Abstract
Background:
Despite expanded access to antiretroviral therapy (ART) and the rollout of the World Health Organization’s (WHO) ‘test-and-treat’ strategy, the proportion of people with HIV (PWH) presenting with advanced HIV disease (AHD) remains unchanged at approximately 30%. Fifty percent of persons with AHD report prior engagement to care. ART failure and insufficient retention in HIV care are major causes of AHD. People living with AHD are at high risk for opportunistic infections and death. In 2017, the WHO published guidelines for the management of AHD that included a comprehensive package of care for screening and prophylaxis of major opportunistic infections (OIs). In the interim, ART regimens have evolved: integrase inhibitors are first-line therapy globally, and the diagnostic landscape is evolving. The objective of this review is to highlight novel point-of-care (POC) diagnostics and treatment strategies that can facilitate OI screening and prophylaxis for persons with AHD.
Methods:
We reviewed the WHO guidelines for recommendations for persons with AHD. We summarized the scientific literature on current and emerging diagnostics, along with emerging treatment strategies for persons with AHD. We also highlight the key research and implementation gaps together with potential solutions.
Results:
While POC CD4 testing is being rolled out in order to identify persons with AHD, this alone is insufficient; implementation of the Visitect CD4 platform has been challenging given operational and test interpretation issues. Numerous non-sputum POC TB diagnostics are being evaluated, many with limited sensitivity. Though imperfect, these tests are designed to provide rapid results (within hours) and are relatively affordable for resource-poor settings. While novel POC diagnostics are being developed for cryptococcal infection, histoplasmosis and talaromycosis, implementation science studies are urgently needed to understand the clinical benefit of these tests in the routine care.
Conclusions:
Despite progress with HIV treatment and prevention, a persistent 20%–30% of PWH present to care with AHD. Unfortunately, these persons with AHD continue to carry the burden of HIV-related morbidity and mortality. Investment in the development of additional POC or near-bedside CD4 platforms is urgently needed. Implementation of POC diagnostics theoretically could improve HIV retention in care and thereby reduce mortality by overcoming delays in laboratory testing and providing patients and healthcare workers with timely same-day results. However, in real-world scenarios, people with AHD have multiple comorbidities and imperfect follow-up. Pragmatic clinical trials are needed to understand whether these POC diagnostics can facilitate timely diagnosis and treatment, thereby improving clinical outcomes such as HIV retention in care.
Keywords: advanced HIV, cryptococcal meningitis, opportunistic infections, point-of-care diagnostics, tuberculosis
INTRODUCTION
In 2016, the United Nations General Assembly set a goal to end AIDS by 2030 with an interim goal to reduce AIDS-related deaths to fewer than 500 000 by 2020; unfortunately that goal has not been met [1]. An estimated 650 000 AIDS-related deaths occurred in 2021 [2], with the majority occurring among people living with advanced HIV disease (AHD). Despite expanded access to antiretroviral therapy (ART) and the rollout of the World Health Organization’s (WHO) ‘test-and-treat’ strategy, the proportion of people with HIV (PWH) presenting with AHD remains unchanged at approximately 30% [3–6]. Fifty percent of persons with AHD report prior engagement to care. ART failure and insufficient retention in HIV care are major causes of AHD [7].
People living with AHD are at high risk for opportunistic infections (OIs) which drive mortality. Common OIs include tuberculosis (TB), cryptococcal disease, severe bacterial infection (SBI), toxoplasmosis, pneumocystis pneumonia (PJP), and, in specific regions, histoplasmosis and talaromycosis. In the REALITY trial, among ambulatory PWH with CD4 <100 cells/mm [3], 13% died within 48 weeks, with the highest mortality occurring during the first 4 weeks on ART. [8] Similarly, in the REMSTART trial, 16% of participants with AHD died by 28 weeks [9]. This likely represents a gross underestimate of routine care mortality among persons with AHD outside of clinical trials [10–12]. Studies of patients hospitalized with AHD report >20% mortality, primarily due to TB [13–15].
In 2017, the WHO published guidelines for the management of AHD that included a comprehensive package of care for screening and prophylaxis of major OIs (Table 1) based on the above-mentioned trials among AHD outpatients [3, 8, 9]. In the interim, ART regimens have evolved: integrase inhibitors are first-line therapy globally, and the diagnostic landscape has changed. In this review, we discuss novel diagnostic and OI prophylaxis strategies for people with AHD and highlight key research and implementation gaps together with potential solutions. Given the breadth of AHD-related OI management, we focus our discussion on emerging diagnostics, prophylactic strategies, and new areas of investigation.
TABLE 1.
World Health Organization recommendations for opportunistic infection screening and prophylaxis, stratified by CD4 cell count.
| Always recommended | CD4 <350 cells/μl | CD4 <200 cells/μl | CD4 <100 cells/μl | |
|---|---|---|---|---|
| Rapid ART initiationa |
|
|||
| TPT (without active TB) |
|
|||
| Xpert MTB/RIF (if TB symptoms present) |
|
|||
| Cotrimoxazole prophylaxis |
|
|||
| Medication adherence support |
|
|||
| CrAg screening |
|
|||
| Fluconazole preemptive therapy if CrAg-positive |
|
|||
| TB LAM |
|
|||
Deferred if clinical symptoms of TB or cryptococcal meningitis present.
Abbreviations: ART, antiretroviral; CrAg, cryptococcal antigen; LAM, lipoarabinomannan; MTB, Mycobacterium tuberculosis; RIF, rifampin; TB, tuberculosis; TPT, tuberculosis preventive therapy.
IDENTIFICATION OF PEOPLE WITH AHD: CD4 TESTING AS THE GATEWAY TO THE AHD PACKAGE OF CARE
In this review, we define AHD as adults with a CD4 cell count <200 cells/mm [3]. While children can have AHD, their risk of OIs and death are different than adults. The referenced clinical trials only include adults and, as a result, the WHO recommendations for OI screening and preemptive treatment are only applicable to adults. The WHO recommends that all adults entering or re-entering HIV care receive CD4 testing to identify AHD and thereafter screen for OIs (Table 1) [3]. However, due to resource constraints and as CD4 counts are no longer used to guide ART initiation, viral load testing is often preferentially performed. [16] Thus, persons with AHD are not always identified and appropriately screened and treated for OIs. Indeed, in an analysis of >500 000 patients in six countries in southern Africa, CD4 testing at ART initiation occurred in 78% in 2008 compared to only 38% in 2017 [17].
Where CD4 testing is performed, due to laboratory-based testing, results are typically reviewed at the next clinic visit, often 2–4 weeks later. The opportunity to perform real-time targeted OI screening with provision of OI treatment/prevention prior to ART initiation or re-initiation is thus missed. Among persons with untreated OIs, initiating ART may lead to unmasking immune reconstitutions inflammatory syndrome (IRIS), which can be fatal. [18] Indeed, lack of pre-ART CD4 testing in persons with AHD is associated with higher 90-day mortality [19].
Point-of-care CD4 testing
Near point-of-care (POC) CD4 testing using PIMA analyser (Abbott) and BD FACS Presto (BD Biosciences) instruments have been available for several years. These CD4 platforms provide accurate results in 20 min through battery or electricity run instruments, allowing for early detection of AHD in outpatient settings [20]. Replacement of cartridges is frequently a barrier to care. Furthermore, production of these POC CD4 machines will be discontinued in 2024 by both BD Biosciences and Abbott.
