Abstract
Sea star wasting—marked in a variety of sea star species as varying degrees of skin lesions followed by disintegration—recently caused one of the largest marine die-offs ever recorded on the west coast of North America, killing billions of sea stars. Despite the important ramifications this mortality had for coastal benthic ecosystems, such as increased abundance of prey, little is known about the causes of the disease or the mechanisms of its progression. Although there have been studies indicating a range of causal mechanisms, including viruses and environmental effects, the broad spatial and depth range of affected populations leaves many questions remaining about either infectious or non-infectious mechanisms. Wasting appears to start with degradation of mutable connective tissue in the body wall, leading to disintegration of the epidermis. Here, we briefly review basic sea star biology in the context of sea star wasting and present our current knowledge and hypotheses related to the symptoms, the microbiome, the viruses, and the associated environmental stressors. We also highlight throughout the article knowledge gaps and the data needed to better understand sea star wasting mechanistically, its causes, and potential management.
Introduction
In the past decade, sea stars (Echinodermata: class Asteroidea, asteroids) have been confronted with a pandemic that has gained increasing attention because of the severity, taxonomic breadth, and geographic extent of the disease. Previously referred to as sea star wasting disease (SSWD), sea star wasting syndrome (SSWS), asteroid idiopathic wasting syndrome (AIWS), or just sea star wasting (SSW), the nomenclature points to a suite of signs observed across a broad range of species that, in the most severe cases, result in death following disintegration of the star (Hewson et al., 2018). Although species-specific variations exist, wasting sea stars generally exhibit modified behavior, such as lethargy and disinterest in nearby prey, pronounced body wall lesions, and arm autotomy (Fig. 1; Eisenlord et al., 2016; Kohl et al., 2016; Menge et al., 2016; Jaffe et al., 2019).
Figure 1.

Examples of sea star species affected by sea star wasting (SSW). The left column represents animals with no or with early symptoms. The right column represents animals with late symptoms. (A) Pycnopodia helianthoides; the right image shows terminal stages of SSW. Photo credit: Mark Nayer. (B) Asterias forbesi; the arrow on the left image shows a small lesion. (C) Pisaster ochraceus; the arrow on the left image shows a small lesion. Photo credit: Melissa Miner. (D) Orthasterias; the arrow on the left image shows a small lesion. Photo credit: Feiro Marine Life Center (left).
While the primary SSW event that is often referred to in current discussion—emerging on the Pacific coast of North America in 2013—is the most extreme documented example, prior asteroid wasting events matching this general description have been periodically observed, with the first known reported over a century ago (Mead, 1898). Over the years, wasting has been noted independently across many sea star species globally, often with regional mortality, including the North American Pacific coast (Dungan et al., 1982; Eckert et al., 2000; Konar et al., 2019), Atlantic coast (Sieling, 1960; Tiffany, 1978; Menge, 1979; Bucci et al., 2017), Mediterranean Sea (Staehli et al., 2009), Atlantic coast of Europe (Thorpe and Spencer, 2000), East China Sea (Suzuki et al., 2012), Yellow Sea (China), and Port Philip Bay, Australia (Hewson et al., 2019). Importantly, most earlier events targeted only a few species in an area. For example, in the summer of 1978, populations of Heliaster kubiniji (sun star) suffered massive mortality in the Gulf of California rocky intertidal zone. The outbreak reduced abundance of H. kubiniji from dense aggregations to near-zero values at sites throughout the Gulf of California in just a few weeks (Dungan et al., 1982); there was similarly high intertidal mortality in the smaller high intertidal sea star Othilia tenuispina, but this species rebounded in numbers quickly. At least four co-occurring species of subtidal sea stars were reportedly unaffected. The event was coincident with exposure to warmer waters in the intertidal (Dungan et al., 1982). In contrast, the occurrence of SSW in the past decade has profoundly affected more than 20 species of sea stars in the northeastern Pacific and other sites—from intertidal to deeper waters, from Baja California to the northern Gulf of Alaska, and beyond (M. Dawson, University of California Merced, LMS, unpubl. data). In this sense, the current prevalence of wasting makes it among the most geographically and taxonomically widespread diseases of invertebrates ever recorded.
Many asteroids are top-level consumers with direct effects on the structure and function of benthic ecosystems (Menge et al., 1994; Vicknair and Estes, 2012; Schultz et al., 2016; Burt et al., 2018). Indeed, the concept of keystone species was first developed from work on populations of the predatory Pisaster ochraceus in the Pacific Northwest (Paine, 1966). When present, P. ochraceus consumes the dominant encrusting invertebrate species, thus freeing up space for other sessile macroinvertebrates and algae (Feder, 1959; McClintock and Robnett, 1986; Robles et al., 2009; Lafferty and Suchanek, 2016). Other asteroid species function similarly, sometimes at different size scales (Gravem and Morgan, 2017). In addition to direct predation, some asteroids have important indirect effects by altering prey behavior (Duggins, 1983; Rogers et al., 2018; Kay et al., 2019; Murie and Bourdeau, 2019). Sea stars also directly compete for marine resources with other high-level consumers (Traiger et al., 2016) and can have substantial effects on aquaculture and restoration endeavors (Silina, 2008; Miyoshi et al., 2018; Wilcox and Jeffs, 2019).
Along with the key ecosystem functions of many echinoderms, mass mortality events can generate cascading effects, ranging from temporary local disruptions to long-term ecological phase shifts (Lessios et al., 1984; Burge et al., 2014; Miner et al., 2018). Mass mortality events affecting critical foundation species, ecosystem engineers, or keystone species have been coined “marine disease emergencies” due to the detrimental, and sometimes irreversible, series of events that often succeed them (Miner et al., 2018). A textbook example of coincident marine disease events with profound ecological effects started with mass die-offs of the sea urchin Diadema antillarum that resulted in overgrowth of algae on coral reefs (Lessios et al., 1984), and the subsequent mass mortality through a variety of causes of Acropora corals led to complete physical restructuring of Caribbean coral reefs (Hughes, 1994; Hughes et al., 2003; Cramer et al., 2020). Notably, all of these changes promote increasing prevalence of microbial and algal nutrient cycles, a feature of many recent climate-driven shifts leading to eutrophication of marine ecosystems (Petraitis and Dudgeon, 2020).
In this context, recent mass mortality events affecting asteroids threaten the health of coastal ecosystems, and additional understanding is needed to manage or mitigate them. As stated above, the most recent SSW event has differed markedly from prior events in geographic and taxonomic extent. This epizootic killed many billions of asteroids across over 20 taxa (indeed, billions are estimated to have been lost in a single species alone; e.g., Pycnopodia helianthoides) (Gravem et al., 2021), primarily along the Pacific coast of North America, where the coastal community is regularly monitored, and is considered to be among the most geographically widespread disease events to sweep through marine wildlife in documented history (Gudenkauf and Hewson, 2015; Eisenlord et al., 2016; Hewson et al., 2018). Sea star wasting has been forecast to reshape community structure throughout the region (Menge et al., 2016; Gravem and Morgan, 2017) and, due to its broad taxonomic effect, is considered a risk for other coastal communities globally (Hewson et al., 2019).
Unfortunately, there is a deficit of fundamental knowledge surrounding the etiology, pathogenesis, and epidemiology of SSW, reflected in the sparse and discontinuous literature on the subject. Thus, in an attempt to generate the framework for a cohesive narrative on SSW for future reference, the aims of our review are to (1) provide a relevant review of normal sea star biology, anatomy, and physiology in the context of the common outward signs of SSW in affected sea stars and what we have learned; (2) highlight our understanding of the biotic factors associated with wasting, including microbial and viral interactions; (3) identify crucial gaps in our understanding of the symptoms and progression of SSW; and (4) point to forthcoming research designed to further our understanding of SSW.
We begin by considering the basic biology of sea stars. Our goal in this first section is to establish which anatomical systems may be initially or overtly affected by SSW, in a way that informs not only the recent and unprecedented mass mortality event but also prior historical examples. Our paper then reviews each of these organismal systems for indicators of cause or response to SSW.
Asteroid Biology as Context for Sea Star Wasting
In this section, we review some basic elements of sea star biology to introduce unique aspects, traits, and physiology that come into play—across large phylogenetic breadth (LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data)—when these animals succumb to SSW. The ecological consequences noted above are intertwined with both the density and distribution of sea stars as well as the broadly defined traits that are affected by SSW. Sea stars are widely distributed throughout the world’s oceans. They are stenohaline and occur from the deep sea to the intertidal zone, and from tropical to polar waters (Binyon, 1961; Gage and Tyler, 1991; Byrne et al., 2017). Across this extensive range, local species richness is highly variable (Cintra-Buenrostro et al., 2005; Iken et al., 2010), with diversity driven by many environmental and ecological factors. Local diversity can encompass deep phylogenetic divergences, because often dozens of genera and several families can be found within any ecoregion (sensu Spalding et al., 2007)—this is key because observed effects of SSW affect such great diversity of sea star species with vastly different life histories and diets (VanVeldhuizen and Oakes, 1981; McClintock et al., 2008; Lawrence, 2013; Martinez et al., 2017).
The size, density, and distribution of sea star populations are highly variable through time (Uthicke et al., 2009), but they are not always closely monitored in the absence of clearly identified economic consequences (Hewson et al., 2019). Nevertheless, demographic variation in sea star populations is associated with recognizable ecological responses, such as overgrowth by mussels in the absence of sea star predation (Paine, 1966; Gravem and Morgan, 2017; Kay et al., 2019). Some distributional patterns are difficult to track because they comprise multiple cryptic species. For instance, species of the Acanthaster complex are found throughout tropical waters and can form high-density feeding fronts that devastate coral reefs (Pratchett et al., 2017), but the individual Acanthaster species and their distributions remain uncertain (Haszprunar et al., 2017). Similar problems have been noted in the taxonomy—and, thus, the precision in evaluating disease effects—of other asteroid genera (Melroy and Cohen, 2021). What is important about these components of phylogenetic diversity and potential density-dependent effects is not yet clear; but if we are to understand the apparent transmissibility of SSW (Hewson et al., 2014), then we must know how and whether the health of any sea star in an ecosystem is predictive of the health of other individuals, both conspecific and across the phylogeny of asteroids (LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data). Whether SSW is primarily driven by infectious or non-infectious etiologies is still unclear—and disease etiology likely involves complex interactions—but the broad taxonomic effect indicates that the shared traits of sea stars could be a first target for clues into mechanisms of pathogenesis.
Body plan and life history
Echinoderms possess four main features that distinguish them from other metazoans: their calcareous endoskeleton composed of ossicles of varying sizes, a water vascular system, mutable collagenous tissues, and pentaradial body organization in adults (Hyman, 1955; Sloan, 1980; Jangoux, 1982; Blake, 1989; Chia and Koss, 1994; Byrne, 1999, 2013; Mooi and David, 2000; McEdward and Miner, 2001; McEdward et al., 2002; Lawrence, 2013; Mercier and Hamel, 2013; Martinez et al., 2016). The present-day global asteroid fauna includes approximately 1900 species in 37 families (Mah and Blake, 2012; Feuda and Smith, 2015; Byrne et al., 2017; Linchangco et al., 2017).
Asteroid life histories have been studied across diverse taxa (Strathmann, 1985; Chia et al., 1993; McEdward and Miner, 2001; McEdward et al., 2002; Byrne, 2013), though most such studies are biased toward those taxa that are most available near prominent marine research facilities. Most species are dioecious free-spawners that release their gametes into the water column for fertilization, followed by development of a planktonic larva (Emlet et al., 1987) and then metamorphosis and settlement back to the sea floor. Some species deposit eggs that adhere to rocks on the shore and have benthic development (Chia, 1968; Byrne, 1995). Other asteroids retain their eggs in brood chambers on or within the body cavity (Chia et al., 1993; McEdward and Janies, 1993). Variation in reproductive strategies such as these may influence the capacity of a species to recover from mass mortality events. There does not appear to be a close link between larval dispersal and SSW (LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data), and it is still unclear whether early life stages are susceptible to—or could transmit—the disease.
Surface and skeleton
The breakdown of the body surface and its integrity is a feature of SSW, and so an understanding of these tissues is important for understanding the disease. The surface of a sea star is one of the more complicated types of external barriers in the Metazoa. The surface can be covered by abundant spines or have no spines in species with a thick, soft epidermis (e.g., Dermasterias imbricata). The surface consists of an external columnar epithelium (containing secretory cells, supporting cells, sensory cells, and glandular cells). The epithelial cells are covered by a thin cuticle that is visible only microscopically (McKenzie and Grigolava, 1996). The body wall connective tissue has mutable properties (mutable connective tissue [MCT]), is capable of reversible rapid changes of tensile strength from stiff to viscous, is under neural control, and is mediated by proteinaceous neurotransmitters (for review see Wilkie, 2002; Barbaglio et al., 2013). Ossicles composed of magnesium calcite are embedded in the MCT and form the endoskeleton. This internal calcareous skeleton is considered a synapomorphy of the echinoderms (but see Cameron and Bishop, 2012).
In sea stars, MCT is found in the body wall dermis, spine joint ligaments, and epithelial cells of the tube feet (Motokawa, 1986, 2011; O’Neill, 1989; Santos et al., 2005) and is composed of spindle-shaped collagen fibers in a proteoglycan and glycosaminoglycan matrix to which they are covalently bound (Motokawa, 1982; O’Neill, 1989; Trotter and Koob, 1989; Wilkie et al., 1992; Erlinger et al., 1993; Barbaglio et al., 2012). Cells in the MCT include immune cells, nerve cells, and juxtaligamental cells that are unique to the MCT. The juxtaligamental cells are recognized by their possession of dark granules, which are thought to have effector molecules involved in the nervous system innervation of MCT (Wilkie, 1979, 2002).
The coelomic cavity is a hemocoel lined by a layer of myoepithelial, secretory, and supporting cells. This epithelium tissue also forms the papulae that project through the thick body wall and into the overlying seawater. Papulae are covered both externally and internally by the two epithelial layers noted above; however, the intervening connective tissues between the epithelial layers are greatly thinned (Hyman, 1955; Cobb, 1978). Papulae also can be retracted in recessed pockets with the use of muscles and cavities at the base of the papula, or they can be extended quickly above the surface by relaxation of the muscles and diversion of the fluid contents of the perivisceral coelom into the papular lumen. Papulae function in oxygen exchange and waste disposal (Binyon, 1972; Cobb, 1978).
Pedicellariae are small pincer-like compound ossicles covered by epithelium and controlled by muscles. Pedicellariae are found primarily on the aboral body surface; they function in removal of small particulates or fouling organisms that settle on the body wall (Campbell and Rainbow, 1977; Ruppert, 2004), in defense (Jensen, 1966; Chia, 1969; VanVeldhuizen and Oakes, 1981), in aggression (Menge and Menge, 1974; Wobber, 1975), and in prey capture (Robilliard, 1971; Chia and Amerongen, 1975; Hendler and Franz, 1982; Dearborn et al., 1991; Emson and Young, 1994; Lauerman, 1998).
On the ventral side of the sea star, the centrally located mouth faces the benthos. Ambulacral grooves bearing the tube feet radiate from the oral surface to the ends of each arm; the highly distensible tube feet enable locomotion and prey capture. These may have terminal adhesive suckers for attachment or be pointed in species that burrow in soft sediments (Paine, 1926; Smith, 1937). Paired ambulacral ossicles (skeletal elements) form an arch-like arcade spanning the entire arm and overlying the water vascular canal and tube feet that project into the ambulacral groove.
Nervous system
The nervous system organization reflects the pentaradial symmetry and is composed of a circumoral nerve ring from which the radial nerve cords originate that run along the arms just internal to the ambulacral grooves. At the end of each arm, the radial nerve cord connects with the ocelli of the eye spot, which is a phototactic sensory organ. The nervous system is divided into ectoneural and hyponeural systems separated from one another by a thin connective tissue layer (Hyman, 1955; Cobb, 1987). The ectoneural system is mainly associated with sensory and motor components. The hyponeural tissue is considered to be exclusively motor and comprises the thinner inner layer of the radial nerve cords (Cobb, 1987). Perception of physical and chemical change in the environment influences hormonal and immune responses in echinoderms (Hamel et al., 2021). Autotomy itself is a component sign of SSW; it is a complex behavioral response and can be induced by a number of diverse stressors (Mladenov et al., 1989; Byrne et al., 2019). For this reason, much research has focused on how autotomy and nerve regeneration interplay, but the details remain elusive (Byrne, 2020).
