SUMMARY
Background
Child-friendly fixed dose combination (FDC) antiretroviral treatment (ART) options are limited. We evaluated the pharmacokinetics, safety, and tolerability of once-daily dispersible and immediate-release FDC abacavir/dolutegravir/lamivudine in young children with HIV.
Methods
IMPAACT 2019 was an international, phase I/II, multi-site, open-label, non-comparative dose confirmation study of abacavir/dolutegravir/lamivudine in children <12 years. Participants were enrolled across five weight bands (WBs): those 6-<25 kg received abacavir/dolutegravir/lamivudine (60/5/30 mg) dispersible tablets (3–6 depending on body weight), and those 25-<40 kg received the immediate-release tablet (600/50/300 mg). At entry, participants were ART-naïve or ART-experienced and virologically suppressed on stable ART for ≥6 months. Dose confirmation was based on pharmacokinetic and safety criteria in the first 5–7 participants in each WB through Week 4; all participants were followed through Week 48. We present results for the primary objectives to assess pharmacokinetics, confirm dosing, and evaluate safety through 24 weeks across all WBs.
Findings
Fifty-seven participants were enrolled (46% female; 65% Black, 32% Asian). Within each WB (6-<10kg, 10-<14 kg, 14-<20 kg, 20-<25 kg, and ≥25 kg), the geometric mean (CV%) dolutegravir area under the concentration time curve over the 24-hour dosing interval (AUC0–24h) was 75.9 (33.7%), 91.0 (36.5%), 71.4 (23.5%), 84.4 (26.3%), and 71.8 (13.9%) μg∙h/mL; dolutegravir concentration at 24 hours post-dose (C24h) was 0.91 (67.6%), 1.22 (77.5%), 0.79 (44.2%), 1.35 (95.5%), and 0.98 (27.9%) μg/mL; abacavir AUC0–24h was 17.7 (38.8%), 19.8 (50.6%), 15.1 (40.3%), 17.4 (19.4%), and 25.7 (14.6%) μg∙h/mL; and lamivudine AUC0–24h was 10.7 (46.0%), 14.2 (23.9%), 13.0 (15.6%), 14.5 (16.6%), and 21.7 (26.2%) μg∙h/mL. Pharmacokinetic targets and safety criteria were met within each WB, and thus dosing of abacavir/dolutegravir/lamivudine was confirmed at the originally selected doses. Ninety-five percent of participants were treatment-experienced and all remained virologically suppressed (<200 copies/mL) through week 24. Virologic suppression was achieved in 2/3 ART-naïve participants by week 24. There were no grade 3 or higher adverse events related to abacavir/dolutegravir/lamivudine and no discontinuations due to toxicity through week 24. Both FDC formulations were well-tolerated.
Interpretation
Dosing of abacavir/dolutegravir/lamivudine was confirmed in children weighing 6-<40 kg, and both FDC formulations were safe, well-tolerated, and efficacious through 24 weeks of treatment. These findings support global efforts to expand the availability of FDC abacavir/dolutegravir/lamivudine to children with HIV.
Funding
National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Mental Health, and ViiV Healthcare/GlaxoSmithKline.
Keywords: Pediatric, fixed dose combination (FDC), antiretroviral (ARV), integrase inhibitor (INSTI), NRTI
BACKGROUND
Children account for 5% of the global population with HIV but constitute 15% of AIDS-related deaths.1 In 2021, 48% of children with HIV globally were not receiving antiretroviral therapy (ART),1 and of those who were on ART, combinations primarily consisted of protease inhibitor (PI)- or non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing regimens.2 Integrase strand transfer inhibitor (INSTI)-based regimens are preferred over PI- and NNRTI-based regimens due to superior safety, efficacy, and resistance profiles.3,4 Single tablet, once-daily fixed dose combination (FDC) regimens are associated with improved adherence and treatment outcomes.5 However, once-daily FDC options are limited for children, which is due in part to challenges in the selection and scaling of individual drug doses across changing weights and developmental processes when formulated in a single FDC. There are currently no INSTI-based FDC options available globally for children weighing less than 14 kg. Thus, there is a need to expand ART access in this population and child-friendly, INSTI-containing ART regimens are critical to addressing this need.
Dolutegravir is a key component of recommended ART options for adults and children with HIV,6,7 and global efforts are underway to support the uptake of dolutegravir-containing regimens across all ages.8 The combination of dolutegravir with abacavir and lamivudine is a first-line6 or recommended treatment option for persons with HIV (PWH),9 including children at least 4 weeks of age weighing at least 3 kg.7 The FDC formulation of abacavir/dolutegravir/lamivudine was identified by the WHO as a priority ART option for development in children with HIV.8 These formulations also do not require cold chain storage, offering logistical benefits for use. The dispersible and immediate-release FDC formulations were approved for children weighing at least 10 kg in the U.S. in March 202210 and at least 14 kg in Europe in February 2023 at doses in alignment with WHO weight band (WB) recommendations.11 However, for children weighing less anm3d those in resource limited settings, use of this combination requires multiple tablets or liquids.
There is a critical need to expand the availability of safe, effective, and child-friendly dolutegravir-containing FDC formulations globally to minimize pill burden and challenges with administration and adherence. The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network 2019 study examined the pharmacokinetics, safety, tolerability, and efficacy of FDC dispersible and immediate-release abacavir/dolutegravir/lamivudine tablets in children with HIV weighing 6 to <40 kg. Selected doses under study aligned with WHO WB dosing for the individual components of dolutegravir, abacavir, and lamivudine in children. We present primary pharmacokinetic, safety, tolerability, and efficacy outcomes through 24 weeks of treatment.
METHODS
Study Population
Children with HIV less than 12 years of age and weighing 6 to <40 kg were eligible. Participants were recruited from study sites in Botswana, South Africa, Thailand, and the U.S. Eligible participants were treatment-naïve or treatment-experienced and virologically suppressed (HIV-1 RNA <200 copies per mL) on a stable non-NNRTI containing regimen for at least six months prior to entry. At screening, participants were required to be HLA-B*5701-negative, have grade 2 or less severe hematology and chemistry laboratory values, and have no evidence of hepatitis B coinfection. Exclusion criteria included documented resistance to abacavir, dolutegravir, or lamivudine (except M184V); known hypersensitivity reaction to abacavir; current evidence of pancreatitis, active tuberculosis, or AIDS-defining opportunistic infections; or any other medical condition that might interfere with participation or interpretation of study outcomes.
Ethics Statement
The study protocol (appendix and https://www.impaactnetwork.org/studies/impaact2019) was reviewed and approved by the Division of AIDS, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health, and site Institutional Review Boards. Written informed consent was obtained from the participant’s parent or legal guardian. Assent was also obtained where applicable in accordance with local requirements.
