Abstract
Background and Aims
Mitral annular calcification (MAC) by computed tomography (CT) is reported as an independent predictor of poor outcomes. However, it currently remains unclear if quantitative MAC parameters provide more value for mitral valve disease (MVD) management, therefore, we examined the prognostic value of MAC scores using noncontrast cardiac‐CT in MVD patients.
Methods
Between January 2020 and December 2021, we prospectively enrolled 300 consecutive patients with MVD (MAC‐present = 80 and MAC‐absent = 220) undergoing preoperative cardiac‐CT and mitral valve (MV) surgery. Noncontrast cardiac‐CT images were used to qualitatively detect MAC (present or absent) and evaluate MAC scores. For analyses, we also collected baseline clinical data, intraoperative conversion (from MV repair to MV replacement), and follow‐up arrhythmia data.
Results
Compared with the MAC‐absent group, MAC‐present patients were older (62 ± 7 vs. 58 ± 9 years, p < .001), mostly women (55% vs. 39.5%, p = .017), and also had aortic valve calcification (57.5% vs. 23.2%, p < .001), mitral stenosis (82.5% vs. 61.8%, p < .001), atrial fibrillation (30% vs. 11.8%, p < .001), and larger left atrial end‐diastolic dimension (LADD, 49 [44–56] versus 46 [41–50], p = .001]. Furthermore, MAC‐present patients underwent more MV replacements (61.8% vs. 82.5%, p = .001) and experienced a higher intraoperative conversion prevalence (11.8% vs. 61.3%, p < .001). Multiple logistic regression analyses showed that the female gender (odds ratio [OR]/95% confidence interval [CI]/p = 2.001/1.042–3.841/0.037) and MAC scores (OR/95% CI/p = 10.153/4.434–23.253/p < .001) were independent predictors of intraoperative conversion. During a follow‐up of 263 ± 134 days, MAC‐present patients had more arrhythmias (42.5% vs. 9.5%, p < .001). Also, MAC‐scores (hazard ratio [HR]/95% CI/p = 6.841/3.322–14.089/p < .001) and LADD (HR/95% CI/p = 1.039/1.018–1.060/p < .001) were independently associated with arrhythmias by Cox regression analyses.
Conclusions
Noncontrast cardiac CT‐derived MAC‐scores showed a high risk for intraoperative conversion and follow‐up arrhythmias in MVD‐patients.
Keywords: arrhythmia, mitral annular calcification, mitral annular calcification score, mitral valve disease, noncontrast cardiac computed tomography
Mitral annular calcification (MAC) score: Distribution, measurement and prognosis. The present analysis is based on noncontrast cardiac computed tomography (CT) to semiautomatically quantitatively measure the MAC score using Agatston's method in mitral valve disease (MVD). Noncontrast cardiac CT‐derived MAC scores showed a high risk for intraoperative conversion and follow‐up arrhythmias in MVD patients. The annular segments: A1, A2, A3, segments of the anterior mitral annulus; P1, P2, P3, segments of the posterior mitral annulus, from lateral to medial.
Abbreviations
- AF
atrial fibrillation
- AS
aortic stenosis
- AVC
aortic valve calcification
- CI
confidence interval
- CT
computed tomography
- ECG
electrocardiography
- HR
hazard ratio
- ICC
intraclass correlation coefficients
- LADD
left atrial end‐diastolic dimension
- LVOT
left ventricular outflow tract
- MAC
mitral annular calcification
- MDCT
multidetector computed tomography
- MR
mitral regurgitation
- MS
mitral stenosis
- MV
mitral valve
- MVD
mitral valve disease
- OR
odds ratio
- ROC
receiver operating characteristic
1. INTRODUCTION
Characterized as a progressive and chronic degenerative process in the fibrous annulus of the mitral valve (MV), mitral annular calcification (MAC) is often an incidental, asymptomatic, and under‐reported finding. 1 , 2 Typically affecting the posterior aspect of the annulus fibrosa, MAC may extend to the anterior aspect, involve the entire annular circumference or myocardium and mitral leaflets, and lead to MV dysfunction. 1 , 2 , 3 , 4 The condition is also associated with elevated left ventricular afterload, including hypertrophic cardiomyopathy with obstruction, hypertension, and valvular aortic stenosis (AS). MAC may lead to mitral stenosis (MS) and/or mitral regurgitation (MR), with concomitant severe AS requiring ameliorative double‐valve intervention. 5 , 6
MAC is associated with elevated perioperative complications and all‐cause mortality risks 1 , 2 , 5 ; it reportedly causes a sixfold increase in operative mortality in patients undergoing isolated MV surgery, 7 while early mortality rates, upon surgical MV replacement in MAC, reportedly as high as 28%. 1 Generally, replacing or repairing MV in severely affected patients with MAC is technically difficult, even when concomitant aortic valve replacement risks are removed. 8 , 9
Multidetector computed tomography (MDCT) generates detailed MAC assessment before surgery and may alter therapeutic strategies. 10 Noncontrast cardiac computed tomography (CT) is reported as viably assessing valve calcification scores. 11 , 12 , 13 The approach, characterized by high X‐ray calcium attenuation, excellent spatial resolution, and three‐dimensional postprocessing analysis, assesses MAC characteristics, total calcium distribution, and coronary artery and aortic valve calcification (AVC). 10 , 14
MAC is reportedly an independent predictor of poor outcomes, 1 , 2 , 5 with prognostic value for atrial fibrillation (AF) ablation 15 and transcatheter aortic valve implantation. 3 Noncontrast cardiac CT is a semi‐automated quantification method for calculating calcium burden (MAC scores), such as coronary calcification scores which are used to quantitatively evaluate calcification, and provide more accurate risk assessments and disease prognosis predictions for multicenter clinical research. However, the value of MAC scores from noncontrast cardiac CT for mitral valve diseases (MVDs) have been rarely reported. The literature is limited in determining if MAC is associated with surgical method choice and predicting postoperative arrhythmia in MVD. Therefore, new investigations must ascertain if MAC provides useful clinical information enabling early intervention and improving treatment strategies. In our retrospective study, we examined MAC incidence, explored the clinical value of MAC scores in selecting surgical methods, and identified its potential predictive power in patients with MVD.
