** | ** |
| Hasselgren et al., 2019 | *** | ** | ** |
| Karp et al., 2004 | *** | ** | ** |
| Letelier et al, 2018 | *** | ** | ** |
| Ouvrard et al., 2020 | *** | ** | ** |
| Paykel et al., 1994 | *** | ** | * |
| Samuel et al, 2020 | *** | ** | * |
| Stern et al., 1994 | *** | ** | ** |
| Takasugi et al., 2019 | *** | ** | *** |
| Yaffe et al., 2013 | *** | ** | ** |
Note: The “Selection” section assesses the case definition, representativeness of the cases, selection of controls and definition of controls. The “Comparability” section assesses the basis of the design or analysis. The “Outcome” section assesses ascertainment of exposure, method of ascertainment for cases and controls, and non-response rate. Higher number of stars (max.=9) corresponds to better quality of the study.
Discussion
In this paper, we have sought to summarise existing knowledge on the association between belonging to a disadvantaged social class and the risk of dementia. We found that indicators of education, income, occupation and neighbourhood social class were all significantly associated with the risk of dementia in prospective, longitudinal studies. Out of these, education and occupation effects remained significant after adjusting for sex, age and the year of the cohort start.
In included studies, social class was defined as education, income, occupation, wealth and the neighbourhood of residence. The operationalisation of social class using this set of variables in studies on dementia is common [7] but increasingly criticised. According to Jones [7], it does not capture the complexity of interactions between social position and dementia in a society facing perpetual changes and where cognitive ability remains the most valuable attribute of an individual. Additionally, as was recently pointed out by Barata et al. [59], the multitude of conceptual frameworks for social class and social divide allows researchers to measure the differences in opportunities and forms of inequality responsible for specific health outcomes. As such, if future studies on dementia considered a more complex view of social stratification [59–61], they could more clearly highlight some of the mechanisms linking social class to dementia.
Out of the indicators of social class used in the retained studies, only education and occupation were significantly associated with dementia risk in the adjusted model. While a recent meta-analysis found a consistent association between educational attainment and dementia risk [62], a previous review did not [63]. The inconsistency of the association in the latter was explained first by the difference in the cut-off point used to quantify higher versus lower education, which can be set across a wide range of years of formal education from 0 to 15 [63], and second by the fact that economic and political contexts might matter [63]. In developing countries, higher education might be associated with lifelong advantages in other areas, such as quality of nutrition or less exposure to infections [63]. This in turn can lead to better survival and longer lifespan, and finally might be associated with a higher crude prevalence of dementia in people with more years of education. Meanwhile, both education and occupational complexity are targets of interventions in public health, aiming to reduce the incidence of dementia [64–67]. The significance of education and occupation in our results shows that at least one casual pathway, linking belonging to a social class and the risk of dementia, might be cognitive/brain reserve [17–20].
Social characteristics of the residential neighbourhood as an indicator of social class also yielded a significant association with the risk of dementia in the crude model. These results support previous findings from systematic review and meta-analysis, where significant associations between neighbourhood/community characteristics and cognitive health outcomes [31,32] and interactions between characteristics of neighbourhood and indicators of individual social class were first reported [31,32]. Despite these findings, there is heterogeneity in the results from individual studies. For example, McCann et al. [68] found a positive association between neighbourhood social status and cognitive decline, while Kim et al. [69] and Mantri et al. [70] did not observe a significant relationship. A likely explanation for this inconsistency across studies is that authors employed different operationalisations of geographical deprivation. For example, Cadar et al. [52] used a composite index of deprivation, counting individual and collective variables (health deprivation and disability, barriers to housing and services, living environment deprivation and crime), while Kim et al. considered revenue level only [69]. Evidently, we cannot measure geographical deprivation in individual characteristics of residents. Recent research on the association between neighbourhood characteristics and dementia risk found positive results for greenness [71–73], walkability [74,75], quality of air [64,65,73] and level of noise [73]. It would be interesting to compare the distribution of these characteristics with overall neighbourhood deprivation, and include these as indicators of the social status of an area in future research on dementia. Finally, interactions between individual indicators of deprivation, neighbourhood characteristics and social policies should be taken into account to understand how proximal environments shape the cognitive health of older people.
