Abstract
Background
In the entire world, acne vulgaris (AV) is the most prevalent skin condition. Approximately 9.4% of people worldwide have acne vulgaris. This study compared the blood levels of chitinase 3-like protein 1 (YKL-40) in acne vulgaris patients before and after oral isotretinoin therapy.
Patients and Methods
The design of the study was cross-sectional case-control. Forty patients with moderate to severe acne vulgaris and twenty healthy participants participated in this study. Using the Global Acne Grading System (GAGS) score, patients with acne vulgaris were evaluated both before and after concluding their treatment. Using the enzyme-linked immunosorbent assay (ELISA), the serum levels of YKL-40 were measured before and after oral isotretinoin therapy in healthy controls and acne patients.
Results
Patients with acne vulgaris had considerably greater serum levels of YKL-40 than healthy control subjects (p 0.001) did. After three months of oral isotretinoin medication, the GAGS score and blood levels of YKL-40 in acne vulgaris patients both significantly decreased.
Conclusion
The conclusion of this study was that reducing the blood levels of YKL-40 and the GAGS score in patients with acne vulgaris who took oral isotretinoin for three months was a crucial strategy.
Keywords: acne vulgaris, YKL-40, isotretinoin, GAGS
Introduction
The pilosebaceous units are harmed by acne vulgaris, a common chronic skin disorder that can manifest as inflammatory, non-inflammatory, or a combination of both types of lesions. It is ranked as the seventh most common disease in the world.1,2
It can affect people of all ages, but is particularly common among teenagers and young adults, with 10% experiencing severe acne. Between the ages of 13 and 17, 90% of males and 80% of females and 40% of children between the ages of 7 and 11 years are affected by acne.3
The multifactorial pathogenesis of acne can be broken down into four key stages: excessive sebum production, follicular hyper-keratinization, P. acnes proliferation, and immunological response. It appears on skin that has an abundance of hair growth, such as the upper chest, back, and face.4,5
The typical early lesions of acne are comedones, either closed or open comedones. The first lesions, which may include inflamed papules, pustules, and inflamed cystic nodules with induration, can lead to severe acne.6
There are two other techniques for assessing the clinical disease of acne: lesion counting and global severity grading. Criteria for descriptive text or pictures are often compared to determine global severity ratings. Using the acne lesion-counting methodology, the number of open and closed comedones, papules, pustules, and nodules is tallied.7
There are abundant topical and oral treatment options available for patients with acne. First-line agents include topical retinoids, azelaic acid, benzoyl peroxide, and combinations of these agents. For recalcitrant or more severe acne, oral medications, including oral antibiotics, isotretinoin, or hormonal therapy, may be considered. This review will also discuss the many advances being made in the treatment of acne vulgaris, from the development of microencapsulated medications to targeted treatments.8
The most effective acne medication is still isotretinoin (13-cis-retinoic acid), which was authorized for the treatment of acne vulgaris in 1982. Numerous randomized controlled trials have demonstrated its superiority to a placebo and oral antibiotics in lowering the number of acne lesions.9
Chitinase-3-like protein 1 (YKL-40) is a member of the classic family of mammalian chitinases, which consists of 18 glycosyl hydrolases. Ten Since YKL-40 lacks enzymatic activity, its biological function remains unknown. It is well known that YKL-40 functions in angiogenesis, proliferation, and inflammation. Numerous inflammatory diseases, such as inflammatory bowel diseases, rheumatoid arthritis, inflammatory lung diseases, osteoarthritis, viral hepatitis, cardiovascular disease, and a number of malignant processes, have been studied with YKL-40.10,11
Serum YKL-40 level was found to be higher in acne patients. This may suggest that YKL-40 has a role in different acute and chronic inflammatory disorders.12
The purpose of the investigation was to compare serum levels of YKL-40 in acne vulgaris patients before and after treatment with oral isotretinoin.
Patient and Methods
The case–control cross-sectional design of this investigation. Twenty healthy controls and 40 patients with moderate to severe acne vulgaris participated in this study. These patients and a control group were drawn from the Aswan University Hospital’s Outpatient Clinic for Dermatology, Venereology, and Andrology. The months of April 2022 and April 2023 were used for this study.
