Abstract
Growth and development of a wild-type Sclerotinia sclerotiorum isolate were examined in the presence of various pharmacological compounds to investigate signal transduction pathways that influence the development of sclerotia. Compounds known to increase endogenous cyclic AMP (cAMP) levels in other organisms by inhibiting phosphodiesterase activity (caffeine and 3-isobutyl-1-methyl xanthine) or by activating adenylate cyclase (NaF) reduced or eliminated sclerotial development in S. sclerotiorum. Growth in the presence of 5 mM caffeine correlated with increased levels of endogenous cAMP in mycelia. In addition, incorporation of cAMP into the growth medium decreased or eliminated the production of sclerotia in a concentration-dependent manner and increased the accumulation of oxalic acid. Inhibition of sclerotial development was cAMP specific, as exogenous cyclic GMP, AMP, and ATP did not influence sclerotial development. Transfer of developing cultures to cAMP-containing medium at successive time points demonstrated that cAMP inhibits development prior to or during sclerotial initiation. Together, these results indicate that cAMP plays a role in the early transition between mycelial growth and sclerotial development.
Fungi have adopted various physiologically and developmentally specialized strategies for dispersal, propagation, and long-term (season-to-season, year-to-year) survival. In Sclerotinia sclerotiorum, these processes are mediated through the sclerotium, a pigmented, multihyphal structure which can remain quiescent for long periods of time under conditions that are unfavorable for vegetative growth. The sclerotium plays a central role in the life and infection cycles of S. sclerotiorum and can serve as a dispersal propagule when carried in contaminated seed lots or infested soil. The importance of sclerotia for survival and propagation of S. sclerotiorum and other sclerotium-forming fungi has stimulated numerous investigations into the structural makeup and developmental regulation of sclerotia (reviewed in references 3, 16, 29, and 30).
The following three stages of sclerotial development have been distinguished and characterized (25): (i) initiation (aggregation of hyphae to form discrete initials), (ii) development (hyphal growth and further aggregation to increase size), and (iii) maturation (surface delimitation, melanin deposition in peripheral rind cells, and internal consolidation). In general, vigorous mycelial growth precedes sclerotial development, with sclerotia produced when there is nutrient limitation (5). Nutritional and environmental factors that affect the development of sclerotia have been extensively reviewed previously (3, 16, 29, 30). Nutritional factors may stimulate (C, N, P, K+, Mg, S, and Zn2+) or inhibit (Al3+) development. Nonnutritional factors that influence sclerotial development include light, temperature, substrate pH, organic acid and stale product accumulation, phenolics, polyphenoloxidase activity, contact with mechanical barriers, -SH group modifiers, and osmotic potential. Although the list of factors known to influence sclerotial development is extensive, studies of these factors have been mostly observational. The underlying molecular mechanisms that regulate and signal this development remain to be elucidated.
We are interested in the molecular events that trigger and coordinate sclerotial morphogenesis. In recent years, signal transduction pathways linked to morphogenesis in phytopathogenic fungi have been studied for involvement in sporulation (8), spore germination (21, 28), appressorial development (13, 17, 28, 31–33), and filamentous or infectious growth (1, 4, 8, 10, 19, 20, 31). The genes and protein activities involved in these morphological processes include pheromone receptors (1), G-proteins (4, 19), mitogen-activated protein kinase (31), protein kinase A (17, 32, 33), and adenylate cyclase (10). Our objective was to examine the effects of various signal transduction effectors on sclerotial development to gain insight into which characterized signal transduction pathways are involved in sclerotial morphogenesis.
MATERIALS AND METHODS
Fungal isolates and growth conditions.
The wild-type isolate of S. sclerotiorum used in this study was isolate 1980 (ATCC 18683), obtained from dry bean culls in western Nebraska (9). In addition, S. sclerotiorum 192 (ATCC 52585) (Canadian thistle, 1985, Montana), 222 (ATCC 18015) (sunflower, North Dakota, 1989), and 278 (ATCC 18687) (oil seed rape, Great Britain, 1995), Sclerotinia trifoliorum 246 (ATCC 34327) (alfalfa, 1992), and Sclerotinia minor 240 (ATCC 52583) (lettuce, 1969, New York) were provided by Jim Steadman (University of Nebraska—Lincoln). A single Rhizoctonia solani isolate, PR45 Ag-1-IB (ATCC 18619) (dry beans, Puerto Rico, 1995), was provided by Graciella Godoy (Ministry of Agriculture, Dominican Republic). Stocks of these isolates were stored as mycelia on desiccated paper discs or as sclerotia at −20°C. Fresh cultures were started from the paper disc stocks or sclerotia by sterile transfer onto potato dextrose agar (PDA) (Difco) plates.