Study Design
IMPAACT 2019 (NCT03760458) was an international phase I/II, multi-site, open-label, non-comparative dose confirmation study. Participants were enrolled in five WBs concurrently (Table 1). The study was designed with a dose confirmation phase over the first four weeks among a subset of participants followed by longer term evaluation of safety, tolerability, and efficacy in all participants through 48 weeks. Dose confirmation was based on the first 5–7 children in each WB who were deemed both pharmacokinetic- and safety-evaluable. Pharmacokinetic-evaluable participants were required to have exclusively received the study drug dose being evaluated for their WB between study entry and the day of intensive pharmacokinetic sampling; an observed dose on the day of intensive pharmacokinetic sampling; blood samples collected across all time points; and concentration data for all three study drug components available from all intensive pharmacokinetic sampling time points. Missing data were reviewed for pharmacokinetic evaluability on a case-by-case basis. Dolutegravir concentrations were analyzed and monitored in real-time for comparison to individual targets and direct feedback to study sites. To be safety-evaluable, participants had to receive only the study drug dose being evaluated for their WB between study entry and week 4 or have experienced a grade 3 or higher adverse event (AE) or permanent study drug discontinuation assessed as related to study drug prior to week 4. For a dose to be confirmed, each WB had to meet protocol-defined pharmacokinetic targets based on historical data (AUC0–24h and C24h for dolutegravir; 12,13 AUC0–24h for abacavir and lamivudine14) (appendix p. 3) and safety guidelines (no fatal or life-threatening AEs related to study drug; no grade 3 or higher AEs or permanent discontinuations related to study drug in two or more participants). For dolutegravir, the AUC0–24h target range was based on the lower and upper bounds of the 90% CIs for geometric mean exposures associated with once- and twice-daily dolutegravir in adults (35.1–134 μg∙h/mL).12,13 The dolutegravir C24h target ranges were based on 60% of once-daily and 140% of twice-daily geometric mean values in adults (0.67–2.97 μg/mL). The minimum individual dolutegravir AUC0–24h (≥25 μg∙h/mL) and C24h (≥0.5 μg/mL) targets were based on the 95% maximum virologic response.12 Abacavir and lamivudine AUC0–24h targets (6.3–50.4 and 6.3–26.5 μg∙h/mL, respectively) were based on the lower and upper bound of the 90% CIs for predicted exposures with once-daily administration in children with HIV.14 Dose confirmation determinations were made by the Clinical Management Committee of the protocol team, with independent review and confirmation obtained from the Study Monitoring Committee.
Table 1.
Dosing by Weight Band
| Weight Band | Formulation (Daily Dose of ABC/DTG/3TC) | Volume of Water | |
|---|---|---|---|
|
| |||
| 1 | 6 to <10 kg | 3 dispersible tablets (180/15/90 mg) | 15 mL |
| 2 | 10 to <14 kg | 4 dispersible tablets (240/20/120 mg) | 20 mL |
| 3 | 14 to <20 kg | 5 dispersible tablets (300/25/150 mg) | 20 mL |
| 4 | 20 to <25 kg | 6 dispersible tablets (360/30/180 mg) | 20 mL |
| 5 | ≥25 kg | 1 immediate release tablet (600/50/300 mg) | n/a |
After the initial 5–7 participants undergoing intensive pharmacokinetic evaluations were enrolled in each WB, subsequent participants underwent sparse pharmacokinetic collections at the week 1 visit. Remaining study visits took place at weeks 4, 12, 24, 36, and 48 for all participants; and weeks 8, 16, and 20 for participants with a known M184V mutation. Participants who grew into a different WB were permitted to increase their dose after Week 4. Adherence and tolerability were assessed via caregiver questionnaires administered at weeks 4, 12, 24, and 48. Hematologic, hepatic, and renal safety evaluations were performed at weeks 4, 12, 24, 36, 48. CD4 cell counts and percentages were performed at weeks 4, 12, 24, and 48. Lipids (non-fasting) were evaluated at weeks 24 and 48. HIV-1 RNA was monitored at all visits except week 1. Site investigators assessed AE relatedness to study drug and AE severity was graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (corrected version 2.1, July 2017).15
Pharmacokinetic Analysis
Intensive pharmacokinetic evaluations were performed at Week 1 (i.e., 5–10 days after entry at steady-state). Dosing was confirmed for at least four days leading up to this visit using video or in-person direct observation. Participants were required to fast for intensive pharmacokinetic evaluations (low fat, light snacks permitted at least 2 hours prior to the observed dose). Participants were instructed to disperse the dispersible tablets in water (3 minutes or less) prior to intake, and to swallow the immediate-release tablet whole. Pharmacokinetic samples were collected at time 0 (pre-dose) and 1, 2, 3, 4, 6, 8, and 24 hours post-dose. Dolutegravir, abacavir, and lamivudine concentrations were analyzed using validated liquid chromatography mass spectrometry methods by the University of Alabama (appendix p. 4). Steady-state intensive pharmacokinetic parameters for all three components were calculated using noncompartmental methods with linear up-log down trapezoidal rule (Phoenix WinNonlin®, Certara, Inc.).
Outcomes
Primary pharmacokinetic outcome measures were the geometric mean area under the concentration time curve over the 24-hour dosing interval (AUC0–24h), maximum concentration (Cmax), and concentration at 24 hours post-dose (C24h) for dolutegravir, abacavir, and lamivudine for each WB. Primary safety outcome measures included all AEs and the proportion of participants with at least one of the following deemed related to study drug: grade 3 or higher, fatal or life-threatening, other serious, or AEs that resulted in permanent discontinuation of study drug through 24 weeks of treatment. Secondary outcome measures included virologic suppression, adherence, tolerability (palatability and acceptability), lipid profiles, CD4+ cell count and percentage, and AEs through 48 and 144 weeks of treatment; population pharmacokinetic modeling through 48 weeks; and antiretroviral resistance mutations at the time of virologic failure. Virologic suppression, adherence, tolerability (palatability and acceptability), lipid profiles, and CD4+ cell count and percentage outcomes through 24 weeks of treatment were included in this submission; all other outcomes and data through 48 and 144 weeks will be reported separately. Virologic suppression was summarized with <50 and <200 copies per mL thresholds and with the U.S. FDA snapshot algorithm. 16 Virologic failure was summarized and defined as two consecutive plasma HIV-1 RNA values ≥200 copies per mL. For participants who were ART-naïve at entry, the two consecutive results were from specimens collected at or after week 24.
Statistical Analysis
Analyses were performed using SAS version 9.4 within two populations: the All-Treated Population, defined as participants who received at least one dose of abacavir/dolutegravir/lamivudine, and the Primary Safety Population (PSP), defined as participants who received abacavir/dolutegravir/lamivudine and remained on the enrollment WB dose or an increased dose in accordance with their weight through either Week 24 or early treatment discontinuation due to toxicity. Pharmacokinetic analyses included participants who underwent intensive pharmacokinetic evaluations. Analyses of safety, virologic response, immunologic response, and lipids were conducted in the PSP, and the FDA snapshot algorithm was in the All-Treated Population. Adherence and tolerability were presented for the All-Treated Population. All analyses were performed by enrollment WB dose and used while on treatment strategy (i.e., participants were censored the day after treatment discontinuation).17 Safety, viral suppression, and virologic success rates using the FDA snapshot algorithm were summarized with exact 95% confidence intervals (CIs). A post hoc sensitivity analysis was performed for the primary safety outcome measure with renal laboratory test results graded based on actual value alone rather than grading from both actual value and change from baseline based on the DAIDS AE Table.15 For viral response analyses of <50 copies per mL, unquantifiable HIV RNA was imputed to lower limit of quantification −1. Consistent with the noncomparative dose confirmation study design, no statistical comparisons were made between WBs.