The Aswan University Faculty of Medicine’s Scientific and Ethical Committees gave their approval to the project. All individuals provided written consents after being fully informed.
The following patients were not included in the study; 1) Pregnant and lactating women and female welling to have pregnancy in the period of study. 2) Patients with any abnormalities in the laboratory assessment at baseline. 3) Immunocompromised patients and those with a history of chronic liver disease, hyperlipidemia, non-inflammatory acne, neurologic disorders, neoplastic disorders, and neurologic disorders. 4) acne patients who use cosmetics, aesthetic medicine or any medications that affect sebaceous gland secretion.
Methodology
All study participants underwent thorough history taking, a thorough general and dermatological examination, an investigation to rule out any other related systemic diseases, and YKL-40 measurement at the baseline.
Clinical Assessment
All of the patients underwent thorough general and local examinations as well as extensive medical histories. Before and after oral isotretinoin treatment, patients with acne vulgaris were assessed using the GAGS score. When the GAGS score was between 1 and 18, intermediate disease severity was assumed, and severe disease was assumed when the score was between 19 and 30.
Treatment Protocol
Isotretinoin Therapy
Prior to starting isotretinoin therapy, measurements of the complete blood count (CBC), triglycerides (TG), cholesterols, and liver functions (SGOT and SGPT) were made. For 12 weeks, Forty patients with acne vulgaris were treated with isotretinoin (0.5–1 mg/kg/day, up to 40 mg daily).
Medical Photography
Using a high resolution digital camera canon EOS 1300D 18 megapixels (manufactured in Taiwan), photography of the patients was done at baseline and after three months of regular treatment.
YKL-40 Serum Level Evaluation
Before and after oral administration of isotretinoin, 5 cc blood samples were collected from the antecubital vessels of acne sufferers and healthy control participants. Five cc of the patient’s blood was extracted from an antecubital vein. The collected specimen was deposited in serum separator tubes (SST). Blood was permitted to clot for 10–20 minutes at ambient temperature before being centrifuged for 20 minutes at 2000–3000 rpm. Before being examined, the serum was removed and frozen at −30 degrees Celsius. Serum levels of human chitinase 3-like 1 (YKL-40) were measured before and after oral isotretinoin treatment in healthy control participants and AV patients using an ELISA reagent from R&D Systems, Minneapolis, MN (Catalog no. DC3L10).
Statistical Analysis
The data were analyzed using the statistical package for social sciences (SPSS) software, version 22. Comparing qualitative variables using the chi-square test, frequencies and percentages were recorded. A Student’s t-test was employed to compare a quantitative measure presented as means standard deviation (SD). As indicated, regression analysis and correlation will be performed between various variables. The P value for significant results will be 0.05.
Results
This study included 40 patients with moderate to severe acne vulgaris and 20 normal subjects who served as control. There was no significant difference regarding age between subjects (p = 0.375) (Table 1). On the other hand, regarding the sex of the patients; males represented 60% (n = 12) and females represented 40% (n = 8) of controls while about three-quarters (n = 28) of AV cases were females and 30% (n = 12) were males. The association between gender and case status was statistically significant (p = 0.025) (Table 1).
Table 1.
Socio-Demographic Data Differences Between Cases and Control
| Control (n =20) | Case (n=40) | P-value | |
|---|---|---|---|
| Age in years | 22.35 ± 3.4 | 21.08 ± 5.9 | = 0.375 |
| Sex | |||
| ● Male | 12 (60%) | 12 (30%) | = 0.025 |
| ● Female | 8 (40%) | 28 (70%) |
As regard to differences in the serum level of YKL_40 between AV cases and control. It was statistically significant (P < 0.001) (Table 2). There was a significant reduction in the median YKL40 level from 128 (28.5–394 ng/mL) before treatment to 62 (18–181 ng/mL) after treatment with isotretinoin (P < 0.001) (Table 3).
Table 2.
Serum Level of YKL_40 Differences Between Cases and Control
| Control (n =20) | Case (n=40) | P-value | |
|---|---|---|---|
| YKL-40 serum Level (ng/mL) | |||
| ● Mean ± SD | 64.65 ± 25.7 | 138.34 ± 72.9 | < 0.001 |
| ● Median (Range) | 59.5 (30–117) | 127.6 (28.5–394) |
Table 3.