Activator and inhibitor studies.
Cultures of isolate 1980 were grown on PDA supplemented with different concentrations of the following compounds known to affect conserved signal transduction pathways: staurosporine, H89, NaF, caffeine, KT5720, 3-isobutyl-1-methyl xanthine (IBMX), forskolin, diacyl glycerol kinase inhibitor I, okadaic acid, mastoparan, cholera toxin, verapamil, nifedipine, neodymium chloride, A23187, KN62, compound 48/80, and EGTA [ethylene-bis(oxyethylenenitrolo)tetraacetic acid]. When available, these compounds were obtained from Sigma Chemical Co. (St. Louis, Mo.). All other compounds except neodymium chloride and information concerning their modes of action were obtained from Calbiochem (San Diego, Calif.); neodymium chloride was purchased from Aldrich Chemical Co. (Milwaukee, Wis.). Cultures were grown in 2-cm-diameter wells of 24-well culture plates containing 2 ml of medium. Chemicals were added to the culture wells first and then thoroughly mixed with molten (45 to 50°C) medium. Control cultures were prepared in the same manner except that an equal volume of water or dimethyl sulfoxide was added depending on the solvent used to make the stock solution of each compound. After the medium had solidified, a mycelial plug (approximately 1 mm3) from a 5-day-old PDA culture was transferred to the center of each culture well. The cultures were incubated at room temperature (24 to 26°C) and then evaluated for sclerotial development at 7 days postinoculation. The effects of cyclic AMP (cAMP) and 8-Br-cAMP were evaluated in the same manner. All treatments and controls were set up in duplicate or triplicate. Treatments which affected sclerotial development in the primary screening were repeated a minimum of three times.
cAMP assays.
The cultures used for cAMP assays were set up in the same manner as the cultures used for inhibitor-activator studies, except that the medium surface was overlaid with cellophane before inoculation with the mycelial plug. The medium was supplemented with 5 mM caffeine for treatments or with an equal volume of water for controls. Cultures were grown for 3 days and then sampled every 24 h thereafter beginning with the 3-day (72-h) time point. Samples were taken by aseptically removing the mycelia growing on the cellophane and immediately submerging them in liquid nitrogen. The samples were stored at −80°C until samples for all time points were collected. Mycelium-cellophane mats were processed by grinding each mat in a 1.5-ml Eppendorf tube with a sealed pipette tip in the presence of liquid nitrogen. The ground samples were extracted with 1 ml of absolute ethanol at −20°C for 20 min and then centrifuged for 20 min at 16,000 × g and 4°C to separate the mycelial debris from the extract. Each extract was evaporated in a SpeedVac concentrator (Savant) and reconstituted in 400 μl of assay buffer (0.05 M acetate buffer). Each pellet was lyophilized to estimate the dry weight. Duplicates of each sample were assayed by a cAMP 125I radioimmunoassay by using the protocol suggested by the manufacturer (Amersham Life Science Inc., Arlington Heights, Ill.).
Oxalic acid quantification.
Cultures of wild-type isolate 1980 were grown as described above for cAMP assays on PDA or PDA supplemented with 5 mM cAMP. After 3 days of growth, each mycelium-cellophane overlay was removed, and the underlying medium was removed from the culture well. Eight milliliters of buffered 10 mM EDTA, pH 7.6 (provided with the oxalate detection kit; Sigma catalog no. 591-C), was added to the agar medium, and the mixture was heated to melt the agar. Samples were cooled to room temperature, and 2 ml was mixed with activated charcoal for 5 min. Samples were centrifuged at 1,500 × g for 5 min, and the supernatant was removed and diluted 10-fold in dilution buffer. The oxalic acid concentration was determined by an enzymatic assay by using the instructions of the manufacturer (Sigma).
Culture transfer experiments.