We did not find any modifying effect of race on this association in the current study. This could be due to the low number of studies reporting racial/ethnic composition of participants (n=4). Additionally, there was a difference in populations marked as ‘minorities’: for example, Yaffe et al. [44] reported the percentages of white and black people, while Samuel et al. [57] also reported Hispanic ethnicity and ‘other’. Nonetheless, previous studies describe the modifying effect of race on the association between some indicators of social class and cognitive health outcomes [70,76–79]. For example, Yaffe et al. [44] and Mantri et al. [70] reported no effect of race on the incidence of dementia [44] and global cognition [70] if adjusted for social deprivation (financial strain and racial segregation of the neighbourhood). In contrast, other studies showed that among racial/ethnic minorities, there was a lower protective effect of higher educational attainment on the risk of dementia [78], as well as other cognitive health outcomes, such as global memory and orientation score [76]. As such, the research on the association between racial discrimination and the risk of dementia should be continued, with special attention to the institutions contributing to the higher unadjusted risk in minorities [44].
Along with race, sex and gender and their association with the risk of dementia are the subjects of ongoing discussion. Certain studies have presented that sexual difference is primordial, as many biological differences were found between women and men [80]. However, more recent studies have emphasised the role of gender. For example, Mazure et al. [81] attributed a part of sex-based differences in the incidence of dementia to social factors, namely, inequalities in the access to education and occupation, and to gender-based differences in behavioural choices in diet, substance consumption and exercise. In turn, Mielke [82] point out that lifelong exposures to risk factors of dementia are different in men and women. In the current study, the female gender was inversely associated with the risk of dementia in the model, including also age, occupation and year of recruitment. These results, together with the finding that housewives share the highest risk of dementia with non-qualified manual workers, should raise concerns about how traditional gender roles may be noxious for the cognitive health of older women. These findings are in line with some previously published work, such as Hasselgern et al. [83] who reported that differences in educational attainment and experiences of psychological distress could mediate the association between gender and the risk of dementia.
We also found that certain groups might not be protected from dementia despite belonging to a higher social class. For example, housewives have dementia risks comparable to those of non-skilled manual workers, even if they have higher education levels than working women [1,2,51]. We can expect that belonging to such groups as those with a history of incarceration [84], sexual minorities [85] and people living with disabilities [86] could also modify the relationships between indicators of social class and dementia that are typically observed in the general population.
We also did not find that there were similar associations between belonging to a disadvantaged social class and the risk of dementia in studies conducted in the same countries and in the same time periods. The search for local particularities in the relationship between social stratification and dementia risk must therefore be continued as societies map their social divides from different cultural and political traditions. Gursky and Weisshaar underlined that possession of different resources may or may not indicate a higher social status across societies, and that different institutions are involved in the distribution of ‘status-defining’ resources in each society [43,87].
The study has several strengths, including good internal validity, as the majority of studies were rated as being of good or excellent quality. However, lack of information on important cofounders (e.g. cardiovascular risks, physical activity, depression) limit the external validity of our results. While some publication bias was identified in the study, this did not affect the results significantly (see Table III).
Several limitations are worthy of mention. First, the representativeness of our findings is limited by the studies included in our analysis, most of which were conducted in high-income countries (e.g. the USA, the UK and Sweden). The association between social divide and dementia risk in low- and middle-income countries remains understudied. Second, we excluded studies reporting social mobility. This is a limitation, given that the association between indicators of baseline social class and dementia at follow-up may present differently in individuals with upward or downward social mobility. Finally, we did not consider a minimal or specific follow-up period in our inclusion criteria, meaning a number of studies had a follow-up periods of less than five years. Since shorter follow-up periods may not capture the onset of dementia, some false-negative results could appear.