Effect of Isotretinoin Level of the Studied AV Cases
| YKL-40 Serum Level (ng/mL) (n = 40) | P-value | ||
|---|---|---|---|
| Before Treatment | 3-m After Treatment | ||
| ● Mean ± SD | 138.35 ± 72.9 | 67.50 ± 34.7 | |
| < 0.001 | |||
| ● Median (Range) | 127.6 (28.5–394) | 62.3 (18–181) | |
There was insignificant (P = 0.105) lower level of YKL40 after treatment among moderately severe AV cases (48.5 (21.5–103.5 ng/mL)) compared with severe cases (63 (18–181 ng/mL) (Table 4).
Table 4.
Effect of Disease Severity on the Level of YKL-40 After Treatment
| YKL-40 Serum Level (ng/mL) After Treatment | P-value | ||
|---|---|---|---|
| Mean ± SD | Median (Range) | ||
| Disease Severity | |||
| ● Moderate | 62.38 ± 33.1 | 48.5 (21.5–103.5) | = 0.105 |
| ● Severe | 68.99 ± 35.5 | 63 (18–181) | |
Comparing before and after therapy, there was a modest to moderately strong positive connection between the various socio-demographic and clinical indicators and the YKL-40 serum level (Table 5).
Table 5.
Correlation Between YKL-40 Before and After Treatment and Socio-Demographic and Clinical Parameters Among Cases (n = 40)
| YKL-40 Before | YKL-40 3-m After | |||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| YKL-40 Before | 1 | 0.784 | < 0.001 | |
| Age in years | 0.052 | = 0.367 | 0.210 | = 0.096 |
| Weight/kg | 0.235 | = 0.072 | 0.216 | = 0.090 |
| Isotretinoin Daily Dose | 0.214 | = 0.092 | 0.136 | = 0.202 |
In the present study, there was statistically significant high positive correlation between YKL-40 serum level before and after treatment with isotretinoin (r = 0.784, P < 0.001). In other words, with increase in the serum level of YKL40 before treatment, there was a decrease in its level after treatment (Table 5) (Figures 1–3).
Figure 1.
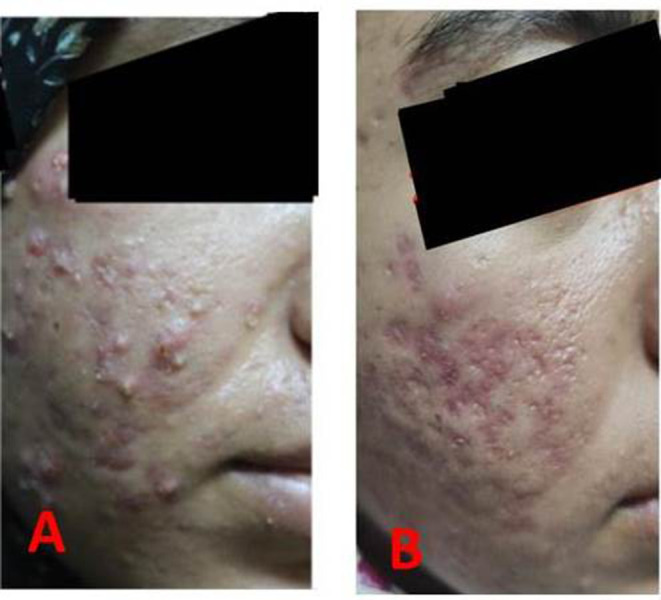
Patient before treatment (A) and after treatment (B).
Figure 2.