Cultures of wild-type isolate 1980 were grown on cellophane overlays of PDA in 24-well culture plates. The cellophane was positioned such that it covered the sides of the wells and the medium surface. At time points beginning with 48 h after inoculation (when the surface was completely colonized) until 96 h (when immature sclerotia were present), colonies were removed with the cellophane and overlaid onto water agar (1.5%) and onto water agar amended with 5 mM cAMP. The percentage of cultures with melanized sclerotia was recorded 7 days after the last culture was transferred.
RESULTS
Pharmacological studies.
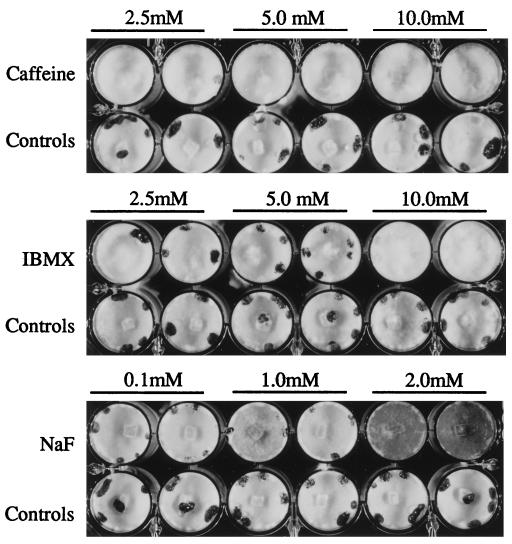
Cultures were grown on PDA in wells of multiwell culture plates. These conditions provided adequate nutrition for vigorous mycelial growth and a uniform, physically limited growth space conducive to sclerotial development. Growth and development of isolate 1980 were examined for 14 days on PDA supplemented with various concentrations of pharmacological compounds. These compounds affect different components of conserved signal transduction pathways, including calcium homeostasis, cAMP metabolism, and protein kinase, phosphatase, and G-protein activities. In control cultures, sclerotial initials were usually visible by day 3 and generally reached maturity within 7 days. No effects on growth or development of sclerotia were observed (data not shown) when preparations were treated with the G-protein activators mastoparan (concentration, 10 μM) and cholera toxin (250 ng/ml to 1 μg/ml), the kinase inhibitors staurosporine (50 nM to 5 mM), H89 (0.5 to 50 mM), KT5720 (50 nM to 10 mM), and diacyl glycerol kinase inhibitor (5 to 20 mM), the adenylate cyclase activator forskolin (100 nM to 1.0 mM), the protein phosphatase 2A inhibitor okadaic acid (10 to 100 nM), the voltage-dependent calcium channel inhibitor verapamil (10 nM to 1 μM), the calcium channel blockers nifedipine (2.5 to 10 μM) and neodymium chloride (10 nM to 1 μM), the calcium ionophore A23187 (2 to 200 nM), the calcium-calmodulin protein kinase inhibitor KN62 (1 to 10 μM), the calmodulin inhibitor 48/80 (5 to 15 μM), or the calcium chelator EGTA (100 μM to 10 mM). Effects on sclerotial development were observed when preparations were treated with caffeine (2.5 to 10 mM), IBMX (2.5 to 10 mM), and NaF (0.5 to 2 mM) (Table 1 and Fig. 1).
TABLE 1.
Signal transduction inhibitors and activators that affect sclerotial developmenta
| Compound | Concn (mM) | Sclerotial dry wt (mg)
|
No. of sclerotia
|
||
|---|---|---|---|---|---|
| Treatment | Controls | Treatment | Controls | ||
| Caffeine | 2.5 | 8.6 ± 6.5 | 26.3 ± 2.2 | 2.3 ± 1.7 | 5.0 ± 2.2 |
| 5.0 | 5.7 ± 5.2 | 27.2 ± 2.1 | 1.5 ± 1.6 | 5.8 ± 2.1 | |
| 10.0 | 0.8 ± 1.3 | 27.5 ± 1.4 | 0.3 ± 0.5 | 5.5 ± 2.4 | |
| IBMX | 2.5 | 16.3 ± 3.6 | 26.4 ± 1.7 | 3.3 ± 1.9 | 6.3 ± 2.7 |
| 5.0 | 14.0 ± 6.6 | 28.4 ± 1.8 | 3.8 ± 1.6 | 6.8 ± 1.2 | |
| 10.0 | 12.4 ± 6.4 | 26.6 ± 2.5 | 3.0 ± 1.9 | 5.3 ± 1.0 | |
| NaF | 0.5 | 5.5 ± 2.5 | 27.1 ± 2.7 | 4.5 ± 1.3 | 4.5 ± 1.7 |
| 1.0 | 5.1 ± 2.6 | 28.9 ± 1.9 | 4.0 ± 1.4 | 5.5 ± 1.0 | |
| 2.0 | 2.5 ± 2.0 | 28.4 ± 3.9 | 1.3 ± 1.0 | 5.0 ± 0.0 | |
S. sclerotiorum 1980 cultures were grown on PDA in 24-well tissue culture plates in the presence of different concentrations of caffeine, IBMX, and NaF. The average sclerotial dry weights and numbers of sclerotia produced per culture were determined by using data from four to six replicates.