Sample Size
The total sample size of at least 50 children, with at least 25 aged <6 years and at least 25 aged 6 to <12 years, was selected based on the previously agreed upon Paediatric Investigation Plan within the European Union and Pediatric Study Plan within the United States for FDC abacavir/dolutegravir/lamivudine. Within the overall target of 50 children, a minimum of 25, of which at least five in each of the weight bands shown in Table 1, were required to be dose-evaluable for abacavir, dolutegravir, and lamivudine based on the previously described pharmacokinetic and safety-evaluable definitions. The sample size for intensive pharmacokinetic sampling was based on the power to detect a difference between the study-defined AUC0–24h WB minimum and maximum targets of 35.1 and 134 μg∙h/mL for dolutegravir and the geometric mean AUC0–24h value of 53.6 μg∙h/mL (CV 26.9%) in adults. A sample size of 25 provided >99.9% power to detect a change between the adult geometric mean and the lower or upper AUC0–24h target for dolutegravir using log-transformed AUC0–24h values and a two-sample t-test at a significance level of 0.05. A sample size of five participants within each WB provided 94.6% and >99.9% power to detect a difference between the adult dolutegravir geometric mean and the lower and upper AUC0–24h WB targets, respectively. For dolutegravir C24h, 25 participants provided >99.9% power to detect a difference between the geometric mean (%CV) C24h in adults of 1.11 μg/mL (46.3%) and both WB targets of 0.67 and 2.97 μg/mL. Five participants provided 71.8% and 99.9% power for the lower and upper target, respectively. Though a minimum of 5 participants was targeted, up to 7 were permitted to be enrolled in each WB for purposes of intensive pharmacokinetic assessments. To allow for additional enrollments if confident pharmacokinetic determinations could not be made, or if alternative dosing strategies needed to be assessed within a WB, up to 75 children total were permitted to be enrolled.
Role of the Funding Source
The U.S. NIH and ViiV/GSK provided funding for the study and ViiV/GSK provided study drug. The study design and interpretation were performed by the protocol team, which includes representatives of the NIH and ViiV/GSK. Data collection and analysis were performed by the IMPAACT Network. All authors contributed to the writing of the manuscript.
RESULTS
Study Population
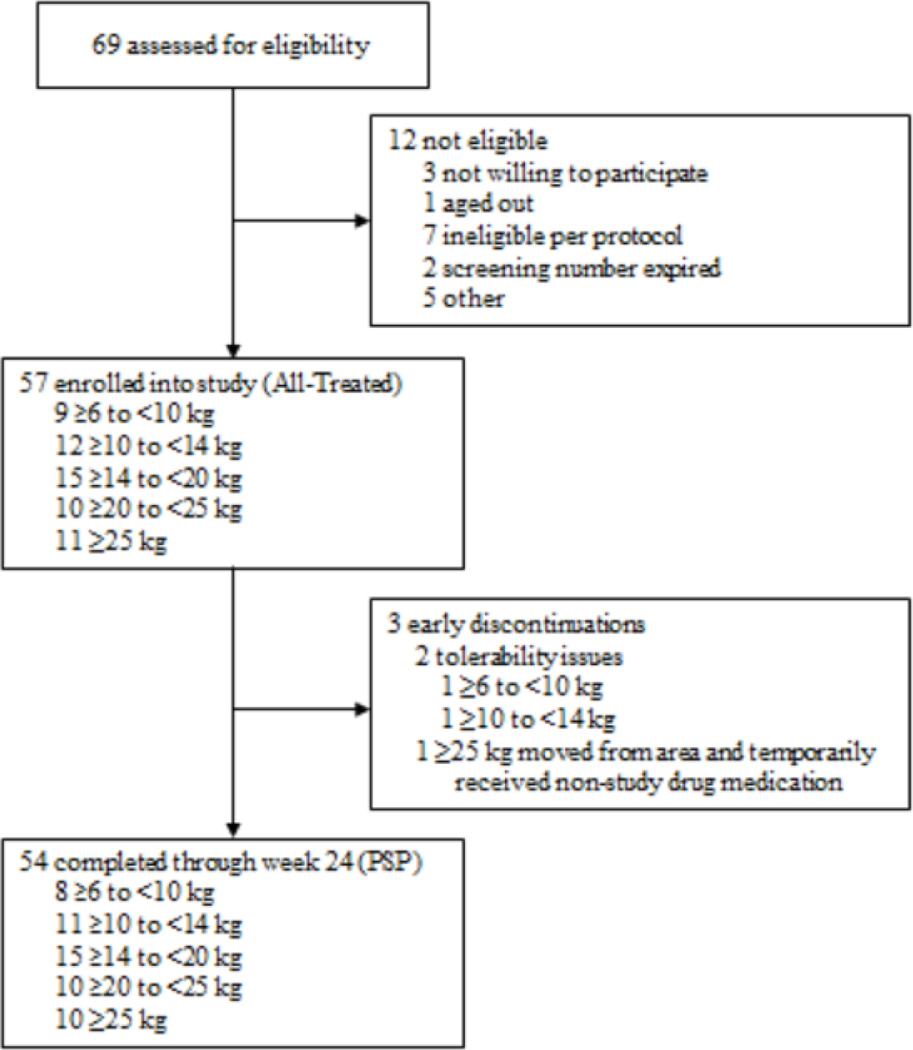
Participants were enrolled from September 9, 2020 through June 28, 2021. The last Week 24 study visit took place on December 14 2021. Sixty-nine children were screened and 57 were enrolled and initiated study drug, composing the All-Treated population (Figure 1 and Table 2). Most (54 [95%]) were treatment-experienced at entry and switched from a regimen containing lopinavir/ritonavir (38 [67%]), dolutegravir (8 [14%]), or raltegravir (7 [12%]) as the anchor medication (appendix p. 5). The three treatment-naïve participants were in the 6 to <10 kg WB. Two ART-experienced participants (one each weighing 6 to <10 kg and 10 to <14 kg) withdrew within eight days of entry due to study drug palatability issues, and a third weighing ≥25 kg relocated then returned to the study prior to week 24, resulting in a temporary interruption of study drug. The remaining 54 participants continued study treatment through 24 weeks and comprise the PSP.
Figure 1. Participant flow chart.
PSP: primary safety population. An individual may have more than one reason for not being enrolled. Reasons for being deemed ineligible per protocol included: >12 years of age at entry (n=1); grade 3 or higher result for hemoglobin (<8.5 g/dL), not expected to be available for 48 weeks of follow up (n=1); did not have documentation of suppressed viral load (<200 copies/mL) for at least 6 months prior to entry; had documented HIV-1 RNA ≥200 copies/mL within 6 months prior to entry; not HLA-B*5701-negative based on documented testing prior to entry.
Table 2.