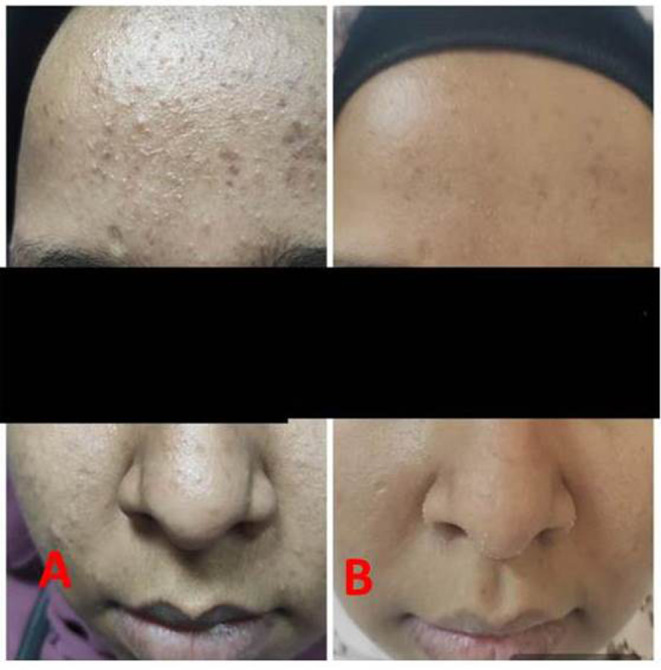
Patient before treatment (A) and after treatment (B).
Figure 3.
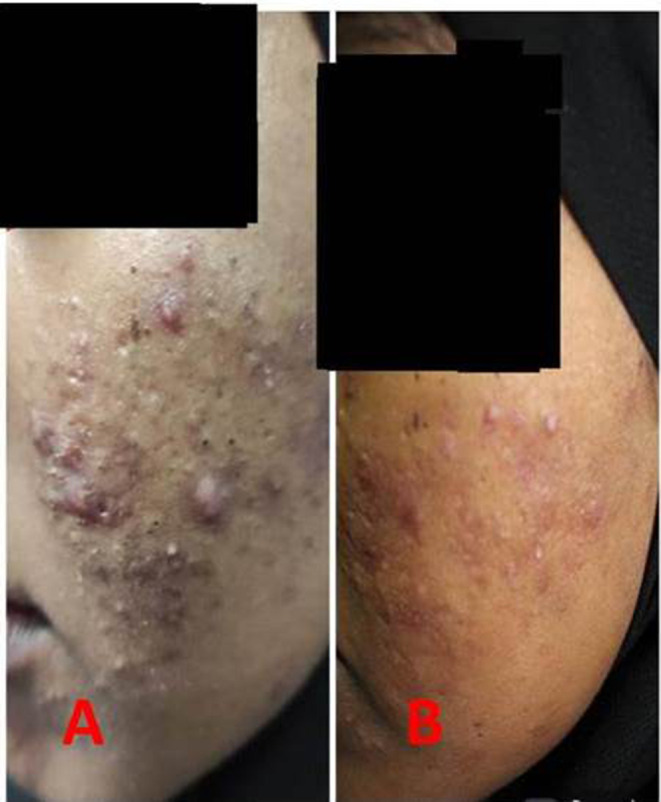
Patient before treatment (A) and after treatment (B).
Diagnostic value of the serum YKL-40 level for diagnosis of AV (AUC = 0.864, p < 0.001; 95% CI 0.77–0.96) (Table 6).
Table 6.
Diagnostic Performance of YKL-40 for Diagnosis of AV, Analysed as Area Under the Curve (95% CI)
| AUC | 95% CI | SE | P-value | |
|---|---|---|---|---|
| Serum Level of YKL-40 | 0.864 | 0.771–0.958 | 0.048 | < 0.001 |
Goodness criteria of the serum YKL-40 level for diagnosis of AV in the studied cohort. Using a cut-off value of 80 ng/mL, the validity criteria were as follows; 85% sensitivity ie, serum YKL-40 correctly identified 85% of positive cases as having the AV. Also, 75% specificity ie, the test correctly identified 75% of healthy individuals as not having the condition). Additionally, the test had 77% precision – Positive Predictive Value (PPV) ie, the ability of the test to predict diseased AV patients among all positively diagnosed cases. It has 83% Negative Predictive Value (NPV) ie, the ability to predict healthy individuals among all those diagnosed as negative. Overall, the test had 80% accuracy (Table 7).
Table 7.
