Abstract
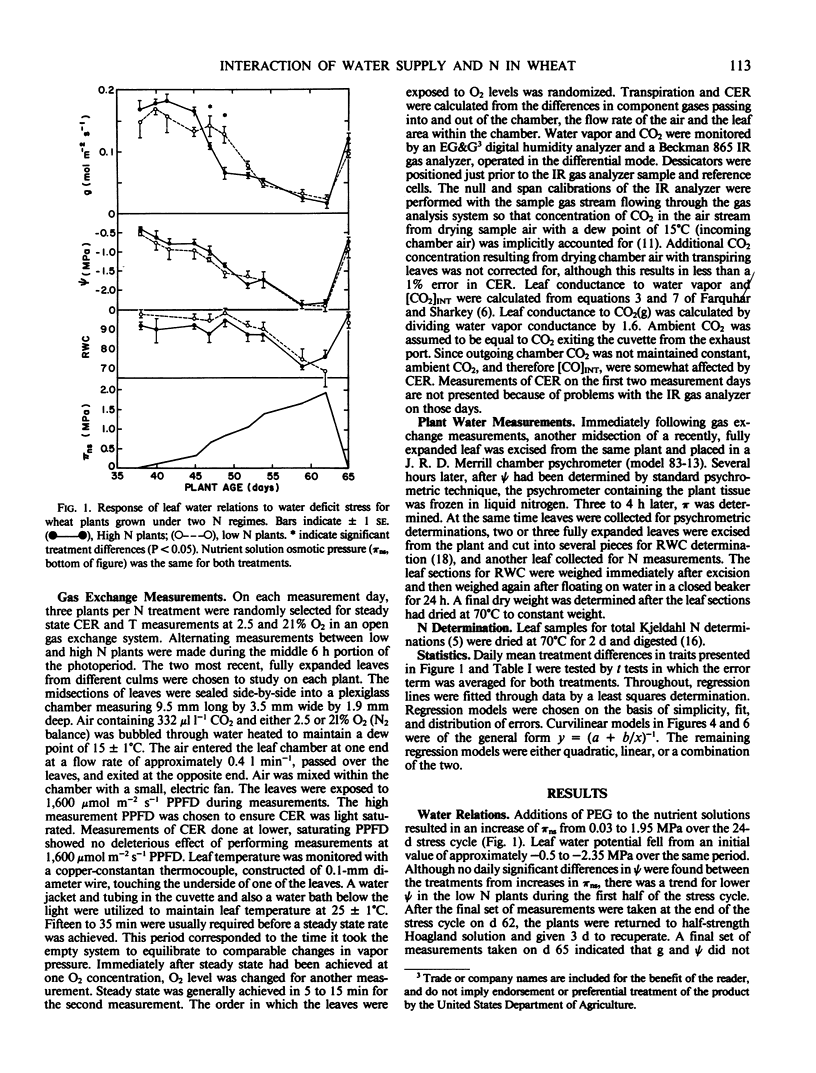
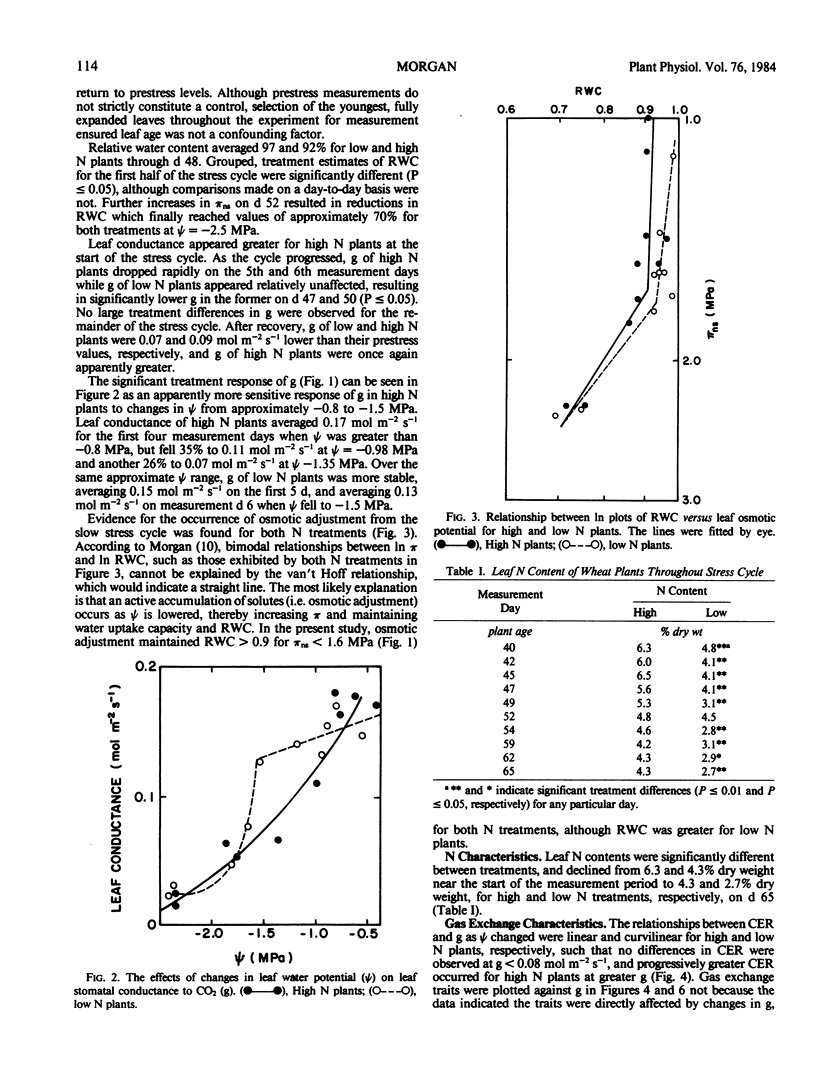
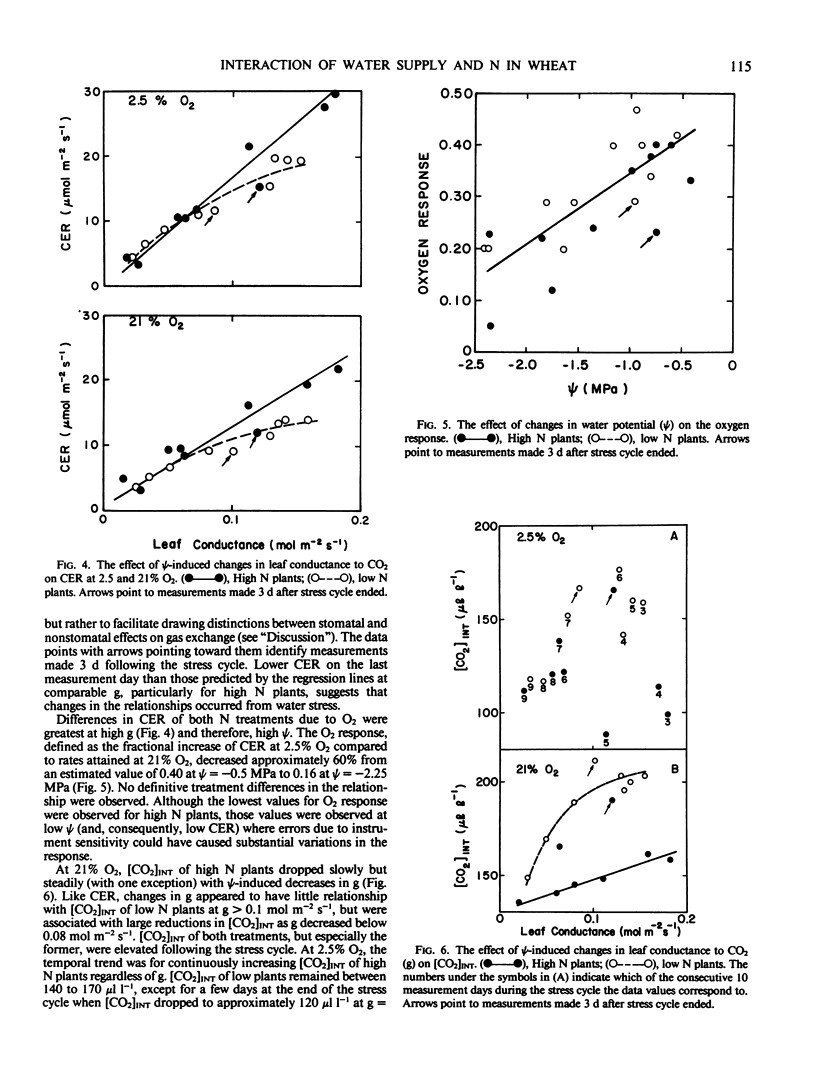
The purpose of this study was to investigate effects of N nutrition and water stress on stomatal behavior and CO2 exchange rate in wheat (Triticum aestivum L. cv Olaf). Wheat plants were grown hydroponically with high (100 milligrams per liter) and low (10 milligrams per liter) N. When plants were 38 days old, a 24-day water stress cycle was begun. A gradual increase in nutrient solution osmotic pressure from 0.03 to 1.95 mega Pascals was achieved by incremental additions of PEG-6,000. Plants in both N treatments adjusted osmotically, although leaf water potential was consistently lower and relative water content greater for low N plants in the first half of the stress cycle. Leaf conductance of high N plants appeared greater than that of low N plants at high water potentials, but showed greater sensitivity to reductions in water potential as indicated by earlier stomatal closure during the stress cycle. The apparent greater stomatal sensitivity of high N plants was associated with a curvilinear relationship between leaf conductance and leaf water potential; low N plants exhibited more of a threshold response. Trends in [CO2]INT throughout the stress cycle indicated nonstomatal effects of water stress on CO2 exchange rate were greater in high N plants. Although estimates of [CO2]INT were generally lower in high N plants, they were relatively insensitive