FIG. 1.
Inhibition of sclerotial development in S. sclerotiorum 1980 cultures grown on PDA supplemented with different concentrations of caffeine, IBMX, and NaF. Cultures were grown in 1.5-cm wells of 24-well tissue culture plates. Each culture was inoculated with a small agar-mycelium plug from a 5-day-old PDA culture. The cultures were photographed 7 days postinoculation.
Caffeine, IBMX, and NaF reduced or prevented mycelial aggregation, sclerotial initial formation, and the development of mature sclerotia in a dose-dependent manner (Table 1). With the caffeine and IBMX treatments, the final mycelial accumulation was comparable to that in the control cultures, but at the highest treatment concentrations, the growth rate was marginally slower. With the NaF treatments, the mycelial growth rate was comparable to the wild-type rate, but growth was accompanied by increased pigmentation of the mycelia, especially at a concentration of 2 mM. NaF concentrations greater than 2 mM inhibited growth. Caffeine, IBMX, and NaF are known to raise cAMP levels by blocking cAMP degradation via inhibiting the activity of cAMP phosphodiesterase (caffeine and IBMX) or by increasing its synthesis by activating adenylate cyclase (NaF).
Endogenous cAMP levels in caffeine-treated and untreated wild-type cultures.
Endogenous levels of cAMP were measured during growth and sclerotial development of isolate 1980 grown on PDA and on PDA supplemented with 5 mM caffeine. In control cultures, mycelial colonization of the substrate surface was complete by 48 h, and sclerotial initials appeared between 72 and 96 h; all sclerotia were melanized by 144 h, and all sclerotia were fully mature by 192 h. New sclerotia were never initiated after 96 h. The cAMP levels were relatively constant during growth and development on PDA (Table 2). The highest levels of cAMP were found in mycelia after sclerotia matured (192 h). The levels of cAMP in cultures amended with 5 mM caffeine were always higher than the levels found in corresponding control cultures.
TABLE 2.
Effect of caffeine on mycelial cAMP concentrationa
| Time (h) | cAMP concn (fmol/mg)
|
|
|---|---|---|
| Treatment | Controls | |
| 72 | 180 ± 30 | 80 ± 30 |
| 96 | 800 ± 30 | 90 ± 30 |
| 120 | 790 ± 50 | 110 ± 10 |
| 144 | 1,000 ± 260 | 170 ± 40 |
| 168 | 550 ± 130 | 100 ± 40 |
| 192 | 460 ± 100 | 340 ± 10 |
| 216 | 440 ± 260 | 50 ± 0 |
S. sclerotiorum 1980 cultures were grown on cellophane discs overlaid on PDA (controls) or PDA containing 5 mM caffeine (treatment) in 24-well tissue culture plates. The data for each time point are the mean ± standard deviation for cAMP concentrations determined by a radioimmunoassay as described in Materials and Methods.
Effects of cAMP, 8-Br-cAMP, and other nucleotides on sclerotial development.
Exogenous cAMP or the more lipophilic analog 8-Br-cAMP inhibited sclerotial development much like the caffeine, IBMX, and NaF treatments; exogenously supplied cAMP was the most effective inhibitor. Exogenously supplied cAMP at final concentrations of 0.01 to 0.1 mM had no effect on sclerotial development, final cAMP concentrations between 1.0 and 2.5 mM reduced but did not eliminate sclerotial development, and cAMP at a concentration of 5 mM or above completely inhibited sclerotial development (Fig. 2) (data not shown). Although 8-Br-cAMP substantially reduced sclerotial development, it never completely inhibited sclerotial development in all replications of a treatment, even at a concentration of 10 mM (data not shown). To determine the specificity of cAMP for mediating effects on sclerotial development, the related nucleotides AMP, ATP, and cyclic GMP were tested. None of these compounds influenced growth or sclerotial development when it was supplied at final concentrations between 2.5 and 10 mM (data not shown).