Conclusions
This study demonstrated that belonging to a disadvantaged social class, measured as educational attainment and occupational class, was statistically significantly associated with a higher risk of dementia over time. Education and occupation, as indicators of social class, were significantly associated with the risk of dementia, while income was not; nor were gender, race and the country of the study.
More importantly, the higher risk of dementia in certain groups translates to ‘excess’ cases which can be framed as inequitable and preventable. Since social class indicators have been well documented as influencing cognitive health outcomes, including dementia risk, it is important that public health policies aim to reduce health inequities such as the risk of dementia associated with exposure to social disadvantages.
To increase public health’s ability to reduce the risk of dementia across social classes, future research on dementia must adopt a wider view of social stratification. In addition to the widespread measurement of indicators rooted in economic capital (e.g. income, socio-economic status), more axes of social stratification should be considered, such as differences in social and cultural capital. Leveraging the social sciences is one way to guide biomedical research in the selection and use of such theoretical frameworks. Finally, those found at the intersections of various types of social stratification and other underrepresented groups should be studied with special attention.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-csv-1-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health
Supplemental material, sj-docx-3-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-R-2-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Yuliya Bodryzlova  https://orcid.org/0000-0001-7083-7250
https://orcid.org/0000-0001-7083-7250
Supplemental material: Supplemental material for this article is available online.
References
- [1]. Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271:1004–10. [PubMed] [Google Scholar]
- [2]. Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol 1997;54:1399–405. [DOI] [PubMed] [Google Scholar]
- [3]. Fritsch T, McClendon MJ, Smyth KA, et al. Effects of educational attainment and occupational status on cognitive and functional decline in persons with Alzheimer-type dementia. Int Psychogeriatr 2002;14:347–63. [DOI] [PubMed] [Google Scholar]
- [4]. Karp A, Kareholt I, Qiu C, et al. Relation of education and occupation-based socioeconomic status to incident Alzheimer’s disease. Am J Epidemiol 2004;159:175–83. [DOI] [PubMed] [Google Scholar]
- [5]. Wilson RS, Scherr PA, Hoganson G, et al. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology 2005;25:8–14. [DOI] [PubMed] [Google Scholar]
- [6]. Fotenos AF, Mintun MA, Snyder AZ, et al. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol 2008;65:113–20. [DOI] [PubMed] [Google Scholar]
- [7]. Jones IR. Social class, dementia and the fourth age. Sociol Health Illn 2017;39:303–17. [DOI] [PubMed] [Google Scholar]
- [8]. Hasselgren C, Ekbrand H, Fassberg MM, et al. APOE epsilon 4 and the long arm of social inequity: estimated effects of socio-economic status and sex on the timing of dementia onset. Ageing Soc 2019;39:1951–75. [Google Scholar]
- [9]. Ouvrard C, Meillon C, Dartigues JF, et al. Do individual and geographical deprivation have the same impact on the risk of dementia? A 25-year follow-up study. J Gerontol B Psychol Sci Soc Sci 2020;75:218–27. [DOI] [PubMed] [Google Scholar]
- [10]. Gugushvili A, Bukodi E, Goldthorpe JH. The direct effect of social origins on social mobility chances: ‘glass floors’ and ‘glass ceilings’ in Britain. Eur Sociol Rev 2017;33:305–16. [Google Scholar]
- [11]. Sen A, Clemente A. Intergenerational correlations in educational attainment: birth order and family size effects using Canadian data. Econ Educ Rev 2010;29:147–55. [Google Scholar]
- [12]. Chow A, Guppy N. Intergenerational educational mobility over the past century in Canada. Can Rev Sociol 2021;58:372–98. [DOI] [PubMed] [Google Scholar]
- [13]. Caparrós Ruiz A. The impact of education on intergenerational occupational mobility in Spain. J Vocat Behav 2016;92:94–104. [Google Scholar]
- [14]. Jonsson J, Grusky D, Di Carlo M, et al. It’s a decent bet that our children will be professors too. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.480–493. [Google Scholar]