2. PATIENTS AND METHODS
After approval from our ethics committee, informed patient consent was waived due to the retrospective nature of our investigation.
2.1. Patient selection
In our single‐center hospital (in the Department of Cardiovascular Surgery), between January 2020 and December 2021, we evaluated 300 consecutively admitted patients with MVD. We adhered to the 2017 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines outlining MVD patient management. 16 Upon admission and before surgery, patients underwent echocardiography and electrocardiography (ECG)/dynamic ECG. 17 Before surgery, patients also underwent cardiac computed tomography angiography to evaluate coronary artery and intracardiac disease. To be included in the study, patients with MVD were ≥18 years or older, had noncontrast cardiac CT detection, and had undergone MV surgery. Patients were excluded if they had previous valvular surgery or ablation for AF, poor image quality, and had not undergone previous surgery. Patient basic characteristics, intraoperative conversion, follow‐up data, and arrhythmia information were retrospectively collected from the 300 patients.
2.2. Imaging
Imaging was performed using a 640‐slice MDCT scanner (Aquilion ONE Vision Edition; Canon Medical Systems Corporation) with prospective ECG gating. Imaging parameters included: tube voltage = 100 kV, tube current = SD32 mAs, field of view = 260 × 260 cm, slice thickness = 0.5 mm, matrix = 512 × 512, detector width = 16 cm, and reconstruction phase = 75%. ECG editing technology was used to reconstruct images in severe arrhythmia.
2.3. MAC
MAC was examined using noncontrast cardiac‐gated CT. MAC scores and distribution were gathered using the Agatston method 18 , 19 (Supporting Information: Figure S1). Noncontrast images (0.5‐mm slices and 0.5‐mm increments) were assessed using semiautomatic software (VScore, Vitrea, Vital Images). We selected the diastolic phase of the cardiac cycle, with maximal MV plane, using 4‐ and 2‐chamber views. We recorded calcific deposit status in MVs or annulus segments. Manual editing was performed to eliminate aortic or coronary calcium.
To define calcium areas, Agatston scores using a CT attenuation threshold = 130 Hounsfield units were used, 12 and the maximum CT attenuation in lesions was used to generate weighting scores. Weight = 1 indicated an attenuation of 130–199; 2 = 200–299; 3 = 300–399; and 4 ≥ 400. 13 The weighting factor was multiplied by lesion area, with the total of lesions values used to determine total Agatston scores 13 , 20 (Figure 1). CT scans were separately and independently analyzed by two experienced and blinded cardioradiologists (≥5 and ≥3 years' experience, respectively). Discordance was settled by discussion and consensus. MAC scores were also recorded by cardioradiologists to identify intraclass correlation coefficients (ICCs) and evaluate the precision and accuracy of the MAC score method.
Figure 1.
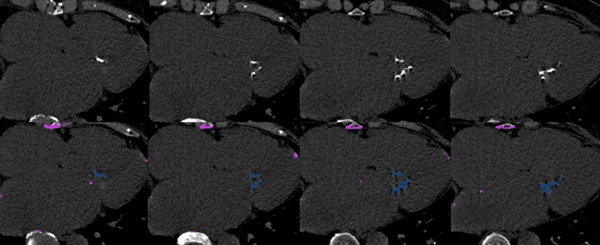
Multiplanar measurement of MAC. Figure 1 showed the case of a mitral valve disease with MAC. MAC score was evaluated by CT. MAC volume was measured at 1372 mm3, MAC score was measured at 1767. CT, computed tomography; MAC, mitral valve calcification.
2.4. Patient follow‐up
Patients were followed up to December 2022. Primary study outcomes were arrhythmia (including atrioventricular block, AF, and bundle branch block) recurrence during routine follow‐up (>3 months postsurgery), which required direct or drug current cardioversion. In the first 3 months postsurgery (“blank period”), arrhythmias were not recorded as adverse events. The period between the surgery date and arrhythmia recurrence was recorded as the time to event (arrhythmia recurrence). Follow‐up at outpatient visits or rehospitalization included echocardiography and ECG evaluations after surgery.
2.5. Statistical analyses
Data were analyzed in SPSS v. 20.0 (IBM Corporation). Continuous data were represented as the mean ± standard deviation or median (quartiles), and analyzed using independent sample t‐ or Mann–Whitney U tests. Normal distributions across continuous variables were examined using Kolmogorov–Smirnov tests. Categorical data (numbers and percentages) were analyzed using Fisher's exact or Pearson's χ 2 tests (Tables 1 and 2). Inter‐ and intraobserver agreement data for subjectively assessing MAC and AVC occurrence were evaluated using cross‐tabulation and kappa (κ) calculations. To determine significant independent predictors, multivariate logistic regression analyses were performed (Table 3). Also, to determine collinear covariates, multicollinearity analyses were performed. We used Cox regression for follow‐up arrhythmia analyses after surgery, and parameters with significant effects in univariate Cox regression analysis underwent multivariate Cox regression (Table 4). We used receiver operating characteristic (ROC) analyses to examine predictive potential factors in a multivariate‐adjusted logistic regression model. A p < .05 value indicated statistical significance.