FIG. 2.
Growth in the presence of exogenously supplied cAMP inhibits sclerotial development in S. sclerotiorum 1980 in a concentration-dependent manner. Cultures were amended with different concentrations of cAMP or an equal volume of water for controls. The culture conditions used are described in Materials and Methods.
Effect of cAMP on sclerotial initiation and development in other fungi.
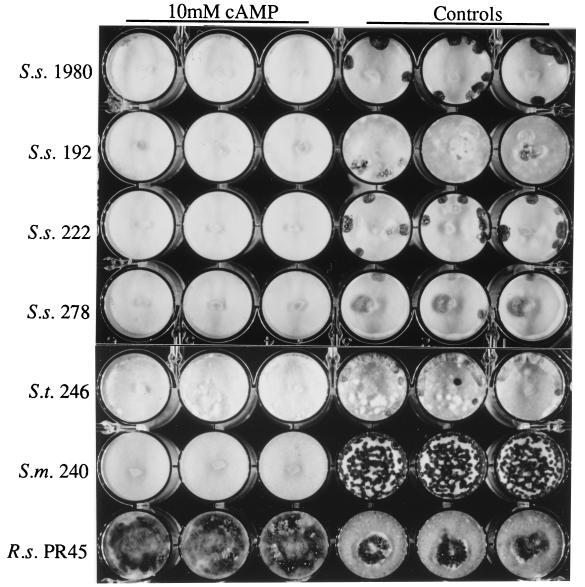
cAMP at a final concentration of 10 mM inhibited sclerotial development in three additional isolates of S. sclerotiorum, an isolate of S. trifoliorum, and an isolate of S. minor (Fig. 3). The inhibition of sclerotial development was similar to that observed with isolate 1980, but in some of the S. trifoliorum cultures sclerotial initials were observed even at the highest cAMP concentration used (10 mM). In contrast, 10 mM cAMP had a stimulatory effect on sclerotial development in the one isolate of R. solani examined (Fig. 3).
FIG. 3.
Effects of exogenously supplied cAMP on sclerotial development in S. sclerotiorum (S. s.) 1980, 192, 222, and 278, S. trifoliorum (S. t.) 246, S. minor (S. m.) 240, and R. solani (R. s.) PR45. All cultures were grown on PDA amended with 10 mM cAMP in 24-well culture plates as described in Materials and Methods.
Culture transfer studies.
Colonies of S. sclerotiorum were grown on PDA overlaid with cellophane. At times corresponding to discrete stages of sclerotial development, colonies were transferred to water agar or to water agar supplemented with 5 mM cAMP. Development of sclerotia was monitored, and the percentage of cultures developing melanized sclerotia was recorded 7 days after the transfer. Transfers were made after 48 h (surface was colonized with mycelia), 60 h (some of the mycelia at the colony periphery were becoming aerial and fluffy), 69 h (fluffy and condensed mycelial aggregates [sclerotia initials] were at the colony peripheries), and 96 h (immature sclerotia with exudate were evident, and there was no pigmentation). cAMP effectively blocked sclerotial development in 100% of the colonies transferred to cAMP-containing medium after 48 h of growth on PDA and in 89% of the cultures transferred after 60 h. At these times sclerotial initials had not yet developed in the colonies. In control cultures, melanized sclerotia developed in 100% of the cultures transferred before 60 h. Transfers after 69 h, when sclerotial initials were present, resulted in sclerotia in 89% of the treated cultures examined and in 100% of the control cultures. Sclerotia developed in all of the treated and control colonies transferred after immature sclerotia appeared (96 h). Thus, cAMP regulates sclerotial development at or before initiation but has no effect on sclerotial development or maturation once initials are present.
cAMP effect on oxalic acid accumulation.