Digestive system
Sea stars have a complete digestive system. The mouth located toward the substrate is surrounded and controlled by a peristomial membrane. The mouth is connected to a short esophagus that leads to the cardiac stomach (Anderson, 1954, 1959). Sea stars can often evert their cardiac stomach to catch and digest their prey and retract the stomach afterward (Semmens et al., 2013; Tinoco et al., 2018). The cardiac stomach is separated from the pyloric stomach by a slight constriction, and the pyloric stomach reaches the anus via a short intestine. The pyloric stomach also connects via a pyloric duct to a pair of pyloric caeca in each arm. These pyloric caeca, which consist of complex, ramified evaginations, have been described as the most important parts of the gut (in both volume and function) in asteroids (Binyon, 1972). Pyloric caeca provide the majority of digestive enzymes and also absorb and store nutrients (Ferguson, 1969). Their structures have been well described in Asterias forbesi and Marthasterias glacialis (Anderson, 1953; Martinez et al., 1989). The connection between the digestive system and SSW is unclear. Sunflower stars (Pycnopodia helianthoides) in captivity that are fed a regular diet will seem to refuse food only when they are in poor condition, such as suffering from a wound, or otherwise stressed, such as shortly after collection. However, stars of this species have been observed to feed even when exhibiting early signs of ultimately fatal cases of SSW (JH, unpubl. data)
Coelomic system
As is typical of echinoderms, asteroids have a hydraulic system known as the water vascular system, used for locomotion as well as particle transportation. This system consists of a central ring canal that connects with the radial canals, which in turn connect with the tube feet. In asteroids, the stone canal connects the water vascular system with environmental water at the sieve plate called the madreporite (Hyman, 1955; Ferguson and Walker, 1991). Tube feet are connected to the radial canals through ampullae that can contract or relax to move water in each tube foot.
Within the body and surrounding all organs, including the water vascular system components, is the perivisceral coelom. The concentrations of electrolytes (Binyon, 1962; Stickle and Denoux, 1976; Held and Harley, 2009; Wahltinez et al., 2019), amino acids, and ammonium (Diehl and Lawrence, 1984) in coelomic fluid are not substantially different from those in seawater (Binyon, 1962). Tube foot activity depends on the water vascular system and seems to be maintained by an active transport of potassium leading to an osmotic influx of water directly through their walls (Prusch and Whoriskey, 1976; Ferguson, 1990). While sea stars are osmoconformers, they are partially able to regulate coelomic fluid volume (Pearse, 1967; Ellington and Lawrence, 1974), though the mechanism remains unknown.
The hyponeural coelom runs parallel to the water vascular system from the madreporic ampulla to the lateral canals. The hyponeural coelom surrounds the vertical axial hemal canal, the hyponeural hemal ring, and the radial and lateral hemal vessels. The hyponeural coelom also forms the genital coelom that surrounds and connects the genital hemal ring and the gonads. The hyponeural canal and the stone canal both connect to the madreporic ampulla, and seawater is thought to supply both systems (Hyman, 1955; Ferguson and Walker, 1991). The flattening that is associated with SSW (see Sea Star Wasting, below) likely involves coelomic processes.
The echinoderm immune system
An important milestone in the identification of an immune system in biological organisms was reached in sea stars over a century ago. Eli Metchnikoff inserted a rose thorn into a sea star larva and to his surprise saw cells migrate to this thorn and eventually encompass the intruder (see, e.g., Gordon, 2016b). This was a novel characterization of the process of phagocytosis in an immune defense and helped earn Metchnikoff the Nobel Prize in 1908. Since then, our understanding of the immune system of this and other echinoderms has increased enormously; later sections will detail what we have learned because of SSW itself.
As with many invertebrates, host defenses comprise behavioral and cellular changes in response to non-self recognition, an intact epidermis with functional pedicellariae, and immune cells. Asteroids only have an innate system of immune defense. That is, cells and molecules are present in the organism that can recognize an invader as non-self and minimize infectivity by encapsulating, consuming through phagocytosis, or killing it (Smith et al., 2010). Evidence for an adaptive system of immune memory is lacking. Limited analyses of available echinoderm genomes show a lack of elements known to adapt to pathogen exposure, for example (Sea Urchin Genome Sequencing Consortium, 2006; Smith et al., 2010; Hall et al., 2017).
Much of the early work on immune function in sea stars was accomplished through grafting experiments. Grafts of a piece of tissue from one sea star to a different conspecific or heterospecific individual result in the host animal killing cells of the donor tissue at the border and eventually releasing the graft (Hildemann and Dix, 1972; Karp and Hildemann, 1976). These similar responses demonstrate that individuals within a species can recognize one another as non-self and can do this in as robust a manner as seen in animals with adaptive immune mechanisms (Silva, 2000; Furukawa et al., 2009).
Subsequent work on sea star immunity has focused on immune cells in the coelomic fluid and tissues, referred to as coelomocytes (Smith et al., 2010). Asteroids have several types of coelomocytes, including phagocytic amoebocytes, phagocytes, amoebocytes, spherule cells, vibratile cells, haemocytes, crystal cells, and progenitor cells (Smith, 1981). Each of these circulating cells appears to have unique characteristics and functions, in combination similar to the innate immune system functions of vertebrates, including clot formation, phagocytosis, encapsulation, clearance of bacteria or other foreign materials, and even oxygen transport (Pinsino and Matranga, 2015; Smith et al., 2018).
Innate immune systems rely on recognition of evolutionarily conserved structures on pathogens, also known as pathogen-associated molecular patterns (PAMPs), which act through a limited number of pattern recognition receptors (PRRs), notably the family of Toll-like receptors (TLRs). As in other invertebrates, analyses of the sea urchin genome revealed a profound complexity of innate immune recognition receptors, regulators, and effectors (Hibino et al., 2006; Rast et al., 2006; Smith et al., 2018), generated perhaps by combinations of gene recombination, gene duplication, and selection for sequence diversification in response to high rates of change in their pathogens. Generally, these gene regions have been identified in asteroid genomes as well (Rast et al., 2006; Ruiz-Ramos et al., 2020). While our insights are still limited to a few species that have been well characterized, echinoderms have more than 10 times the number of gene copies in these families than are found in vertebrates, providing a diverse innate immune complexity.
Like other ectotherms, environmental factors such as temperature, salinity, heavy metals, and UV light (Pinsino and Matranga, 2015) can affect the immune response of sea stars. Recent work has shown that the interaction of an echinoderm with stressors such as perceived predation rapidly influences the hormonal regulation of coelomocytes and other components of the innate immune system (Hamel et al., 2021) and that nutrient deprivation can drive wasting-like responses in some sea stars (Van Volkom et al., 2021). Thus, interpreting experimental data on immune and disease responses in echinoderms in the context of SSW would need to account and control for these environmental variables.
A challenge in our understanding of disease in echinoderms is the lack of basic knowledge of cell physiology and mechanisms of host cell responses to insults. Moreover, there is a lack of functional evidence to understand host cell gene expression and its role in cell death and genesis of lesions, all rich avenues for future investigative efforts.
Virology and microbiology of grossly normal asteroids
Early microscopic study of apparently healthy asteroids found an abundance of subcuticular bacteria of varying morphologies (Holland and Nealson, 1978; Kelly et al., 1995; Foret and Lawrence, 2001). However, these studies highlighted the large abundance of spiral-shaped bacteria (which were distinctive for Spirochaetes) beneath the outer epidermis and embedded within body wall tissues (Holland and Nealson, 1978; Kelly et al., 1995; Foret and Lawrence, 2001). Cultivation-based surveys, which are highly biased toward taxa that grow on surfaces and in nutrient-rich conditions, reflected mostly copiotrophic taxa (i.e., microorganisms capable of rapid assimilation of compounds and subsequent rapid cell division) (e.g., Narita et al., 1987; Choi et al., 2003; Beleneva and Zhukova, 2009; Rivera-Posada et al., 2011b; Luo et al., 2013; Hewson et al., 2018; Table A1). However, most bacterial taxa in marine habitats cannot yet be cultivated; as a consequence, less biased molecular barcoding approaches are typically used to examine the structure of microbial communities inhabiting marine habitats.
Despite the known cultivation biases, many commonly reported orders, families, and genera of bacteria in culture collections (Table A1) are also common constituents of cultivation-independent surveys (Nakagawa et al., 2017; Hewson et al., 2018; Hoj et al., 2018; Jackson et al., 2018; Lloyd and Pespeni, 2018; Nunez-Pons et al., 2018; Table A2). These taxonomic groupings represent microorganisms that are typically found in organic nutrient-rich environments in marine habitats.
By contrast, other aspects of asteroid-associated micro-diversity (e.g., fungi and protists) have not been extensively documented. Nunez-Pons et al. (2018) examined fungal communities associated with grossly normal Odontaster validus and found that communities were dominated by asco- and basidiomycetes (notably, Saccharomycetes, Eurotiomycetes, Dothideomycetes, and Agaricomycetes). The parasitic ciliate Orchitophrya stellarum is known to infect testes of males and castrate them (Cepede, 1907; Peters, 1992; Lawrence, 2013) in a range of species (Vevers, 1951; Leighton et al., 1991; Byrne et al., 1997, 1998; Stickle et al., 2001; Stickle and Kozloff, 2008; Sunday et al., 2008). While Orchitophrya does not cause gross disease signs (Cepede, 1907; Bang, 1982), it may lead to mortality, especially for males (Leighton et al., 1991; Claereboudt and Bouland, 1994), and reduced gamete abundance or fertility. Otherwise asymptomatic individuals may also be infected by an unidentified apicomplexan parasite reported in Asterias amurensis (Goggin and Bouland, 1997). Asteroids may host labyrinthulids (FioRito et al., 2016) and mesomycetozoa (Kerk et al., 1995; Ragan et al., 1996; Glockling et al., 2013; Paps et al., 2013; Hewson and Sewell, 2021). The functions of these less well-studied organisms in sea star health and disease are unclear, and they raise questions of whether such diversity is involved in SSW transmission or symptomatic progression.
Viruses inhabiting tissues of grossly normal sea stars are diverse and include both DNA and RNA viruses (Hewson et al., 2014, 2018; Jackson et al., 2016, 2020a). While much early attention in the SSW outbreak focused on the sea star-associated densovirus (SSaDV, renamed Asteroid ambidensovirus-1 by the International Committee on Taxonomy of Viruses; Hewson et al., 2014), this viral genotype was one of many inhabiting clinically normal asteroids and appears to be a common constituent across grossly normal echinoderms worldwide (Gudenkauf et al., 2014; Hewson et al., 2018; Jackson et al., 2020a, b; Hewson and Sewell, 2021). Recent work has demonstrated that these taxa may develop persistent infections (Jackson et al., 2020a). Asymptomatic individuals also bear picornaviruses, dicistroviruses (Hewson et al., 2018, 2020a), circular rep-encoding single-stranded DNA viruses (Jackson et al., 2016), and nucleocytoplasmic large DNA viruses (NCLDVs), as well as abundant bacteriophage (Hewson et al., 2014). As such, due to the diversity of potential pathogens identified in affected organisms, and the inherent challenges of fulfilling Koch’s postulates—even using molecular data (Fredericks and Relman, 1996)—in an invertebrate for which no cell cultures for viral isolation are available, it has become very difficult to point to any virus as a cause of SSW; hence, additional mechanisms are being considered.
Sea Star Wasting
Sea star wasting has affected dozens of sea star species, spanning a broad phylogenetic breadth (LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data). There are also numerous instances preceding the 2013–2014 outbreak, and comparing the signs and etiology across such instances can be difficult (Hewson et al., 2019). For this reason, it is not clear whether the 2013–2014 outbreak is mechanistically related to prior or ongoing observations of wasting; we do not know whether the causes are the same across species or across events. A focus in this paper is on common structural traits of sea stars that may provide clues as to the signs and mortality that follow multiple potential causes, ranging from apparent pathogens to environmental stressors (Hewson et al., 2018).
Sea stars, like other invertebrates, have a limited host response repertoire, so it would not be surprising that they respond to insults by manifesting lesions in the tissues that are most abundant (epidermis, MCT). Because many of the affected species during the 2013–2014 SSW event are sympatric, they likely have similar exposure rates to the causal agent(s) of the disease (Eisenlord et al., 2016) or environmental stressors (M. Dawson, University of California Merced, LMS, unpubl. data). This indicates that differences in the appearance or outcome among species may, instead, be driven by variations in the host immune response or other physical and physiological vulnerabilities. Understanding such variation may guide researchers in interpreting prior and ongoing asteroid wasting events that have similar signs or outcomes.
Regardless of why particular species were disproportionately affected by SSW over others, the die-off noted in 2013–2014 led to ecological shifts in echinoderm populations (M. Dawson, University of California Merced, LMS, unpubl. data). For example, Dermasterias imbricata (the leather star) has increased in abundance at many sites (Montecino-Latorre et al., 2016), and Kay et al. (2019, p. 1) document “reciprocal abundance shifts” in the Salish Sea between the heavily affected Pisaster ochraceus and the less affected Evasterias troschelii. Other species, such as the sunflower star (Pycnopodia helianthoides), experienced sharp declines across their entire range, resulting in a nearly complete loss south of Oregon and functional depletion in much of their northern range (Harvell et al., 2019). Pycnopodia helianthoides was recently listed by the International Union for Conservation of Nature as critically endangered, with greater than 90% of individuals lost (Gravem et al., 2021). By contrast, it is unclear why species like D. imbricata persisted so successfully, but one hypothesis is that this species may have morphological or life-history characteristics that affect disease severity (see LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data). As an example of structural or ontogenetic variation that may be important, P. ochraceus ochre morphs appear to be affected more than purple morphs (Raimondi et al., 2007; Work et al., 2021). This trait itself may be plastic (Harley et al., 2006), serving as an indicator of other factors in the life of an asteroid that may be associated with severity of response.
Disease signs
While minor visible and pathological differences exist among host species, the onset of SSW is generally characterized by a progression of key visual signs. Sea stars first appear lethargic and uninterested in nearby prey (Kohl et al., 2016), followed by an overall loss of body turgor and the ability to attach (Schultz et al., 2016). Signs become more pronounced as arm rays twist and contort, and the sea stars quickly develop characteristic white lesions that mottle the epidermis (Figs. 1, 2; Hewson et al., 2014; Eisenlord et al., 2016; Bucci et al., 2017).
Figure 2.