The Visitect CD4 test (AccuBio Ltd) is a heat-stable, electricity and instrument-free, POC CD4 test that yields visual semiquantitative results (above or below 200 cells/mm [3]) in 40 min at a cost of US$4 per test. [21] A recent study among 1053 venous samples from Botswana demonstrated 94% (112/119) sensitivity compared to flow cytometry but positive predictive value for CD4 <200 cells/mm [3] was only 46% [22]. If the goal is to identify and screen persons at highest risk of undiagnosed OIs, false-positive results may be a reasonable trade-off for a highly sensitive POC test that can allow for rapid OI screening prior to ART initiation [23]. One additional drawback is that healthcare workers often prefer a precise CD4 value over a semiquantitative CD4 range [24, 21].
As of June 2022, a total of 44 countries are implementing or evaluating Visitect CD4 tests in facilities (Clinton Health Access Initiative, personal communication, January 2023). The Visitect CD4 test has been included in the PEPFAR Country Operational Plan (COP) Guidance for 2022 and listed as a priority diagnostic for 2023–2025 by the Global Fund. Operational challenges include numerous steps must be performed within 40 min; buffer needs to be added after 3 min, and again after an additional 17 min. The practicalities of performing multiple tests in a busy clinical laboratory have not yet been established. Interpreting the results of the test remains challenging in real-world settings (unpublished data).
AHD-ASSOCIATED OPPORTUNISTIC INFECTIONS
Tuberculosis
Tuberculosis (TB) is the leading cause of mortality among PWH, contributing to up to one-third of AIDS-related deaths [25]. Sputum-based diagnostics are the WHO-recommended initial test for TB diagnosis. However, fewer than 60% of persons initiate TB treatment with a confirmed microbiological diagnosis [26]. In people with AHD, diagnosis of TB is challenging due to atypical clinical presentation and difficulty producing sputum. In a meta-analysis of autopsy studies among PWH in resource-limited settings, approximately 45% of TB cases remained undiagnosed and untreated at the time of death [27]. Furthermore, subclinical TB, where patients may be asymptomatic for 6 months prior to symptom onset, may occur in ~50% of microbiologically confirmed TB cases and drives transmission [28] [29]. Thus, in persons with AHD, TB screening is essential, and more sensitive, nonsputum POC diagnostics are required to reduce TB transmission and deaths.
Point-of-care TB diagnostics
TB-lipoarabinomannan testing
Liporabinomannan (LAM) is a lipopolysaccharide component of the Mycobacterium tuberculosis (MTB) cell wall that is detectable in the urine of PWH with active TB disease. The Alere Determine TB-LAM Antigen test (TB-LAM; Alere) is a cheap, rapid, POC lateral flow test that requires no electricity or laboratory infrastructure (Table 2) [30]. The diagnostic sensitivity of the Alere TBLAM is highest in inpatients with low CD4 cell counts.
TABLE 2.
Summary of tuberculosis point-of-care diagnostics performance, costs and recommendation
| Test name | Specimen | Sensitivity/specificity | WHO recommendations [8] | Time to result | Cost (US$) | Comments |
|---|---|---|---|---|---|---|
| Alere LAM Abbott |
Urine | CD4 <200 cells/μl: • Sensitivity 45% (95% CI 31% to 61%) • Specificity 89% (95% CI 11% to 94%) [93] |
Inpatient: WHO strongly recommends LF-LAM to assist in diagnosis of active TB in PWH with signs/symptoms of TB with AHD | <1 h | $3.70 | • Commercially available • No electricity needed • Higher sensitivity in disseminated TB disease |
| ||||||
| CD4 <100 cells/μl: | OR | |||||
| • Sensitivity 54% (95% CI 38% to 69%) • Specificity 88% (95% CI 11% to 94%) [93] |
Seriously ill PWH with CD4 count ≤200 cells/mm3 irrespective of signs/symptoms of TB (Strong recommendation) | |||||
| Outpatient: WHO suggests using LF-LAM to assist in diagnosis of active TB in PWH with signs/symptoms of TB | ||||||
| OR | ||||||
| PWH who are seriously ill with CD4 count <100 cells/mm3 irrespective of signs/symptoms of TB (Conditional recommendation) | ||||||
| Fujifilm SILVAMP FujiLAM | Urine | Sensitivity 53% (95% CI 44% to 62%), specificity 99% (95% CI 91% to >99%) [94] Inpatient: 70% sensitivity, 90% specificity for hospitalized PWH Outpatient: 15% sensitivity, 89% specificity in PWH [95] |
NA | <1 h | $6.00 [96] | • Not commercially available • No electricity needed • Higher sensitivity in persons with HIV |
|
|
||||||
| Xpert MTB/RIF Ultra Cepheid | Sputum Synovial fluid Pleural fluid Lymph node Blood [34] CSF [33] Oral swab |
Pulmonary TB [38]: Sputum: sensitivity 68%, specificity 98% Tongue: sensitivity 91.8% [35] Buccal: sensitivity 50% [37, 38] Extrapulmonary TB [38]: |
• First use in adults and children for pulmonary TB evaluation (Strong recommendation) • First use in adults and children for TB meningitis CSF evaluation (Strong recommendation) |
<2 h | $12.63 [98] | • Commercially available • Requires GeneXpert machine • Requires electrical supply • Can test for rifampin resistance |

|
Pleural: sensitivity 38%- 61%, specificity 96%- 99% Lymph node biopsy: sensitivity 67%, specificity 96% Synovial: sensitivity 96%, specificity 97% CSF: sensitivity 87%, specificity 88% Blood: sensitivity 37% [34] Stool (children): sensitivity 63%, specificity 100% [97] |
• May be used in PWH with suspected disseminated TB on blood (Conditional recommendation) | • Increased sensitivity in blood with lower CD4 or haemoglobin • Increased sensitivity in TB endemic countries |
|||
Xpert MTB Host Response (MTB-HR)
|
Sputum | Pulmonary TB: sensitivity 87%, specificity 94% [34] | NA | <2 h | • Not commercially available • Uses 3-gene signature to create a TB Score • 10% in study population HIV infection, limited numbers with advanced disease |
|
TrueNAT/TrueNAT Plus
|
Sputum | • AFB smear + patients: Sensitivity 73% (95% CI 68–78) Specificity 80% (95% CI 75–84) [37] • AFB smear negative patients: Sensitivity 37% (95% CI 27–48) Specificity 46% (95% CI 36–57) [37] |
• May be used in exchange for Xpert MTB/RIF Ultra for suspected pulmonary TB (Conditional recommendation) | 1 h | $13.20 [34] | • Commercially available • No electricity needed • Can be performed in peripheral laboratories and primary health centres • Can test for rifampin resistance • No data in children • Temperature stable |
| Cell-free MTB DNA detection | Plasma Urine |
• Plasma: sensitivity 97% (80%-100%), specificity 94% (71%-100%) [32] | NA | 1 day | • Not commercially available • Limited data in PWH |
|
| • Urine: sensitivity 83.7% (71%-91%), specificity 100% (86%-100%) [43] | • Sensitive for pulmonary and extra- pulmonary TB in children with HIV |
Abbreviations: AFB, acid-fast bacilli; AHD, advanced HIV disease; CI, confidence interval; CSF, cerebrospinal fluid; LAM, lipoarabinomannan; LF, lateral flow; MTB, Mycobacterium tuberculosis; NA, not available; PWH, people with HIV; RIF, rifampin; TB, tuberculosis; WHO, World Health Organization.
The STAMP trial examined systematic urine TB-screening with Alere TB-LAM and Xpert MTB/RIF assay (Xpert; Cepheid) among hospitalized adult PWH and found an increase in TB diagnoses and treatment frequency (adjusted hazard ratio 1.56, 95% CI 1.29 to 1.88; p < 0.0001) [14]. Among adults with CD4 <100 cells/mm [3] there was a survival benefit at day 56 [14]. Accordingly, WHO 2021 guidelines recommend urine TB-LAM testing in all persons with CD4 <100 cells/mm [3] regardless of symptoms, and in PWH with signs/symptoms of TB [31].