Wild-type cultures grown on PDA and PDA supplemented with 5 mM cAMP were quantitatively assayed for oxalic acid. Cultures of S. sclerotiorum 1980 grown on PDA contained 1,600 ± 140 mg of oxalate per liter, compared to the 3,900 ± 110 mg of oxalate per liter present in cultures grown on PDA containing 5 mM cAMP. Thus, cAMP increased oxalic acid accumulation in addition to inhibiting sclerotial development. None of the compounds tested in this study blocked oxalic acid accumulation (data not shown) when qualitative assays were performed on oxalate indicator plates (9).
DISCUSSION
Cells of organisms as diverse as single-celled prokaryotes and multicellular vertebrates communicate through remarkably well-conserved molecules and signal transduction pathways. These pathways are essential for sensing and responding to internal and external environments and ultimately control cell proliferation and differentiation. In the filamentous fungi, components of conserved signal transduction pathways, including G-proteins, protein kinases, and adenylate cyclases, have been identified and have been shown to regulate growth, differentiation, and pathogenesis. In this study, we examined a broad range of compounds with diverse effects on eukaryotic signal transduction pathways for their effects on sclerotial development in S. sclerotiorum. Of the 18 compounds examined, only those that influenced cAMP metabolism (caffeine, IBMX, and NaF) affected sclerotial development.
Caffeine, IBMX, and NaF all block sclerotial development and are expected to increase cAMP levels. Elevated cAMP levels appear to block early sclerotial development since sclerotial initials are not observed or are greatly reduced in number in cultures amended with cAMP. Developing cultures transferred to cAMP-containing medium before sclerotial initiation did not produce sclerotia, whereas cultures transferred after sclerotial initiation continued to develop mature sclerotia. Although cAMP regulates sclerotial development, the nature of this regulation remains uncertain. cAMP regulation may be indirect in that signaling through a cAMP-dependent pathway may stimulate filamentous growth, resulting in hyphae which fail to differentiate into sclerotia.
One compound, forskolin, a known activator of adenylate cyclase, had no effect on sclerotial development. Forskolin does not influence cAMP-dependent development in Magnaporthe grisea (15) or Colletotrichum trifolii (33). Forskolin may not be readily taken up by the cells, or it may not activate adenylate cyclase in these fungi. Impermeability or lack of activity also may account for the ineffectiveness of other compounds which we tested. The effective concentrations of the compounds tested were sought by using concentrations within and above the reported Ki, 50% effective concentrations, or 50% inhibitory concentrations (Calbiochem) and by using concentrations effective in other filamentous fungi (13, 18, 22, 28, 33). We cannot rule out the possibility of participation of G-protein signaling, calcium homeostasis, or other kinases in the regulation of sclerotial development. We can only conclude that compounds known to affect these molecules were ineffective in S. sclerotiorum under the conditions used in this study.
Although sclerotial development has been studied for over a century (2, 7) and an extensive list of environmental and nutritional factors which influence sclerotial development has been compiled (reviewed in references 3, 16, 29, and 30), molecular mechanisms that regulate sclerotial development have rarely been studied. cAMP affects sclerotial production in Sclerotium rolfsii (11) and Rhizoctonia solani (12, 23). In both of these fungi, intracellular cAMP levels were highest prior to (12) or at the time of (11) sclerotial initiation. In R. solani (12), cAMP stimulated sclerotial development in otherwise non-sclerotium-forming isolates. We showed that addition of exogenous cAMP to the growth medium of a wild-type R. solani isolate increased sclerotial development. As in other fungi, sclerotial development in S. sclerotiorum appears to be regulated by cAMP. Unlike these other fungi, however, cAMP inhibited the development of sclerotia in S. sclerotiorum rather than stimulating development. The inhibition of sclerotial development in other isolates of S. sclerotiorum and other Sclerotinia species indicates that cAMP regulation of sclerotial development is highly conserved among Sclerotinia spp.