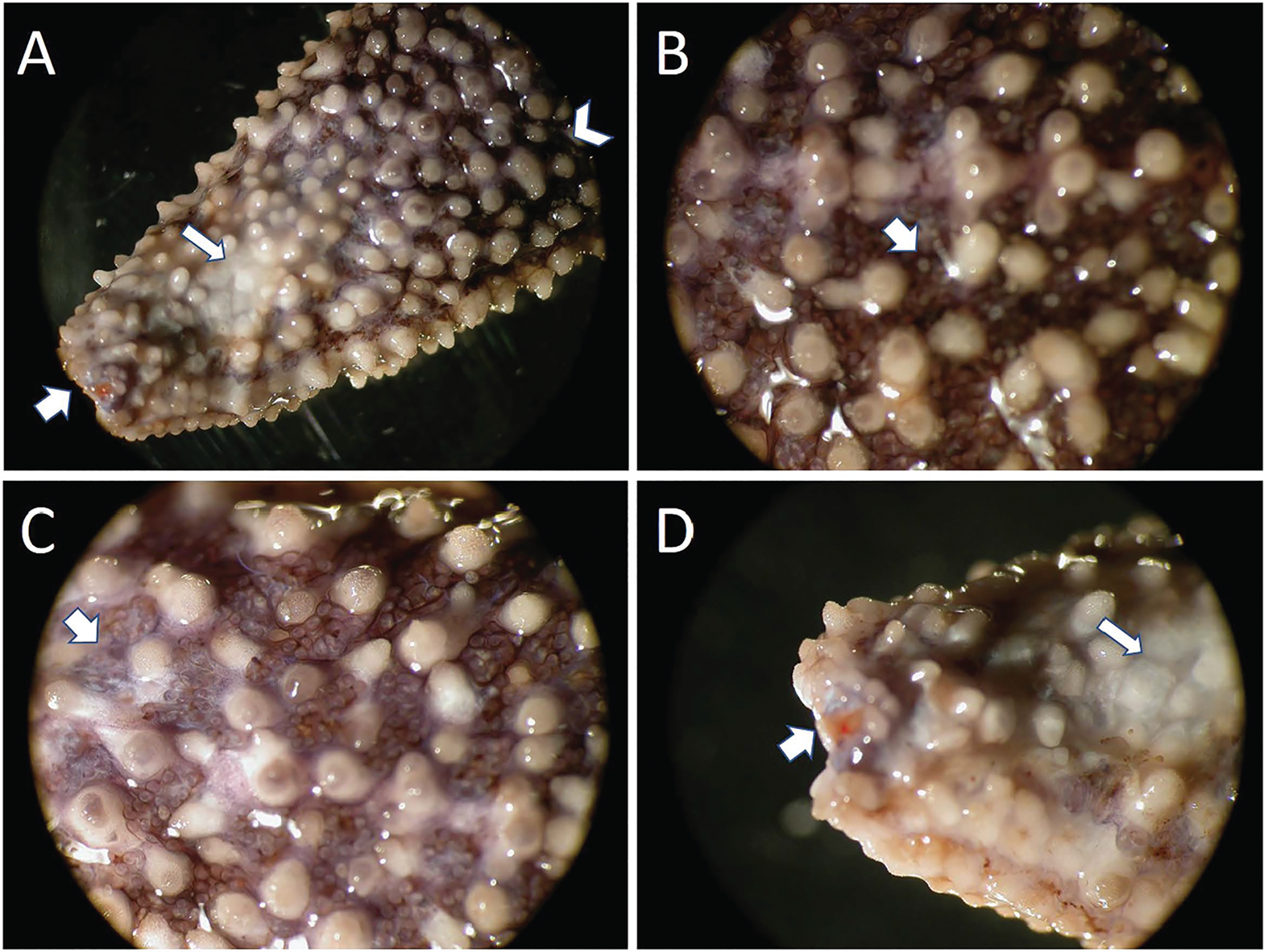
Progression of gross lesions in the aboral side of sea star wasting in Asterias forbesi. (A) Arm tip of an affected sea star showing a progression from healthy-looking tissue (chevron, top right) to broad areas of discoloration and loss of cuticle and epithelial tissue (narrow arrow) and severe ulcers leading to loss of tissue at the tip of the arm and exposure of underlying ossicle and the tip of the radial canal (broad arrow). (B) Close-up of early-stage lesion with small pinpoint white lesions (broad arrow) and early degradation of the spines. (C) Close-up of a more advanced lesion showing further development of pinpoint white lesions (broad arrow) and spine degradation. (D) Close-up of the tip of the arm in (A) showing advanced lesions with total loss of epithelium (narrow arrow) and exposure of the underlying tissues and ossicles and the tip of the radial canal (broad arrow).
Autotomy, or the detachment of arms (Fig. 1), is a regular occurrence as lesions extend more deeply and wider into the tissues. Body wall tissues degrade and slough off, and the sea stars are often described as dissolving or melting from the outside inward as the animal dies (Eisenlord et al., 2016). Progression of the disease may occur at a rapid pace: Menge et al. (2016) observed one star struggling to maintain its grip during a changing tide; four hours later they returned to find the animal in the final stages of disintegration and death.
Of course, SSW does not always progress linearly; there are cases documented where lesions form and then go away, limbs autotomize prior to lesions, or the lesions can form in the absence of deflation or arm twisting (Kohl et al., 2016; Menge et al., 2016). The progression can vary between species or even between individuals within a species (Wares and Schiebelhut, 2016). The signs of SSW can also differ between species. For example, as opposed to the deflated Pycnopodia presented in Figure 1, Dermasterias can appear bloated and may have blue lesions rather than white. In some cases, the gonads are larger than in healthy animals and protrude out of the body through the lesions (SJW and Lesanna L. Lahner, Seattle Aquarium, pers. comm., June 2020). This gonadal bloating is suggestive of a problem with osmotic homeostasis, and this hypothesis is also supported by the increase in chloride concentration detected in the coelomic fluid of affected sea stars (see Wahltinez et al., 2020 and Immune responses in sea star wasting).
The aforementioned outward signs of SSW are not by themselves definitive proof that the animal has SSW. For example, loss of body turgor can occur normally in intertidal sea stars when exposed at low tide. Arm curling appears to be a normal response to a physical wound (Fig. 3A, B) and may be observed in healthy, unwounded sea stars as well (Fig. 3C). Arm autotomy also occurs following predation attempts (Ramsay et al., 2000) and temperature stress (Pincebourde et al., 2013). A related natural process of asexual reproduction, by splitting the body in half (fission) followed by regeneration, is seen in a few taxa such as Coscinasterias muricata (Byrne, 2020). Interestingly, SSW seems to be an extreme expression of these behaviors that occur typically in sea stars.
Figure 3.

Arm twisting is a behavior that can be present in both healthy and wasting sea stars and, thus, cannot always be attributed to sea star wasting (SSW). (A, B) Pycnopodia helianthoides. (A) shows the wound, and (B) shows how the non-wasting sea star covers this wound by twisting its arms. (C) Pisaster ochraceus. The twisting behavior is here observed in a sea star that showed no other signs of SSW. Photo credits: (A, B) Julia Kobelt; (C) Mike Dawson.
Histology
During the summer of 1991, an epizootic of SSW occurred in Asterias forbesi, with numerous animals washing up on the beaches along the coast of Long Island Sound and the Gulf of Maine (RS, unpubl. data). The animals had not autotomized arms but did show flaccidity and extensive erosive, ulcerative, and penetrating lesions of the dorsal and dorsolateral surfaces of the arms. The ambulacral groove and tube feet were not affected, and no internal abnormalities were apparent. Gonads were shrunken in evaluated specimens; however, gonad maturity is seasonal, and it is not clear whether this is a sign associated with the SSW event. In terms of histology, internal organs showed no lesions; however, examination of the dorsal epithelium showed areas of progressively necrotizing dermatitis, characterized by an accumulation of coelomocytes in the superficial dermis and the base of the overlying epithelium, with vacuolation of epithelial cells and sloughing of the epithelial layer. Edema and necrosis of the deeper dermal connective tissues and underlying ossicles occurred in the ulcerated regions.
During the SSW epizootic that occurred on the Pacific coast in 2013, histology was evaluated for symptomatic and asymptomatic individuals of 13 asteroid species affected by the event (ALN, unpubl. data). Histologic lesions in P. ochraceus and P. helianthoides were similar to those described above for A. forbesi (Figs. 4, 5; as in Bucci et al., 2017). Some areas exhibited epidermal degeneration and necrosis with ulceration. Areas of epidermal loss were associated with edema in the subjacent dermal connective tissue and coelomocyte infiltrates. In addition to the epidermal and dermal changes, coelomocyte aggregates were apparent within the coelomic cavity, vascular system, and papulae, with frequent adhesion of coelomocytes to the coelomic epithelial lining. There were no primary lesions noted within the coelomic viscera or dermal ossicles, and no infectious agents were apparent (ALN, unpubl. data). More recent studies looking at experimental induction of SSW in P. ochraceus revealed that pathology involved a basal to surface process starting with infiltration, breakdown of MCT, and lysis, leading to epidermal ulceration that had a greater effect on the purple than the ochre morphs. Indeed, ochre stars survived longer, with more apparent and extensive signs of SSW, than those that are purple (Work et al., 2021).
Figure 4.
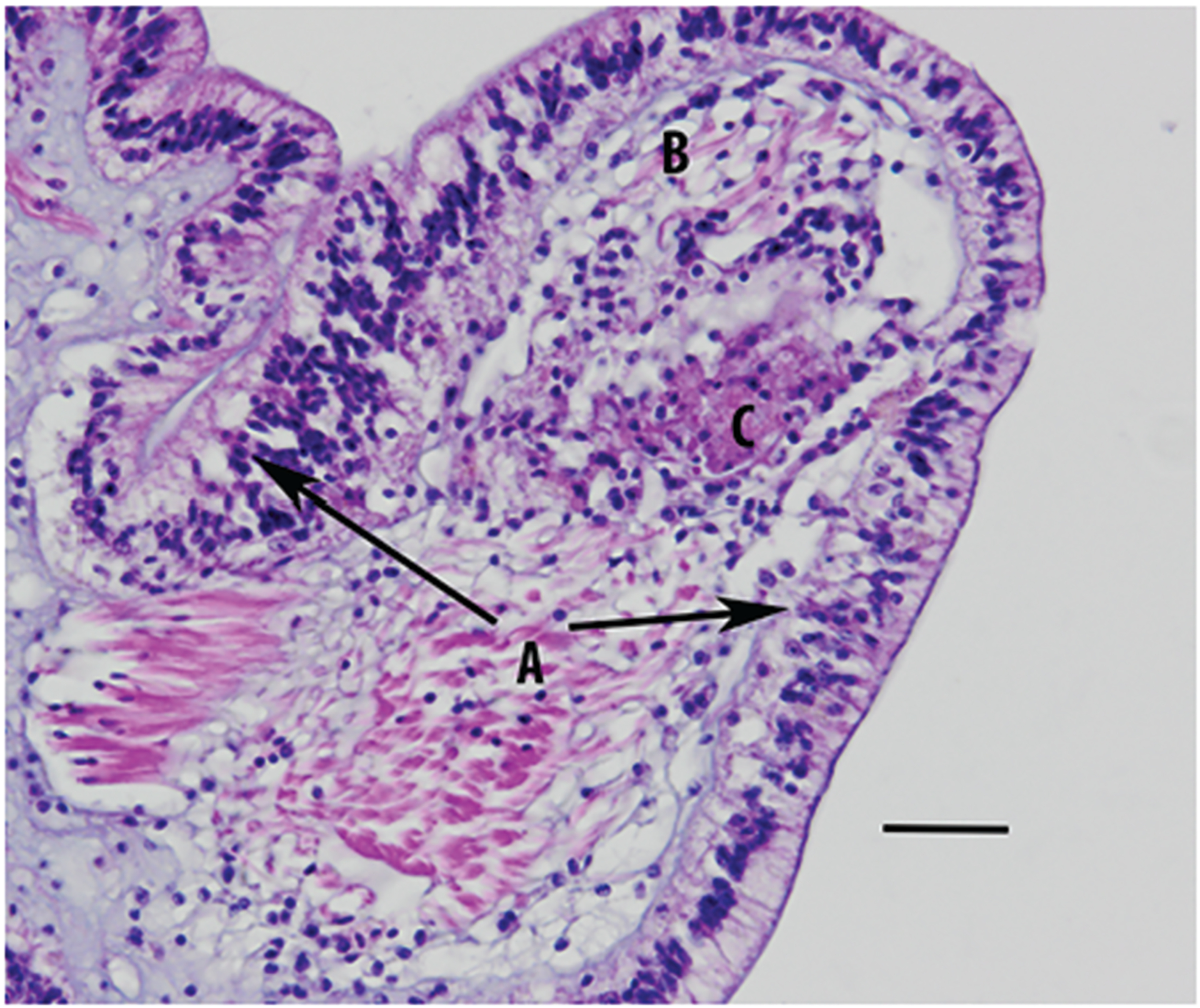
Early lesions of the dorsal epithelium of an Asterias forbesi individual with sea star wasting (SSW). (A) Vacuolation and necrosis of epithelial cells with influx of hemocytes (arrows) and disruption of the cuticle. (B) Edema of underlying connective tissues. (C) Necrosis of muscle and associated underlying connective tissues. Scale bars = 32 μm.
Figure 5.
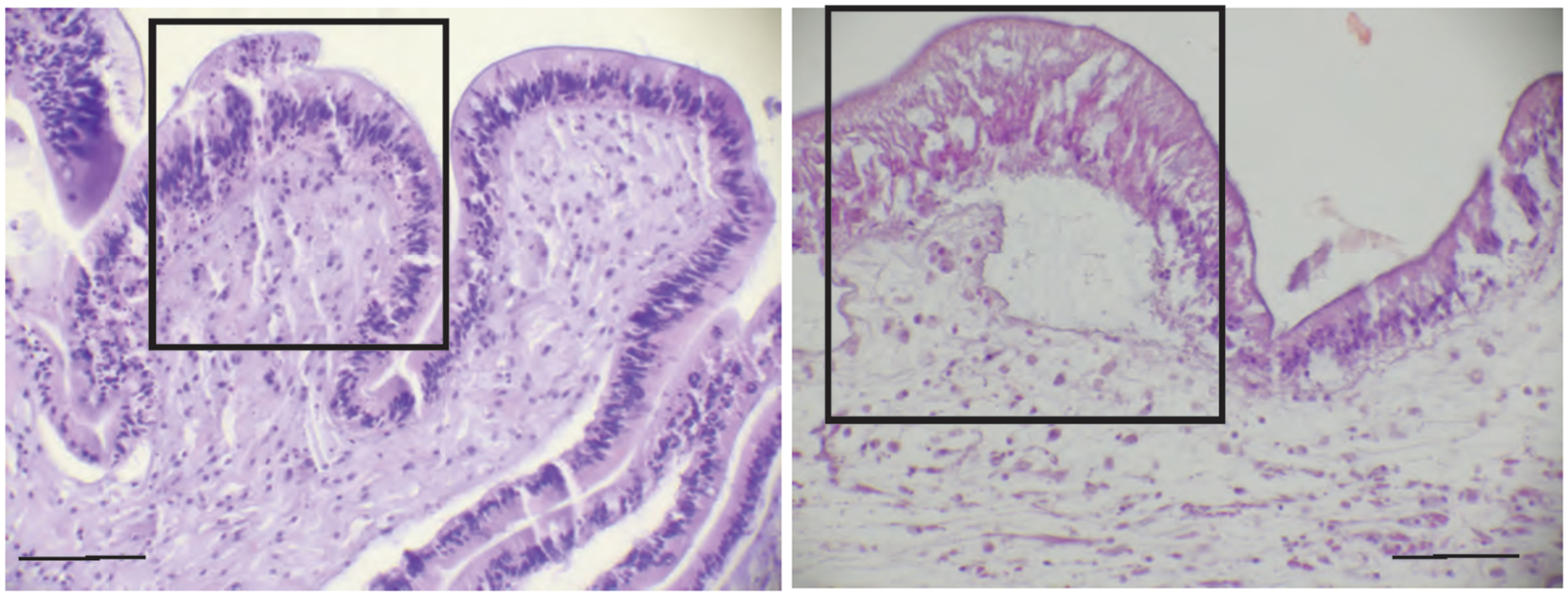
Common lesions noted in the epidermis and dermis. On the left (Pisaster ochraceus), within the box, there is multifocal epidermal degeneration and lytic necrosis characterized by a loss of cell distinction, nuclear condensation, and fragmentation (karyorrhexis). On the right (Pycnopodia helianthoides), there is coagulation-type epidermal necrosis characterized by loss of cellular detail, particularly nuclear and cell margin distinction with increased uptake of eosin stain (pink staining) by the cytoplasm. Both types of lesions were associated with microscopic epidermal loss or ulceration in some specimens. The right image also shows the two most common lesions within the dermis: separation of the connective tissue fibers by edema and infiltrations of coelomocytes consistent with inflammation. A low instance of dermal degeneration and necrosis also occurred. Scale bars = 50 μm.