The Fujifilm SILVAMP TB LAM (FujiLAM; Fujifilm) combines a pair of high-affinity monoclonal antibodies directed toward MTB-specific LAM epitopes and a silver-amplification step. This design enables the detection of urinary LAM concentrations that are approximately 30 times lower than that detected by the Alere TB-LAM assay. While the FujiLAM performance appears promising (Table 2) it is not yet commercially available.
Molecular TB tests
Xpert MTB/RIF Ultra is a cartridge-based, automated, real-time polymerase chain reaction (PCR) assay that provides results (including rifampin resistance) in under 2 h. This test is a re-engineered version of the original Xpert MTB/RIF assay and is recommended by the WHO as the first diagnostic test for TB [32]. Xpert Ultra has been tested extensively on non-sputum samples; however, interpretation of sensitivity remains challenging (Table 2). For example, among 264 hospitalized adults with AHD and suspected meningitis who were systematically screened for TB, 11% (29/264) had evidence of disseminated TB disease by urine Xpert Ultra and 23% (75/327) by urine Alere TB-LAM, with poor concordance between the two tests [33]. Blood Xpert Ultra results have also been closely associated with mortality (odds ratio 2.39, 95% CI 1.5–3.9) adding prognostic utility [34]. Xpert testing of multiple tongue swabs appears promising and may be an option for those with suspected pulmonary TB who cannot produce sputum [35, 36].
Implementation of Xpert and Xpert Ultra are limited by cost and requirements for a stable electrical supply. In contrast, the WHO-endorsed sputum Truenat assay (Molbio Diagnostics) is a chip-based, real-time PCR that is battery powered and heat stable up to 40°C with comparable accuracy to Xpert Ultra in sputum and nonsputum samples (Table 2) [37–39].
New horizons for TB diagnostics
A strategy using a combination of multiple TB diagnostic tests in persons with AHD may be valuable. Prospective data from Tanzania and South Africa in PWH clearly show the incremental diagnostic yield when urine Alere TB-LAM testing is added to Xpert testing on sputum [40, 41]. Further promising approaches under development focused on bacilli component detection include plasma or urine cell-free MTB DNA, next-generation sequencing and CRISPR-mediated technologies (Table 2) [42, 43]. Additionally, the Xpert MTB/XDR assay uses the GeneXpert instrument to rapidly detect MTB and resistance to first- and second-line in sputum specimens [44].
An alternative approach is to use the body’s immune response as a TB diagnostic test. The Xpert MTB Host Response (Xpert MTB-HR; Cepheid) generates a TB score based on messenger RNA expression of three genes using the GeneXpert platform. [45] Compared to Xpert Ultra, Xpert MTB-HR had 87% sensitivity and 94% specificity for persons with pulmonary TB, although only 10% of the study population had HIV infection. Although the MTB-HR test has potential as a POC triage test, performance data are needed in combination with microbiologic or molecular tests among persons with AHD.
TB preventive therapy
In PWH, if active TB disease has been excluded, TB preventive therapy (TPT) is indicated to prevent progression of latent TB infection to active TB disease [3]. The risk of developing active TB within 6 months of ART initiation in people with AHD is as high as 20% [46]. Short-course TPT regimens (1 or 3 months of rifapentine-based therapy) are non-inferior to 9 months with improved adherence and fewer adverse events, though these studies included very few persons with AHD [47]. Preliminary data from an ongoing pharmacokinetic clinical trial of doubling the dose of dolutegravir while on 1HP demonstrated no serious adverse effects (ACTG A5372), though final trial results are pending [48]. WHO recommendations for TPT are summarized in Table 3.
TABLE 3.
Summary of tuberculosis preventive therapy option
| Side effects Hepatotoxicity Hypersensitivity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
Costs (US$) [9] total direct cost includes medication costs, supply costs, clinic costs and health system costs | Literature highlights |
|
||||||
| Regimen | Drug | Adult dose | Duration | Advantages | Disadvantages | ||||
| 6H, 9H Isoniazid preventive therapy (IPT) | Isoniazid | 300 mg daily | 6 months OR 9 months |
Increased risk of hepatotoxicity with 9H |
6H: $0.19/dose $25 total $374 total direct costs 9H: $0.19/dose $52 total $499 total direct costs |
Systematic review shows IPT reduces the overall risk for TB by 33% (RR 0.67; 95% CI 0.51; 0.87), with a 64% risk reduction for people with a positive TST (RR 0.36; 95% CI 0.22; 0.61) [99] No controlled clinical trials comparing 9H vs. 6H Systematic review and metaanalysis of PWH in settings with high TB prevalence and transmission showed that continuous IPT can reduce the risk for active TB by 38% more than 6 months’ isoniazid [100] |
Daily |
Affordable, well studied Low pill burden | Long duration Lower completion rates |
| 3HP | 3 months | $11.12/dose | Shorter duration | Drug interactions: | |||||
| Rifapentine + Isoniazid | 900 mg weekly + 900 mg weekly |
Greatest hepatotoxicity risk |
$133 total $412 total direct costs In a high-burden setting, the cost of successful delivery of 3HP was lower than that of 6H, driven by higher completion [101] |
No significant difference in the incidence of active TB between participants given a 3HP and 6H or 9H (RR 0.73, 95% CI 0.23; 2.30) The risk for hepatotoxicity was significantly lower with 3HP in adult PWH (RR 0.26, 95% Cl 0.12; 0.55) [102] |
Weekly | Weekly administration Higher completion rates Less hepatotoxicity |
• Double dolutegravir dose during TPT • Avoid rifamycin containing regimens in patients on protease inhibitors and nevirapine Variable access to rifapentine Higher costs |
||
| 3HR | Rifampin + isoniazid | 300 mg daily + 600 mg daily | 3 months |
|
$0.85/dose $76 total $355 total direct costs |
A systematic review showed that the efficacy and the safety profile of 3– 4 months’ daily rifampicin plus isoniazid were similar to those on 6 months of isoniazid [103] | Daily |
Shorter duration | Drug interactions: • Double dolutegravir dose during TPT • Avoid rifamycin- containing regimens in patients on protease inhibitors and nevirapine |
| 4R | Rifampin | 600 mg daily | 4 months |
|
$0.65/dose $78 total $365 total direct costs |
A systematic review found similar efficacy for 3–4 months’ daily rifampicin and 6H (OR 0.78; 95% CI 0.41 ;1.46) Individuals given rifampicin daily for 3–4 months had a lower risk for hepatotoxicity than those treated with isoniazid monotherapy (OR 0.03; 95% Cl 0.00;0.48) [104] |
Daily |
Shorter duration Single drug Low pill burden | Variable access to rifapentine Higher costs |
| 1HP | Daily rifapentine + isoniazid | 600 mg daily + 300 mg daily *Not studied in pregnant participants | 1 month |
|
$7.23/dose $202.44 total $421 total direct costs (estimated from 3HP data) | An RCT showed non-inferiority comparing 1HP to 9H for active TB or death. Serious adverse events were equivocal between groups. Treatment completion was higher in 1HP group [47] | Daily |
Shortest duration |
Abbreviations: CI, confidence interval; IPT, isoniazid preventive therapy; OR, odds ratio; RCT, randomized controlled trial; RR, relative risk; TB, tuberculosis; TST, tuberculin skin test.