to leaf water potential-induced changes in leaf conductance. In contrast, [CO2]INT of low N plants dropped concomitantly with leaf conductance at low leaf water potentials. Oxygen response of CO2 exchange rate for both treatments was affected less by reductions in water potential than was CO2 exchange rate at 2.5% O2, suggesting that CO2 assimilation capacity of the leaves was affected more by reductions in leaf water potential than were processes related to photorespiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. K., Brown R. H. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways: V. RESPONSE OF PANICUM MAXIMUM, PANICUM MILIOIDES, AND TALL FESCUE (FESTUCA ARUNDINACEA) TO NITROGEN NUTRITION. Plant Physiol. 1980 Jul;66(1):97–100. doi: 10.1104/pp.66.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C., Cheesbrough J. K., Walker D. A. Effects of mannose on photosynthetic gas exchange in spinach leaf discs. Plant Physiol. 1983 Jan;71(1):108–111. doi: 10.1104/pp.71.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Parker L. L. Water Relations of Cotton Plants under Nitrogen Deficiency: I. Dependence upon Leaf Structure. Plant Physiol. 1979 Sep;64(3):495–498. doi: 10.1104/pp.64.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin J. W., Parker L. L. Water Relations of Cotton Plants under Nitrogen Deficiency: II. Environmental Interactions on Stomata. Plant Physiol. 1979 Sep;64(3):499–501. doi: 10.1104/pp.64.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]