The function and pathophysiological changes seen in the body wall in afflicted stars are similar to those observed with the breakdown and autolysis of MCT during arm autotomy and at the separation plane during asexual reproduction by fission in sea stars, processes that are modulated by the nervous system (Mladenov and Burke, 1994; Wilkie, 2002; Byrne et al., 2019). Hence, the changes in the body wall during SSW might, fundamentally, be a neurological disease leading to inability to control softening of the MCT to the point that total dissolution occurs. Additional research is needed to test this hypothesis and indeed to better understand neural control of MCT tensility. Fuess et al. (2015) noted changes in transcript abundance for genes associated with nervous system processes when comparing wasting versus non-wasting stars. These differentially expressed transcripts included many associated with MCT, supporting concern that the arm twisting and autotomy could be related to dysregulation of this system.
Pedicellariae
As noted previously, a difficulty in defining disease in organisms like sea stars is to separate abnormal condition from injury—this is not easy when body structures such as pedicellariae are involved. In 2014, a mixed-species group of sea stars, including Pisaster brevispinus, Pisaster ochraceus, Evasterias troschelii, Dermasterias imbricata, and Orthasterias koehleri, were placed in tanks for a study on SSW (SJW and Lesanna L. Lahner, oral comm., June 2020). Detached pedicellariae from P. ochraceus were noted independently moving and penetrating the coelomic cavity of D. imbricata. This observation was associated with clinical signs of SSW in D. imbricata (epidermal lesions, drooping limbs). Removal of the pedicellariae reversed these clinical signs (SJW and Lesanna L. Lahner, oral comm., June 2020). Though a sudden and dramatic uptick in agonistic interactions would itself be of interest, these interactions became a focus of study into mechanisms driving SSW.
To investigate the potential connection between pedicellaria attack and SSW, flow-through holding tanks were set up, each with two adult D. imbricata individuals and two adult P. ochraceus individuals (SJW and Lesanna L. Lahner, oral comm., June 2020). Both species initiated contact, and physical contact was required for pedicellariae transfer (i.e., defense response) from P. ochraceus to D. imbricata, with the majority of the pedicellariae (88.9%) transferred to the oral surface. In the area surrounding the transferred pedicellariae, the ambulacral groove closed, and tube foot dysfunction was noted. These signs immediately resolved following manual removal of the pedicellariae (SJW and Lesanna L. Lahner, oral communication, June 2020). These signs are consistent with what has been noted after pedicellariae transfer from Pisaster giganteus and P. brevispinus to Pycnopodia helianthoides (Wobber, 1975) and from P. ochraceus to Solaster dawsoni (VanVeldhuizen and Oakes, 1981). The disuse of a limb following pedicellariae transfer could be due to a toxin, as seen in echinoid globiferous pedicellariae (Jensen, 1966; Chia, 1969), irritation, or another mechanism. Transfer of pedicellariae could represent a novel pathway for pathogen transmission or may predispose the recipient to infection through disruption of the epidermis or could contain an agent that causes the MCT to break down.
Immune responses in sea star wasting
Very limited information is available on the immune responses of wasting stars. This is in part because of unclear associations of a putative pathogen across taxa—the densovirus initially proposed as a causal agent (Hewson et al., 2014) is now known to be as frequently found in asymptomatic individuals as in those showing signs of SSW. The overall class of viruses that have been primarily focused on (SSaDV) is still potentially associated, in terms of viral load, with wasting disease in P. helianthoides (Hewson et al., 2018) but does not correspond with the outcome of other challenged species—yet we still lack mechanistic knowledge of the immune systems of the highly diverse group of species that are affected by wasting (LMS, M. Giakoumis, M. Dawson, University of California Merced, unpubl. data). Coelomic fluid in P. ochraceus with SSW had significantly increased chloride concentration and increased osmolality and coelomocyte count, suggestive of an osmotic breakdown (Wahltinez et al., 2020), when compared to clinically normal conspecifics (Wahltinez et al., 2019). The increased chloride concentrations and hypertonic coelomic fluid may be due, at least in part, to wound healing or inability to regulate osmolality of the coelomic fluid secondary to skin ulceration. An increase in chloride permeability was noted in sea star oocytes after wounding (Fein and Terasaki, 2005). In a more recent study, P. ochraceus manifesting experimental SSW showed a significant increase in a heretofore undescribed type of coelomocyte, illustrating our very limited knowledge of sea star host responses to insults (Work et al., 2021).
Another approach of great relevance to SSW is to measure the transcriptional profile in the coelomocytes of a wasting animal compared to healthy individuals. Fuess et al. (2015) used P. helianthoides to explore the transcriptomic response of coelomocytes from sea stars injected with a <0.2-μm tissue filtrate from an afflicted sea star. Within several days, disease signs were seen in the test animals, but not in the control injected animals. Remarkably, over 1000 immune-related genes were identified that were significantly changed in expression profile compared to the coelomocytes of the control animals. These included PRRs and other immune receptors, intracellular signaling, transcription factors, cytokines and growth factors, and immune effector genes, as well as genes involved in coagulation and the complement system and homologs of genes that function in the vertebrate adaptive immune system.
The study of Fuess et al. (2015) raises a series of questions. Specifically, which of these genes are most important to SSW pathogenesis, and just how does this gene expression profile relate to host pathology at the cellular level? Also lacking in this experiment was a control for allorecognition reactions, such as a heat-killed tissue homogenate. Recall that the immune system of the sea star was defined early on by allorecognition and tissue rejection (Smith et al., 2010; Gordon, 2016a). Indeed, Aquino et al. (2021) argued that the <0.2-μm size fraction of sea star-derived filtered homogenate used in similar challenge studies may contain a broad array of dissolved organic matter, which ultimately may lead to allorecognition but also may stimulate heterotrophic bacteria in subcuticular space or on or near surfaces, which ultimately may cause oxygen depletion and tissue degradation.
Regardless, the study of Fuess et al. (2015), as one of the very few contemporary studies on sea star molecular immunity, yielded valuable information on the transcriptomic response of sea stars to non-self that could be used to develop more precise tests. An important follow-up experiment could involve exposure of healthy stars to recombinantly expressed proteins of candidate virus particles associated with SSW, as well as with specific antigens (PAMPs), environmental toxins, pedicellaria attack, or isolated pathogens (e.g., bacteria) to test immune responses. The signature of SSW in these transcriptomic data has already been partially picked up in independent post-selection and heat stress experiments that are mapped to the genome of P. ochraceus (Ruiz-Ramos et al., 2020). The search for consilience across several studies exploring wasting or stressors is a hopeful guide toward key mechanisms involved in the response.
As an alternative to whole-animal studies, in vitro approaches have been performed by exposing purified immune cells (coelomocytes in absence of serum from the water vascular system) to various insults and then measuring in real time their responses, using medium- to high-throughput assays (Matranga et al., 2002; Pinsino and Matranga, 2015). It may be more efficient to study responses in sea star larvae; immunocytes of sea star larvae share several similarities with adult coelomocytes, showing the capability for phagocytosis of cellular debris and foreign agents that invade blastocoel. Furukawa et al. (2009) showed that sea star larval immune cells phagocytose small foreign particles injected into the blastocoel within two hours. When small amounts are inserted, few cells respond. However, when larger particles are inserted into the blastocoel, multiple cells converge onto the site from within the blastocoel and undergo cell-cell fusion to form a syncytial multinucleated giant cell that encapsulates the aggregated particles. Similar syncytial formations have also been noted for adult phagocytes in vitro (Majeske et al., 2013). The number of recruited immune cells is dependent on the amount and size of the foreign substance, indicating that the process of clearing the blastocoel is strictly regulated.
Candidate genes involved in this regulation appear to be two macrophage migration inhibitory factors (MIFs): ApMIF2 and ApMIF1 (Furukawa et al., 2016). These immune effectors are evolutionarily ancient and highly conserved (Furukawa et al., 2016). Therefore, the immune cell behaviors of chemotaxis, cytoskeletal modifications, and syncytia formation provide excellent nodes to investigate how the innate immune system functions generally, in a larva, and likely elements that are shared in an adult. Intriguingly, in sea cucumbers, the virus associated with the acute peristome edema disease has been detected in both adults and 30-day-old diseased larvae (Wang et al., 2007). Currently, it is unknown whether embryos and larvae of sea stars are affected by SSW as well.
Potential Agents Associated with Sea Star Wasting
It is difficult to isolate the cause of SSW, which remains unknown in all spatial, temporal, and taxonomic context, from the proliferation of microbial or viral agents that may be found in compromised sea star tissues. There are often changes in microbial communities of affected individuals that indicate proliferation of several bacterial taxonomic groups and viruses (Hewson et al., 2014, 2018, 2020a; Lloyd and Pespeni, 2018; Aquino et al., 2021). Comparative viral metagenomic study of asymptomatic and wasting-affected tissues revealed a proliferation of a virus (SSaDV; Hewson et al., 2014). However, further study with redesigned quantitative polymerase chain reaction (qPCR) primers targeting only SSaDV and excluding homologous non-SSaDV densoviruses revealed no association with wasting (Hewson et al., 2018). More recent approaches also found no association between viruses and disease and found that densoviruses form persistent infections in their hosts (Jackson et al., 2020a, b). More recent work tracking viral populations during progression of SSW in controlled laboratory experiments has revealed that wasting is associated with elevated richness of all viral genotypes, which may indicate a general proliferation of viruses in affected tissues or a down-regulation of the immune responses (Hewson et al., 2020a).
Screening of SSW-affected tissues by histopathology in 2013 yielded no protist cells associated with lesions (Hewson et al., 2014). Comparisons of fungal communities in Odontaster validus revealed no difference in associates between asymptomatic and SSW-affected states (Nunez-Pons et al., 2018). While there have been no pan-eukarya surveys of protistan diversity in wasting asteroids, viral metagenomes also yield sequences of co-extracted and amplified ribosome-bound rRNAs (Hewson and Sewell, 2021). A survey of unicellular fungal and other eukaryotic 18S and 28S rRNAs in Pisaster ochraceus during wasting progression (Hewson et al., 2020a) did not reveal any single operational taxonomic unit (OTU) consistently associated with wasting (Fig. A1). In summary, there is little consistent evidence that infectious agents were responsible for the 2013–2014 mortality event on the west coast of North America. Though the information available sometimes suggests association of viral load with signs of disease in certain populations (Hewson et al., 2018), the diverse and complex observations of SSW across the breadth of environmental and viral and prokaryotic communities that can be detected have made it challenging to narrow the likely sources.
Bacteria and Archaea also change in abundance and composition during SSW. It is believed that this is mainly due to stimulation of taxa that degrade organic matter released from decaying tissues. It is therefore unsurprising that the bacterial orders that associate with wasting tissues (Table A2)—notably Flavobacterales (Lloyd and Pespeni, 2018), Rhodobacteriales (Nunez-Pons et al., 2018), and Campylobacterales (Hoj et al., 2018)—are copiotrophs (Haggerty and Dinsdale, 2016). In terms of overall community structure, assemblages are different between healthy and diseased states (Nunez-Pons et al., 2018). In Acanthaster sp., disease was associated with increasing dominance by fewer OTUs in the pyloric caeca but decreasing dominance (i.e., greater evenness) in the body wall tissues (Hoj et al., 2018). Indeed, injection of bacterial culture media into Acanthaster sp. causes wasting-like lesions and animal death; isolated bacterial cultures from lesions included taxa in the genera Vibrio and Photobacterium (Rivera-Posada et al., 2011a, c). Moreover, healthy animals in contact with these infected animals also displayed signs of disease and died within 24 hours, indicating that these first observations were not due to a toxic shock after injection of the media. Because SSW is mainly defined by gross lesions that could have multiple etiologies, sorting out causes of sea star mortalities will require more systematic effort combining careful observations of tissues at the cellular level coupled with studies to relate molecular processes to tissue changes for each species.
Environment
Environmental effects on agents
Environmental conditions potentially affect the abundance and composition of microorganisms associated with SSW as both commensals and opportunists. Although much attention has been given to environmental correlates with SSW since 2013 (Eisenlord et al., 2016; Kohl et al., 2016; Menge et al., 2016; Hewson et al., 2018; Miner et al., 2018; Aalto et al., 2020), no link has been found between the microbiome composition in affected asteroids and an environmental perturbation. Environmental stressors may be linked to dysbiosis, a change in microbiome composition relative to the normal state (Egan and Gardiner, 2016); they could induce a proliferation of opportunistic pathogens when host tissues are compromised (Burge et al., 2014). These stressors could lead to hypoxia through bacterial heterotrophic respiration (Haas et al., 2011; Gregg et al., 2013; Aquino et al., 2021). Environmental conditions could directly affect the ability of the host to respond to insults. Although viruses do not bear innate metabolism, their replication in tissues may be influenced by the intracellular conditions of their hosts. Thus, their proliferation may reflect breakdown in homeostatic properties as a consequence of environmental stressors in their poikilothermic hosts (Frakolaki et al., 2018; Hewson et al., 2020a). It is worth noting that common taxa inhabiting asymptomatic asteroid specimens, including Rhodobacter (Erythrobacter), flavobacteria, and alteromonads, include some of the largest genome-bearing and fastest growth-rate taxa in the picoplankton, which emphasizes their ability to rapidly adapt to changing conditions. The proliferation of these taxa during SSW may reflect their ability to rapidly assimilate organic matter from the environment surrounding asteroids (Aquino et al., 2021).
Environmental stressors
A common hypothesis is that anomalies in temperature may elicit or exacerbate SSW. Although most studies have revealed that SSW is positively correlated to elevated sea surface temperatures (Bates et al., 2009; Eisenlord et al., 2016; Kohl et al., 2016; Harvell et al., 2019; Aalto et al., 2020), other studies either have found no empirical relationship between the two variables (Hewson et al., 2018) or concluded instead that a reduction in sea surface temperature (Menge et al., 2016) is inducing or exacerbating the wasting disease. The context-dependent relationship between temperature and SSW may result from an integration of both short- and long-term stressors but may also instead indicate that the magnitude of the temperature anomaly induces wasting, regardless of the direction. In addition, some basic environmental variables can serve as proxies to environmental stressors, such as low-tide exposure and wave action (M. Dawson, University of California Merced, LMS, unpubl. data). Early symptoms of SSW are similar to those resulting from other sources of stress in sea stars, such as starvation, desiccation, or injury (Miner et al., 2018; Van Volkom et al., 2021). What is most intriguing about SSW is that in many locations, predictive environments for sea star assemblages are changing rapidly (Kay et al., 2019; Konar et al., 2019).
Recently, Aquino et al. (2021) provided evidence that SSW could be related to microbial processes—driven by temperature and organic matter availability—at the animal-water interface. Microorganisms inhabiting the diffusive boundary layer around individual specimens may respond to inputs from surrounding waters. In marine environments, a large proportion of this organic matter is in the form of phytoplankton exudates (Ogawa and Tanoue, 2003). Aquino et al. (2021) observed correspondence between chlorophyll a peak (and decline) and SSW mass mortality at a field site; and Hewson (2021) noted correspondence between SSW and upwelling at several sites, so it is plausible that SSW is influenced by water column processes, such as stratification, nutrient availability, and temperature. Linking SSW to biological oceanographic phenomena may also help explain the multi-host nature of SSW and the apparent discontinuous geographic appearance of SSW in 2013–2014, as well as the seasonal nature of the condition in years since (Bates et al., 2009). We note here that mass mortalities of echinoderms from other possible environmental mechanisms, such as the toxins from harmful algal blooms, can be contrasted in parts of California (Jurgens et al., 2015) with those of SSW. Related toxins were evaluated in sea stars, with no relationship to wasting status (Hewson et al., 2018).
Finally, asteroids produce chemical compounds under stress that may induce wasting-like conditions in grossly normal specimens when challenged via direct injection. In response to short exposure (45–90-s) to heat stress, asteroids release proteinaceous compounds within their coelomic fluid that cause almost immediate body wall softening, limb autotomy, and death when injected into healthy specimens (Chaet, 1962; Mladenov et al., 1989). Although these factors were obtained by immersing specimens at 76 °C, an extreme that these species would never experience, these results illustrate that stressed asteroids have the potential to affect the health of surrounding individuals. Because these compounds also occur within the material tested during challenge experiments (Hewson et al., 2014, 2018; Bucci et al., 2017; Aquino et al., 2021), it is possible that such factors may have been responsible for the observation of apparent transmission and bring us full circle with questions about changing density and abundance.