As in other eukaryotes, cAMP presumably functions through a cAMP-dependent protein kinase A (PKA) phosphorylation cascade in Sclerotinia spp. (24). The PKA holoenzyme is a tetramer consisting of a dimeric catalytic subunit and a dimeric regulatory subunit. The binding of cAMP to the regulatory subunit forces release from the catalytic subunit and results in activation of the catalytic subunit. The activated PKA catalytic subunit phosphorylates targeted proteins and ultimately controls the transcriptional activation of selected genes. The metabolism of cAMP must be tightly regulated to ensure that appropriate genes are expressed and repressed where and when they are needed. Interference with this regulation can profoundly affect fungal growth and development (18, 22). Two key enzymes in the cell, adenylate cyclase (which synthesizes cAMP from ATP) and phosphodiesterase (which degrades cAMP to AMP), regulate levels of cAMP. Pharmacological compounds that activate adenylate cyclase or inhibit phosphodiesterase effectively disrupt sclerotial development. Furthermore, addition of exogenous cAMP to growing cultures also disrupts sclerotial development. These data indicate that cAMP accumulation disrupts sclerotial development. Because PKA activity is positively regulated by cAMP, we infer that PKA is constitutively active when organisms are grown in the presence of exogenous cAMP. In a simplistic model, active PKA may signal the expression of genes involved in filamentous hyphal growth, which renders the hyphae incompetent for sclerotial development even when other factors conducive for sclerotial development are present. The apparent cAMP-mediated up-regulation of sclerotial development in R. solani and S. rolfsii presumably reflects differences in genes targeted for transcriptional activation by the PKA signaling pathway.
An apparent positive correlation between oxalic acid production and sclerotial development has been noted by previous researchers (6, 9, 14, 26, 27). Our results, however, revealed that exogenously supplied cAMP inhibited sclerotial development and substantially increased oxalic acid accumulation. Two possible hypotheses for these apparently contradictory results are (i) that oxalate metabolism and sclerotial development are part of a bifurcating pathway which branches prior to oxalic acid biosynthesis and cAMP-dependent regulation of sclerotial development and (ii) that cAMP regulates early sclerotial development, whereas oxalic acid biosynthesis contributes to sclerotial development in a separate pathway that converges at a later point in sclerotial development. Further studies on the molecular regulation of sclerotial development and oxalic acid biosynthesis may provide insight into the molecular linkage between these two important metabolic processes.
This study identified a specific endogenous factor, cAMP, which regulates sclerotial development. This factor is more than a simple addition to the list of chemicals which influence sclerotial development because its mechanism of action is reasonably well-understood. This mechanism involves regulation of a conserved cAMP-dependent PKA phosphorylation cascade. Evidence that this regulatory pathway is present in S. sclerotiorum and details of its effects on sclerotial development are being sought through molecular cloning and manipulation of homologous and heterologous cAMP-dependent PKA pathway components. Through these studies, we hope to better understand how the myriad different factors which influence sclerotial development affect this conserved signaling pathway and how this pathway coordinates regulation of sclerotial development and oxalic acid production.
ACKNOWLEDGMENT
This work was supported in part by grant BARD 2473-94 from the United States-Israel Binational Agricultural Research and Development Fund.
REFERENCES
- 1.Bolker M, Urban M, Kahmann R. The a mating type locus of U. maydis specifies cell signalling components. Cell. 1992;68:441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- 2.Brefeld O. Botanische Untersuchungen uber Schimmelpilze. III. Leipzig, Germany: Felix; 1877. [Google Scholar]
- 3.Chet I, Henis Y. Sclerotial morphogenesis in fungi. Annu Rev Phytopathol. 1975;13:169–192. [Google Scholar]
- 4.Choi G H, Chen B, Nuss D L. Virus-mediated or transgenic suppression of a G-protein α subunit and attenuation of fungal virulence. Proc Natl Acad Sci USA. 1995;92:305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christias C, Lockwood J L. Conversion of mycelial constituents in four sclerotium-forming fungi in nutrient deprived conditions. Phytopathology. 1973;63:602–605. [Google Scholar]