Baseline Demographics in the All-Treated Population
| Characteristic | 6 to <10 kg (n=9) | 10 to <14 kg (n=12) | 14 to <20 kg (n=15) | 20 to <25 kg (n=10) | ≥25 kg (n=11) | Total (n=57) |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (years) | ||||||
|
| ||||||
| Median | 1·4 | 3·6 | 6·4 | 8·4 | 9.7 | 6·4 |
|
| ||||||
| Q1, Q3 | (1.1, 1.9) | (2.2, 3.7) | (4.7, 7.5) | (7.8, 8.9) | (9.5, 10.5) | (3.5, 8.8) |
|
| ||||||
| Range | 1·0-2·0 | 1·5-·5 | 3·9-9·6 | 6·4-8·9 | 8·7-11·3 | 1·0-11·3 |
|
| ||||||
| Age Category | ||||||
|
| ||||||
| <6 years | 9 (100%) | 12 (100%) | 7 (47%) | 0 (0%) | 0 (0%) | 28 (49%) |
|
| ||||||
| 6 to <12 years | 0 (0%) | 0 (0%) | 8 (53%) | 10 (100%) | 11 (100%) | 29 (51%) |
| Weight (kg) | ||||||
| Median | 9·2 | 12·9 | 17·0 | 21·5 | 28·5 | 17·0 |
| Q1, Q3 | (8.5, 9.5) | (12.5, 13.7) | (15.9, 18.8) | (21.0, 23.3) | (26.0, 35.6) | (12.8, 22.1) |
| Range | 8·2-9·6 | 10·3-13·8 | 14·4-19·6 | 20·0-24·6 | 25·6-39·3 | 8·2, 39·3 |
| Sex | ||||||
| Female | 5 (56%) | 7 (58%) | 5 (33%) | 3 (30%) | 6 (55%) | 26 (46%) |
| Male | 4 (44%) | 5 (42%) | 10 (67%) | 7 (70%) | 5 (46%) | 31 (54%) |
| Race | ||||||
| Black | 6 (67%) | 10 (83%) | 7 (47%) | 6 (60%) | 8 (73%) | 37 (65%) |
| Asian | 3 (33%) | 1 (8%) | 7 (47%) | 4 (40%) | 3 (27%) | 18 (32%) |
| White | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Unknown | 0 (0%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Ethnicity | ||||||
| Hispanic or Latino | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (27%) | 3 (5%) |
| Not Hispanic or Latino | 9 (100%) | 12 (100%) | 15 (100%) | 10 (100%) | 7 (64%) | 53 (93%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) | 1 (2%) |
| Region | ||||||
| Botswana | 4 (44%) | 2 (17%) | 3 (20%) | 2 (20%) | 2 (18%) | 13 (23%) |
| South Africa | 2 (22%) | 7 (58%) | 4 (27%) | 3 (30%) | 1 (9%) | 17 (30%) |
| Thailand | 3 (33%) | 1 (8%) | 6 (40%) | 4 (40%) | 3 (27%) | 17 (30%) |
| United States | 0 (0%) | 2 (17%) | 2 (13%) | 1 (10%) | 5 (46%) | 10 (18%) |
| Treatment History | ||||||
| ART-experienced | 6 (67%) | 12 (100%) | 15 (100%) | 10 (100%) | 11 (100%) | 54 (95%) |
| ART-naïve | 3 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5%) |
| Pre-study ART experience by class | ||||||
| NRTI | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) | 1 (2%) |
| PI and NRTI | 6 (67%) | 10 (83%) | 10 (67%) | 8 (80%) | 4 (36%) | 38 (67%) |
| INSTI and NRTI | 0 (0%) | 2 (17%) | 4 (27%) | 2 (20%) | 5 (46%) | 13 (23%) |
| PI, INSTI, and NRTI | 0 (0%) | 0 (0%) | 1 (7%) | 0 (0%) | 1 (9%) | 2 (4%) |
| Known Presence of M184V mutation | 0 (0%) | 0 (0%) | 2 (13%) | 0 (0%) | 2 (18%) | 4 (7%) |
| CD4 Cell Count (cells/mm3) | ||||||
| Median | 2321 | 1452 | 886 | 1155 | 915 | 1201 |
| Q1, Q3 | (1390, 3514) | (1161, 2615) | (761, 1412) | (801, 1251) | (753, 1450) | (880, 1535) |
| Range | 663-4636 | 1038-3073 | 522-2374 | 479-1480 | 320-1530 | 320-4636 |
| CD4 Cell Count Category | ||||||
| <350 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9%) | 1 (2%) |
| 350 - <500 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (2%) |
| 500 - <750 | 1 (11%) | 0 (0%) | 3 (20%) | 1 (10%) | 1 (9%) | 6 (11%) |
| ≥750 | 8 (89%) | 11 (100%) | 12 (80%) | 8 (80%) | 9 (82%) | 48 (86%) |
| CD4 Percentage (%) | ||||||
| Median | 34·0 | 33·9 | 32·9 | 32·6 | 37·7 | 35·1 |
| Q1, Q3 | (32·3, 49·1) | (31·6, 45·6) | (31·6, 39·2) | (26·2, 43·0) | (35·6, 43·0) | (31·9, 43·0) |
| Range | 18·0-55·0 | 23·9-49·0 | 25·3-47·6 | 25·8-46·7 | 32·2-49·5 | 18·0-55·0 |
| WHO Clinical Stage | ||||||
| Stage 1 | 8 (89%) | 8 (67%) | 12 (80%) | 8 (80%) | 7 (64%) | 43 (75%) |
| Stage 2 | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) | 1 (9%) | 2 (4%) |
| Stage 3 | 1 (11%) | 1 (8%) | 3 (20%) | 1 (10%) | 3 (27%) | 9 (16%) |
| Stage 4 | 0 (0%) | 2 (17%) | 0 (0%) | 1 (10%) | 0 (0%) | 3 (5%) |
Categorical data presented as n(%); continuous data presented as median, 25th percentile (Q1), 75th percentile (Q3), range
Dose Confirmation
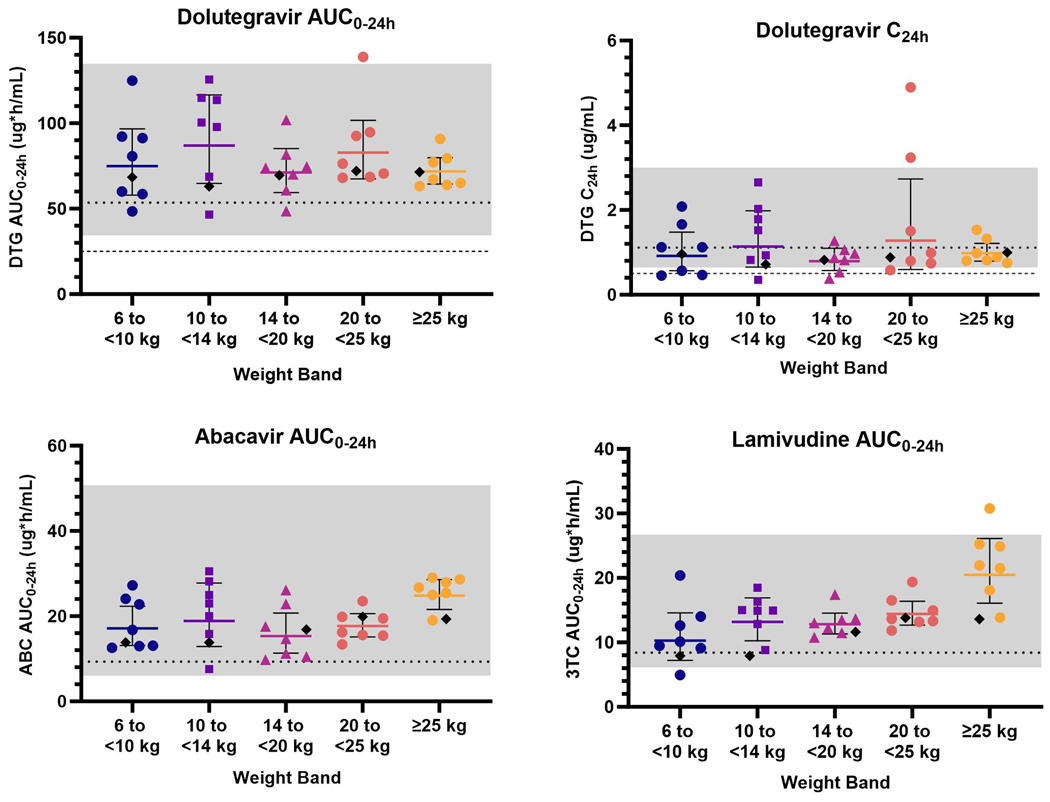
Thirty-five participants (seven per WB) underwent intensive pharmacokinetic evaluations. Two participants in the 10 to <14 kg WB underwent repeat intensive pharmacokinetic assessments at ~7 weeks post-entry due to questionable integrity of the original Week 1 samples. All 35 participants were both pharmacokinetic- and safety-evaluable. Concentration-time profiles were comparable across all WBs for all three agents (appendix p. 6). Protocol-specified pharmacokinetic targets were met in all five WBs with the originally selected dosing from Table 1 (Figure 2 and Table 3). Four participants (two weighing 6 to <10 kg, one weighing 10 to <14 kg, one weighing 14 to <20 kg) had individual dolutegravir C24h values below the target of 0.5 ug/mL but met the individual AUC0–24h target and were maintained on their original doses. Two participants in the 20 to <25 kg WB had elevated dolutegravir C24h of 3.24 and 4.90 μg/mL, the latter of whom also had dolutegravir AUC0–24h of 138.8 μg∙h/mL, which was above the individual target range. These two participants were also maintained on their original doses. Post hoc dose confirmation analyses were performed with these two participants excluded given their higher AUC0–24h and C24h results, and WB targets were still met (appendix p. 7). Safety guidelines were met within each WB and thus dosing was confirmed for all five WBs at the originally selected dosing.