Contrasts with other marine diseases and mass mortality events
Unfortunately, SSW is not a unique phenomenon in terms of huge, sudden impacts on marine communities. Our growing recognition of mass mortality events in the ocean (e.g., coral bleaching and death) (Hughes et al., 2018a, b) dovetails with the associated recognition of how climate change will ultimately affect many taxa, for example, dolphins (Duignan et al., 2020), manatees (Hardy et al., 2019), and other invertebrates (Petraitis and Dudgeon, 2020). The difficulties in pinpointing a cause for disease are increased when the environmental background is rapidly changing (Burge et al., 2014; Groner et al., 2016; Tracy et al., 2019).
Extraordinary waves of disease are now more frequently the focus of marine research (Harvell et al., 1999). Multiple outbreaks of seagrass wasting are being explored in the western Atlantic, northeastern Pacific, and elsewhere (Short et al., 1987; Muehlstein et al., 1991; Sullivan et al., 2018). A series of devastating diseases have reduced coral abundance in the Caribbean—the most recent wave being stony coral tissue loss disease (Aeby et al., 2019; Landsberg et al., 2020)—concomitant with increasing frequency of climate-driven bleaching. Sponges (Olson et al., 2006), shrimp (Frischer et al., 2017), and salmon (Asche et al., 2009) were also affected by major disease outbreaks that, together with climate change, are shifting entire ecosystems (Tracy et al., 2019).
Focusing on echinoderms, we are left to look back on previous mass mortality events for lessons of what comes next. Echinoids are known to be affected by bald sea urchin disease (Johnson, 1971; Maes and Jangoux, 1984). They present symptoms such as body wall lesions, where spines, tube feet, pedicellaria, and epidermis are lost. Sometimes necrosis leads to the perforation of the test and the death of the animals. The bacteria later detected in this disease were found to be opportunistic instead of specific (Becker et al., 2008). In the case of Diadema antillarum, no mechanism is known for the mass mortality in the Caribbean; current study is focused on how and where it is recovering its numbers (Myhre and Acevedo-Gutiérrez, 2007; Lessios, 2016). In the Sea of Cortez, the recovery of Heliaster kubiniji has been spatially variable (Eckert et al., 2000).
Currently, some species have not recovered from SSW (Gravem et al., 2021), while others are returning to their former abundance (M. Dawson, University of California Merced, LMS, unpubl. data) but with localized die-offs from this unknown cause (Moritsch and Raimondi, 2018). We know there have been similar outbreaks and apparent recovery (Hewson et al., 2018), and we know that asteroids cycle demographically over long-time scales (Uthicke et al., 2009). Finally, there are current studies documenting new losses in holothuroids (Hewson et al., 2020b), and the ecological effects of loss are likely to have substantial effects on coastal benthic communities (Harrold and Pearse, 1987; McPherson et al., 2021).
Conclusions and Future Directions
Sea star wasting, characterized by behavioral changes, body lesions, and arm autotomy, has triggered periodic mass mortality events across a broad range of sea star species—and possibly with similar mechanisms across disparate events. To better characterize the etiology, the epidemiology, and the effect of SSW, it is essential to build a community of investigators that can share knowledge, samples, and protocols (Table A3) and monitor the corresponding environmental parameters around the world.
Understanding SSW—especially the most recent iterations—is challenging because it is now affecting so much diversity, the ecological consequences of which have yet to be fully appreciated. Our primary challenge is that the basic biology of the sea stars is surprisingly poorly known, making it difficult to directly compare healthy and symptomatic animals. This challenge is further compounded by studying these dynamics in a rapidly changing environment and with increased scrutiny of species and populations that have previously not been closely monitored. A deeper knowledge of the basic biology, health, disease, and life stage dynamics and of the growth of sea stars, with insights from a range of disciplines, will be essential to understand how and why these animals are so profoundly affected by or react to SSW conditions.
Acknowledgments
The virtual workshop supporting development of this project and participation of JPW, Michael N. Dawson (University of California, Merced), LMS, PD, and IH was funded by National Science Foundation (NSF) grants OCE-1737091, OCE-1737381, and OCE-1737127. We thank the reviewers for their comments and suggestions. We appreciate the encouragement of our many colleagues who have shared their previously unpublished data or results and photographers who have allowed us to use their images. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US government.
Abbreviations:
- MCT
mutable connective tissue
- OTU
operational taxonomic unit
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- SSaDV
sea star-associated densovirus
- SSW
sea star wasting
Appendix
Figure A1.
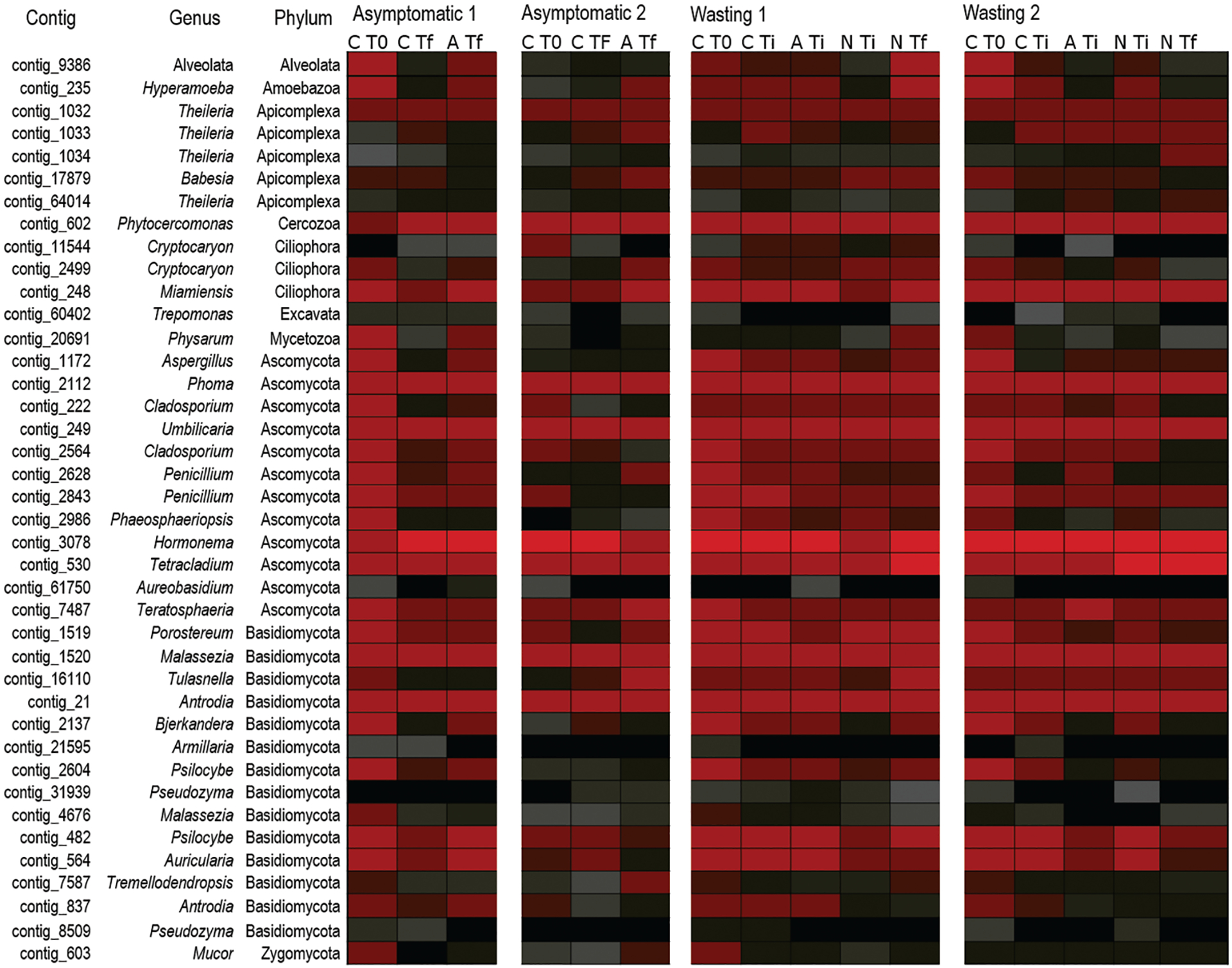
Heat map representation of 18S and 28S rRNAs recovered from metagenomes targeting material <0.2 μm during longitudinal study of asteroid wasting progression in control tissues, artificial scars, and wasting lesion margins. Red hues indicate greater numbers of library reads associated with taxonomy; black indicates no reads associated with taxonomy. There were no operational taxonomic units (OTUs) that were consistently present in wasting tissues across individuals but that were also absent from asymptomatic tissues on the same animal at the same time or in artificial scar tissues. C, asymptomatic tissue; A, artificial scar tissue; N, wasting lesion margin; T0, experiment initiation; Tf, time at death or for controls the end of observation period; Ti, time of appearance of lesion. Data derived from Hewson et al. (2020a).
Table A1.
Cultivated bacterial isolates from grossly normal asteroids
| Phylum | Class | Order | Family | Genus | Reference(s) |
|---|---|---|---|---|---|
| Proteobacteria | Gammaproteobacteria | Vibrionales | Vibrionaceae | Vibrio, Photobacterium, Aliivibrio | Narita et al., 1987; Rivera-Posada et al., 2011b; Hewson et al., 2018 |
| Alteromonadales | Pseudoalteromonadaceae | Pseudoalteromonas | Hewson et al., 2018; Luo et al., 2013 | ||
| Shewanellaceae | Shewanella | Hewson et al., 2018; Beleneva and Zhukova, 2009 | |||
| Colwelliaceae | Colwellia | Choi et al., 2010a | |||
| Alteromonadaceae | Marinobacter | Choi et al., 2003 | |||
| Pseudomonadales | Pseudomonadaceae | Pseudomonas | |||
| Moraxellaceae | Psychobacter | Choi et al., 2003 | |||
| Oceanospirillales | Halomonadaceae | Halomonas | Beleneva and Zhukova, 2009 | ||
| Hahellaceae | Kistomonas | Choi et al., 2010b | |||
| Alphaproteobacteria | Rhodobacteriales | Rhodobacteriaceae | Sulfitobacter, Lutimaribacter, Paracoccus | Choi et al., 2003; Ivanova et al., 2004; Zhang et al., 2016 | |
| Sphingomonadales | Sphingomonadaceaea | Erythrobacter | Choi et al., 2003; Ivanova et al., 2004 | ||
| Betaprotobacteria | Rhodocylales | Rhodocyclaceae | Zoogtea | Choi et al., 2003 | |
| Actinobacteria | Actinobacteria | Cotynebacterales | Beleneva and Zhukova, 2009 | ||
| Actinomycetales | Micorcoccaceae | Arthrobacter, Microbacterium, Kocuria | Beleneva and Zhukova, 2009; Choi et al., 2003 | ||
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | Beleneva and Zhukova, 2009; Choi et al., 2003 |
| Staphylococcaceae | Staphylococcus | Beleneva and Zhukova, 2009; Choi et al., 2003 | |||
| Bacteroidetes | Flavobacteria | Flavobacterales | Flavobacteraceae | Psychoserpens | Hewson et al., 2018; Beleneva and Zhukova, 2009 |
| Planctomyceles | Beleneva and Zhukova, 2009 |
Table A2.
Dominant bacterial taxa represented in cultivation-independent surveys
| Phylum | Class | Family | Asterias amuruneis a | Acanthaster planci b | Pisaster orchraceus c | Odontaster balidus d | Multiple speciese |
|---|---|---|---|---|---|---|---|
| Proteobacteria | Alphaproteobacteria | Rhodobacteraceae | * | ** | * | ** | *** |
| Caulobacterales | * | * | |||||
| Rhizobacterales | * | * | ** | ||||
| Sphingomonadales | * | * | |||||
| Rhodospirillales | * | ||||||
| Rickettsiales | * | ||||||
| Betaproteobacteria | Nitrosomonadaceae | * | |||||
| Burholderales | * | * | * | ||||
| Neisseriales | * | ||||||
| Gammaproteobacteria | Thiotrichales | *** | |||||
| Vibrionaceae | * | * | * | ||||
| Alcanivoracacaea | * | ||||||
| Alteromonadales | * | ** | * | ** | |||
| Enterobacterales | * | ** | |||||
| Oceanospirillales | *** | * | * | ||||
| Pseudomonadales | * | * | * | * | |||
| Xanthomonadales | * | * | |||||
| Chromatiales | * | ||||||
| Deltaproteobacteria | SAR324 | * | |||||
| Epsilonproteobacteria | Helicobacteraceae | *** | |||||
| Campylobacteraceae | * | * | * | ||||
| Oligoflexia | |||||||
| Bacteroides | Flavobacteriia | Flayobactericeae | ** | ** | ** | ** | |
| Cryomorphceae | * | ||||||
| Bacteroidea | * | ||||||
| Shpingobacteriia | * | ||||||
| Cytophagales | * | ||||||
| Cyanobacteria | Cyanophaceae | Oscillatoriacaea | * | ||||
| Firmicutes | Bacilli | Lactobacillales | * | ||||
| Clostridia | Clostridiales | * | * | ||||
| Bacillia | Bacillales | * | * | ||||
| Spirochaetes | Spirochetia | *** | *** | ** | |||
| Tenericutes | Mollicutes | *** | *** | *** | |||
| Verrucomocrobia | Verrucomicrobiae | Verrucomicrobiaceae | * | ||||
| Actinobacteria | Actinobacteria | Actinomycetaceae | * | * | |||
| Corynebacterales | *** |
One asterisk indicates taxon comprised <20% of sequences surveyed; two asterisks indicate taxon comprised >20% of sequences surveyed; three asterisks indicate taxon comprised >50% of sequences surveyed.
Table A3.
How to store the samples?
| Preservation method | Cost | IATA shipping regulation | Compatibility for hosts studies | Compatibility for molecular microbial studies | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA | DNA | Microscopy/TEM | Histology | RNA viruses | DNA viruses | Bacteria and Archaea | Eukaryotic microorganisms | |||
| Freezing at −20 °C | $ | Dry ice; packing instruction 904 | N | Y | N | N | N | Y | Y | Y |
| Freezing at −80 °C | $ $ $ | Liquid N2 not permitted; dry shippers exemption A152 | Y | Y | N | N | Y | Y | Y | Y |
| RNALater (×freezing) | $ $ | None | Y | N | N | N | N* | N | Y | Y |
| TriZol(+freezing) | $ $ | Corrosive liquid n.o.s., hazard class 8 | Y | Y | N | N | ** | ** | ** | ** |
| Formalin | $ | DG class II | N | N | Y | Y | N | N | N | N |
| ≥95% ethanol | $ | Exempted quantities | Y* | Y | N | N | Y* | Y | Y | Y |
Appropriate preservation and storage of the samples are key elements in these studies, and consistent sampling and storage are critical for current and future studies of wasting. In particular, we would like to offer simple sampling and storage protocols so that researchers anywhere can take advantage of wasting (and non-wasting) specimens to be most useful to the research community at large. Here, we provide best practices for characterizing and analyzing individuals that are encountered with wasting-like symptoms. One asterisk indicates significant loss of target organism or intact particles suitable for metagenomic approaches; two asterisks indicate that the effects of TriZol with freezing on the stability of microbial nucleic acids has not been assessed. In the cost column, the expected price of the method is described from the cheapest ($) to the most expensive ($$$). DG, dangerous goods; IATA, International Air Transport Association; n.o.s., not otherwise specified; TEM, transmission electron microscopy.