Cryptococcal infection
Cryptococcosis causes 19% of AIDS-related mortality globally, with approximately 152 000 (IQR 111 000–185 000) cases of cryptococcal meningitis and 112 000 (IQR 79 000–134 000) HIV-associated cryptococcal deaths annually [49]. Cryptococcal meningitis is preceded by asymptomatic cryptococcal antigenemia, where patients are without meningitis symptoms, but have cryptococcal antigen (CrAg) in their blood. Among persons with AHD, mean prevalence of cryptococcal antigenemia is 4.4% (95% CI 1.6% to 7.4%) [49]. This period of antigenemia prior to onset of meningitis symptoms provides a crucial window for screening and prevention of invasive cryptococcal disease. CrAg-positive persons are at high risk for meningitis and death within weeks to months [50]. Screening and identification of CrAg-positive persons and effective preemptive antifungal therapy as recommended by WHO is a key strategy in reducing cryptococcosis-related morbidity and mortality [3].
Diagnostics
The CrAg lateral flow assay (CrAg LFA; IMMY) is an inexpensive, rapid, simple, heat-stable test that is ideal for resource-limited settings where disease burden is highest. The CrAg LFA is highly sensitive (>98%) and specific (>94%) in both cerebrospinal fluid (CSF) and blood [51, 52]. The recently developed IMMY CrAg semiquantitative LFA provides CrAg titres in real time [53]. As high blood CrAg titres (≥1:160) predict meningitis and death, a rapid test with titre information may assist in key management decisions such as triage to hospital for lumbar puncture [51]. Implementation of the CrAg semiquantitative test in clinical practice including acceptability, feasibility assessments by laboratory versus clinical staff, and measurement of clinical outcomes has not been evaluated.
Molecular cryptococcal nucleic amplification through PCR within CSF is highly sensitive and specific, with the capability to detect a low level of fungal burden. Cryptococcal PCR is only commercially available through a multiplex meningitis panel. [54] Such a panel may be useful for meningitis that is difficult to diagnose. However, with widespread availability of the CrAg LFA, along with its cost of US$3 to US$5 per test, makes the LFA the ideal first test in meningitis diagnosis among people with AHD.
Preemptive therapy for CrAg-positive persons
In asymptomatic CrAg-positive patients, preemptive fluconazole (800–1200 mg/day) reduces incidence of cryptococcal meningitis and death in combination with measures to improve ART adherence [9]. Despite this WHO-recommended preemptive therapy, 25% of asymptomatic CrAg-positive persons develop meningitis or die within 6 months of screening [55]. Risk factors for mortality are low CD4 count and high blood CrAg titres [56, 57]. Specifically, CrAg titres ≥1:640 in blood are associated with 10 times the risk of death compared with titres ≤1:80 [56]. Though preemptive antifungal fluconazole therapy is effective in participants with a CrAg titre ≤1:80, fluconazole is inadequate for persons with high CrAg titres.
Histoplasmosis
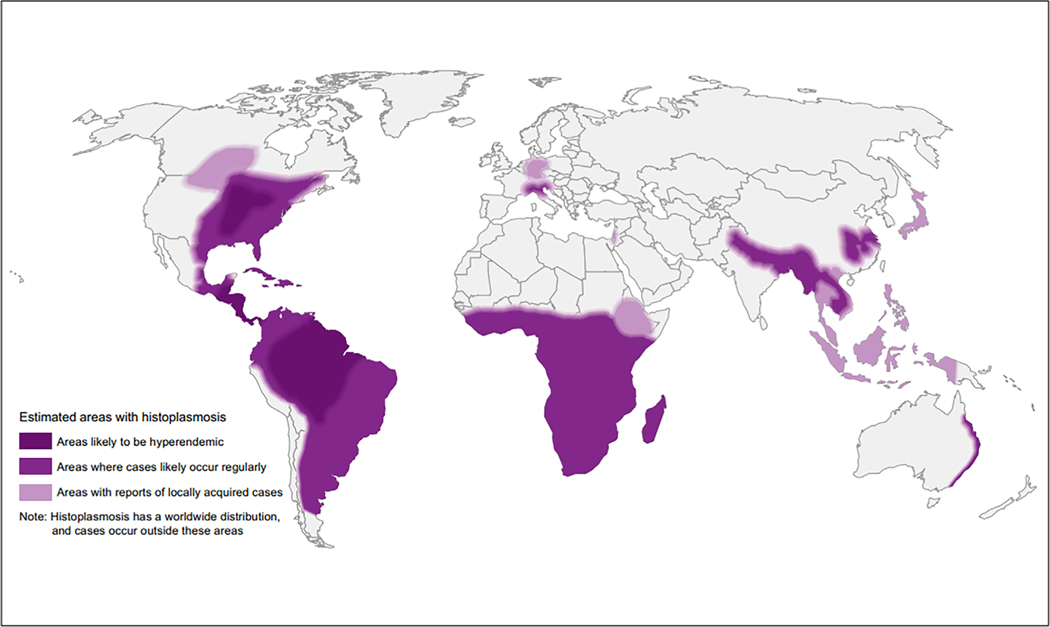
Histoplasmosis has recently been identified globally and is not just endemic in certain parts of the Americas (Figure 1) [58]. The burden of histoplasmosis among people with AHD is unknown, though a recent study from Nigeria identified Histoplasma antigen prevalence in the urine of 7.7% (76/998) among persons with AHD [59]. Mathematical models suggest that among PWH in Latin America, mortality from histoplasmosis may be higher than from TB [60]. In persons with AHD, histoplasmosis is frequently disseminated, presenting as a multisystem febrile illness (similar to TB), and carries ≥20% mortality [61]. Unfortunately, histoplasmosis is often not diagnosed until autopsy, as it is either: (1) not considered outside of traditionally endemic areas, (2) considered less likely than TB or (3) not considered due to lack of diagnostics or antifungal therapeutics [62].
FIGURE 1.
World map estimating regions most likely to have histoplasmosis based on literature review [58].
Diagnosis of disseminated histoplasmosis remains challenging. Histopathology reveals yeast in tissue samples but sensitivity is variable [63]. Fungal culture from blood or bronchoalveolar lavage is the gold standard; however, the fastidious organism requires a biosafety level (BSL)-3 laboratory, thus limiting widespread implementation [64]. Serologic testing, while easier to perform in the laboratory, has inadequate sensitivity (58%) compared to culture [65].
However, recent advances include the Histoplasma galactomannan enzyme immunoassay (EIA) (IMMY), which is commercially available and can be performed without laboratory infrastructure, although refrigeration is required. This assay has 91% sensitivity and specificity when compared to culture or histopathology [66]. A LFA to detect Histoplasma galactomannan (Miravista) has the advantage of requiring minimal laboratory infrastructure but is not yet commercially available. The assay has 90% (95% CI 83%–95%) sensitivity and 92% (95% CI 89%–95%) specificity compared to culture or histopathology [66]. There are insufficient data to know whether these measures are maintained for H. duboisii (the only reported testing is two samples from patients with H. duboisii, both had positive results), although MiraVista believes its likely performance would be similar [67]. In addition, the Miravista LFA compared well to the current standard-ofcare Miravista EIA antigen test in the United States in two studies (kappa 0.656–0.84) [68, 69]. Cross-reaction with other fungi (Blastomyces, Talaromyces, Paracoccidiodes, Coccidioides) is common with these assays [69].
The Infectious Diseases Society of America guidelines recommend itraconazole prophylaxis for CD4 <150 cells/mm [3] where disease incidence is >10 cases/100 patientyears [70]. In a prospective randomized trial among adults with AHD, itraconazole prophylaxis (200 mg daily) reduced the incidence of histoplasmosis but did not improve survival. [71] Pan American Health Organization (PAHO)/WHO guidance for histoplasmosis recommends (1) Histoplasma antigen as the preferred diagnostic and (2) early ART initiation but makes no recommendation on screening or prevention [72].