Table 1.
Clinical characteristics of all patients.
| Variable | All patients (n = 300) | Patients grouped by MAC | p‐Value | |
|---|---|---|---|---|
| Present (n = 80) | Absent (n = 220) | |||
| Clinical characteristics | ||||
| Age (mean ± SD, years) | 59 ± 9 | 62 ± 7 | 58 ± 9 | <.001 |
| Female, n (%) | 131 (43.7) | 44 (55) | 87 (39.5) | .017 |
| BMI (kg/m2) | 24 ± 3.5 | 24 ± 3.8 | 24 ± 3.5 | .981 |
| Heart rate (bpm) | 84 ± 14 | 85 ± 17 | 83 ± 12 | .383 |
| Chronic kidney disease, n (%) | 3 (1) | 0 (0) | 3 (1.4) | .567 |
| Hypertension, n (%) | 168 (56) | 35 (43.8) | 133 (60.5) | .010 |
| Diabetes mellitus, n (%) | 22 (7.3) | 8 (10) | 14 (6.4) | .285 |
| Smoking, n (%) | 79 (26.3) | 20 (25) | 59 (26.8) | .752 |
| Alcohol, n (%) | 75 (25) | 16 (20) | 59 (26.8) | .228 |
| Prior stroke/TIA history, n (%) | 15 (5) | 4 (5.0) | 11 (5.0) | 1.000 |
| Coronary heart disease, n (%) | 82 (27.3) | 22 (27.5) | 60 (27.2) | .969 |
| Paroxysmal or chronic AF, n (%) | 50 (16.7) | 24 (30) | 26 (11.8) | <.001 |
| Conduction system disease, n (%) | 18 (6) | 5 (6.3) | 13 (5.9) | 1.000 |
| NYHA class on admission ≥ III, n (%) | 293 (97.7) | 80 (100) | 213 (96.8) | .237 |
| NT‐ProBNP (pg/mL, median [IQR]) | 604 (184–1584) | 836 (326–1669) | 548 (167–1504) | .032 |
| MAC score | 911.6 ± 1852 | – | – | |
| AVC present on CT, n (%) | 97 (32.3) | 46 (57.5) | 51 (23.2) | <.001 |
| Echocardiography on admission | ||||
| LADD (mm, median [IQR]) | 47 (42–51) | 49 (44–56) | 46 (41–50) | 0.001 |
| LVEDV (mL) | 141 ± 56 | 127 ± 53 | 146 ± 56 | 0.008 |
| LVESV (mL) | 62 ± 31 | 57 ± 29 | 64 ± 31 | 0.076 |
| LVEF (%) | 56.4 ± 5.3 | 55.7 ± 4.8 | 56.7 ± 5.5 | 0.166 |
| Aortic stenosis, n (%) | 56 (18.7) | 27 (33.8) | 29 (13.2) | <0.001 |
| Aortic regurgitation, n (%) | 91 (30.3) | 33 (41.3) | 58 (26.4) | 0.013 |
| Mitral stenosis, n (%) | 73 (24.3) | 43 (53.8) | 30 (13.6) | <0.001 |
| Mitral regurgitation, n (%) | 300 (100) | 80 (100) | 220 (100) | – |
| Tricuspid regurgitation, n (%) | 186 (62) | 55 (68.8) | 131 (59.5) | .146 |
| Mitral valve prolapses | 161 (53.7) | 15 (18.8) | 146 (66.4) | <.001 |
| Surgery, n (%) | ||||
| Mitral valve repair | 98 (32.7) | 14 (17.5) | 84 (38.2) | .001 |
| Mitral valve replacement | 202 (67.3) | 66 (82.5) | 136 (61.8) | .001 |
| Aortic valve replacement | 73 (24.3) | 26 (32.5) | 47 (21.4) | .047 |
| Tricuspid valve repair | 151 (50.3) | 49 (61.3) | 102 (46.4) | .023 |
| Tricuspid valve replacement | 2 (0.7) | 1 (1.3) | 1 (0.5) | .463 |
| Coronary artery bypass grafting | 29 (9.7) | 8 (10) | 21 (9.5) | .906 |
| Maze procedure | 15 (5) | 6 (7.5) | 9 (4.1) | .369 |
| Intraoperative conversion | 75 (25) | 49 (61.3) | 26 (11.8) | <.001 |
| Followed‐up echocardiography | ||||
| LADD (mm, median [IQR]) | 40 (38–44) | 43 (40–51)a | 40 (37–42)b | <.001 |
| LVEDV (mL) | 100 ± 29 | 99 ± 24a | 101 ± 30b | .531 |
| LVESV (mL) | 45 ± 16 | 44 ± 14a | 45 ± 17b | .646 |
| LVEF (%) | 56 ± 4.3 | 56.1 ± 4.2 | 56.1 ± 4.3b | .505 |
| Admission time (day) | 19.8 ± 6.5 | 19.3 ± 7.1 | 19.9 ± 6.3 | .508 |
| Follow‐up time (day) | 263 ± 134 | 242 ± 104 | 268 ± 143 | .145 |
| Arrhythmia, n (%) | 55 (18.3) | 34 (42.5) | 21 (9.5) | <.001 |
Note: “a”/“b” indicates statistical significance between the echocardiographic findings on admission and the followed‐up findings in MAC‐present/MAC‐absent group.