- 6.Corsini D L, Le Tourneau D. Organic acid metabolism in Sclerotinia sclerotiorum. Arch Mikrobiol. 1973;90:59–64. doi: 10.1007/BF00424824. [DOI] [PubMed] [Google Scholar]
- 7.De Bary A. Comparative morphology and biology of the fungi, mycetozoa and bacteria. Oxford, United Kingdom: Clarendon Press; 1884. [Google Scholar]
- 8.Gao S, Nuss D L. Distinct roles for two G protein α subunits in fungal virulence, morphology and reproduction revealed by targeted gene disruption. Proc Natl Acad Sci USA. 1996;93:14122–14127. doi: 10.1073/pnas.93.24.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godoy G, Steadman J R, Dickman M B, Dam R. Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol. 1990;37:179–191. [Google Scholar]
- 10.Gold S, Duncan G, Barret K, Kronstad J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994;8:2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]
- 11.Hadar Y, Pines M, Chet I, Henis Y. The regulation of sclerotium initiation in Sclerotium rolfsii by glucose and cyclic AMP. Can J Microbiol. 1983;29:21–26. [Google Scholar]
- 12.Hashiba T, Ishikawa T. Effect of adenosine 3′,5′-cyclic monophosphate on induction of sclerotia in Rhizoctonia solani. Phytopathology. 1978;68:1723–1727. [Google Scholar]
- 13.Hoch H C, Staples R C. Evidence that cAMP initiates nuclear division and infection structure formation in the bean rust fungus, Uromyces phaseoli. Exp Mycol. 1984;8:37–46. [Google Scholar]
- 14.Humpherson-Jones F M, Cooke R C. Induction of sclerotium formation by acid staling compounds in Sclerotinia sclerotiorum and Sclerotium rolfsii. Trans Br Mycol Soc. 1977;68:413–420. [Google Scholar]
- 15.Lee Y-H, Dean R A. cAMP regulates infection structure formation in the plant pathogenic fungus Magnaporthe grisea. Plant Cell. 1993;5:693–700. doi: 10.1105/tpc.5.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Tourneau D. Morphology, cytology, and physiology of Sclerotinia species in culture. Phytopathology. 1979;69:887–890. [Google Scholar]
- 17.Mitchell T K, Dean R A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995;7:1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pall M L. Adenosine 3′,5′-phosphate in fungi. Microbiol Rev. 1981;45:462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regenfelder E, Spellig T, Hartmann A, Lauenstein S, Bolker M, Kahmann R. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 1997;16:1934–1942. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robson G D, Wiebe M G, Trinci A P J. Exogenous cAMP and cGMP modulate branching in Fusarium graminearum. J Gen Microbiol. 1991;137:963–969. doi: 10.1099/00221287-137-4-963. [DOI] [PubMed] [Google Scholar]
- 21.Ruan Y, Kotraiah V, Straney D C. Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Mol Plant Microbe Interact. 1995;8:929–938. [Google Scholar]
- 22.Scott A W, Solomon B. Adenosine 3′,5′-cyclic monophosphate and morphology in Neurospora crassa: drug-induced alterations. J Bacteriol. 1975;122:454–463. doi: 10.1128/jb.122.2.454-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharada K, Ikegami H, Hyakumachi M. 2,4-D induced, c-AMP mediated, sclerotial formation in Rhizoctonia solani. Mycol Res. 1992;96:863–866. [Google Scholar]
- 24.Taylor S S, Buechler J A, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 25.Townsend B B. Nutritional factors influencing the production of sclerotia by certain fungi. Ann Bot. 1957;21:153–166. [Google Scholar]
- 26.Vega R R, Corsini D, Le Tourneau D. Nonvolatile organic acids produced by Sclerotinia sclerotiorum in synthetic liquid media. Mycologia. 1970;62:332–338. [PubMed] [Google Scholar]
- 27.Wang S-Y, Le Tourneau D. Amino acids as nitrogen sources for growth and sclerotium formation in Sclerotinia sclerotiorum. Trans Br Mycol Soc. 1972;59:509–512. [Google Scholar]
- 28.Warwar V, Dickman M B. Effects of calcium and calmodulin on spore germination and appressorium development in Colletotrichum trifolii. Appl Environ Microbiol. 1996;62:74–79. doi: 10.1128/aem.62.1.74-79.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willetts H J, Bullock S. Developmental biology of sclerotia. Mycol Res. 1992;96:801–816. [Google Scholar]
- 30.Willetts H J, Wong J A-L. The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot Rev. 1980;46:101–165. [Google Scholar]
- 31.Xu J-R, Hamer J E. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 32.Xu J-R, Urban M, Sweigard J A, Hamer J E. The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Mol Plant Microbe Interact. 1997;10:187–194. [Google Scholar]
- 33.Yang Z, Dickman M B. Regulation of cAMP and cAMP dependent protein kinase during conidial germination and appressorium formation in Colletotrichum trifolii. Physiol Mol Plant Pathol. 1997;50:117–127. [Google Scholar]