Figure 2. Comparison of dolutegravir, abacavir, and lamivudine pharmacokinetic outcomes with dose confirmation targets & historical data.
Data presented as geometric mean (95% CI). Dashed lines (--) indicate individual dolutegravir minimum targets (25.0 μg∙h/mL for AUC0–24h and 0.5 μg/mL for C24h). Gray shading ( ) indicates weight band (WB) target ranges for each pharmacokinetic outcome (35.1–134 μg∙h/mL for dolutegravir AUC0–24h; 0.67–2.97 μg/mL for dolutegravir C24h; 6.3–50.4 μg∙h/mL for abacavir AUC0–24h; 6.3–26.5 μg∙h/mL for lamivudine AUC0–24h). Dotted lines (•••) indicate GM measures in adults with HIV receiving once-daily dolutegravir 50 mg (53.6 μg∙h/mL for AUC0–24h and 1.11 μg/mL for C24h), abacavir 600 mg (9.3 μg∙h/mL for AUC0–24h), or lamivudine 300 mg (8.4 μg∙h/mL for AUC0–24h). Black diamonds (♦) indicate predicted geometric mean measures in children with HIV receiving the same WB doses of each component (compiled from FDA Clinical Pharmacology Reviews).
Table 3.
Primary PK Outcomes for Dolutegravir, Abacavir, and Lamivudine by Weight Band
| Weight Band | N | Dose by Weight (mg/kg) | AUC0-24h (μg*h/mL) | Cmax (μg/mL) | C24h (μg/mL) |
|---|---|---|---|---|---|
|
| |||||
| Dolutegravir | |||||
|
| |||||
| 6 to <10 kg | 7 | 1.58 | 75.9 | 7.4 | 0.91 |
| (1.54, 1.80) | (33.7%) | (28.0%) | (67.6%) | ||
| 10 to <14 kg | 7 | 1.53 | 91.0 | 8.85 | 1.22 |
| (1.41, 1.79) | (36.5%) | (21.3%) | (77.5%) | ||
| 14 to <20 kg | 7 | 1.33 | 71.4 | 7.04 | 0.79 |
| (1.28, 1.52) | (23.5%) | (17.0%) | (44.2%) | ||
| 20 to <25 kg | 7 | 1.39 | 84.4 | 7.29 | 1.35 |
| (1.23, 1.52) | (26.3%) | (16.7%) | (95.5%) | ||
| ≥25 to <40 kg | 7 | 1.79 | 71.8 | 6.25 | 0.98 |
| (1.35, 1.93) | (13.9%) | (20.6%) | (27.9%) | ||
|
| |||||
| Abacavir | |||||
|
| |||||
| 6 to <10 kg | 7 | 18.9 | 17.7 | 7.3 | 0.003 |
| (18.4, 21.6) | (33.8%) | (20.5%) | (128%) | ||
| 10 to <14 kg | 7 | 18.3 | 19.8 | 8.36 | 0.005 |
| (16.9, 21.4) | (50.6%) | (43.7%) | (127%) | ||
| 14 to <20 kg | 7 | 16.0 | 15.1 | 6.26 | 0.003 |
| (15.4, 18.2) | (40.3%) | (31.0%) | (108%) | ||
| 20 to <25 kg | 7 | 16.7 | 17.4 | 6.65 | 0.004 |
| (14.8, 18.2) | (19.4%) | (27.7%) | (84.9%) | ||
| ≥25 to <40 kg | 7 | 21.4 | 25.7 | 9.04 | 0.011 |
| (16.2, 23.2) | (14.6%) | (21.9%) | (229%) | ||
|
| |||||
| Lamivudine | |||||
|
| |||||
| 6 to <10 kg | 7 | 9.47 | 10.7 | 2.29 | 0.055 |
| (9.22, 10.8) | (46.0%) | (39.8%) | (39.5%) | ||
| 10 to <14 kg | 7 | 9.16 | 14.2 | 3.55 | 0.046 |
| (8.45, 10.7) | (23.9%) | (18.7%) | (48.3%) | ||
| 14 to <20 kg | 7 | 7.98 | 13.0 | 2.92 | 0.058 |
| (7.69, 9.09) | (15.6%) | (23.0%) | (36.7%) | ||
| 20 to <25 kg | 7 | 8.33 | 14.5 | 2.99 | 0.06 |
| (7.38, 9.09) | (16.6%) | (31.9%) | (18.3%) | ||
| ≥25 to <40 kg | 7 | 10.7 | 21.7 | 4.15 | 0.084 |
| (8.09, 11.6) | (26.2%) | (29.3%) | (35.0%) | ||
Data presented as median (range) for dose by weight and geometric mean (CV%) for PK parameters; AUC0–24h: area under the concentration-time curve through 24 hours post-dose; Cmax: maximum concentration; C24h: concentration at 24 hours post-dose.
Safety
A summary of AEs by WB is shown in Table 4 and the appendix (p. 8–12). No drug-related AEs were grade 3 or higher, life-threatening, or resulted in abacavir/dolutegravir/lamivudine discontinuation. Participants experiencing drug-related AEs included 10 (19%) with grade 2 decreased estimated glomerular filtration rate (eGFR), four (7%) with grade 2 increased serum creatinine (SCr), two (4%) with grade 1 increased alanine aminotransferase, and one (2%) with grade 2 nightmares. Related grade 2 SCr increases were confirmed within 2–4 weeks in 2 participants and had resolved in 50% by Week 24. The participant with nightmares reported onset approximately 4 days after starting therapy and resolved 4 weeks later. Of participants with increases in SCr or decreases eGFR, four (33%) and 15 (50%) experienced changes by week 4, respectively, and seven (58%) and 25 (80%) experienced changes by week 12. When grading SCr based on actual value or change from baseline, eight participants had grade 2 and three had grade 3 elevations. However, when grading based on actual values only, there were no participants with grade 2 or higher elevations. Similarly, twenty-seven participants had grade 2 and four had grade 3 reductions in eGFR based on actual value or change from baseline. When grading based on actual values, eight had grade 2 eGFR reductions, none of which were related to study drug.
Table 4.