Talaromycosis
Talaromycosis (previously penicilliosis) is the leading cause of HIV-associated mortality in persons with AHD in Southeast Asia, specifically in Thailand, Vietnam, Hong Kong and southern China [3]. Mortality rates despite antifungal therapy with amphotericin B are as high as 30%, with up to 100% mortality with delayed diagnosis and treatment [73].
Lack of diagnostics remains a critical barrier to reducing talaromycosis mortality. Culture of blood or tissue specimen is the gold standard diagnostic, but results may take up to 14 days [74]. Additionally, blood culture is estimated to miss infection in 30% of PWH [75]. Microscopy of lymph nodes and skin lesions can yield diagnosis when these lesions are present [75]. A TaqMan real-time PCR assay targeting the MP1 gene encoding a cell wall protein specific to Talaromyces marneffei has 70% sensitivity, and 100% specificity compared to blood culture [74]. Given the rapid turnaround (<6 h) and high specificity, this test could be a rapid rule-in test to reduce time to antifungal therapy initiation, although it requires significant laboratory infrastructure [74]. A recent advance in diagnostics for talaromycosis is the Mp1p (cell wall mannoprotein) antigen EIA, which is highly specific and more sensitive than blood culture (86% vs. 72%) [76]. This novel diagnostic test gives results in 6 h and could improve early detection of talaromycosis, though implementation studies to determine whether the Mp1p antigen EIA can reduce mortality are lacking.
Regarding prevention, a retrospective study from China examined cotrimoxazole use and incidence of Talaromyces and found that persons with AHD who started cotrimoxazole within 6 months of ART initiation had significantly lower incidence of Talaromyces infection than those not taking cotrimoxazole (4.1% vs. 7.5%; adjusted hazard ratio 0.50, 95% CI 0.35–0.73) [77]. This effect was most pronounced in patients with CD4 <50 cells/mm [3], suggesting that cotrimoxazole could be used as potential prophylaxis in persons with AHD in Southeast Asia.
Severe bacterial infections
HIV infection increases susceptibility to SBI by preexisting activation and exhaustion of the immune system, thereby leading to an unbalanced immune response to sepsis [78]. Incidence of and mortality from SBI is higher among PWH, with the highest relative risk of mortality among PWH in low-income settings [79, 80]. Differences in HIV prevalence, ART access and medical resources leads to significant variation in recorded SBI mortality across settings in PWH, with mortality rates as low as 16% in high-income settings, and as high as 43% in Asia and 46% in Africa [81]. The aetiology of SBI is poorly characterized in persons with AHD due to lack of diagnostics. However, a systematic review of bloodstream infections among adults admitted to hospital in Africa noted the most common etiologies were Salmonella enterica (42%), followed by Brucella (13%), Streptococcus pneumonia (10%) and other non-Salmonella (7%) [82].
Bacterial sepsis diagnostics
Blood culture is the gold standard for diagnosis of SBI but requires substantial laboratory capacity, therefore limiting use in low- and middle-income countries. In the absence of cultures, the use of extended differential parameters (generated routinely on automated haematology analysers) are of interest but negative predictive value is inadequate to rule out bacterial infection [83]. Molecular diagnostics for sepsis carry the potential to increase yield and decrease time to result. The TaqMan Array Card allows for simultaneous, single-plex quantitative polymerase chain reaction (qPCR)-based amplification of multiple targets including bacteria [84]. In 336 samples from persons with SBI in Uganda, the TaqMan qPCR sensitivity was 61% and specificity was 98% compared to culture [85]. Plasma metagenomic nextgeneration sequencing platforms can detect >2000 DNA pathogen targets directly from blood samples thus avoiding cultures [86]. The cost, maintenance and work force to support sequencing technology limits widespread adoption of molecular diagnostics. However, with WHO support of increased genomic surveillance and innovative sequencing technologies such as the pocket-sized, portable MinION (Oxford Nanopore), targeted molecular SBI diagnostics may be an emerging tool that can be regionally adapted [87, 88].
Prophylaxis
Antimicrobial prophylaxis among people with AHD to prevent SBI is not recommended due to concerns relating to antimicrobial resistance. In PWH, trimethoprim–sulfamethoxazole prophylaxis is widely used to prevent PJP but may also prevent bacterial infection. The REALITY trial randomized 1805 persons with HIV and CD4 <100 cells/mm [3] in sub-Saharan Africa to standard of care (trimethoprim–sulfamethoxazole alone) plus or minus additional preventive agents including azithromycin. While there was no change in documented SBIs between groups nor attributable mortality from SBI reported, there was an overall 27% relative reduction in 6-month mortality. Given the major challenges in diagnosing SBIs in resource-limited settings and the high proportion of deaths due to unknown causes (39%, 88/225), it is possible that a proportion of averted deaths were due to azithromycin [8]. Vaccination in PWH also decreases the incidence of vaccine-preventable diseases such as S. pneumoniae [89, 90].
AREAS FOR FUTURE RESEARCH
Burden of disease studies requires access to POC diagnostics
While the Joint United Nations Programme on HIV and AIDS (UNAIDS) publishes number of PWH by country and region, numbers with CD4 <200 cells/mm [3] are no longer consistently recorded. Collecting and reporting number of people with AHD by country and region, which requires widespread CD4 testing, would be a first step to allow stakeholders and ministries of health to plan and budget for the required OI screening and prophylaxis treatments. Additionally, POC CD4 implementation studies and evaluation of subsequent retention-in-care, survival and cost-effectiveness are essential to improve access to such diagnostics. The ENCORE trial (clinicaltrials.gov NCT05085171) seeks to randomize 24 clinics to Visitect CD4 testing versus traditional CD4 testing to evaluate 6-month retention-in-care. Without POC CD4 testing, patients may get CD4 testing but never present for follow-up for the result. Thus, they may have AHD but never be screened for OIs. Such persons are not counted in HIV programmes because they have not yet fully engaged with clinic. Thus, a clinic may report high retention in care (of PWH who are engaged in clinic), while simultaneously not engaging PWH who present for laboratory testing. Not accounting for PWH who present for initial CD4 testing in the laboratory without further engagement in the clinic for ART, OI screening and follow-up is a failure of the HIV care system; POC CD4 testing with same-day results is an attempt to bridge that gap.
We have highlighted the operational challenges with the Visitect POC CD4 test platform including difficulty with test interpretation, and an intensive 40-min test requiring multiple intermittent steps, which is difficult to carry out accurately in a busy laboratory. In 2024, both Abbott and BD Biosciences will discontinue production of their POC CD4 platforms. Thus, PIMA and BD FacsPresto will no longer be available, leaving only the Visitect to fill the role of POC CD4 testing. Development of POC (or near-bedside) CD4 platforms that are rapid (<30 min), without requiring electricity or laboratory infrastructure, that are highly accurate and easy to interpret is a critical gap that needs to be filled.
Quantification of the burden of histoplasmosis is needed, especially in African low- and middle-income countries where the burden of AHD is greatest. However, estimating the burden of histoplasmosis and talaromycosis is reliant on access to and implementation of POC antigen testing for screening and early detection.
