Abstract
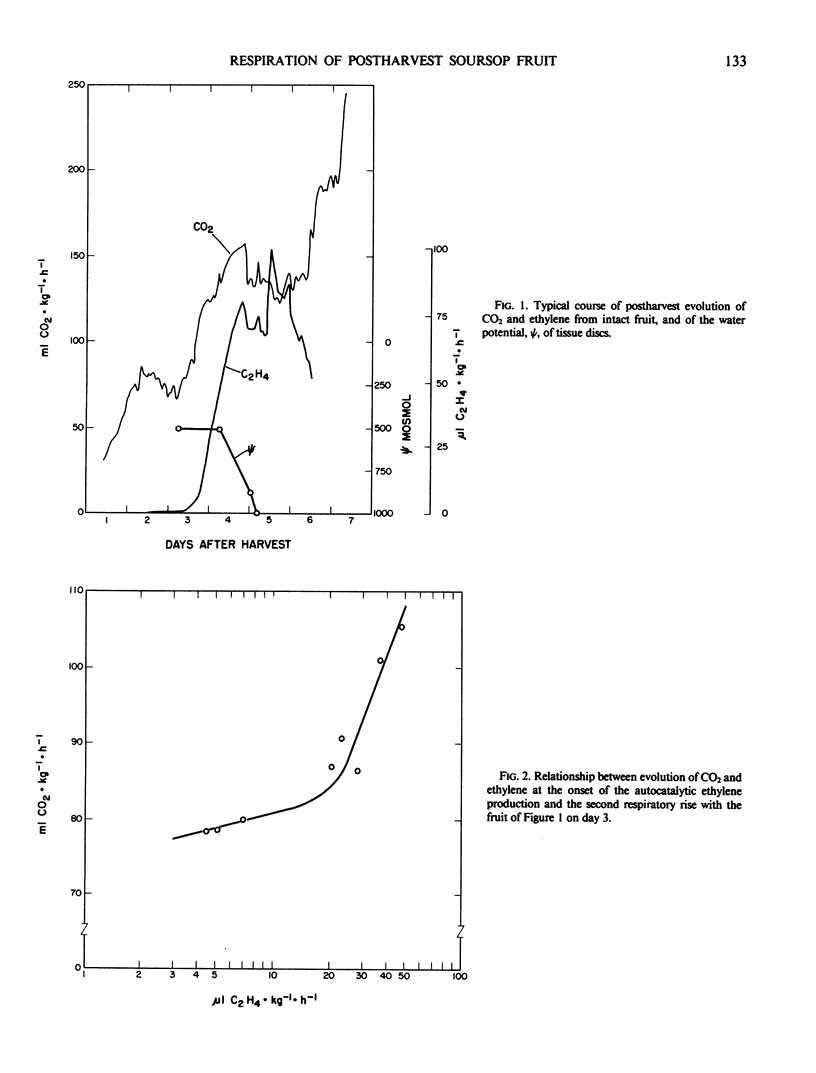
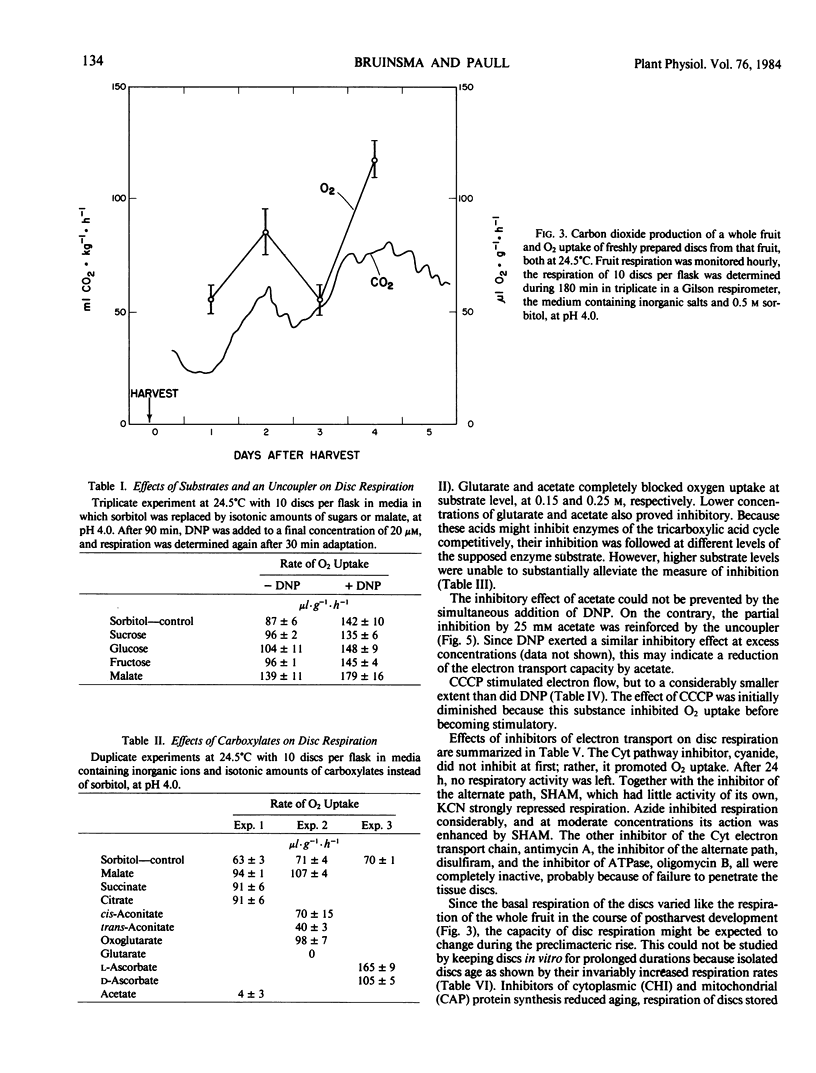
Fruit of soursop, Annona muricata L., showed increased CO2 production 2 days after harvest, preceding the respiratory increase that coincided with autocatalytic ethylene evolution and other ripening phenomena. Experiments to alter gas exchange patterns of postharvest fruit parts and tissue cylinders had little success.
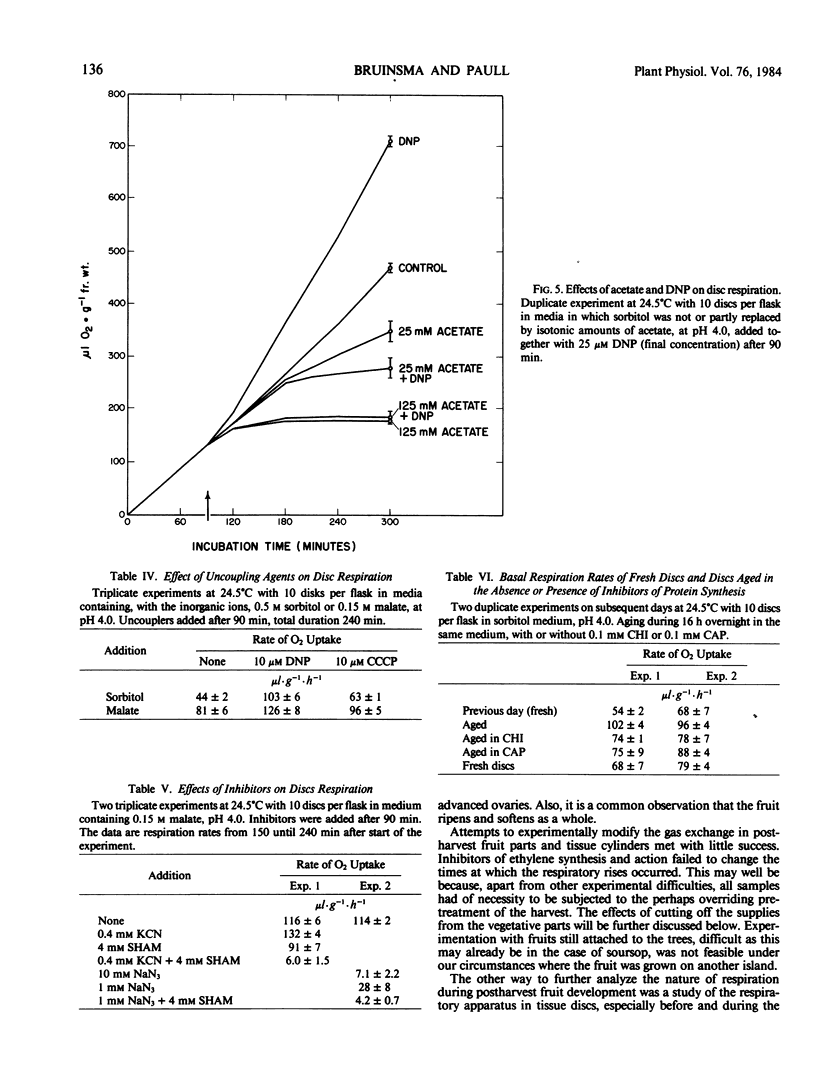
The respiratory quotient of tissue discs was near unity throughout development. 2,4-Dinitrophenol uncoupled respiration more effectively than carbonylcyanide m-chlorophenylhydrazone; 0.4 millimolar KCN stimulated, 4 millimolar salicylhydroxamic acid slightly inhibited, and their combination strongly inhibited respiration, as did 10 millimolar NaN3. Tricarboxylic acid cycle members and ascorbate were more effective substrates than sugars, but acetate and glutarate strongly inhibited.