Summary of Adverse Events (AE) by Weight Band through Week 24 in the Primary Safety Population
| Weight Band | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||||
| 6 to <10 kg (N=8) | 10 to <14 kg (N=11) | 14 to <20 kg (N=15) | 20 to <25 kg (N=10) | ≥25 kg (N=10) | Total (N=54) | |||||||
| 95% | 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | |||||||
| n (%) | CI (%) | n (%) | (%) | n (%) | (%) | n (%) | (%) | n (%) | (%) | n (%) | (%) | |
|
| ||||||||||||
| Any adverse event | 8 (100·0) | (63·1, 100·0) | 10 (90·9) | (58·7, 99·8) | 15 (100·0) | (78·2, 100·0) | 8 (80·0) | (44·4, 97·5) | 8 (80·0) | (44·4, 97·5) | 49 (90·7) | (79·7, 96·9) |
| Adverse event related to study drugA | 4 (50·0) | (15·7, 84·3) | 2 (18·2) | (2·3, 51·8) | 0 | (0·0, 21·8) | 2 (20·0) | (2·5, 55·6) | 2 (20·0) | (2·5, 55·6) | 10 (18·5) | (9·3, 31·4) |
| Grade 3 or higher adverse event | 2 (25·0) | (3·2, 65·1) | 2 (18·2) | (2·3, 51·8) | 4 (26·7) | (7·8, 55·1) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 8 (14·8) | (6·6, 27·1) |
| Grade 3 or Grade 4 adverse event related to study drugA | 0 | (0·0, 36·9) | 0 | (0·0, 28·5) | 0 | (0·0, 21·8) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 0 | (0·0, 6·6) |
| Grade 5 adverse event related to study drugA | 0 | (0·0, 36·9) | 0 | (0·0, 28·5) | 0 | (0·0, 21·8) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 0 | (0·0, 6·6) |
| SeriousB adverse event related to study drugA | 0 | (0·0, 36·9) | 0 | (0·0, 28·5) | 0 | (0·0, 21·8) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 0 | (0·0, 6·6) |
| Life-threatening adverse event related to study drugA | 0 | (0·0, 36·9) | 0 | (0·0, 28·5) | 0 | (0·0, 21·8) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 0 | (0·0, 6·6) |
| Adverse event related to study drugA that led to permanent discontinuation of study drug | 0 | (0·0, 36·9) | 0 | (0·0, 28·5) | 0 | (0·0, 21·8) | 0 | (0·0, 30·8) | 0 | (0·0, 30·8) | 0 | (0·0, 6·6) |
Drug-relatedness of adverse events was determined by the site.
Serious adverse events were defined according to Version 2.0 of the DAIDS EAE Manual.
N = Number of primary safety participants in each weight band
n (%) = Number (percent) of participants in each subcategory who experienced at least one such event (with respect to the number of primary safety participants in each weight band)
95% CI = Exact 95% Confidence Interval (Clopper-Pearson)
Grade: 3 = Severe, 4 = Potentially Life-Threatening, 5 = Death
Adherence and Tolerability
Adherence.
Across all WBs, caregivers reported that participants received at least 90% of their study drug within the 30 days prior to their study visit. In the few instances with missed doses reported, the primary reason was the caregiver forgetting to administer the dose. All but one caregiver reported “good,” “very good,” or “excellent” administration across all timepoints.
Tolerability, Palatability and Acceptability.
The number of dispersible tablets required to be taken in each WB was deemed acceptable by 80–100% of caregivers at Weeks 4, 12, and 24 (appendix p. 13–27). At least 80% of caregivers reported the dispersible tablets dispersed within 3 minutes. At least 67% of caregivers in each of WB 3–5 reported the participant took study drug “easily by themselves.” In WB1, 75% of caregivers at Week 4 and 100% at Weeks 12 and 24 reported the participant took their study drug “easily with help.” In WB2, 91% of caregivers at Weeks 4 and 12, and 82% at Week 24 reported the participant took study drug “easily by themselves” or “easily with help.” Of those in the ≥25 kg WB, one (9%) reportedly broke the tablet before swallowing at Weeks 4 and 12, with the other 10 (91%) reporting intake as a whole tablet across visits. Most caregivers in each WB (70–100%) reported the child’s facial expression corresponded to “average, good, or very good” when taking abacavir/dolutegravir/lamivudine between Weeks 4, 12, and 24, which was slightly lower but overall comparable to facial ratings of the participant’s favorite food (90–100%).
Virologic and Immunologic Response
In the PSP, 52 participants (96%; 95% CI, 87%, 100%) were suppressed to <200 copies per mL at Week 4 and 53 (98%; 95% CI, 90%, 100%) at Week 24. All 51 ART-experienced participants, including the four with a known M184V mutation, maintained suppression (<200 copies per mL) through 24 weeks. Two of the three ART-naïve participants achieved virologic suppression (<200 copies per mL) by Week 24. The third did not achieve suppression <200 copies per mL by Week 24 and subsequently met the protocol definition of virologic failure. This participant was 1 year of age and entered the study with a viral load of over 3.5 million copies per mL, CD4 count of 2425 cells per mm3, and was WHO Stage 1. Their viral load steadily decreased while on abacavir/dolutegravir/lamivudine to 419 copies per mL at Week 24, and they remained on study treatment after Week 24 given their positive treatment response. The caregiver reported 100% adherence and no issues with medication administration throughout this period. Using the FDA snapshot algorithm and a threshold of 200 copies per mL in the All-Treated population, virologic success was achieved in 54 (95%; 95% CI, 85%, 99%) at both Weeks 4 and 24. Using a threshold of 50 copies/mL, virologic success was achieved in 47 (83%; 95% CI, 70%, 91%) at Week 4 and 52 (91%; 95% CI, 81%, 97%) at Week 24. Both CD4 count and percentage remained consistent with age-appropriate norms over time across all WBs (appendix p. 28).
Lipid Profiles
At Week 24, median (Q1, Q3) total cholesterol was 3·91 (3·55, 4·64) mmol/L, HDL cholesterol was 1·26 (1·09, 1·54) mmol/L, LDL cholesterol was 2·25 (1·81, 2·63) mmol/L, and triglyceride levels were 1·00 (0·69, 1·27) mmol/L across all WBs. There was a general decline in all types of cholesterol from baseline through Week 24 across WBs in the PSP (appendix p. 29–33).
DISCUSSION
Dosing of dispersible and immediate-release once daily abacavir/dolutegravir/lamivudine was confirmed in children weighing 6 to <40 kg in alignment with WHO WB dosing recommendations. All ART-experienced participants maintained viral suppression below 200 copies/mL through 24 weeks of treatment. Two of three ART-naïve participants achieved suppression by Week 24. Immunologic outcomes were also favorable. Both dispersible and immediate-release formulations were safe; and adherence, acceptability, and palatability ratings were overall positive across all WBs through 24 weeks of treatment.
Protocol-defined pharmacokinetic targets were met for all three agents, and the AUC0–24h, Cmax, and C24h were comparable or slightly higher than historical pharmacokinetic data with these medications in children and adults with HIV.12–14,18–20 The dispersible formulations generally have resulted in higher AUC and Cmax results for dolutegravir in comparison to the film coated tablets. However, these exposures are still within the range of once- to twice-daily exposures in adults and children.12–14,18–20 Four participants fell below the individual dolutegravir C24h target but were maintained on their original WB dose as they met the individual AUC0–24h targets, thus dose increases may have increased overall drug exposures relative to other participants. Additionally, all C24h results were above the in vivo 90% effective concentration (EC90) of 0.3 μg/mL and within the range of simulated prediction intervals for dolutegravir dispersible tablet exposures upon which FDA approval was based. Prior exposure-response analyses of dolutegravir in children with AUC0–24h, C24h, and the average concentration over the dosing interval showed none of these parameters were associated with decreased virologic response, suggesting drug exposures were on the plateau portion of the exposure-response curve.12 The only predictor of failure to achieve virologic suppression in this previous analysis was a baseline HIV viral load above 100,000 copies per mL. Consistent with these previous findings, all four participants remained suppressed throughout the study, and the single treatment-naïve participant who did not achieve virologic suppression at Week 24 had a high baseline viral load. For the two participants with elevated dolutegravir C24h, both were maintained on their original doses with no safety concerns identified.