Clinical trials studying novel screening and preventive strategies
Additional predictors of mortality are needed among asymptomatic CrAg-positive persons. While high titre and low CD4 cell count both predict meningitis and death, are there other biomarkers such as C-reactive protein (CRP) that could predict who will progress to meningitis? In addition, more effective preemptive therapy for CrAg-positive persons is needed to reduce mortality in persons with high serum CrAg titres. Clinical trials are underway evaluating single-dose liposomal amphotericin in combination with standard fluconazole to prevent cryptococcal meningitis in CrAg-positive persons [91], and another trial is evaluating fluconazole with flucytosine for preemptive treatment [92]. Should either of these strategies improve survival, global equitable access to flucytosine and/or liposomal amphotericin will need to be expanded. CrAg screening and preemptive treatment is a success story among AIDS-related mycoses, and a model for OI screening and prevention for histoplasmosis and talaromycosis. Whether similar screening and preemptive treatment strategies could reduce mortality in Talaromyces or Histoplasma infection is an area of much needed research. Finally, the role of azithromycin or other antibacterial agents to prevent SBI and thereby improve survival is a promising area of future study. Whether such antibacterial agents for prophylaxis promote antimicrobial resistance will need to be investigated in tandem.
Despite the many diagnostic advances and prophylactic strategies for AIDS-related OIs, implementation strategies that facilitate the uptake of these diagnostics and integration of prophylactic treatments into routine HIV care remains a major challenge. A major barrier is inadequate availability of diagnostic and treatment commodities. The national supplies of diagnostics and preemptive therapy are not only inadequate but only intermittently available. Furthermore, national programmes have competing priorities as other diseases are prioritized and resources allocated to health areas that are considered more crucial.
Implementation studies in ambulatory and hospital settings are needed to translate clinical trial findings into real-world context of multiple co-infections and diagnostic uncertainty. Hospital management of AHD in particular remains largely neglected despite advances in TB and cryptococcal meningitis treatment regimens. As a result, mortality among hospitalized persons with AHD remains high [10]. Diagnostic combination strategy studies utilizing emerging TB devices in conjunction with other POC diagnostics in hospitalized patients are needed. In community-based laboratory settings, implementation studies assessing the feasibility of POC tests such as the semiquantitative CrAg LFA in community-based laboratory settings will determine the true impact of such a test of clinical management. Real-world pragmatic clinical trials to measure the effectiveness and cost-effectiveness of packages of novel diagnostics and prophylactic regimens will be essential for the development of policies and funding mechanisms to improve mortality. Advocacy and investment from global health stakeholders in health care systems, including training of frontline healthcare workers and laboratory technicians, and health system strengthening, is critical alongside equitable access to essential tests and medicines for AHD.
CONCLUSIONS
Despite progress with HIV treatment and prevention, a persistent 20%–30% of PWH present to care with AHD. Unfortunately, these persons with AHD continue to carry the burden of HIV-related morbidity and mortality. Implementation of existing WHO-recommended guidelines for AHD need to be scaled up urgently in low- and middle-income countries where disease burden is greatest. However, access to existing tests and medicines for diagnosis and prophylaxis of strategies for AHD remains a significant barrier to roll out of WHO-recommended AHD packages of care in resource-limited settings. In addition, implementation science studies are needed to assess the acceptability, feasibility, generalizability and cost-effectiveness of AHD packages of care. Feasibility studies of diagnostic tests such as semiquantitative CrAg LFAs are also needed to determine the appropriate setting for effective testing in routine care settings. Burden of disease studies are also urgently needed to assess whether the current WHO-recommended AHD package of care requires regional adaptation to include, for example, screening for histoplasmosis in Latin America or if widespread geographical screening is needed. Lastly, trials are required to determine prophylactic strategies for OIs including talaromyces and histoplasmosis in line with CrAg screening studies for cryptococcal disease.
Over the last decade there has been much needed progress toward development of POC diagnostics for CD4 testing and OI screening. Development and implementation of POC diagnostics have the potential to facilitate rapid diagnosis of AHD at the time of entry or reentry into HIV care, and screening for OIs. POC diagnostics theoretically could improve HIV retention-in-care and thereby reduce mortality by overcoming delays in laboratory testing and providing patients and healthcare workers with timely same-day results. Investment in the development of additional CD4 platforms is critically needed including advocacy for retention of existing platforms within routine care laboratory health systems. Additional clinical trials and implementation science studies are essential to evaluate the effectiveness of such diagnostics in combination and in real-world scenarios, where patients with AHD have multiple comorbidities and imperfect follow-up. The combination of expanded ART, timely OI diagnosis with short, tolerable prophylaxis is fundamental to reduce mortality among persons with AHD.
Funding information
National Institute of Allergy and Infectious Diseases
Footnotes
CONFLICT OF INTEREST STATEMENT
No conflicts of interest declared.
REFERENCES
- 1.Joint United Nations Programme on HIV and AIDS (UNAIDS). Fast-track commitments to end AIDS by 2030. 2020. Accessed March 22, 2021. https://www.unaids.org/sites/default/files/media_asset/fast-track-commitments_en.pdf
- 2.Joint United Nations Programme on HIV and AIDS (UNAIDS). AIDSinfo. 2020. Accessed March 22, 2021. https://aidsinfo.unaids.org/
- 3.World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy (p.56). 2017. Accessed March 22, 2021. https://www.who.int/publications/i/item/9789241550062. [PubMed]
- 4.Carmona S, Bor J, Nattey C, et al. Persistent high burden of advanced HIV disease among patients seeking care in South Africa’s national HIV program: data from a nationwide laboratory cohort. Clin Infect Dis. 2018;66(Suppl_2):S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalintya E, Sekar P, Kavuma P, Kigozi J, et al. Effect of Covid-19 lockdowns on identification of advanced HIV disease in outpatient clinics in Uganda. Clin Infect Dis. 2023;Feb 17:ciad087. doi: 10.1093/cid/ciad087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerschberger B, Jobanputra K, Schomaker M, et al. Feasibility of antiretroviral therapy initiation under the treat-all policy under routine conditions: a prospective cohort study from Eswatini. J Int AIDS Soc. 2019;22(10):e25401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebelonyane R, Mills LA, Mogorosi C, et al. Advanced HIV disease in the Botswana combination prevention project: prevalence, risk factors, and outcomes. AIDS. 2020;34(15): 2223–2230. [DOI] [PubMed] [Google Scholar]
- 8.Hakim J, Musiime V, Szubert AJ, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med. 2017;377(3):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mfinanga S, Chanda D, Kivuyo SL, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385(9983):2173–2182. [DOI] [PubMed] [Google Scholar]
- 10.Ford N, Patten G, Rangaraj A, Davies MA, Meintjes G, Ellman T. Outcomes of people living with HIV after hospital discharge: a systematic review and meta-analysis. Lancet HIV. 2022;9(3):e150–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangaraj A, Connor S, Harding R, Pinto C, Chitembo L, Ford N. Advanced HIV disease and health-related suffering-exploring the unmet need of palliative care. Lancet HIV. 2022; 10(2):e126–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izco S, Murias-Closas A, Jordan AM, et al. Improved detection and management of advanced HIV disease through a community adult TB-contact tracing intervention with same-day provision of the WHO-recommended package of care including ART initiation in a rural district of Mozambique. J Int AIDS Soc. 2021;24(8):e25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke RM, Henrion MYR, Mallewa J, et al. Incidence of HIV-positive admission and inpatient mortality in Malawi (2012–2019). AIDS. 2021;35(13):2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. 2018;392(10144):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. 2016;19(1):20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States President’s Emergency Plan For AIDS Relief (PEPFAR)PEPFAR 2019 Country Operational Plan Guidance for all PEPFAR Countries. 2019. Accessed March 22, 2021. https://www.state.gov/wp-content/uploads/2019/08/PEPFARFiscal-Year-2019-Country-Operational-Plan-Guidance.pdf.