Abbreviations: AF, atrial fibrillation; AVC, aortic valve calcification; BMI, body mass index; IQR, interquartile range; LADD, left atrial end‐diastolic dimension; LVEDV/LVESV, left ventricular end‐diastolic/end‐systolic volume; LVEF, left ventricle ejection fraction; MAC, mitral annular calcification; n, number; NYHA, New York Heart Association; SD, standard deviation; TIA, transient ischemic attack.
Table 2.
Clinical characteristics of patients with intraoperative conversion from mitral valve repair to replacement.
| Variable | Intraoperative conversion | p‐Value | |
|---|---|---|---|
| With (n = 75) | Without (n = 225) | ||
| Clinical characteristics | |||
| Age (mean ± SD, years) | 59.6 ± 9.94 | 59.4 ± 8.72 | .296 |
| Female, n (%) | 44 (58.7) | 87 (38.7) | .002 |
| BMI (kg/m2) | 23.9 ± 3.86 | 24.1 ± 3.44 | .590 |
| Hypertension, n (%) | 38 (50.7) | 130 (57.8) | .283 |
| Diabetes mellitus, n (%) | 8 (10.7) | 14 (6.2) | .201 |
| Smoking, n (%) | 18 (24) | 61 (27.1) | .596 |
| Alcohol, n (%) | 14 (18.7) | 61 (27.1) | .144 |
| Prior stroke/TIA history, n (%) | 4 (5.3) | 11 (4.9) | 1.000 |
| Coronary heart disease, n (%) | 20 (26.7) | 62 (27.6) | .881 |
| Paroxysmal or chronic AF, n (%) | 22 (29.3) | 28 (12.4) | .001 |
| Conduction system disease, n (%) | 7 (9.3) | 11 (4.9) | .261 |
| NT‐ProBNP (pg/mL, median [IQR]) | 936.5 (423.5–1827) | 547 (174–1497.5) | .008 |
| MAC present on CT, n (%) | 49 (65.3) | 31 (13.8) | <.001 |
| MAC score | 324.3 ± 889.1 | 183.5 ± 957.8 | .003 |
| AVC present on CT, n (%) | 29 (38.7) | 68 (30.2) | .176 |
| Echocardiography on admission | |||
| LADD (mm, median [IQR]) | 48 (45–56) | 40 (37–43) | .001 |
| LVEDV (mL) | 127.5 ± 52.7 | 145.4 ± 56.1 | .016 |
| LVESV (mL) | 57.2 ± 28.9 | 63.7 ± 31.4 | .117 |
| LVEF (%) | 55.8 ± 5.04 | 56.7 ± 5.40 | .237 |
| Aortic stenosis, n (%) | 21 (28) | 35 (15.6) | .017 |
| Aortic regurgitation, n (%) | 27 (36) | 64 (28.4) | .218 |
| Mitral stenosis, n (%) | 35 (46.7) | 38 (16.9) | <.001 |
| Mitral regurgitation, n (%) | 75 (100) | 225 (100) | – |
| Tricuspid regurgitation, n (%) | 55 (73.3) | 131 (58.2) | .020 |
Abbreviations: AF, atrial fibrillation; AVC, aortic valve calcification; BMI, body mass index; IQR, interquartile range; LADD, left atrial end‐diastolic dimension; LVEDV/LVESV, left ventricular end‐diastolic/end‐systolic volume; LVEF, left ventricle ejection fraction; MAC, mitral annular calcification; n, number; NYHA, New York Heart Association; SD, standard deviation; TIA, transient ischemic attack.
Table 3.
Factors associated with intraoperative conversion from mitral valve repair to replacement.
| Variables | VIF | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p‐Value | OR (95% CI) | p‐Value | ||
| Female | 1.124 | 2.251 (1.322, 3.833) | .003 | 2.040 (1.061, 3.924) | .033 |
| AF | 1.305 | 2.043 (1.113, 3.749) | .021 | 0.764 (0.319, 1.830) | .546 |
| MAC scorec | 1.239 | <.001 | <.001 | ||
| >268 | 13.786 (6.396, 29.714) | <.001 | 10.153 (4.434, 23.253) | <.001 | |
| ≤268 | 10.095 (4.775, 21.343) | <.001 | 7.942 (3.575, 17.642) | <.001 | |
| LADD | 1.360 | 1.053 (1.021, 1.086) | .001 | 1.034 (0.993, 1.077) | .108 |
| AS | 1.149 | 2.111 (1.136, 3.923) | .018 | 0.973 (0.452, 2.093) | .943 |
| MS | 1.448 | 4.306 (2.430, 7.631) | <.001 | 1.479 (0.679, 3.220) | .325 |
Note: “c” indicates that patients were divided into negative MAC (MAC score = 0, n = 220), lower MAC score (0 < MAC score ≤ 268, n = 40) and higher MAC score (MAC score > 268, n = 40) subgroups.
Abbreviations: AF, atrial fibrillation; AS, aortic stenosis; CI, confidence interval; LADD, left atrial end‐diastolic dimension; MAC, mitral annular calcification; MS, mitral stenosis; OR, odds ratio; VIF, variance inflation factor.
Table 4.
Predictors of follow‐up arrythmia with Cox regression analysis in patients with MVD.