Goodness Criteria of YKL-40 for Diagnosis of AV for the Studied Cohort
| Goodness Criteria for YKL-40 | |
|---|---|
|
0.864 |
|
80 ng/mL |
|
80% |
|
85% |
|
75% |
|
77% |
|
83% |
Discussion
In our investigation, acne vulgaris patients’ GAGS scores and responses to oral isotretinoin medication were evaluated together with their serum levels of YKL-40. Patients with acne vulgaris in this study exhibited significantly higher serum levels of YKL-40 than healthy control volunteers. This outcome was consistent with the findings of Khaled et al4 and Ebrahim et al,12 who found that acne vulgaris patients had higher serum levels of YKL-40 than healthy control individuals. The median YKL40 level significantly (P value 0.001) decreased before and after receiving isotretinoin. This outcomes contest with Kutlu et al,13 as they conveyed that the levels of the inflammatory markers in patients with acne vulgaris decreased statistically significantly after receiving isotretinoin for 3 months (p 0.05).
AlJasser et al14 also found that MMP-8 and MMP-9 as inflammatory biomarkers were significantly lower among cases receiving isotretinoin therapy compared to the group not taking the medication (P value < 0.0001). In divergence from that, Metin et al15 instituted that monocyte-to-high-density lipoprotein ratio (MHR) as inflammatory biomarker its level increased significantly (p value 0.042) decrease after isotretinoin therapy. Our analysis displayed that there is insignificant difference between serum level of YKL-40 and disease severity (P value = 0.105) with lower level of YKL40 among moderate AV cases (48.5 (21.5–103.5 ng/mL)) compared with severe AV cases (63 (18–181 ng/mL)).
Our results were aligned with Ebrahim et al,12 that noted that the higher level of YKL-40 may only reflect the level of systemic inflammation in AV patients, and its concentration does not relate with the severity of skin changes in the study.
This observation, which may be explained by the fact that pro-inflammatory biomarkers are typically more active and elevated at sites of tissue inflammation than in circulation, warrants additional research to determine the level of YKL-40 in skin lesions and correlate it with serum levels of YKL-40 and disease severity in acne vulgaris patients. Unlike wise Hedetoft et al16 stated that high plasma YKL-40 to be associated with disease severity in patients with Necrotizing soft-tissue infection (NSTI). Also Khashaba et al17 and Agamia et al18 discovered that there was a statistical positive correlation between serum levels of YKL-40 and disease severity scoring by (PASI) score in patients with moderate to severe acne.
Conclusion
The present study found significantly higher level of serum YKL-40 in acne patients than normal control. No significant correlation was found between the disease severity serum level of YKL-40. After oral isotretinoin, serum levels of YKL-40 decreased significantly. In addition, we can conclude from our study that YKL-40 testing was beneficial for acne patients prior to initiating isotretinoin therapy. The rise of this sensitive biomarker of inflammation in acne patients may indicate its involvement in the pathogenesis of acne. We concluded that a greater level of YKL-40 was utilized to predict acne susceptibility.
Acknowledgments
The study’s participants are acknowledged by the writers.
Funding Statement
There is no funding to report.
Medical Writing and Editorial Assistance
No other writers’ assistance or editorial support was required.
Data Sharing Statement
The corresponding author, [Ali, MA], may provide the data that support the study’s conclusions upon request. Because [the data contain information that could jeopardize the privacy of research participants], they are not publicly available.
Ethics Statement
The patient who is the topic of this manuscript provided written, explicit agreement for the publication of the case details and photos.
Compliance of Ethics Guidelines
This investigation has received permission from the ethics committees of the Aswan University Faculty of Medicine (551/7/21). The Institutional Review Board-Ethics committee of the Faculty of Medicine, Aswan University approved the study, which was conducted in conformance with the standards of the Declaration of Helsinki. This research registered on clinical trial with ID NCT05218486.
Consent for Publication
The subject provided written, explicit agreement for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors reported no potential conflicts of interest.