Literature Cited
- Aalto EA, Lafferty KD, Sokolow SH, Grewelle RE, Ben-Horin T, Boch CA, Raimondi PT, Bograd SJ, Hazen EL, Jacox MG et al. 2020. Models with environmental drivers offer a plausible mechanism for the rapid spread of infectious disease outbreaks in marine organisms. Sci. Rep 10: 5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeby GS, Ushijima B, Campbell JE, Jones S, Williams G, Meyer JL, Häse C, and Paul VJ. 2019. Pathogenesis of a tissue loss disease affecting multiple species of corals along the Florida reef tract. Front. Mar. Sci 6: 18. [Google Scholar]
- Anderson JM 1953. Structure and function in the pyloric caeca of Asterias forbesi. Biol. Bull 105: 47–61. [Google Scholar]
- Anderson JM 1954. Studies on the cardiac stomach of the starfish, Asterias forbesi. Biol. Bull 107: 157–173. [Google Scholar]
- Anderson JM 1959. Studies on the cardiac stomach of a starfish, Patira miniata. Biol. Bull 117: 185–201. [Google Scholar]
- Aquino CA, Besemer RM, DeRito CM, Kocian J, Porter IR, Raimondi PT, Rede JE, Schiebelhut LM, Sparks JP, Wares JP et al. 2021. Evidence that microorganisms at the animal-water interface drive sea star wasting disease. Front. Microbiol 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asche F, Hansen H, Tveterås R, and Tveterås S. 2009. The salmon disease crisis in Chile. Mar. Resour. Econ 24: 405–411. [Google Scholar]
- Bang FB 1982. Disease processes in seastars: a Metchnikovian challenge. Biol. Bull 162: 135–148. [Google Scholar]
- Barbaglio A, Tricarico S, Ribeiro A, Ribeiro C, Sugni M, Di Benedetto C, Wilkie I, Barbosa M, Bonasoro F, and Candia Carnevali MD. 2012. The mechanically adaptive connective tissue of echinoderms: its potential for bio-innovation in applied technology and ecology. Mar. Environ. Res 76: 108–113. [DOI] [PubMed] [Google Scholar]
- Barbaglio A, Tricarico SC, Di Benedetto D, Fassini AP, Lima AR, Ribeiro CC, Ribeiro M, Sugni F, Bonasoro L, and Wilkie EA. 2013. The smart connective tissue of echinoderms: a materializing promise for biotech applications. Cah. Biol. Mar 54: 713–720. [Google Scholar]
- Bates AE, Hilton BJ, and Harley CD. 2009. Effects of temperature, season and locality on wasting disease in the keystone predatory sea star Pisaster ochraceus. Dis. Aquat. Organ 86: 245–251. [DOI] [PubMed] [Google Scholar]
- Becker PT, Egea E, and Eeckhaut I. 2008. Characterization of the bacterial communities associated with the bald sea urchin disease of the echinoid Paracentrotus lividus. J. Invertebr. Pathol 98: 136–147. [DOI] [PubMed] [Google Scholar]
- Beleneva IA, and Zhukova NV. 2009. Seasonal dynamics of cell numbers and biodiversity of marine heterotrophic bacteria inhabiting invertebrates and water ecosystems of the Peter the Great Bay, Sea of Japan. Microbiology 78: 369–375. [PubMed] [Google Scholar]
- Binyon J 1961. Salinity tolerance and permeability to water of the starfish Asterias rubens. J. Mar. Biol. Assoc. U.K 41: 161–174. [Google Scholar]
- Binyon J 1962. Ionic regulation and mode of adjustment to reduced salinity of the starfish Asterias rubens. J. Mar. Biol. Assoc. U.K 42: 49. [Google Scholar]
- Binyon J 1972. Physiology of Echinoderms. Pergamon, Oxford. [Google Scholar]
- Blake DB 1989. Asteroidea: Functional Morphology, Classification and Phylogeny. A.A. Balkema, Rotterdam. [Google Scholar]
- Bucci C, Francoeur M, McGreal J, Smolowitz R, Zazueta-Novoa V, Wessel GM, and Gomez-Chiarri M. 2017. Sea star wasting disease in Asterias forbesi along the Atlantic Coast of North America. PLoS One 12: e0188523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge CA, Eakin CM, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, Petes LE, Prager KC, Weil E, Willis BL et al. 2014. Climate change influences on marine infectious diseases: implications for management and society. Annu. Rev. Mar. Sci 6: 249–277. [DOI] [PubMed] [Google Scholar]
- Burt JM, Tinker MT, Okamoto DK, Demes KW, Holmes K, and Salomon AK. 2018. Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts. Proc. R Soc. B Biol. Sci 285: 20180553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M 1995. Changes in larval morphology in the evolution of benthic development by Patiriella exigua (Asteroidea: Asterinidae), a comparison with the larvae of Patiriella species with planktonic development. Biol. Bull 188: 293–305. [DOI] [PubMed] [Google Scholar]
- Byrne M 1999. Echinodermata. Pp. 940–954 in Encyclopedia of Reproduction, Knobil E and Neill J, eds. Academic Press, New York. [Google Scholar]
- Byrne M 2013. Asteroid evolutionary developmental biology and ecology. Pp. 51–58 in The Ecology of the Asteroidea, Lawrence J, ed. Johns Hopkins University Press, Baltimore. [Google Scholar]
- Byrne M 2020. The link between autotomy and CNS regeneration: echinoderms as non-model species for regenerative biology. Bioessays 42: e1900219. [DOI] [PubMed] [Google Scholar]
- Byrne M, Cerra A, Nishigak IT, and Hoshi M. 1997. Infestation of the testes of the Japanese sea star Asterias amurensis by the ciliate Orchitophrya stellarum: a caution against the use of this ciliate for biological control. Dis. Aquat. Org 28: 235–239. [Google Scholar]
- Byrne M, Cerra T, Nishigaki T, and Hoshi M. 1998. Male infertility: a new phenomenon affecting Japanese populations of the sea star Asterias amurensis (Asteroidea) due to the introduction of the parasitic ciliate Orchitophrya stellarum to Japan. Pp. 203–207 in Echinoderms, Mooi R and Telford M, eds. A.A. Balkema, Rotterdam. [Google Scholar]
- Byrne M, Rowe FEW, Marsh LM, Mah CL, and T. D. 2017. Class Asteroidea. Pp. 231–294 in Australian Echinoderms: Biology Ecology and Evolution, Byrne M and O’Hara TD, eds. CSIRO, Clayton, Victoria, Australia. [Google Scholar]
- Byrne M, Mazzone F, Elphick MR, Thorndyke MC, and Cisternas P. 2019. Expression of the neuropeptide SALMFamide-1 during regeneration of the seastar radial nerve cord following arm autotomy. Proc. R. Soc. B Biol. Sci 286: 20182701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CB, and Bishop CD. 2012. Biomineral ultrastructure, elemental constitution and genomic analysis of biomineralization-related proteins in hemichordates. Proc. R. Soc. B Biol. Sci 279: 3041–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AC, and Rainbow PS. 1977. The role of pedicellariae in preventing barnacle settlement on the sea-urchin test. Mar. Behav. Physiol 4: 253–260. [Google Scholar]
- Cepede C 1907. La castration parasitaire des étoiles de mer males par un nouvel infuoire astome: Orchitophrya stellarum, n.g., n. sp. C. R. Hebd. Seances Acad. Sci . Paris 145: 1305–1306. [Google Scholar]
- Chaet AB 1962. A toxin in the coelomic fluid of scalded starfish (Asterias forbesi). Exp. Biol. Med 109: 791–794. [DOI] [PubMed] [Google Scholar]
- Chia F-S 1968. Embryology of a brooding starfish Leptasterias hexactis (Stimpson). Acta Zool. 49: 321–354. [Google Scholar]
- Chia F-S 1969. Response of the globiferous pedicellariae to inorganic salts in three regular echinoids. Ophelia 6: 203–210. [Google Scholar]
- Chia F-S, and Amerongen H. 1975. On the prey-catching pedicellariae of a starfish, Stylasterias forreri (de Loriol). Can. J. Zool 53: 748–755. [Google Scholar]
- Chia F-S, and Koss R. 1994. Asteroidea. Pp. 169–245 in Microscopic Anatomy of the Invertebrates (Echinodermata), Vol. 14, Harrison FW and Chia F-S, eds. Wiley-Liss, New York. [Google Scholar]
- Chia F-S, Oguro C, and Komatsu M. 1993. Sea-star (asteroid) development. Oceanogr. Mar. Biol 31: 223–257. [Google Scholar]
- Choi EJ, Kwon HC, Koh HY, Kim YS, and Yang HO. 2010a. Colwellia asteriadis sp. nov., a marine bacterium isolated from the starfish Asterias amurensis. Int. J. Syst. Evol. Microbiol 60: 1952–1957. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Kwon HC, Sohn YC, and Yang HO. 2010b. Kistimonas asteriae gen. nov., sp. nov., a gammaproteobacterium isolated from Asterias amurensis. Int. J. Syst. Evol. Microbiol 60: 938–943. [DOI] [PubMed] [Google Scholar]
- Choi G-G, Lee O-H, and Lee G-H. 2003. The diversity of heterotrophic bacteria isolated from intestine of starfish (Asterias amurensis) by analysis of 16S rDNA sequence. Korean J. Ecol 26: 307–312. [Google Scholar]
- Cintra-Buenrostro CE, Reyes-Bonilla H, and Herrero-Perezrul MD. 2005. Oceanographic conditions and diversity of sea stars (Echinodermata: Asteroidea) in the Gulf of California, Mexico. Rev. Biol. Trop 53 (suppl.): 245–261. [PubMed] [Google Scholar]
- Claereboudt M, and Bouland C. 1994. The effect of parasitic castration by a ciliate on a population of Asterias vulgaris. J. Invertebr. Pathol 63: 172–177. [Google Scholar]
- Cobb JL 1987. Neurobiology of the Echinodermata. Pp. 483–525 in Nervous Systems in Invertebrates, Ali MA, ed. Springer, Boston. [Google Scholar]
- Cobb JL 1978. An ultrastructural study of the dermal papulae of the starfish, Asterias rubens, with special reference to innervation of the muscles. Cell Tissue Res. 187: 515–523. [DOI] [PubMed] [Google Scholar]
- Cramer KL, Jackson JBC, Donovan MK, Greenstein BJ, Korpanty CA, Cook GM, and Pandolfi JM. 2020. Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv 6: eaax9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn JH, Edwards KC, and Fratt DB. 1991. Diet, feeding behavior, and surface morphology of the multi-armed Antarctic sea star Labidiaster annulatus (Echinodermata: Asteroidea). Mar. Ecol. Prog. Ser 77: 65–84. [Google Scholar]
- Diehl WJ, and Lawrence JM. 1984. The effect of salinity on coelomic fluid osmolyte concentration and intracellular water content in Luidia clathrata (Say) (Echinodermata: Asteroidea). Comp. Biochem. Physiol. A Physiol 79: 119–126. [Google Scholar]
- Duggins DO 1983. Starfish predation and the creation of mosaic patterns in a kelp-dominated community. Ecology 64: 1610–1619. [Google Scholar]
- Duignan PJ, Stephens NS, and Robb K. 2020. Fresh water skin disease in dolphins: a case definition based on pathology and environmental factors in Australia. Sci. Rep 10: 21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan ML, Miller TE, and Thomson DA. 1982. Catastrophic decline of a top carnivore in the Gulf of California rocky intertidal zone. Science 216: 989–991. [DOI] [PubMed] [Google Scholar]
- Eckert GL, Engle JM, and Kushner DJ. 2000. Sea star disease and population declines at the Channel Islands. Pp. 390–393 in Proceedings of the Fifth California Islands Symposium, US Department of the Interior, Minerals Management Service, Pacific OCS Region, Camarillo, CA. [Google Scholar]
- Egan S, and Gardiner M. 2016. Microbial dysbiosis: rethinking disease in marine ecosystems. Front. Microbiol 7: 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlord ME, Groner ML, Yoshioka RM, Elliott J, Maynard J, Fradkin S, Turner M, Pyne K, Rivlin N, van Hooidonk R et al. 2016. Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Philos. Trans. R. Soc. B Biol. Sci 371: 20150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington WR, and Lawrence JM. 1974. Coelomic fluid volume regulation and isosmotic intracellular regulation by Luidia clathrata (Echinodermata: Asteroidea) in response to hyposmotic stress. Biol. Bull 146: 20–31. [DOI] [PubMed] [Google Scholar]
- Emlet RB, McEdward LR, and Strathmann RR. 1987. Echinoderm larval ecology viewed from the egg. Echinoderm Stud. 2: 55–136. [Google Scholar]
- Emson RH, and Young CM. 1994. Feeding mechanism of the brisingid starfish Novodinia antillensis. Mar. Biol 118: 433–442. [Google Scholar]
- Erlinger R, Welsch U, and Scott JE. 1993. Ultrastructural and biochemical observations on proteoglycans and collagen in the mutable connective tissue of the feather star Antedon bifida (Echinodermata, Crinoidea). J. Anat 183: 1–11. [PMC free article] [PubMed] [Google Scholar]
- Feder HM 1959. The food of the starfish, Pisaster ochraceus along the California coast. Ecology 40: 721–724. [Google Scholar]
- Fein A, and Terasaki M. 2005. Rapid increase in plasma membrane chloride permeability during wound resealing in starfish oocytes. J. Gen. Physiol 126: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JC 1969. Feeding, digestion and nutrition in Echinodermata. Pp. 71–100 in Chemical Zoology, Florkin Mand Scheer BT, eds. Academic Press, London. [Google Scholar]
- Ferguson JC 1990. Hyperosmotic properties of the fluids of the perivisceral coelom and water vascular system of starfish kept under stable conditions. Comp. Biochem. Physiol. A Mol. Integr. Physiol 95: 245–248. [Google Scholar]
- Ferguson JC, and Walker CW. 1991. Cytology and function of the madreporite systems of the starfish Henricia sanguinolenta and Asterias vulgaris. J. Morphol 210: 1–11. [DOI] [PubMed] [Google Scholar]
- Feuda R, and Smith AB. 2015. Phylogenetic signal dissection identifies the root of starfishes. PLoS One 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FioRito R, Leander C, and Leander B. 2016. Characterization of three novel species of Labyrinthulomycota isolated from ochre sea stars (Pisaster ochraceus). Mar. Biol 163: 10. [Google Scholar]
- Foret TW, and Lawrence JM. 2001. Variation in abundance of subcuticular bacteria in Florida echinoderms. Symbiosis 31: 309–322. [Google Scholar]
- Frakolaki E, Kaimou P, Moraiti M, Kalliampakou KI, Karampetsou K, Dotsika E, Liakos P, Vassilacopoulou D, Mavromara P, Bartenschlager R et al. 2018. The role of tissue oxygen tension in Dengue virus replication. Cells 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks DN, and Relman DA. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin. Microbiol. Rev 9: 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer M, Lee RF, Price AR, Walters TL, Bassette MA, Verdiyev R, Torris MC, Bulski K, Geer PJ, Powell SA et al. 2017. Causes, diagnostics, and distribution of an ongoing penaeid shrimp black gill epidemic in the U.S. South Atlantic Bight. J. Shellfish Res 36: 487–500. [Google Scholar]
- Fuess LE, Eisenlord ME, Closek CJ, Tracy AM, Mauntz R, Gignoux-Wolfsohn S, Moritsch MM, Yoshioka R, Burge CA, Harvell CD et al. 2015. Up in arms: immune and nervous system response to sea star wasting disease. PLoS One 10: e0133053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa R, Takahashi Y, Nakajima Y, Dan-Sohkawa M, and Kaneko H. 2009. Defense system by mesenchyme cells in bipinnaria larvae of the starfish, Asterina pectinifera. Dev. Comp. Immunol 33: 205–215. [DOI] [PubMed] [Google Scholar]
- Furukawa R, Tamaki K, and Kaneko H. 2016. Two macrophage migration inhibitory factors regulate starfish larval immune cell chemotaxis. Immunol. Cell. Biol 94: 315–321. [DOI] [PubMed] [Google Scholar]
- Gage JD, and Tyler PA. 1991. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press, Cambridge. [Google Scholar]
- Glockling SL, Marshall WL, and Gleason FH. 2013. Phylogenetic interpretations and ecological potentials of the Mesomycetozoea (Ichthyosporea). Fungal Ecol. 6: 237–247. [Google Scholar]