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| Hypertension | 0.573 (0.334, 0.984) | .043 | 0.966 (0.533, 1.752) | .910 |
| MAC scorec | <.001 | <.001 | ||
| >268 | 7.948 (4.382, 14.415) | <.001 | 6.897 (3.349, 14.207) | <.001 |
| ≤268 | 2.653 (1.202, 5.858) | .016 | 2.952 (1.289, 6.764) | .010 |
| LADD | 1.057 (1.039, 1.075) | <.001 | 1.038 (1.018, 1.059) | <.001 |
| AS | 2.308 (1.313, 4.057) | .004 | 1.303 (0.703, 2.417) | .400 |
| MS | 1.734 (1.012, 2.972) | .045 | 0.518 (0.266, 1.011) | .054 |
Note: “c” indicates that patients were divided into negative MAC (MAC score = 0, n = 220), lower MAC score (0 < MAC score ≤ 268, n = 40) and higher MAC score (MAC score > 268, n = 40) subgroups.
Abbreviations: AF, atrial fibrillation; AS, aortic stenosis; CI, confidence interval; HR, hazard ratio; LADD, left atrial end‐diastolic dimension; MAC, mitral annular calcification; MS, mitral stenosis; MVD, mitral valve disease.
3. RESULTS
3.1. Study population
Of the 300 patients (mean age = 59 ± 9 years and 43.7% were females), 80/300 (26.7%) were assigned to the MAC‐present group and 220/300 (73.3%) to the MAC‐absent group. When compared with the MAC‐absent group, patients with MAC were older (58 ± 9 vs. 62 ± 7 years, t = 3.353, p < .001), mainly female (39.5% vs. 55%, χ 2 = 5.696, p = .017), and had hypertension (χ 2 = 13.964, p < .001), paroxysmal or chronic AF (χ 2 = 13.964, p < .001), AVC (χ 2 = 31.58, p < .001), AS (χ 2 = 16.347, p < .001), aortic regurgitation (AR, χ 2 = 6.152, p = .013), MS (χ 2 = 51.271, p < .001), and larger left atrial end‐diastolic dimension (LADD) at admission (Z = −3.336, p = .001).
From surgery data, MV replacement (χ 2 = 11.409, p = .001), aortic valve replacement (χ 2 = 3.952, p = .047), and intraoperative conversion from MV repair to replacement ratios were higher in the MAC‐present group when compared with the MAC‐absent group (χ 2 = 76.455, p < .001). No significant differences (p > .05) in follow‐up duration were observed between groups. During follow‐up (263 ± 134 days), patients with MAC had a higher arrhythmia prevalence (χ 2 = 42.554, p < .001), while follow‐up echocardiographic findings were significantly improved when compared with those at admission (p < .05). Additionally, a significant difference was observed in follow‐up LADD between groups after follow‐up (Z = −5.051, p < .001; Table 1).
3.2. MV calcification assessment
We identified 80 patients (26.7%) with MAC and observed that calcific deposits were more frequent on the posterior mitral annulus when compared with the anterior. MAC location: A1/A2/A3/P1/P2/P3 = 27 (33.8%)/20 (25%)/28 (35%)/44 (55%)/34 (42.5%)/30 (37.5%). MAC thickness = 4.1 ± 2.5 mm, MAC volume = 703 ± 1348 mm3, left ventricular outflow tract (LVOT) calcification was identified in 25 cases (25/80, 31.3%), and LVOT calcification volume = 447 ± 1293 mm3. In patients with MAC, the mean score of MAC was 911.6 ± 1852. Excellent intra‐ (κ = 0.98) and interobserver (κ = 0.97) agreement scores were recorded between operators assessing MAC on the same noncontrast cardiac‐gated CT images. Patients with MAC had a higher MS incidence when compared with patients without MAC (53.8% vs. 13.6%, p < .001).
3.3. Reproducibility of MAC scores
Intra‐ and interobserver MAC‐score reproducibility was examined using semiquantitative analyses. Excellent intra‐ (ICC = .998; .998–.999) and interobserver reproducibility (ICC = .996; .995–.997) scores were recorded.
3.4. Factors related to intraoperative conversion from MV repair to replacement
Patients were classified into two groups: those with intraoperative (75/300, 25%) and those without intraoperative conversion (225/300, 75%). Intraoperative conversion was more prevalent in females (58.7% vs. 38.7%, χ 2 = 9.147, p = .002), and patients had a greater incidence of paroxysmal or chronic AF (29.3% vs. 12.4%, χ 2 = 11.552, p = .001), AS (28% vs. 15.6%, χ 2 = 5.738, p = .017), MS (46.7% vs. 16.9%, χ 2 = 27.089, p < .001), MAC (65.3% vs. 13.8%, χ 2 = 76.455, p < .001), higher MAC scores (324.3 ± 889.1 vs. 183.5 ± 957.8, Z = − 2.951, p = .003), and larger LADD (48 [45–56] versus 40 [37–43], Z = − 3.339, p = .001) (Table 2). Univariate logistic regression analyses indicated that female gender, AF, MAC, MAC scores, LADD, AS, and MS had significant associations with intraoperative conversion in binary analyses. Multiple logistic regression analyses identified female gender (odds ratio [OR] = 2.001; 95% confidence interval [CI]: 1.042–3.841; p = .037) and MAC scores (OR = 10.153; 95% CI: 4.434–23.253; p < .001) as independently associated with intraoperative conversion after adjusting for AF, LADD, AS, and MS (Table 3). ROC curves showed that MAC scores exhibited a relatively good capacity in predicting intraoperative conversion (area under the ROC curve [AUC] = 0.76; 95% CI: 0.692–0.831; p < .001), followed closely by female gender (AUC = 0.6; 95% CI: 0.526–0.674; p = .009).