- 17.Zaniewski E, Dao Ostinelli CH, Chammartin F, et al. Trends in CD4 and viral load testing 2005 to 2018: multi-cohort study of people living with HIV in southern Africa. J Int AIDS Soc. 2020;23(7):e25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhein J, Hullsiek KH, Evans EE, et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis. 2018;5(8):ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikombe K, Eshun-Wilson I, Koyuncu A, et al. Early mortality in HIV-infected patients initiating ART without a pretherapy CD4. Conference on Retroviruses and Opportunistic Infections. March 4–7 2019 Seattle, Washington. [Google Scholar]
- 20.Diaw PA, Daneau G, Coly AA, et al. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr (1999). 2011;58(4):e103–e111. [DOI] [PubMed] [Google Scholar]
- 21.Ndlovu Z, Massaquoi L, Bangwen NE, et al. Diagnostic performance and usability of the VISITECT CD4 semi-quantitative test for advanced HIV disease screening. PloS One. 2020;15(4):e0230453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechiile K, Leeme TB, Tenforde MW, et al. Laboratory evaluation of the VISITECT advanced disease semiquantitative point-of-care CD4 test. J Acquir Immune Defic Syndr (1999). 2022;91(5):502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Turha L, Maharaj K, Rose A, et al. Point-of-care CD4+ technology implementation in Free State, South Africa, was associated with improved patient health outcomes. S Afr Med J. 2020;110(2):126–131. [DOI] [PubMed] [Google Scholar]
- 24.Scorgie F, Mohamed Y, Anderson D, Crowe SM, Luchters S, Chersich MF. Qualitative assessment of South African healthcare worker perspectives on an instrument-free rapid CD4 test. BMC Health Serv Res. 2019;19(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO). Global tuberculosis report 2022. World Health Organization; 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globaltuberculosis-report-2022 [Google Scholar]
- 26.World Health Organization (WHO). Global tuberculosis report 2021. 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021
- 27.Gupta RK, Lucas SB, Fielding KL, Lawn SDJA. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease-a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. 2021;73(3):e830–e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ku CC, MacPherson P, Khundi M, et al. Durations of asymptomatic, symptomatic, and care-seeking phases of tuberculosis disease with a Bayesian analysis of prevalence survey and notification data. BMC Med. 2021;19(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branigan D Pipeline Report: Tuberculosis Diagnostics. 2021. https://www.treatmentactiongroup.org/wp-content/uploads/2021/11/pipeline_TB_diagnostics_2021_final.pdf
- 31.World Health Organization (WHO). WHO guidelines on tuberculosis infection prevention and control: 2019 update. 2019. Accessed March 22, 2021. https://www.who.int/publications/i/item/9789241550512 [PubMed]
- 32.World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection. 2021. Accessed June 27, 2022. https://www.who.int/publications/i/item/9789240029415 [PubMed]
- 33.Cresswell FV, Ellis J, Kagimu E, et al. Standardized urine-based tuberculosis (TB) screening with TB-lipoarabinomannan and Xpert MTB/RIF Ultra in Ugandan adults with advanced human immunodeficiency virus disease and suspected meningitis. Open Forum Infect Dis. 2020;7(4):ofaa100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boloko L, Schutz C, Sibiya N, et al. Xpert Ultra testing of blood in severe HIV-associated tuberculosis to detect and measure Mycobacterium tuberculosis blood stream infection: a diagnostic and disease biomarker cohort study. Lancet Microbe. 2022;3(7):e521–e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luabeya AK, Wood RC, Shenje J, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol. 2019; 57(3):e01847–e01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Y, Ma Y, Liu R, et al. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Infect Drug Resist. 2021;14:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foundation for Innovative New Diagnostics (FIND). World Health Organization endorses Truenat tests for initial diagnosis of tuberculosis and detection of rifampicin resistance. Accessed June 27, 2022. https://www.finddx.org/publications-and-statements/world-health-organization-endorses-truenat-tests-for-initial-diagnosis-of-tuberculosis-and-detection-of-rifampicin-resistance/
- 38.World Health Organization (WHO). WHO consolidated guidelines on tuberculosis: module 3: diagnosis -rapid diagnostics for tuberculosis detection. World Health Organization; 2021. Accessed December 15, 2022. https://www.who.int/publications/i/item/9789240029415 [Google Scholar]
- 39.Sharma K, Sharma M, Modi M, et al. Comparative analysis of Truenat™ MTB plus and Xpert(®) ultra in diagnosing tuberculous meningitis. Int J Tuberc Lung Dis. 2021;25(8):626–631. [DOI] [PubMed] [Google Scholar]
- 40.Byashalira KMP, Semvua H, Chilongola J, et al. Clinical outcomes of new algorithm for diagnosis and treatment of tuberculosis sepsis in HIV patients. 2019. Accessed June 27, 2022. https://www.ijmyco.org/article.asp?issn=2212-5531;year=2019;volume=8;issue=4;spage=313;epage=319;aulast=Byashalir a#ref16 [DOI] [PubMed]
- 41.Boyles TH, Griesel R, Stewart A, Mendelson M, Maartens G. Incremental yield and cost of urine determine TB-LAM and sputum induction in seriously ill adults with HIV. Int J Infect Dis. 2018;75:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z, LaCourse SM, Kay AW, et al. CRISPR detection of circulating cell-free mycobacterium tuberculosis DNA in adults and children, including children with HIV: a molecular diagnostics study. Lancet Microbe. 2022;3(7):e482–e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oreskovic A, Panpradist N, Marangu D, et al. Diagnosing pulmonary tuberculosis by using sequence-specific purification of urine cell-free DNA. J Clin Microbiol. 2021;59(8):e0007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cepheid. Xpert MTB-XDR English Package Insert. Cepheid; 2021. [Google Scholar]
- 45.Sutherland JS, van der Spuy G, Gindeh A, et al. Diagnostic accuracy of the Cepheid 3-gene host response fingerstick blood test in a prospective, multi-site study: interim results. Clin Infect Dis. 2022;74(12):2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaisson RE. Empirical antituberculosis therapy in advanced HIV disease – too much, too late. N Engl J Med. 2020;382(25): 2459–2460. [DOI] [PubMed] [Google Scholar]
- 47.Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380(11):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institute of Allergy and Infectious Diseases (NIAID). Drug-drug interactions between rifapentine and dolutegravir in HIV/LTBI co-infected individuals. 2020. Accessed September 2, 2022. https://clinicaltrials.gov/ct2/show/NCT04272242
- 49.Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meya DB, Manabe YC, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resourcelimited settings. Clin Infect Dis. 2010;51(4):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal meningitis diagnostics and screening in the era of point-of-care laboratory testing. J Clin Microbiol. 2019;57(1):e01238–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temfack E, Rim JJB, Spijker R, et al. Cryptococcal antigen in serum and cerebrospinal fluid for detecting cryptococcal meningitis in adults living with human immunodeficiency virus: systematic review and metaanalysis of diagnostic test accuracy studies. Clin Infect Dis. 2021;72(7):1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarvis JN, Tenforde MW, Lechiile K, et al. Evaluation of a novel semiquantitative cryptococcal antigen lateral flow assay in patients with advanced HIV disease. J Clin Microbiol. 2020; 58(9):e00441–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhein J, Bahr NC, Hemmert AC, et al. Diagnostic performance of a multiplex PCR assay for meningitis in an HIVinfected population in Uganda. Diagn Microbiol Infect Dis. 2016;84(3):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nalintya E, Meya DB, Lofgren S, Huppler Hullsiek K, Boulware DR, Rajasingham R. A prospective evaluation of a multisite cryptococcal screening and treatment program in HIV clinics in Uganda. J Acquir Immune Defic Syndr (1999). 2018;78(2):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meya DB, Kiragga AN, Nalintya E, et al. Reflexive laboratorybased cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4 <100 cells/microL: a stepped-wedge, cluster-randomized trial. J Acquir Immune Defic Syndr (1999). 2019;80(2):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wake RM, Britz E, Sriruttan C, et al. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis. 2018;66(5):686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185(5):843–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oladele RO, Osaigbovo II, Akanmu AS, et al. Prevalence of histoplasmosis among persons with advanced HIV disease, Nigeria. Emerg Infect Dis. 2022;28(11):2261–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adenis AA, Valdes A, Cropet C, et al. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis. 2018; 18(10):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nacher M, Valdes A, Adenis A, et al. Heterogeneity of clinical presentations and paraclinical explorations to diagnose disseminated histoplasmosis in patients with advanced HIV: 34 years of experience in French Guiana. J Fungi. 2020;6(3):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rakislova N, Hurtado JC, Palhares AEM, et al. High prevalence and mortality due to Histoplasma capsulatum in the Brazilian Amazon: an autopsy study. PLoS Negl Trop Dis. 2021;15(4):e0009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med. 2015;36(5):729–745. [DOI] [PubMed] [Google Scholar]
- 64.Bongomin F. Histoplasma and Histoplasmosis. 2020. https://www.intechopen.com/books/9487. Published September 16, 2020. ISBN 978–1-83962–962-4.