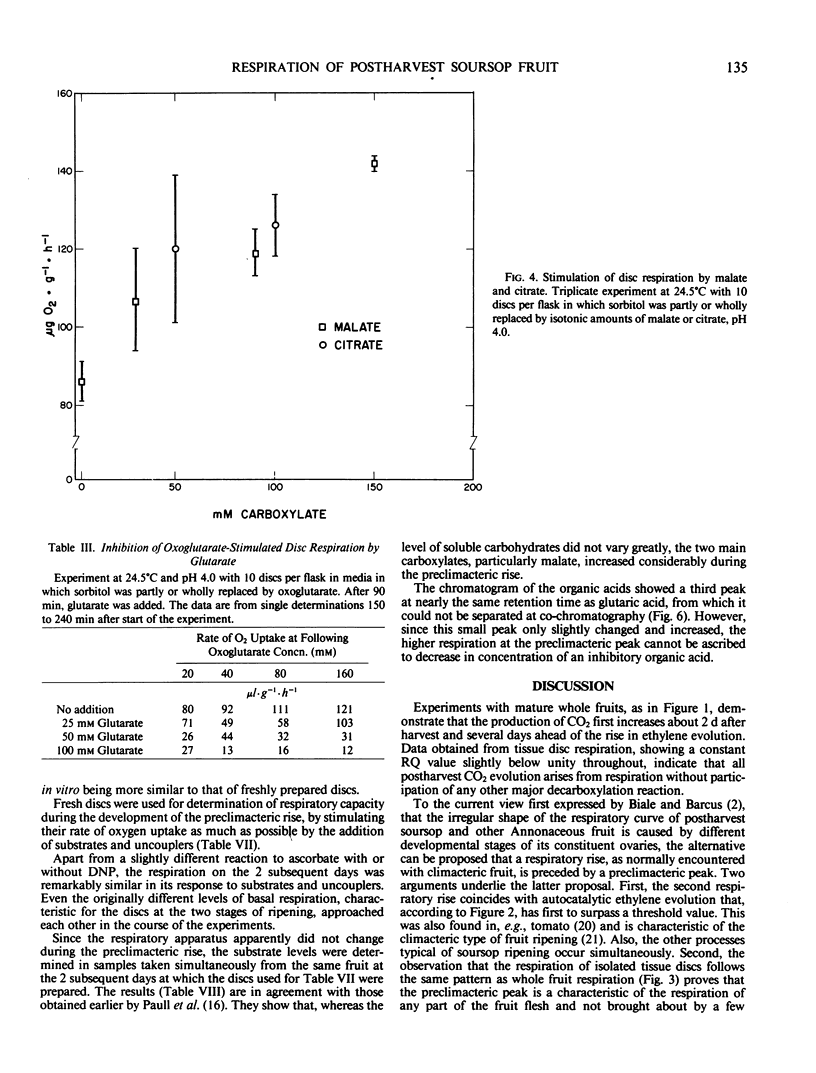
Disc respiration showed the same early peak as whole fruit respiration; this peak is thus an inherent characteristic of postharvest development and cannot be ascribed to differences between ovaries of the aggregatetype fruit. The capacity of the respiratory apparatus did not change during this preclimacteric peak, but the contents of rate-limiting malate and citrate increased after harvest.
It is concluded that the preclimacteric rise in CO2 evolution reflects increased mitochondrial respiration because of enhanced supply of carboxylates as a substrate, probably induced by detachment from the tree. The second rise corresponds with the respiration during ripening of other climacteric fruits.