3.5. Follow‐up arrhythmias
In total, 55 (55/300, 18.3%) patients had follow‐up arrhythmias (including AF, atrioventricular block, and bundle branch block) postsurgery, over a 219 ± 133 days follow‐up. Using univariate Cox regression analyses, hypertension, MAC scores, LADD, AS, and MS were univariate predictors of recurrent arrhythmias in patients with MVD postsurgery. Multivariate Cox regression analyses indicated that MAC scores (hazard ratio [HR] = 6.841; 95% CI: 3.322–14.089; p < .001) and LADD (HR = 1.039; 95% CI: 1.018–1.060; p < .001) remained significant (Table 4). ROC curves showed that LADD had a relatively good capacity in predicting arrhythmia (AUC = 0.772; 95% CI: 0.706–0.837; p < .001), followed closely by MAC scores (AUC = 0.739; 95% CI: 0.656–0.822; p < .001).
4. DISCUSSION
When compared with the MAC‐absent group, patients with MAC were older and mainly female, and had AF, AVC, AS, MS, and larger LADD. Patients with MAC had a higher prevalence of intraoperative conversion from MV repair to replacement. MAC scores and female gender were independent predictors of intraoperative conversion. During follow‐up, MAC patients had an increased arrhythmia incidence. MAC scores and LADD were independent arrhythmia predictors. Thus, MAC was an important imaging index in MVD prognosis outcomes and treatment. MAC scores, based on quantitative nonenhanced cardiac CT evaluations, were important in predicting intraoperative conversion and postoperative arrhythmia events in patients with MVD.
Recent studies reported that MAC is an active and controlled molecular event associated with microscopic and macroscopic injury, lipid deposition, hemodynamic stress, chronic kidney disease, dysregulated bone and mineral metabolism regulators, and local inflammation. 21 , 22 Baseline MAC burden was also related to disease activity and disease progression rates. 21 MAC appears to induce anatomical changes which culminate in either MS or combined MS and MR, while MS in severe MAC settings is caused by encroaching orifice areas, and rheumatic MS arises due to an absence of leaflet commissural union. 23 MR is generated by an altered annulus during systole or leaflet coaptation distortion, which cause left atrium volume and pressure overload, leading to enlargement. 24 , 25 Pawade et al. 13 reported that AVC should be measured using noncontrast CT and the Agatston approach. In the valve, the majority of data are related to Agatston scores and not calcium volume measurements. Density weighting is likely advantageous, the denser the calcium deposits, the more likely they will cause hemodynamic obstruction and valve‐leaflet stiffening. We recorded excellent inter‐ and intraobserver agreements between operators who measured MAC from cardiac CT images, consistent with previous studies. 2 , 26 We showed that noncontrast cardiac‐gated CT is a good semiquantitative method assessing MAC severity. MAC is common in cardiovascular imaging and postmortem and surgical samples, with an estimated 8%–42% prevalence. 11 We also showed that MAC prevalence in patients with MVD was 25.9%, consistent with previous results. 11 Patients with MAC were advanced in age and more likely to be female, with hypertension and valvular heart disease. Critically, similar results were reported in previous studies. 27 , 28 , 29 MAC was also associated with cardiovascular risk factors. 27 , 28 , 29 These observations suggested overlapping but distinct mechanisms underlying these pathologies.
Interestingly, MAC scores and female gender were independent risk markers for intraoperative conversion; indeed, the literature indicated that MAC was more prevalent in females. 21 , 30 While surgical treatment in patients with MAC is technically complex, there is a need for annular reconstruction and adequate debridement before MV replacement or repair. 31 In such cases, MV repair may not be undertaken due to difficulties suturing calcified sites and severe calcification, thereby requiring prosthetic valve replacement. Patients with MAC experience significant comorbidities and have worse survival outcomes, although MAC is not a mortality risk factor. 32 MAC alone, irrespective of severity, is independently related to adverse postoperative outcomes and elevated operative mortality. 33 Potentially, MAC may provide preoperative evaluations, with MAC scores routinely incorporated as integral to prevalve surgery evaluations.
Additionally, MAC and LADD were independent risk markers for follow‐up arrhythmias. Framingham Heart and Multi‐Ethnic Atherosclerosis investigations reported that MAC independently predicted AF development. 25 , 34 The Strong Heart Study 35 reported that left atrium enlargement was key to relationships between AF and MAC. Also, MAC may interrupt inter‐ and intra‐atrial conduction, causing atrial conduction system defects, thus causing AF. 29 Lewicka et al. 15 reported that MAC predicted paroxysmal AF recurrence after ablation. We suggest that early AF detection and treatment in patients with MAC should be performed to prevent related stroke, while high conduction system abnormality risks warrant closer monitoring. Thus, MAC may not just be an AF risk factor, but an important prognostic predictor and potential postoperative evaluation index. In patients with MAC, doctors should inaugurate AF preventative measures and reduce adverse outcomes and associated burden if AF is evident. We observed that AF was more common in patients with MAC, consistent with left atrial dilatation. 36 , 37 MAC patients susceptible to AF may require rhythm control strategies, while patients with complicated AF may require more rigorous anticoagulation regimens. MAC occurrence should increase suspicion for arrhythmia, thus close postprocedural monitoring is strongly advised in MVD patients with MAC.