- [15]. Singh-Manoux A, Richards M, Marmot M. Leisure activities and cognitive function in middle age: evidence from the Whitehall II study. J Epidemiol Commun Health 2003;57:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Clyde White R. Social class differences in the uses of leisure. Am J Sociol 1955;61:145–50. [Google Scholar]
- [17]. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Glei DA, Landau DA, Goldman N, et al. Participating in social activities helps preserve cognitive function: an analysis of a longitudinal, population-based study of the elderly. Int J Epidemiol 2005;34:864–71. [DOI] [PubMed] [Google Scholar]
- [19]. Wang HX, MacDonald SWS, Dekhtyar S, et al. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med 2017;14:e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Reed BR, Dowling M, Tomaszewski Farias S, et al. Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc 2011;17:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Kopp MS, Skrabski A, Székely A, et al. Chronic stress and social changes: socioeconomic determination of chronic stress. Ann N Y Acad Sci 2007;1113:325–38. [DOI] [PubMed] [Google Scholar]
- [22]. Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci 1999;896:131–44. [DOI] [PubMed] [Google Scholar]
- [23]. Hicks B, Veronesi G, Ferrario MM, et al. Roles of allostatic load, lifestyle and clinical risk factors in mediating the association between education and coronary heart disease risk in Europe. J Epidemiol Commun Health 2021;75:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Oken BS, Chamine I, Wakeland W. A systems approach to stress, stressors and resilience in humans. Behav Brain Res 2015;282:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Purnell C, Gao S, Callahan CM, et al. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord 2009;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Matos TM, Souza-Talarico JND. How stress mediators can cumulatively contribute to Alzheimer’s disease: an allostatic load approach. Dement Neuropsychol 2019;13:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Alesina A, La Ferrara E. Who trusts others? J Public Econ 2002;85:207–34. [Google Scholar]
- [28]. Vineis P, Fornero G, Magnino A, et al. Diagnostic delay, clinical stage, and social class: a hospital based study. J Epidemiol Commun Health 1993;47:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Alemu T, Biadgilign S, Deribe K, et al. Experience of stigma and discrimination and the implications for healthcare seeking behavior among people living with HIV/AIDS in resource-limited setting. SAHARA J 2013;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Xian W, Xu X, Li J, et al. Health care inequality under different medical insurance schemes in a socioeconomically underdeveloped region of China: a propensity score matching analysis. BMC Public Health 2019;19:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Wu YT, Prina AM, Brayne C. The association between community environment and cognitive function: a systematic review. Soc Psychiatry Psychiatr Epidemiol 2015;50:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Besser LM, McDonald NC, Song Y, et al. Neighbourhood environment and cognition in older adults: a systematic review. Am J Prev Med 2017;53:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Hammond JM. Multiple jeopardy or multiple resources? J Women Aging 1995;7:5–24. [Google Scholar]
- [34]. Yang Y, Yeung WJ, Feng Q. Social exclusion and cognitive impairment – a triple jeopardy for Chinese rural elderly women. Health Place 2018;53:117–27. [DOI] [PubMed] [Google Scholar]
- [35]. Marx K. Alienation and social classes. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.127–131. [Google Scholar]
- [36]. Marx K. Classes in capitalism and pre-capitalism. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.131–141. [Google Scholar]
- [37]. Miyamoto Y. Culture and social class. Curr Opin Psychol 2017;18:67–72. [DOI] [PubMed] [Google Scholar]
- [38]. Muntaner C, Gunn V. In defense of class wars in population health: the new landscape of social class with Bourdieu, Neo-Marxists, and the Kohn/Schooler/Wright integrative models. Int J Health Serv 2018;49:102–7. [DOI] [PubMed] [Google Scholar]
- [39]. Abel T. Cultural capital in health promotion. In: McQueen DV, Kickbusch I. (eds) Health and modernity: the role of theory in health promotion. New York: Springer, 2007, pp.77–82. [Google Scholar]
- [40]. Weber M. Class, Status, Party (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.165–174. [Google Scholar]