Children receiving NNRTIs were excluded to minimize pharmacokinetic variability as efavirenz and nevirapine cause metabolic enzyme induction and decrease dolutegravir concentrations;21 however, other studies in children have not identified loss of virologic control after switching from an NNRTI- to a dolutegravir-containing regimen.
Adult switch studies of efavirenz to dolutegravir showed the absolute concentrations of dolutegravir remained above the protein adjusted-90% inhibitory concentration (IC90) (0.064 μg/mL) and stabilized four weeks post-switch, thus no washout is needed.22 Children receiving rifampin at entry were also excluded over similar concerns for induction. No participants developed active tuberculosis during this study, but separate pharmacokinetic studies in infants and children have demonstrated this interaction can be overcome by increasing to twice-daily dolutegravir dosing.23,24 A similar approach could be considered with a combination of the FDC formulations and single-entity dolutegravir.
Reported adherence was high across all WBs. Most (89%) caregivers rated palatability of the FDC dispersible tablet formulation as average or better, which is similar to ratings of the FDC immediate release tablet formulation in adults (>88%) and slightly lower than ratings for dolutegravir dispersible tablets in children (99%).18,25 Two participants withdrew from the study due to palatability issues within 8 days of treatment with the FDC dispersible tablet formulation. This may have been due in part to the bitter taste of abacavir and regional differences in taste perception as one was in the U.S. and the other was in Thailand. Though the immediate release tablet formulation is now available to children weighing at least 25 kg,7 challenges with swallowing due to the large tablet size (22×11 mm) have been reported.26 Nearly all participants in our study reported intake of whole intact tablets. However, for those with challenges swallowing this tablet, it can be crushed and mixed with food as an alternative. Use of a greater number of dispersible tablets (instead of the immediate release tablet) in this WB was not possible as dolutegravir is 60% more bioavailable in the dispersible formulation than the immediate release formulation whereas abacavir and lamivudine are bioequivalent, which would result in exceeding adult dolutegravir dosing while providing less than the currently approved doses of abacavir and lamivudine.25 Assessments in those weighing 3 to <6 kg were not performed as the recommended dolutegravir dose is 5 mg once daily. To be consistent with this dosing, only a single dispersible FDC tablet could be administered, which would result in underdosing of the abacavir/lamivudine components at 60/30 mg comparatively to the WHO WB doses of 120/60 mg.
Both FDC formulations were overall safe and well-tolerated in our study population through 24 weeks of treatment. No grade 3 or higher AEs deemed related to study drug occurred and most lower grade AEs involved renal function changes that were asymptomatic and not of clinical concern. Dolutegravir can cause benign increases in SCr through inhibition of renal transporters,27 thus our findings were not unexpected. One participant reported grade 2 nightmares deemed related to study drug. Dolutegravir has been associated with central nervous system side effects in a small percentage of people, some risk factors for which may include pre-existing psychiatric disorders, higher plasma concentrations and abacavir coadministration.28
Safety, tolerability, and efficacy findings should be interpreted with some caution due to our limited sample size and there is limited precision for event rate estimates within individual WBs. Most enrolled participants were treatment-experienced, on a stable ART regimen, and virologically suppressed at entry. This may have selected for individuals more likely to tolerate ART in various formulations for extended periods. Results may not be generalizable to ART-naïve or ART-experienced children who are not virologically suppressed on a stable ART regimen. However, the demographics of our study population are consistent with the global population of children with HIV, and we would not expect pharmacokinetic differences between naïve and experienced populations. Exposure matching to adult populations is the basis upon which regulatory approvals for ART in children with HIV have been historically based. Prior pharmacokinetic, safety, and efficacy data with the individual agents,12,14,18,29 bioequivalence/relative bioavailability studies in adults,25 and modeling and simulation data resulted in U.S. FDA approval of FDC abacavir/dolutegravir/lamivudine dispersible and immediate release tablets for children weighing 10 to less than 40 kg in March 2022.10 The EMA also approved these formulations in children weighing more than 14 kg in February 2023 based in part on our pharmacokinetic data. 11 Full treatment histories were not collected prior to entry; thus, there is a possibility of undocumented resistance prior to switching. All treatment-experienced participants maintained suppression for 24 weeks in our study, but review of full treatment histories should still be considered when switching to this FDC. We also screened for hepatitis B coinfection in addition to HLA-B*5701 for entry into the study. If laboratory screenings are required prior to use, this may be a barrier to larger scale rollout. However, this is not typically done in resource-limited settings, and the WHO does not currently recommend these screenings prior to using this combination. 6 Furthermore, studies in children with HIV have shown abacavir is safe and well-tolerated even in the absence of HLA-B*5701 testing. 30 Finally, we have only presented results through 24 weeks of treatment; additional data from longer follow-up and larger studies are important for understanding the benefits and risks of these FDC options alongside other available ART regimens.
Our favorable pharmacokinetic, safety, tolerability, and efficacy results support the use of these FDCs in children and align with other recent studies that have shown superior outcomes with dolutegravir-containing regimens. These collective findings represent a significant advancement in pediatric HIV care as the same once-daily FDC formulation can be used in children weighing 6 to less than 25 kg without the need to change between multiple medications and formulations as the child grows. These results are expected to inform regulatory decisions for use of the dispersible tablet in children 6 to less than 10 kg in the U.S. and children weighing at least 6 kg globally, providing a critical step towards ensuring the global availability of dolutegravir-containing, child-friendly FDC options in children with HIV.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Dolutegravir is an HIV integrase strand transfer inhibitor (INSTI) and a key component of recommended antiretroviral treatment (ART) regimens. This is due to its superior safety and efficacy compared with protease and non-nucleoside reverse transcriptase inhibitors and higher barrier to resistance in comparison to the first-generation INSTIs, raltegravir and elvitegravir. The combination of dolutegravir, abacavir, and lamivudine is recommended globally but is not widely available for children in a fixed dose combination (FDC) formulation. FDC tablets only recently became available within the U.S. in immediate-release form for children weighing at least 25 kg and dispersible form for children weighing at least 10 kg. However, no child-friendly dolutegravir-containing FDC formulations are available outside the U.S. or for children weighing <10 kg within the U.S., thus requiring the use of multiple tablets and formulations to provide a complete ART regimen for most children with HIV globally. High pill burden can present adherence and administration challenges in children, often resulting in poor treatment outcomes for this vulnerable population. A child friendly FDC formulation could have a significant impact on treatment outcomes in children globally through improvements in adherence and durable virologic suppression, but supporting data on appropriate dosing are needed. We performed a literature search through PubMed for publications including “dolutegravir”, “abacavir”, “lamivudine”, “children”, and “pediatrics” through March 15, 2023 and identified no other studies reporting the pharmacokinetics, safety, tolerability, and efficacy of FDC abacavir/dolutegravir/lamivudine in children with HIV.
Added value of this study
We evaluated the pharmacokinetics, safety, tolerability, and efficacy of dispersible and immediate-release FDC abacavir/dolutegravir/lamivudine in children less than 12 years who weighed 6 to less than 40 kg. Participants weighing 6 to less than 25 kg received the dispersible tablet formulation (3–6 tablets once daily of ABC 60 mg/DTG 5 mg/3TC 30 mg dispersed in 15–20 mL of water; total dose dependent on weight) and those weighing 25 to less than 40 kg received the immediate-release formulation (once-daily single tablet of ABC 600 mg/DTG 50 mg/3TC 300 mg). Dosing was confirmed based on pharmacokinetics and safety in each weight band at the originally selected dosing, which aligned with WHO weight band dosing for the individual agents. Follow-up through 24 weeks confirmed the safety, tolerability, and virologic efficacy of both formulations.