Surgical MV repair or replacement is generally considered as the gold standard treatment in patients with established indications. 38 , 39 Recently, transcatheter intervention therapy has achieved good safety and efficacy in high‐risk surgical patients. 40 , 41 Guerrero et al. 8 performed transcatheter MV replacement for patients with severe MAC who were not surgical candidates, they found cardiac‐CT based score provided a systematic method to grade MAC severity which may assist in predicting valve embolization/migration. A meta‐analysis showed that the feasibility of transcatheter technology in serious MAC needed to be further explored and improved. 42 The experience in this aspect is still limited and general recommendations cannot yet be made. 16 But what is certain is that imaging is critical to the success of these surgical and transcatheter therapies. 43 Cardiac‐CT can provide the entire mitral valvular and subvalvular structures details (e.g., calcification) before and after operation, it can be an additional important evaluation tool in deciding for the best operation method and evaluating the MV disease pre‐operatively and predicting the prognosis.
4.1. Limitations
Our investigation had several limitations. As a retrospective single‐center investigation with a small sample size, selection bias was a possibility. Also, no standard methods categorizing MAC severity using CT have been established. 8 , 10 Therefore, larger multicenter studies with larger sample sizes are required to assess quantitative MAC score assessments for predicting disease outcomes.
5. CONCLUSIONS
MAC scores from noncontrast cardiac‐gated CT provide clinically important information before valve surgery, and warrant closer monitoring for arrhythmia.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Huishan Wang and Benqiang Yang contributed to the conception and design of the study. Jie Hou and Yu Sun contributed significantly to manuscript preparation. Jie Hou and Yu Sun analyzed the data. Jie Hou wrote the manuscript. Libo Zhang, Jinglong Shi, Hongrui You, and Rongrong Zhang helped perform the analysis with constructive discussions and revised the manuscript. All authors received the final approval of the manuscript submitted. This work was supported by general projects of the National Natural Science Foundation of China (Grant No. 82070239), the Project of Science and Technology of Liaoning Province of China (Grant No. 2018225024) and Key Research and Development Project of Liaoning Province of China (Grant No. 2020JH2/10300119).
Hou J, Sun Y, Wang H, et al. Noncontrast cardiac computed tomography‐derived mitral annular calcification scores in mitral valve disease. Clin Cardiol. 2023;46:1310‐1318. 10.1002/clc.24110
Contributor Information
Huishan Wang, Email: huishanw@126.com.
Benqiang Yang, Email: bqyang888@sina.com.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Alexis SL, Malik AH, El‐Eshmawi A, et al. Surgical and transcatheter mitral valve replacement in mitral annular calcification: a systematic review. J Am Heart Assoc. 2021;10(7):e018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eberhard M, Schönenberger ALN, Hinzpeter R, et al. Mitral annular calcification in the elderly—quantitative assessment. J Cardiovasc Comput Tomogr. 2021;15(2):161‐166. [DOI] [PubMed] [Google Scholar]
- 3. Okuno T, Asami M, Khan F, et al. Does isolated mitral annular calcification in the absence of mitral valve disease affect clinical outcomes after transcatheter aortic valve replacement? Eur Heart J Cardiovasc Imaging. 2020;21(5):522‐532. [DOI] [PubMed] [Google Scholar]
- 4. Massera D, Kizer JR, Dweck MR. Mechanisms of mitral annular calcification. Trends Cardiovasc Med. 2020;30(5):289‐295. [DOI] [PubMed] [Google Scholar]
- 5. Çetin M, Duman H, Özer S, et al. Mitral annular calcification predicted major cardiovascular events in patients presented with acute coronary syndrome and underwent percutaneous coronary intervention. Acta Cardiol. 2020;75(8):767‐773. [DOI] [PubMed] [Google Scholar]
- 6. Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification. JACC Cardiovasc Imaging. 2016;9(11):1318‐1337. [DOI] [PubMed] [Google Scholar]
- 7. Feindel CM, Tufail Z, David TE, Ivanov J, Armstrong S. Mitral valve surgery in patients with extensive calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2003;126(3):777‐781. [DOI] [PubMed] [Google Scholar]
- 8. Guerrero M, Wang DD, Pursnani A, et al. A cardiac computed tomography‐based score to categorize mitral annular calcification severity and predict valve embolization. JACC Cardiovasc Imaging. 2020;13(9):1945‐1957. [DOI] [PubMed] [Google Scholar]
- 9. Hamandi M, Lanfear AT, Squiers JJ, et al. Double‐valve replacement in patients with mitral annular calcification and aortic stenosis. Ann Thorac Surg. 2021;111(5):e311‐e313. [DOI] [PubMed] [Google Scholar]
- 10. Mejean S, Bouvier E, Bataille V, et al. Mitral annular calcium and mitral stenosis determined by multidetector computed tomography in patients referred for aortic stenosis. Am J Cardiol. 2016;118(8):1251‐1257. [DOI] [PubMed] [Google Scholar]
- 11. Massera D, Trivieri MG, Andrews JPM, et al. Disease activity in mitral annular calcification. Circ Cardiovasc Imaging. 2019;12(2):e008513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams MC, Massera D, Moss AJ, et al. Prevalence and clinical implications of valvular calcification on coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2021;22(3):262‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel MA. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019;12(9):1835‐1848. [DOI] [PubMed] [Google Scholar]