- [41]. Guiddens A. Elites and Power. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.292–296. [Google Scholar]
- [42]. Grusky D, Weisshaar K. A compressed history of inequality. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.44–51. [Google Scholar]
- [43]. Grusky D, Weisshaar K. The questions we ask about inequality. In: Grusky D, Weisshaar K. (eds) Social stratification: class, race, and gender in sociological perspective. London: Taylor & Francis, 2014, pp.1–15. [Google Scholar]
- [44]. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Takasugi T, Tsuji T, Nagamine Y, et al. Socio-economic status and dementia onset among older Japanese: a 6-year prospective cohort study from the Japan Gerontological Evaluation Study. Int J Geriatr Psychiatry 2019;34:1642–50. [DOI] [PubMed] [Google Scholar]
- [46]. Manstead ASR. The psychology of social class: how socioeconomic status impacts thought, feelings, and behaviour. Br J Soc Psychol 2018;57:267–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg 2011;128:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Hartling L, Hamm M, Milne A, et al. Decision rules for application of the Newcastle–Ottawa scale. In: Validity and inter-rater reliability testing of quality assessment instruments [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US), 2012. [PubMed] [Google Scholar]
- [49]. Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Softw 2010;36. [Google Scholar]
- [50]. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Bickel H, Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med 1994;24:179–92. [DOI] [PubMed] [Google Scholar]
- [52]. Cadar D, Lassale C, Davies H, et al. Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English Longitudinal Study of Ageing. JAMA Psychiatry 2018;75:723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Chen R, Hu Z, Wei L, et al. Incident dementia in a defined older Chinese population. PLoS One 2011;6:e24817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Contador I, Bermejo-Pareja F, Puertas-Martin V, et al. Childhood and adulthood rural residence increases the risk of dementia: NEDICES Study. Curr Alzheimer Res 2015;12:350–7. [DOI] [PubMed] [Google Scholar]
- [55]. De Deyn PP, Goeman J, Vervaet A, et al. Prevalence and incidence of dementia among 75–80-year-old community-dwelling elderly in different districts of Antwerp, Belgium: the Antwerp Cognition (ANCOG) Study. Clin Neurol Neurosurg 2011;113:736–45. [DOI] [PubMed] [Google Scholar]
- [56]. Paykel ES, Brayne C, Huppert FA, et al. Incidence of dementia in a population older than 75 years in the United-Kingdom. Arch Gen Psychiatry 1994;51:325–32. [DOI] [PubMed] [Google Scholar]
- [57]. Samuel L, Szanton S, Wolff J, et al. Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: an examination of financial resources. BMC Geriatr 2020;20:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Letellier N, Gutierrez LA, Carrière I, et al. Sex-specific association between neighbourhood characteristics and dementia: the Three-City cohort. Alzheimers Dement 2018;14:473–82. [DOI] [PubMed] [Google Scholar]
- [59]. Barata RB, Ribeiro MCSdA, Silva ZPd, et al. Social class: concepts and operationalization models in health research. Rev Saúde Pública 2013;47:647–55. [DOI] [PubMed] [Google Scholar]
- [60]. Bouchard G. Collective imaginaries and population health: how health data can highlight cultural history. In: Hall P, Lamont M. (eds) Successful societies: how institutions and culture affect health. New York: Cambridge University Press, 2009, pp.169–200. [Google Scholar]
- [61]. Lamont Ml, Mizrachi N. Ordinary people doing extraordinary things: responses to stigmatization in comparative perspective. Ethn Racial Stud 2012;35:365–81. [Google Scholar]
- [62]. Maccora J, Peters R, Anstey KJ. What does (low) education mean in terms of dementia risk? A systematic review and meta-analysis highlighting inconsistency in measuring and operationalising education. SSM Popul Health 2020;12:100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Sharp ES, Gatz M. The relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 2011;25:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. World Health Organization. Global status report on the public health response to dementia. Geneva: World Health Organization, 2021. [Google Scholar]