- Goggin CL, and Bouland C. 1997. The ciliate Orchitophrya cf. stellarum and other parasites and commensals of the northern Pacific seastar Asterias amurensis from Japan. Int. J. Parasitol 27: 1415–1418. [DOI] [PubMed] [Google Scholar]
- Gordon S 2016a. Phagocytosis: an immunobiologic process. Immunity 44: 463–475. [DOI] [PubMed] [Google Scholar]
- Gordon S 2016b. Phagocytosis: the legacy of Metchnikoff. Cell 166: 1065–1068. [DOI] [PubMed] [Google Scholar]
- Gravem SA, and Morgan SG. 2017. Shifts in intertidal zonation and refuge use by prey after mass mortalities of two predators. Ecology 98: 1006–1015. [DOI] [PubMed] [Google Scholar]
- Gravem SA, Heady WN, Saccomanno VR, Alvstad KF, Gehman ALM, Frierson TN, and Hamilton SL. 2021. Pycnopodia helianthoides. [Online]. IUCN Red List of Threatened Species 2021. Available: https://static1.squarespace.com/static/5b071ddea2772cebc1662831/t/5ffe0633b15beb25b9187695/1610483256712/IUCN+Assessment+of+Pycnopodia+helianthoides+2021.pdf [2022, July 11]. [Google Scholar]
- Gregg A, Hatay M, Haas A, Robinett N, Barott K, Vermeij M, Marhaver K, Meirelles P, Thompson F, and Rohwer F. 2013. Biological oxygen demand optode analysis of coral reef-associated microbial communities exposed to algal exudates. PeerJ 1: e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner ML, Maynard J, Breyta R, Carnegie RB, Dobson A, Friedman CS, Froelich B, Garren M, Gulland FM, Heron SF et al. 2016. Managing marine disease emergencies in an era of rapid change. Philos. Trans. R. Soc. B Biol. Sci 371: 20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf BM, and Hewson I. 2015. Metatranscriptomic analysis of Pycnopodia helianthoides (Asteroidea) affected by sea star wasting disease. PLoS One 10: e0128150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf BM, Eaglesham JB, Aragundi WM, and Hewson I. 2014. Discovery of urchin-associated densoviruses (family Parvoviridae) in coastal waters of the Big Island, Hawaii. J. Gen. Virol 95: 652–658. [DOI] [PubMed] [Google Scholar]
- Haas AF, Nelson CE, Wegley Kelly L, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, and Smith JE. 2011. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS One 6: e27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty JM, and Dinsdale EA. 2016. Distinct biogeographical patterns of marine bacterial taxonomy and functional genes. Glob. Ecol. Biogeogr 26: 177–190. [Google Scholar]
- Hall MR, Kocot KM, Baughman KW, Fernandez-Valverde SL, Gauthier MEA, Hatleberg WL, Krishnan A, McDougall C, Motti CA, Shoguchi E et al. 2017. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 544: 231–234. [DOI] [PubMed] [Google Scholar]
- Hamel JF, Jobson S, Caulier G, and Mercier A. 2021. Evidence of anticipatory immune and hormonal responses to predation risk in an echinoderm. Sci. Rep 11: 10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SK, Deutsch CJ, Cross TA, de Wit M, and Hostetler JA. 2019. Cold-related Florida manatee mortality in relation to air and water temperatures. PLoS One 14: e0225048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CDG, Pankey MS, Wares JP, Grosberg RK, and Wonham MJ. 2006. Color polymorphism and genetic structure in the sea star Pisaster ochraceus. Biol. Bull 211: 248–262. [DOI] [PubMed] [Google Scholar]
- Harrold C, and Pearse JS. 1987. The ecological role of echinoderms in kelp forests. Pp. 137–233 in Echinoderm Studies, Vol. 2, Jangoux M, and Lawrence JM, eds. A.A. Balkema, Rotterdam. [Google Scholar]
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus AD, Overstreet RM et al. 1999. Emerging marine diseases—climate links and anthropogenic factors. Science 285: 1505–1510. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Montecino-Latorre D, Caldwell JM, Burt JM, Bosley K, Keller A, Heron SF, Salomon AK, Lee L, Pontier O et al. 2019. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haszprunar G, Vogler C, and Wörheide G. 2017. Persistent gaps of knowledge for naming and distinguishing multiple species of crown-of-thorns seastar in the Acanthaster planci species complex. Diversity 9: 22. [Google Scholar]
- Held MBE, and Harley CDG. 2009. Responses to low salinity by the sea star Pisaster ochraceus from high- and low-salinity populations. Invertebr. Biol 128: 381–390. [Google Scholar]
- Hendler G, and Franz DR. 1982. The biology of a brooding seastar, Leptasterias tenera, in Block Island Sound. Biol. Bull 162: 273–289. [Google Scholar]
- Hewson I 2021. Microbial respiration in the asteroid diffusive boundary layer influenced sea star wasting disease during the 2013–2014 northeast Pacific Ocean mass mortality event. Mar. Ecol. Progr. Ser 668: 231–237. [Google Scholar]
- Hewson I, and Sewell MA. 2021. Surveillance of densoviruses and mesomycetozoans inhabiting grossly normal tissues of three Aotearoa New Zealand asteroid species. PLoS One 16: e0241026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson I, Button JB, Gudenkauf BM, Miner B, Newton AL, Gaydos JK, Wynne J, Groves CL, Hendler G, Murray M et al. 2014. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl. Acad. Sci. U.S.A 111: 17278–17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson I, Bistolas KSI, Quijano Carde EM, Button JB, Foster PJ, Flanzenbaum JM, Kocian J, and Lewis CK. 2018. Investigating the complex association between viral ecology, environment and northeast Pacific sea star wasting. Front. Mar. Sci 5: 14. [Google Scholar]
- Hewson I, Sullivan B, Jackson EW, Xu Q, Long H, Lin C, Quijano Cardé EM, Seymour J, Siboni N, Jones MRL et al. 2019. Perspective: something old, something new? Review of wasting and other mortality in Asteroidea (Echinodermata). Front. Mar. Sci 6: 1–8.36817748 [Google Scholar]
- Hewson I, Aquino CA, and DeRito CM. 2020a. Virome variation during sea star wasting disease progression in Pisaster ochraceus (Asteroidea, Echinodermata). Viruses 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson I, Johnson MR, and Tibbetts IR. 2020b. An unconventional flavivirus and other RNA viruses in the sea cucumber (Holothuroidea; Echinodermata) virome. Viruses 12: 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, Buckley KM, Brockton V, Nair SV, Berney K et al. 2006. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol 300: 349–365. [DOI] [PubMed] [Google Scholar]
- Hildemann WH, and Dix TG. 1972. Transplantation reactions of tropical Australian echinoderms. Transplantation 14: 624–633. [DOI] [PubMed] [Google Scholar]
- Hoj L, Levy N, Baillie BK, Clode PL, Strohmaier RC, Siboni N, Webster NS, Uthicke S, and Bourne DG. 2018. Crown-of-thorns sea star Acanthaster cf. solaris has tissue-characteristic microbiomes with potential roles in health and reproduction. Appl. Environ. Microbiol 84: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland ND, and Nealson KH. 1978. Fine-structure of the echinoderm cuticle and the subcuticular bacteria of echinoderms. Acta Zool. 59: 169–185. [Google Scholar]
- Hughes TP 1994. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265: 1547–1551. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JB, Kleypas J et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC et al. 2018a. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359: 80–83. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Kerry JT, and Simpson T. 2018b. Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99: 501. [DOI] [PubMed] [Google Scholar]
- Hyman L 1955. The Invertebrates: Echinodermata. McGraw-Hill, New York. [Google Scholar]
- Iken K, Konar B, Benedetti-Cecchi L, Cruz-Motta JJ, Knowlton A, Pohle G, Mead A, Miloslavich P, Wong M, Trott T et al. 2010. Large-scale spatial distribution patterns of echinoderms in nearshore rocky habitats. PLoS One 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova EP, Gorshkova NM, Sawabe T, Zhukova NV, Hayashi K, Kurilenko VV, Alexeeva Y, Buljan V, Nicolau DV, Mikhailov VV et al. 2004. Sulfitobacter delicatus sp. nov. and Sulfitobacter dubius sp. nov., respectively from a starfish (Stellaster equestris) and sea grass (Zostera marina). Int. J. Syst. Evol. Microbiol 54: 475–480. [DOI] [PubMed] [Google Scholar]
- Jackson EW, Bistolas KS, Button JB, and Hewson I. 2016. Novel circular single-stranded DNA viruses among an asteroid, echinoid and holothurian (Phylum: Echinodermata). PLoS One 11: e0166093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EW, Pepe-Ranney C, Debenport SJ, Buckley DH, and Hewson I. 2018. The microbial landscape of sea stars and the anatomical and interspecies variability of their microbiome. Front. Microbiol 9: 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EW, Pepe-Ranney C, Johnson MR, Distel DL, and Hewson I. 2020a. A highly prevalent and pervasive densovirus discovered among sea stars from the North American Atlantic coast. Appl. Environ. Microbiol 86: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EW, Wilhelm RC, Johnson MR, Lutz HL, Danforth I, Gaydos JK, Hart MW, and Hewson I. 2020b. Diversity of sea star-associated densoviruses and transcribed endogenous viral elements of densovirus origin. J. Virol 95: e01594–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe N, Eberl R, Bucholz J, and Cohen CS. 2019. Sea star wasting disease demography and etiology in the brooding sea star Leptasterias spp. PLoS One 14: e0225248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangoux M 1982. Food and feeding mechanisms. Pp. 112–155 in Echinoderm Nutrition, Jangoux Mand Lawrence JM, eds. A.A. Balkema, Rotterdam. [Google Scholar]
- Jensen M 1966. The response of two sea-urchins to the sea-star Marthasterias glacialis (L.) and other stimuli. Ophelia 3: 209–219. [Google Scholar]
- Johnson PT 1971. Studies on diseased urchins from Point Loma. Pp. 82–90 in Kelp Habitat Improvement Project, Annual Report 1970–1971, North WJ, ed. California Institute of Technology, Pasadena. [Google Scholar]
- Jurgens LJ, Rogers-Bennett L, Raimondi PT, Schiebelhut LM, Dawson MN, Grosberg RK, and Gaylord B. 2015. Patterns of mass mortality among rocky shore invertebrates across 100km of northeastern Pacific coastline. PLoS One 10: e0131969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp RD, and Hildemann WH. 1976. Specific allograft reactivity in the sea star Dermasterias imbricata. Transplantation 22: 434–439. [DOI] [PubMed] [Google Scholar]
- Kay SWC, Gehman AM, and Harley CDG. 2019. Reciprocal abundance shifts of the intertidal sea stars, Evasterias troschelii and Pisaster ochraceus, following sea star wasting disease. Proc. R. Soc. B Biol. Sci 286: 20182766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MS, Barker MF, McKenzie JD, and Powell J. 1995. The incidence and morphology of subcuticular bacteria in the echinoderm fauna of New Zealand. Biol. Bull 189: 91–105. [DOI] [PubMed] [Google Scholar]
- Kerk D, Gee A, Standish M, Wainright PO, and Drum AS. 1995. The rosette agent of Chinook salmon (Oncorhyncus tshawytscha) is closely related to choanoflagellates, as determined by phylogenetic analyses of its small ribosomal subunit RNA. Mar. Biol 122: 187–192. [Google Scholar]
- Kohl WT, McClure TI, and Miner BG. 2016. Decreased temperature facilitates short-term sea star wasting disease survival in the Keystone intertidal sea star Pisaster ochraceus. PLoS One 11: e0153670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konar B, Mitchell TJ, Iken K, Coletti H, Dean T, Esler D, Lindeberg M, Pister B, and Weitzman B. 2019. Wasting disease and static environmental variables drive sea star assemblages in the northern Gulf of Alaska. J. Exp. Mar. Biol. Ecol 520: 151–209. [Google Scholar]
- Lafferty K, and Suchanek T. 2016. Revisiting Paine’s 1966 sea star removal experiment, the most-cited empirical article in the American Naturalist. Am. Nat 188: 365–378. [DOI] [PubMed] [Google Scholar]
- Landsberg JH, Kiryu Y, Peters EC, Wilson PW, Perry N, Waters Y, Maxwell KE, Huebner LK, and Work TM. 2020. Stony coral tissue loss disease in Florida is associated with disruption of host-zooxanthellae physiology. Front. Mar. Sci 7: 24. [Google Scholar]
- Lauerman LML 1998. Diet and feeding behavior of the deep-water sea star Rathbunaster californicus (Fisher) in the Monterey Submarine Canyon. Bull. Mar. Sci 63: 523–530. [Google Scholar]
- Lawrence JM 2013. The asteroid arm. Pp. 15–23 in Starfish: Biology and Ecology of the Asteroidea, Lawrence JM, ed. Johns Hopkins University Press, Baltimore. [Google Scholar]
- Leighton B, Boom JDG, Bouland C, and Smith MJ. 1991. Castration and mortality in Pisaster ochraceus parasitized by Orchitophrya stellarum (Ciliophora). Dis. Aquat. Org 10: 71–73. [Google Scholar]
- Lessios HA 2016. The great Diadema antillarum die-off: 30 years later. Annu. Rev. Mar. Sci 8: 267–283. [DOI] [PubMed] [Google Scholar]
- Lessios HA, Robertson DR, and Cubit JD. 1984. Spread of Diadema mass mortality through the Caribbean. Science 226: 335–337. [DOI] [PubMed] [Google Scholar]
- Linchangco GV Jr., Foltz DW, Reid R, Williams J, Nodzak C, Kerr AM, Miller AK, Hunter R, Wilson NG, Nielsen WJ et al. 2017. The phylogeny of extant starfish (Asteroidea: Echinodermata) including Xyloplax, based on comparative transcriptomics. Mol. Phylogenet. Evol 115: 161–170. [DOI] [PubMed] [Google Scholar]
- Lloyd MM, and Pespeni MH. 2018. Microbiome shifts with onset and progression of sea star wasting disease revealed through time course sampling. Sci. Rep 8: 16476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Wang W, Wang Y, Hu C, and He X. 2013. Pseudoalteromonas xishaensis sp. nov., isolated from Acanthaster planci in the Xisha islands. Antonie Leeuwenhoek 104: 779–785. [DOI] [PubMed] [Google Scholar]
- Maes P, and Jangoux M. 1984. The bald-sea-urchin disease: a biopathological approach. Helgol. Meeresunters 37: 217–224. [Google Scholar]
- Mah CL, and Blake DB. 2012. Global diversity and phylogeny of the Asteroidea (Echinodermata). PLoS One 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeske AJ, Bayne CJ, and Smith LC. 2013. Aggregation of sea urchin phagocytes is augmented in vitro by lipopolysaccharide. PLoS One 8: e61419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Villaro AC, and Sesma P. 1989. Microscopic study of the pyloric caeca of the starfish Marthasterias glacialis (Echinodermata): finding of endocrine cells. J. Morphol 202: 151–164. [DOI] [PubMed] [Google Scholar]
- Martinez AS, Byrne M, and Coleman RA. 2016. What and when to eat? Investigating the feeding habits of an intertidal herbivorous starfish. Mar. Biol 163: 1–13. [Google Scholar]
- Martinez AS, Byrne M, and Coleman RA. 2017. Filling in the grazing puzzle: a synthesis of herbivory in starfish. Oceanogr. Mar. Biol 55: 11–44. [Google Scholar]
- Matranga V, Bonaventura R, and Di Bella G. 2002. Hsp70 as a stress marker of sea urchin coelomocytes in short term cultures. Cell. Mol. Biol 48: 345–349. [PubMed] [Google Scholar]