- 14. Little SH, Bapat V, Blanke P, Guerrero M, Rajagopal V, Siegel R. Imaging guidance for transcatheter mitral valve intervention on prosthetic valves, rings, and annular calcification. JACC Cardiovasc Imaging. 2021;14(1):22‐40. [DOI] [PubMed] [Google Scholar]
- 15. Lewicka E. Prediction of recurrence after cryoballoon ablation therapy in patients with paroxysmal atrial fibrillation. Anatol J Cardiol. 2016;16(7):489‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739‐2791. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Han J, Wang Z, et al. A prospective randomized trial of the cut‐and‐sew maze procedure in patients undergoing surgery for rheumatic mitral valve disease. J Thorac Cardiovasc Surg. 2018;155(2):608‐617. [DOI] [PubMed] [Google Scholar]
- 18. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827‐832. [DOI] [PubMed] [Google Scholar]
- 19. Brodov Y, Konen E, Di Segni M, et al. Mitral annulus calcium score. Circulation: Cardiovascular Imaging. 2019;12(1):e007508. [DOI] [PubMed] [Google Scholar]
- 20. Blaha MJ, Mortensen MB, Kianoush S, Tota‐Maharaj R, Cainzos‐Achirica M. Coronary artery calcium scoring. JACC Cardiovasc Imaging. 2017;10(8):923‐937. [DOI] [PubMed] [Google Scholar]
- 21. Cavalcanti LRP, Sá MPBO, Perazzo ÁM, et al. Mitral annular calcification: association with atherosclerosis and clinical implications. Curr Atheroscler Rep. 2020;22:9. [DOI] [PubMed] [Google Scholar]
- 22. Lyle MA, Snipelisky DF, Aggarwal NR, Miller FA, Anavekar NS. Exuberant mitral annular calcification. Int J Cardiovasc Imaging. 2017;33(5):615‐621. [DOI] [PubMed] [Google Scholar]
- 23. Akram MR, Chan T, McAuliffe S, Chenzbraun A. Non‐rheumatic annular mitral stenosis: prevalence and characteristics. Eur J Echocardiogr. 2009;10(1):103‐105. [DOI] [PubMed] [Google Scholar]
- 24. Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 2012;164(2):163‐176. [DOI] [PubMed] [Google Scholar]
- 25. Fox CS, Parise H, Vasan RS, et al. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173(2):291‐294. [DOI] [PubMed] [Google Scholar]
- 26. O'Neal WT, Efird JT, Nazarian S, et al. Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA. Eur Heart J Cardiovasc Imaging. 2018;19(3):279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengupta SP, Mohan JC. Calcific mitral stenosis. J Am Coll Cardiol. 2020;75(24):3058‐3060. [DOI] [PubMed] [Google Scholar]
- 28. Kanjanauthai S, Nasir K, Katz R, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis. 2010;213(2):558‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66(17):1934‐1941. [DOI] [PubMed] [Google Scholar]
- 30. Deslandes M, Paquin A, Guzzetti E, et al. Sex‐specific correlates of valvular and arterial calcification burden in patients with moderate aortic stenosis. Open Heart. 2022;9(2):e002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grossi EA, Galloway AC, Steinberg BM, et al. Severe calcification does not affect long‐term outcome of mitral valve repair. Ann Thorac Surg. 1994;58(3):685‐688. [DOI] [PubMed] [Google Scholar]
- 32. Saran N, Greason KL, Schaff HV, et al. Does mitral valve calcium in patients undergoing mitral valve replacement portend worse survival? Ann Thorac Surg. 2019;107(2):444‐452. [DOI] [PubMed] [Google Scholar]
- 33. Kaneko T, Hirji S, Percy E, et al. Characterizing risks associated with mitral annular calcification in mitral valve replacement. Ann Thorac Surg. 2019;108(6):1761‐1767. [DOI] [PubMed] [Google Scholar]
- 34. O'Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Mitral annular calcification and incident atrial fibrillation in the multi‐ethnic study of atherosclerosis. Europace. 2015;17(3):358‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kizer JR, Bella JN, Palmieri V, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle‐aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006;151(2):412‐418. [DOI] [PubMed] [Google Scholar]
- 36. Ariyarajah V, Apiyasawat S, Barac I, Spodick DH. Is the presence of mitral annular calcification associated with poor left atrial function? Echocardiography. 2009;26(8):877‐884. [DOI] [PubMed] [Google Scholar]
- 37. Aksoy F, Guler S, Kahraman F, et al. The relationship between mitral annular calcification, metabolic syndrome and thromboembolic risk. Braz J Cardiovasc Surg. 2019;34(5):535‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Enta Y, Nakamura M. Transcatheter mitral valve replacement. J Cardiol. 2021;77(6):555‐564. [DOI] [PubMed] [Google Scholar]
- 39. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561‐632. [DOI] [PubMed] [Google Scholar]
- 40. Oguz D, Eleid MF, Dhesi S, et al. Quantitative three‐dimensional echocardiographic correlates of optimal mitral regurgitation reduction during transcatheter mitral valve repair. J Am Soc Echocardiogr. 2019;32(11):1426‐1435.e1. [DOI] [PubMed] [Google Scholar]
- 41. Muller DWM, Sorajja P, Duncan A, et al. 2‐Year outcomes of transcatheter mitral valve replacement in patients with severe symptomatic mitral regurgitation. J Am Coll Cardiol. 2021;78(19):1847‐1859. [DOI] [PubMed] [Google Scholar]
- 42. You T, Wang W, Yi K, et al. Transcatheter mitral valve replacement for degenerated mitral valve bioprostheses, failure of mitral valvuloplasty and native valve with severe mitral annulus calcification: a systematic review and meta‐analysis. J Cardiothorac Surg. 2021;16(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gheorghe LL, Mobasseri S, Agricola E, et al. Imaging for native mitral valve surgical and transcatheter interventions. JACC Cardiovasc Imaging. 2021;14(1):112‐127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