Full text
PDF

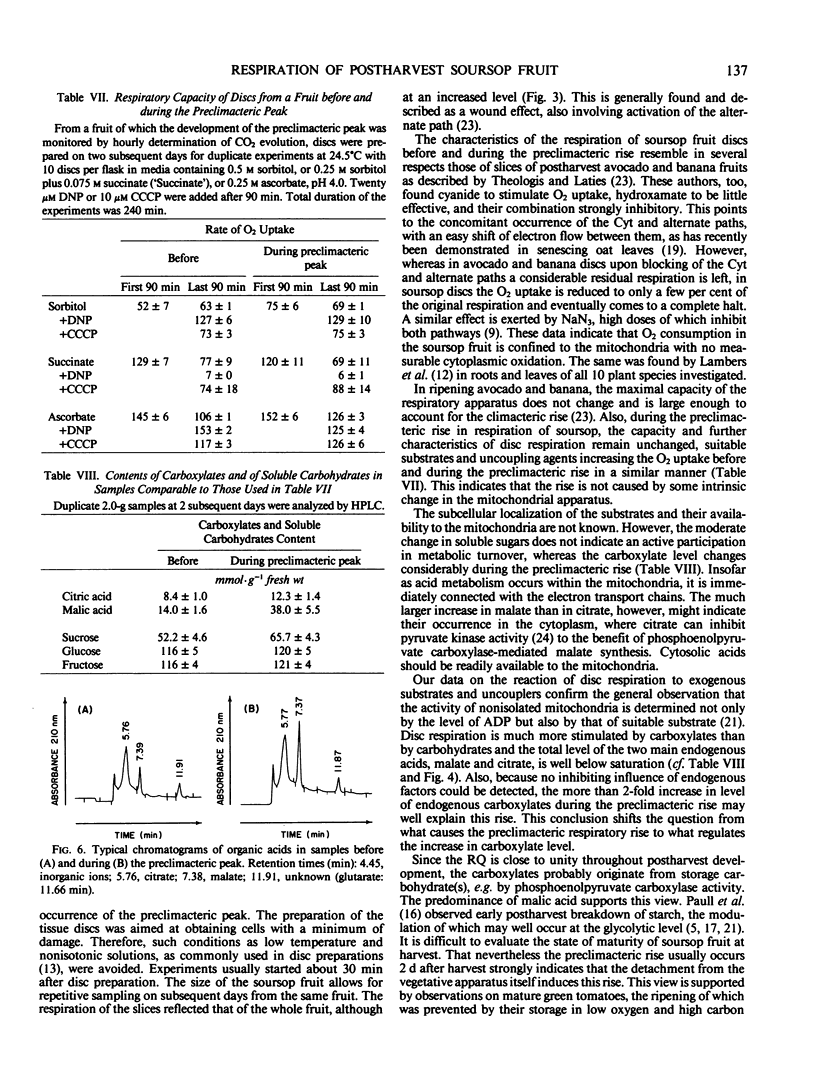

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt-Hansen H. P., Grant N. G., Olson L. W. Respiration of Gametangia of the Aquatic Phycomycete Allomyces macrogynus: Inhibition by Cyanide or Antimycin and Salicylhydroxamic Acid or Propyl Gallate. Plant Physiol. 1983 Sep;73(1):111–117. doi: 10.1104/pp.73.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery D., Smith C., Goodenough P., Prosser I., Grierson D. Ethylene-independent and ethylene-dependent biochemical changes in ripening tomatoes. Plant Physiol. 1984 Jan;74(1):32–38. doi: 10.1104/pp.74.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R. J., Bos T. J., Pech J. C. Cycloheximide stimulation of cyanide-resistant respiration in suspension cultures of senescent pear fruit cells. Plant Physiol. 1981 Oct;68(4):823–826. doi: 10.1104/pp.68.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler S. O., Thimann K. V. Relation between Respiration and Senescence in Oat Leaves. Plant Physiol. 1983 Jun;72(2):540–546. doi: 10.1104/pp.72.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwwan M. A., Poovaiah B. W. Association between Elemental Content and Fruit Ripening in rin and Normal Tomatoes. Plant Physiol. 1978 Jun;61(6):883–885. doi: 10.1104/pp.61.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Respiratory Contribution of the Alternate Path during Various Stages of Ripening in Avocado and Banana Fruits. Plant Physiol. 1978 Aug;62(2):249–255. doi: 10.1104/pp.62.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