Implications of all the available evidence
Dosing has been confirmed for the first dispersible-release dolutegravir-containing FDC for children weighing 6 to less than 25 kg. Based on previous data for the individual agents and bioequivalence/relative bioavailability studies in adults along with modeling and simulation, the U.S. Food and Drug Administration (FDA) expanded its approval for the immediate-release tablet to include children weighing more than 25 kg and approved the dispersible tablet for children 10 to less than 25 kg in March 2022. The European Medicines Agency also issued marketing authorization for the immediate and dispersible release tablets down to 14 kg based in part on the pharmacokinetic findings from our study. Our study will further inform regulatory decisions on use of the dispersible tablets in children weighing down to 6 kg in the U.S. and across all weight bands globally. Ultimately, these findings will support global initiatives to expand the availability of dolutegravir-containing FDC options for children with HIV.
ACKNOWLEDGEMENTS
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support was provided by ViiV Healthcare/GlaxoSmithKline. The University of Colorado is a Certara Center of Excellence. The Center of Excellence program supports leading institutions with Certara’s state-of-the-art model-informed drug development software. We thank and acknowledge the study participants; the site investigators, site staff, and collaborating institutions, and the local and IMPAACT community advisory board members who supported this study. Additional acknowledgements are included in the appendix (pg. 2).
Footnotes
DECLARATION OF INTERESTS
KMB has received consulting fees from ViiV Healthcare. JJK is employed at Merck. LZ, SW, YR received ViiV Healthcare funding paid to their institution. At his prior institution, DEY was an investigator on studies unrelated to HIV that were supported by Astellas, Chimerix, and Viracor-Eurofins, with funding paid to his institution. DEY is an employee of the National Institutes of Health (National Institute of Allergy and Infectious Diseases), who sponsored the study, and was formerly an unpaid technical advisor for the non-profits Cover the Globe and Maipelo Trust. HC holds stock in GlaxoSmithKline and is an employee of GlaxoSmithKline. AMB and CB hold stock in GlaxoSmithKline and are employees of ViiV Healthcare. The remaining authors have nothing to declare.
Trial Registration
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA SHARING STATEMENT
The data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (email address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
REFERENCES
- 1.UNICEF. Paediatric care and treatment. 2022. https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/ (accessed 24 Apr 2023). [Google Scholar]
- 2.Clinton Health Access Initiative. 2021. HIV Market Report: The state of HIV treatment, testing, and prevention in low- and middle-income countries. Available at: http://clintonhealthaccess.org/wp-content/uploads/2021/10/2021-CHAI-HIV-Market-Report.pdf. [Google Scholar]
- 3.Turkova A, White E, Mujuru HA, et al. Dolutegravir as First- or Second-Line Treatment for HIV-1 Infection in Children. N Engl J Med 2021; 385(27): 2531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naive patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis 2021; 21(1): 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther 2018; 15(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed 16 Mar 2023. [Google Scholar]
- 8.Organization WH. Paediatric Antiretroviral Drug Optimization (PADO) 4 Meeting Report, 2018. [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 2023 Apr 24. [Google Scholar]
- 10.Triumeq and Triumeq PD (abacavir, dolutegravir and lamivudine) [Package Insert]. Research Triangle Park, NC: ViiV Healthcare/GlaxoSmithKline; 2022. [Google Scholar]
- 11.European Medicines Agency. Triumeq, ViiV Healthcare. INN-dolutegravir-abacavir-lamivudine. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/triumeq. Accessed 2023 Mar 16. [Google Scholar]
- 12.Center for Drug Evaluation and Research, US Food & Drug Administration. Clinical Pharmacology Review. Tivicay (dolutegravir) PD®. https://www.fda.gov/media/140939/download [Google Scholar]
- 13.Zhang J, Hayes S, Sadler BM, et al. Population pharmacokinetics of dolutegravir in HIV-infected treatment-naive patients. Br J Clin Pharmacol 2015; 80(3): 502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for Drug Evaluation and Research, US Food & Drug Administration. Clinical Pharmacology Review for Ziagen® (abacavir sulfate) and Epivir® (lamivudine). https://fda.report/media/92051/N20-977S027-Abacavir-sulfate-Clinpharm-PREA.pdf [Google Scholar]
- 15.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1. [July 2017]. Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf [Google Scholar]
- 16.Center for Drug Evaluation and Research, Food and Drug Administration, U.S. Department of Health and Human Services. Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral Drugs for Treatment (Guidance for Industry). 2015. [Google Scholar]
- 17.ICH E9 statistical principles for clinical trials. European Medicines Agency, 17 September 2018, https://www.e-ma.europa.eu/en/ich-e9-statistical-principles-clinical-trials (accessed 05 October 2022). [Google Scholar]
- 18.Ruel TD, Acosta EP, Liu JP, et al. Pharmacokinetics, safety, tolerability, and antiviral activity of dolutegravir dispersible tablets in infants and children with HIV-1 (IMPAACT P1093): results of an open-label, phase 1–2 trial. Lancet HIV 2022; 9(5): e332–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waalewijn H, Chan MK, Bollen PDJ, et al. Dolutegravir dosing for children with HIV weighing less than 20 kg: pharmacokinetic and safety substudies nested in the open-label, multicentre, randomised, non-inferiority ODYSSEY trial. Lancet HIV 2022; 9(5): e341–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollen PDJ, Moore CL, Mujuru HA, et al. Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial. Lancet HIV 2020; 7(8): e533–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song I, Borland J, Chen S, et al. Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir. Eur J Clin Pharmacol 2014; 70(10): 1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wet J, DeJesus E, Sloan L, et al. Pharmacokinetics of Dolutegravir After Switching to Abacavir/Dolutegravir/Lamivudine From an Efavirenz-Based Regimen: A PK Sub-Study From STRIIVING 17th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington DC; 2016. [Google Scholar]
- 23.Turkova A, Waalewijn H, Chan MK, et al. Dolutegravir twice-daily dosing in children with HIV-associated tuberculosis: a pharmacokinetic and safety study within the open-label, multicentre, randomised, non-inferiority ODYSSEY trial. Lancet HIV 2022; 9(9): e627–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs TG, Mumbiro V, Cassia U, et al. Adequate dolutegravir exposures in infants on rifampicin treatment receiving twice-daily dolutegravir. Conference on Retroviruses and Opportunistic Infections. Seattle, WA; 2023. [Google Scholar]
- 25.Singh RP, Adkison KK, Baker M, et al. Development of Dolutegravir Single-entity and Fixed-dose Combination Formulations for Children. Pediatr Infect Dis J 2022; 41(3): 230–7. [DOI] [PubMed] [Google Scholar]
- 26.Bossacoma Busquets F, Noguera-Julian A, Sanchez E, Fortuny C. Dolutegravir plus abacavir/lamivudine works in adolescents, but size matters. J Antimicrob Chemother 2017; 72(10): 2958–60. [DOI] [PubMed] [Google Scholar]
- 27.Koteff J, Borland J, Chen S, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol 2013; 75(4): 990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS 2018; 13(2): 102–11. [DOI] [PubMed] [Google Scholar]
- 29.Viani RM, Ruel T, Alvero C, et al. Long-Term Safety and Efficacy of Dolutegravir in Treatment-Experienced Adolescents With Human Immunodeficiency Virus Infection: Results of the IMPAACT P1093 Study. J Pediatric Infect Dis Soc 2020; 9(2): 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulenga V, Musiime V, Kekitiinwa A, et al. Abacavir, zidovudine, or stavudine as paediatric tablets for African HIV-infected children (CHAPAS-3): an open-label, parallel-group, randomised controlled trial. Lancet Infect Dis 2016; 16(2): 169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (email address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.