- 65.Caceres DH, Knuth M, Derado G, Lindsley MD. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: a meta-analysis of assay analytical performance. J Fungi. 2019;5 (3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez-Gamboa A, Niembro-Ortega MD, Torres-Gonzalez P, et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: a prospective multicenter study in Mexico. PLoS Negl Trop Dis. 2021;15(3): e0009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diagnostics MV. Histoplasma Urine Antigen LFA Test Kit. 2021. [Google Scholar]
- 68.Caceres DH, Gómez BL, Tob on AM, et al. Validation and con-cordance analysis of a new lateral flow assay for detection of Histoplasma antigen in urine. J Fungi. 2021;7(10):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdallah W, Myint T, LaRue R, et al. Diagnosis of histoplasmosis using the MVista Histoplasma galactomannan antigen qualitative lateral flow-based immunoassay: a multicenter study. Open Forum Infect Dis. 2021;8(9):ofab454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-infected Adults and Adolescents: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2016. Accessed April 8, 2021. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/cryptococcosis
- 71.McKinsey DS, Wheat LJ, Cloud GA, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis. 1999;28(5):1049–1056. [DOI] [PubMed] [Google Scholar]
- 72.Pan American Health Organization and World Health Organization. Diagnosing and Managing Disseminated Histoplasmosis Among People Living with HIV. Pan American Health Organization and World Health Organization; 2020. [PubMed] [Google Scholar]
- 73.Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013; 175(1–2):57–67. [DOI] [PubMed] [Google Scholar]
- 74.Hien HTA, Thanh TT, Thu NTM, et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses. 2016;59(12):773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thu NTM, Chan JFW, Ly VT, et al. Superiority of a novel Mp1p antigen detection enzyme immunoassay compared to standard BACTEC blood culture in the diagnosis of talaromycosis. Clin Infect Dis. 2021;73(2):e330–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang J, Qin F, Meng S, et al. Effects of cotrimoxazole prophylaxis on Talaromyces marneffei infection in HIV/AIDS patients receiving antiretroviral therapy: a retrospective cohort study. Emerg Microbes Infect. 2019;8(1):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huson MA, Grobusch MP, van der Poll T. The effect of HIV infection on the host response to bacterial sepsis. Lancet Infect Dis. 2015;15(1):95–108. [DOI] [PubMed] [Google Scholar]
- 79.Mayanja BN, Todd J, Hughes P, et al. Septicaemia in a population-based HIV clinical cohort in rural Uganda, 1996–2007: incidence, aetiology, antimicrobial drug resistance and impact of antiretroviral therapy. Trop Med Int Health. 2010;15(6):697–705. [DOI] [PubMed] [Google Scholar]
- 80.Pyarali FF, Iordanov R, Palacio A, Tamariz L. Excess mortality risk from sepsis in patients with HIV – a meta-analysis. J Crit Care. 2020;59:101–107. [DOI] [PubMed] [Google Scholar]
- 81.Huson MA, Stolp SM, van der Poll T, Grobusch MP. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clin Infect Dis. 2014;58(1):79–92. [DOI] [PubMed] [Google Scholar]
- 82.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(6):417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lemkus L, Lawrie D, Vaughan J. The utility of extended differential parameters as a biomarker of bacteremia at a tertiary academic hospital in persons with and without HIV infection in South Africa. PloS One. 2022;17(2):e0262938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Ochieng C, Wiersma S, et al. Development of a TaqMan Array Card for acute-febrile-illness outbreak investigation and surveillance of emerging pathogens, including Ebola virus. J Clin Microbiol. 2016;54(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore CC, Jacob ST, Banura P, et al. Etiology of sepsis in Uganda using a quantitative polymerase chain reaction-based TaqMan Array Card. Clin Infect Dis. 2019;68(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–674. [DOI] [PubMed] [Google Scholar]
- 87.Mongan AE, Tuda JSB, Runtuwene LR. Portable sequencer in the fight against infectious disease. J Hum Genet. 2020;65(1): 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organization (WHO). Global genomic surveillance strategy for pathogens with pandemic and epidemic potential, 2022–2032. World Health Organization; 2022. Accessed September 2, 2022. https://www.who.int/publications/i/item/9789240046979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grau I, Pallares R, Tubau F, et al. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med. 2005;165(13):1533–1540. [DOI] [PubMed] [Google Scholar]
- 90.Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC. Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine. 2004;22(15–16):2006–2012. [DOI] [PubMed] [Google Scholar]
- 91.Meya DBNE. Single dose liposomal amphotericin for asymptomatic cryptococcal antigenemia. 2019. Accessed September 2, 2022. https://clinicaltrials.gov/ct2/show/NCT03945448
- 92.Govender NMS. Treatment of cryptococcal antigen-positive patients identified through screening using fluconazole plus flucytosine vs. fluconazole alone. 2021. Accessed September 2, 2022. https://www.isrctn.com/ISRCTN30579828
- 93.Bjerrum SSI, Dendukuri N, Eisenhut M, et al. Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: an updated systematic review. World Health Organization; 2019. [Google Scholar]
- 94.Broger T, Nicol MP, Sigal GB, et al. Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest. 2020;130(11):5756–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muyoyeta M, Kerkhoff AD, Chilukutu L, Moreau E, Schumacher SG, Ruhwald M. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: a prospective cross-sectional study. Eur Respir J. 2021;58(5):2003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reddy KP, Denkinger CM, Broger T, et al. Cost-effectiveness of a novel lipoarabinomannan test for tuberculosis in patients with human immunodeficiency virus. Clin Infect Dis. 2021;73 (7):e2077–e2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ainan S, Furia FF, Mhimbira F, et al. Xpert® MTB/RIF assay testing on stool for the diagnosis of paediatric pulmonary TB in Tanzania. Public Health Action. 2021;11(2):75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee DJ, Kumarasamy N, Resch SC, et al. Rapid, point-of-care diagnosis of tuberculosis with novel Truenat assay: Cost-effectiveness analysis for India’s public sector. PLoS One. 2019;14 (7):e0218890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;2010(1):Cd000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Den Boon S, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV. AIDS. 2016;30(5):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuen CM, Majidulla A, Jaswal M, et al. Cost of delivering 12dose isoniazid and rifapentine versus 6 months of isoniazid for tuberculosis infection in a high-burden setting. Clin Infect Dis. 2021;73(5):e1135–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–2166. [DOI] [PubMed] [Google Scholar]
- 103.Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161(6):419–428. [DOI] [PubMed] [Google Scholar]
- 104.Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med. 2017;167(4):248–255. [DOI] [PubMed] [Google Scholar]