- [66]. Fratiglioni L, Winblad B, von Strauss E. Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen Project. Physiol Behav 2007;92:98–104. [DOI] [PubMed] [Google Scholar]
- [67]. Solomon A, Mangialasche F, Richard E, et al. Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med 2014;275:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. McCann A, McNulty H, Rigby J, et al. Effect of area-level socioeconomic deprivation on risk of cognitive dysfunction in older adults. J Am Geriatr Soc 2018;66:1269–75. [DOI] [PubMed] [Google Scholar]
- [69]. Kim GH, Lee HA, Park H, et al. Effect of individual and district-level socioeconomic disparities on cognitive decline in community-dwelling elderly in Seoul. J Korean Med Sci 2017;32:1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Mantri S, Nwadiogbu C, Fitts W, et al. Quality of education impacts late-life cognition. Int J Geriatr Psychiatry 2019;34:855–62. [DOI] [PubMed] [Google Scholar]
- [71]. Jin X, Shu C, Zeng Y, et al. Interaction of greenness and polygenic risk score of Alzheimer’s disease on risk of cognitive impairment. Sci Total Environ 2021;796:148767. [DOI] [PubMed] [Google Scholar]
- [72]. De Keijzer C, Tonne C, Basagana X, et al. Residential surrounding greenness and cognitive decline: a 10-year follow-up of the Whitehall II cohort. Environ Health Perspect 2018;126:077003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Oudin A. Short review: air pollution, noise and lack of greenness as risk factors for Alzheimer’s disease – epidemiologic and experimental evidence. Neurochem Int 2020;134:104646. [DOI] [PubMed] [Google Scholar]
- [74]. Rosso AL, Harding AB, Clarke PJ, et al. Associations of neighbourhood walkability and walking behaviors by cognitive trajectory in older adults. Gerontologist 2021;61:1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Guo Y, Chan CH, Chang Q, et al. neighbourhood environment and cognitive function in older adults: a multilevel analysis in Hong Kong. Health Place 2019;58:102146. [DOI] [PubMed] [Google Scholar]
- [76]. Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol 2002;57B:163–72. [DOI] [PubMed] [Google Scholar]
- [77]. Parlevliet JL, Uysal-Bozkir Ö, Goudsmit M, et al. Prevalence of mild cognitive impairment and dementia in older non-western immigrants in the Netherlands: a cross-sectional study. Int J Geriatr Psychiatry 2016;31:1040–9. [DOI] [PubMed] [Google Scholar]
- [78]. Shadlen MF, Siscovick D, Fitzpatrick AL, et al. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc 2006;54:898–905. [DOI] [PubMed] [Google Scholar]
- [79]. Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 2011;25:289–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007;62:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol 2016;15:451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Mielke MM. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr Times 2018;35:14–7. [PMC free article] [PubMed] [Google Scholar]
- [83]. Hasselgren C, Ekbrand H, Halleröd B, et al. Sex differences in dementia: on the potentially mediating effects of educational attainment and experiences of psychological distress. BMC Psychiatry 2020;20:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Kuffel R, Byers A, Williams B, et al. High occurrence of dementia in older adults returning to community from prison. Am J Geriatr Psychiatry 2021;29:S45–7. [Google Scholar]
- [85]. Correro AN, Nielson KA. A review of minority stress as a risk factor for cognitive decline in lesbian, gay, bisexual, and transgender (LGBT) elders. J Gay Lesbian Mental Health 2020;24:2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Fauth EB, Schwartz S, Tschanz JT, et al. Baseline disability in activities of daily living predicts dementia risk even after controlling for baseline global cognitive ability and depressive symptoms. Int J Geriatr Psychiatry 2013;28:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Grusky DB, Weisshaar KR. Social stratification: class, race, and gender in sociological perspective. New York: Routledge, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-csv-1-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health
Supplemental material, sj-docx-3-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
sj-R-2-sjp-10.1177_14034948221110019 for Social class and the risk of dementia: A systematic review and meta-analysis of the prospective longitudinal studies by Yuliya Bodryzlova, Alexie Kim, Xavier Michaud, Claire André, Emmanuelle Bélanger and Grégory Moullec in Scandinavian Journal of Public Health