References
- 1.Sharara M, Diab H, Reda AER. A pilot study on serum lactoferrin in patients with mild versus severe acne in correlation with disease duration. J Egypt Women Dermatol Soc. 2019;16(3):193–197. doi: 10.4103/JEWD.JEWD_35_19 [DOI] [Google Scholar]
- 2.Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(1):1–29. doi: 10.1038/s41598-020-62715-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greydanus DE, Azmeh R, Cabral MD, Dickson CA, Patel DR. Acne in the first three decades of life: an update of a disorder with profound implications for all decades of life. Dis Month. 2021;67(4):101103. doi: 10.1016/j.disamonth.2020.101103 [DOI] [PubMed] [Google Scholar]
- 4.Khaled HN, Hamid WGA, Alghobashy Y. Chitinase-3 protein-like-1 as inflammatory marker and disturbed lipid-profile risk in acne vulgaris. Menoufia Med J. 2022;35(2):477. doi: 10.4103/mmj.mmj_271_21 [DOI] [Google Scholar]
- 5.Patnaik S, Purohit D, Biswasroy P, Diab WM, Dubey A. Recent advances for commedonal acne treatment by employing lipid nanocarriers topically. Int J Health Sci. 2022;6:180–205. doi: 10.53730/ijhs.v6nS8.9671 [DOI] [Google Scholar]
- 6.Stamu‐O’Brien C, Jafferany M, Carniciu S, Abdelmaksoud A. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20(4):1080–1083. doi: 10.1111/jocd.13765 [DOI] [PubMed] [Google Scholar]
- 7.Becker M, Wild T, Zouboulis CC. Objective assessment of acne. Clin Dermatol. 2017;35(2):147–155. doi: 10.1016/j.clindermatol.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 8.Mohsin N, Hernandez LE, Martin MR, Does AV, Nouri K. Acne treatment review and future perspectives. Dermatol Ther. 2022;35(9):e15719. PMID: 35841269. doi: 10.1111/dth.15719 [DOI] [PubMed] [Google Scholar]
- 9.Fallah H, Rademaker M. Isotretinoin in the management of acne vulgaris: practical prescribing. Int J Dermatol. 2021;60(4):451–460. doi: 10.1111/ijd.15089 [DOI] [PubMed] [Google Scholar]
- 10.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mamma- lian chitinase(-like) members of family 18 glycosyl hydro- lases. Genetics. 2007;177(2):959–997. doi: 10.1534/genetics.107.075846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CG, Da Silva CA, la Cruz CS. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73(1):479–501. doi: 10.1146/annurev-physiol-012110-142250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahim A, Mustafa AI, El‐Shimi OS, Fathy MA. Serum YKL40: a novel potential link between inflammation and dyslipidemia in Acne Vulgaris. J Cosmet Dermatol. 2020;19(5):1219–1223. doi: 10.1111/jocd.13124 [DOI] [PubMed] [Google Scholar]
- 13.Kutlu Ö. Effect of isotretinoin treatment on the inflammatory markers in patients with Acne Vulgaris: can monocyte/HDL be a new indicator for inflammatory activity of isotretinoin treatment? Cutan Ocul Toxicol. 2019;39(1):67–70. doi: 10.1080/15569527.2019.1701485 [DOI] [PubMed] [Google Scholar]
- 14.AlJasser RN, Alaqeely RS, Al-Hoqail IA, et al. Association between isotretinoin use and changes in periodontal clinical parameters and MMP-8 and MMP-9 salivary levels. Front Biosci. 2021;26(7):191–197. [DOI] [PubMed] [Google Scholar]
- 15.Metin N, Turan C. The correlation of monocyte to high-density lipoprotein ratio with uric acid and possible atherosclerotic pathway in patients treated with isotretinoin. Authorea Preprints; 2020.
- 16.Hedetoft M, Hansen MB, Madsen MB, Johansen JS, Hyldegaard O. Associations between YKL-40 and markers of disease severity and death in patients with necrotizing soft-tissue infection. BMC Infect Dis. 2021;21(1):1–9. doi: 10.1186/s12879-021-06760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khashaba SA, Attwa E, Said N, Ahmed S, Khattab F. Serum YKL 40 and IL 17 in Psoriasis: reliability as prognostic markers for disease severity and responsiveness to treatment. Dermatol Ther. 2021;34(1):e14606. doi: 10.1111/dth.14606 [DOI] [PubMed] [Google Scholar]
- 18.Agamia NF, Aly EA, El-Hadidy A, El-Abd AM, Gaber D. YKL-40, fetuin-A plasma levels, and carotid intima-media thickness: do they have relations with subclinical atherosclerosis associated with psoriasis and the disease severity? J Egypt Women’s Dermatol Soc. 2019;16(2):133. doi: 10.4103/JEWD.JEWD_23_19 [DOI] [Google Scholar]