- McClintock JB, and Robnett TJ. 1986. Size selective predation by the asteroid Pisaster ochraceus on the bivalve Mytilus californianus: a cost-benefit analysis. Mar. Ecol 7: 321–332. [Google Scholar]
- McClintock JB, Angus RA, Ho C, Amsler CD, and Baker BJ. 2008. A laboratory study of behavioral interactions of the Antarctic keystone sea star Odontaster validus with three sympatric predatory sea stars. Mar. Biol 154: 1077–1084. [Google Scholar]
- McEdward LR, and Janies DA. 1993. Life cycle evolution in asteroids: What is a larva? Biol. Bull 184: 255–268. [DOI] [PubMed] [Google Scholar]
- McEdward LR, and Miner BG. 2001. Larval and life cycle patterns in echinoderms. Can. J. Zool 79: 1125–1170. [Google Scholar]
- McEdward LR, Jaeckle WB, and Komatsu M. 2002. Phylum Echinodermata: Asteroidea. Pp. 499–512 in Atlas of Marine Invertebrate Larvae, Young CM, Sewell MA, and Rice ME, eds. Academic Press, London. [Google Scholar]
- McKenzie JD, and Grigolava IV. 1996. The echinoderm surface and its role in preventing microfouling. Biofouling 10: 261–272. [DOI] [PubMed] [Google Scholar]
- McPherson ML, Finger DJI, Houskeeper HF, Bell TW, Carr MH, Rogers-Bennett L, and Kudela RM. 2021. Large-scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Commun. Biol 4: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AD 1898. Annual Report of the Commissioners of Inland Fisheries Made to the General Assembly/State of Rhode Island. Wentworth Press, St. Albans, UK. [Google Scholar]
- Melroy LM, and Cohen CS. 2021. Temporal and spatial variation in population structure among brooding sea stars in the genus Leptasterias. Ecol. Evol 11: 3313–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge B 1979. Coexistence between the seastars Asterias vulgaris and A. forbesi in a heterogenous environment: a non-equilibrium explanation. Oecologia 272: 245–272. [DOI] [PubMed] [Google Scholar]
- Menge B, Berlow EL, Blanchette CA, Navarrete SA, and Yamada SB. 1994. The Keystone species concept: variation in interaction strength in a rocky intertidal habitat. Ecol. Monogr 64: 249–286. [Google Scholar]
- Menge BA, Cerny-Chipman EB, Johnson A, Sullivan J, Gravem S, and Chan F. 2016. Sea star wasting disease in the Keystone predator Pisaster ochraceus in Oregon: insights into differential population impacts, recovery, predation rate, and temperature effects from long-term research. PLoS One 11: e0153994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge JL, and Menge BA. 1974. Role of resource allocation, aggression and spatial heterogeneity in coexistence of two competing intertidal starfish. Ecol. Monogr 44: 189–209. [Google Scholar]
- Mercier A, and Hamel JF. 2013. Reproduction in Asteroidea. Pp. 37–50 in Starfish: Biology and Ecology of the Asteroidea, Lawrence JL, ed. Johns Hopkins University Press, Baltimore. [Google Scholar]
- Miner CM, Burnaford JL, Ambrose RF, Antrim L, Bohlmann H, Blanchette CA, Engle JM, Fradkin SC, Gaddam R, Harley CDG et al. 2018. Large-scale impacts of sea star wasting disease (SSWD) on intertidal sea stars and implications for recovery. PLoS One 13: e0192870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Kuwahara Y, and Miyashita K. 2018. Tracking the northern Pacific sea star Asterias amurensis with acoustic transmitters in the scallop mariculture field of Hokkaido, Japan. Fish. Sci 84: 349–355. [Google Scholar]
- Mladenov PV, and Burke RD. 1994. Echinodermata: asexual propagation. Pp. 339–383 in Reproductive Biology of Invertebrates, Vol. 6, Pt. B, Asexual Propagation and Reproductive Strategies, Adiyodi KG and Adiyodi RG, eds. IBH Publishing, New Delhi. [Google Scholar]
- Mladenov PV, Igdoura S, Asotra S, and Burke RD. 1989. Purification and partial characterization of an autotomy-promoting factor from the sea star Pycnopodia helianthoides. Biol. Bull 176: 169–175. [Google Scholar]
- Montecino-Latorre D, Eisenlord ME, Turner M, Yoshioka R, Harvell CD, Pattengill-Semmens CV, Nichols JD, and Gaydos JK. 2016. Devastating transboundary impacts of sea star wasting disease on subtidal asteroids. PLoS One 11: e0163190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi R, and David B. 2000. What a new model of skeletal homologies tells us about asteroid evolution. Am. Zool 40: 326–339. [Google Scholar]
- Moritsch MM, and Raimondi P. 2018. Reduction and recovery of keystone predation pressure after disease-related mass mortality. Ecol. Evol 8: 3952–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa T 1982. Rapid change in mechanical properties of echinoderm connective tissues caused by coelomic fluid. Comp. Biochem. Physiol. C Toxicol. Pharmacol 73: 223–229. [Google Scholar]
- Motokawa T 1986. Morphology of the spines and spine joint in the crown-of-thorns starfish Acanthaster planci (Echinodermata, Asteroida). Zoomorphology 106: 247–253. [Google Scholar]
- Motokawa T 2011. Mechanical mutability in connective tissue of starfish body wall. Biol. Bull 221: 280–289. [DOI] [PubMed] [Google Scholar]
- Muehlstein LK, Porter D, and Short FT. 1991. Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass, Zostera marina. Mycologia 83: 180–191. [Google Scholar]
- Murie KA, and Bourdeau PE. 2019. Predator identity dominates non-consumptive effects in a disease-impacted rocky shore food web. Oecologia 191: 945–956. [DOI] [PubMed] [Google Scholar]
- Myhre S, and Acevedo-Gutiérrez A. 2007. Recovery of sea urchin Diadema antillarum populations is correlated to increased coral and reduced macroalgal cover. Mar. Ecol. Progr. Ser 329: 205–210. [Google Scholar]
- Nakagawa S, Saito H, Tame A, Hirai M, Yamaguchi H, Sunata T, Aida M, Muto H, Sawayama S, and Takaki Y. 2017. Microbiota in the coelomic fluid of two common coastal starfish species and characterization of an abundant Helicobacter-related taxon. Sci. Rep 7: 8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita H, Matsubara S, Miwa N, Akahane S, Murakami M, Goto T, Nara M, Noguchi T, Saito T, Shida Y et al. 1987. Vibrio alginolyticus, a tetrodotoxin-producing bacterium isolated from the sea star Astropecten polyancanthus. Bull. Jpn. Soc. Sci. Fish 53: 617–621. [Google Scholar]
- Nunez-Pons L, Work TM, Angulo-Preckler C, Moles J, and Avila C. 2018. Exploring the pathology of an epidermal disease affecting a circum-Antarctic sea star. Sci. Rep 8: 11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, and Tanoue E. 2003. Dissolved organic matter in oceanic waters. J. Oceanogr 59: 129–147. [Google Scholar]
- Olson JB, Gochfeld DJ, and Slattery M. 2006. Aplysina red band syndrome: a new threat to Caribbean sponges. Dis. Aquat. Organ 71: 163–168. [DOI] [PubMed] [Google Scholar]
- O’Neill P 1989. Structure and mechanics of starfish body wall. J. Exp. Biol 147: 53–89. [DOI] [PubMed] [Google Scholar]
- Paine RT 1966. Food web complexity and species diversity. Am. Nat 100: 65–75. [Google Scholar]
- Paine VL 1926. Adhesion of the tube feet in starfishes. J. Exp. Zool 45: 361–366. [Google Scholar]
- Paps J, Medina-Chacon LA, Marshall W, Suga H, and Ruiz-Trillo I. 2013. Molecular phylogeny of unikonts: new insights into the position of apusomonads and ancyromonads and the internal relationships of opisthokonts. Protist 164: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse JS 1967. Coelomic water volume control in the Antarctic sea-star Odontaster validus. Nature 216: 1118–1119. [Google Scholar]
- Peters EC 1992. Diseases of other invertebrate phyla: Porifera, Cnidaria, Ctenophora, Annelida, Echinodermata. Pp. 393–449 in Pathobiology of Marine and Estuarine Organisms, Couch JA, ed. CRC Press, Boca Raton, FL. [Google Scholar]
- Petraitis PS, and Dudgeon SR. 2020. Declines over the last two decades of five intertidal invertebrate species in the western North Atlantic. Commun. Biol 3: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincebourde S, Sanford E, and Helmuth B. 2013. Survival and arm abscission are linked to regional homeothermy in an intertidal sea star. J. Exp. Biol 216: 2183–2191. [DOI] [PubMed] [Google Scholar]
- Pinsino A, and Matranga V. 2015. Sea urchin immune cells as sentinels of environmental stress. Dev. Comp. Immunol 49: 198–205. [DOI] [PubMed] [Google Scholar]
- Pratchett MS, Caballes CF, Wilmes JC, Matthews S, Mellin C, Sweatman HPA, Nadler LE, Brodie J, Thompson CA, Hoey J et al. 2017. Thirty years of research on crown-of-thorns starfish (1986–2016): scientific advances and emerging opportunities. Diversity 9: 41. [Google Scholar]
- Prusch RD, and Whoriskey F. 1976. Maintenance of fluid volume in the starfish water vascular system. Nature 262: 577–578. [DOI] [PubMed] [Google Scholar]
- Ragan MA, Goggin CL, Cawthorn RJ, Cerenius L, Jamieson AV, Plourde SM, Rand TG, Soderhall K, and Gutell RR. 1996. A novel clade of protistan parasites near the animal-fungal divergence. Proc. Natl. Acad. Sci. U.S.A 93: 11907–11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi P, Sagarin R, Ambrose R, Bell C, George M, Lee S, Lohse D, Miner C, and Murray S. 2007. Consistent frequency of color morphs in the sea star Pisaster ochraceus (Echinodermata: Asteriidae) across open-coast habitats in the northeastern Pacific. Pac. Sci 61: 201–210. [Google Scholar]
- Ramsay K, Turner J, Vize S, and Richardson C. 2000. A link between predator density and arm loss in the starfish Marthasterias glacialis and Asterias rubens. J. Mar. Biol. Assoc. U.K 80: 565–566. [Google Scholar]
- Rast JP, Smith LC, Loza-Coll M, Hibino T, and Litman GW. 2006. Genomic insights into the immune system of the sea urchin. Science 314: 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Posada JA, Pratchett M, Cano-Gomez A, Arango-Gomez JD, and Owens L. 2011a. Injection of Acanthaster planci with thiosulfate-citrate-bile-sucrose agar (TCBS). I. Disease induction. Dis. Aquat. Organ 97: 85–94. [DOI] [PubMed] [Google Scholar]
- Rivera-Posada JA, Pratchett M, Cano-Gomez A, Arango-Gomez JD, and Owens L. 2011b. Refined identification of Vibrio bacterial flora from Acanthasther planci based on biochemical profiling and analysis of housekeeping genes. Dis. Aquat. Organ 96: 113–123. [DOI] [PubMed] [Google Scholar]
- Rivera-Posada JA, Pratchett M, and Owens L. 2011c. Injection of Acanthaster planci with thiosulfate-citrate-bile-sucrose agar (TCBS). II. Histopathological changes. Dis. Aquat. Organ 97: 95–102. [DOI] [PubMed] [Google Scholar]
- Robilliard GA 1971. Feeding behaviour and prey capture in an asteroid, Stylasterias forreri. Syesis 4: 191–195. [Google Scholar]
- Robles CD, Desharnais RA, Garza C, Donahue MJ, and Martinez CA. 2009. Complex equilibria in the maintenance of boundaries: experiments with mussel beds. Ecology 90: 985–995. [DOI] [PubMed] [Google Scholar]
- Rogers TL, Schultz HK, and Elliott JK. 2018. Size-dependent interference competition between two sea star species demographically affected by wasting disease. Mar. Ecol. Progr. Ser 589: 167–177. [Google Scholar]
- Ruiz-Ramos DV, Schiebelhut LM, Hoff KJ, Wares JP, and Dawson MN. 2020. An initial comparative genomic autopsy of wasting disease in sea stars. Mol. Ecol 29: 1087–1102. [DOI] [PubMed] [Google Scholar]
- Ruppert EE, Fox RS, and Barnes RD. 2004. Echinodermata. Pp. 872–929 in Invertebrate Zoology, 7th ed., Ruppert EE, Fox R, and Barnes RD, eds. Brooks/Cole-Thomson Learning, Belmont, CA. [Google Scholar]
- Santos R, Haesaerts D, Jangoux M, and Flammang P. 2005. The tube feet of sea urchins and sea stars contain functionally different mutable collagenous tissues. J. Exp. Biol 208: 2277–2288. [DOI] [PubMed] [Google Scholar]
- Schultz JA, Cloutier RN, and Cote IM. 2016. Evidence for a trophic cascade on rocky reefs following sea star mass mortality in British Columbia. PeerJ 4: e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. 2006. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314: 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmens DC, Dane RE, Pancholi MR, Slade SE, Scrivens JH, and Elphick MR. 2013. Discovery of a novel neurophysin-associated neuropeptide that triggers cardiac stomach contraction and retraction in starfish. J. Exp. Biol 216: 4047–4053. [DOI] [PubMed] [Google Scholar]
- Short FT, Muehlstein LK, and Porter D. 1987. Eelgrass wasting disease: cause and recurrence of a marine epidemic. Biol. Bull 173: 557–562. [DOI] [PubMed] [Google Scholar]
- Sieling FW 1960. Mass mortality of the starfish, Asterias forbesi, on the Atlantic Coast of Maryland. Chesapeake Sci. 1: 73–74. [Google Scholar]
- Silina AV 2008. Long-term changes in intra- and inter-specific relationships in a community of scallops and sea stars under bottom scallop mariculture. J. Shellfish Res 27: 1189–1194. [Google Scholar]
- Silva JR 2000. The onset of phagocytosis and identity in the embryo of Lytechinus variegatus. Dev. Comp. Immunol 24: 733–739. [DOI] [PubMed] [Google Scholar]
- Sloan NA 1980. Aspects of the feeding biology of asteroids. Oceanogr. Mar. Biol. Annu. Rev 18: 57–124. [Google Scholar]
- Smith JE 1937. The structure and function of the tube feet in certain echinoderms. J. Mar. Biol. Assoc. U.K 22: 345–357. [Google Scholar]
- Smith LC, Ghosh J, Buckley KM, Clow LA, Dheilly NM, Haug T, Henson JH, Li C, Lun CM, Majeske AJ et al. 2010. Echinoderm immunity. Adv. Exp. Med. Biol 708: 260–301. [DOI] [PubMed] [Google Scholar]
- Smith LC, Arizza V, Barela Hudgell MA, Barone G, Bodnar AG, Buckley KM, Cunsolo V, Dheilly NM, Franchi N, Fugmann SD et al. 2018. Echinodermata: the complex immune system in echinoderms. Pp. 409–501 in Advances in Comparative Immunology, Cooper E, ed. Springer, Cham, Switzerland. [Google Scholar]
- Smith VJ 1981. The echinoderms. Pp. 534–562 in Invertebrate Blood Cells, Vol. II, RatclifIe NA and Rowley AF, eds. Academic Press, New York. [Google Scholar]
- Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA et al. 2007. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57: 573–583. [Google Scholar]
- Staehli A, Schaerer R, Hoelzle K, and Ribi G. 2009. Temperature induced disease in the starfish Astropecten jonstoni. Mar. Biodivers. Rec 2: 1–6. [Google Scholar]
- Stickle WB, and Denoux GJ. 1976. Effects of in situ tidal salinity fluctuations on osmotic and ionic composition of body fluid in Southeastern Alaska Rocky intertidal fauna. Mar. Biol 37: 125–135. [Google Scholar]
- Stickle WB, and Kozloff EN. 2008. Association and distribution of the ciliate Orchitophrya stellarum with asteriid sea stars on the west coast of North America. Dis. Aquat. Organ 80: 37–43. [DOI] [PubMed] [Google Scholar]
- Stickle WB, Weidner EH, and Kozloff EN. 2001. Parasitism of Leptasterias spp. (Echinodermata: Asteroidea) by the ciliated protozoan Orchitophrya stellarum (Scuticociliata). Invertebr. Biol 120: 88–95. [Google Scholar]
- Strathmann RR 1985. Feeding and nonfeeding larval development and life history evolution in marine invertebrates. Annu. Rev. Ecol. Syst 16: 339–361. [Google Scholar]
- Sullivan BK, Trevathan-Tackett SM, Neuhauser S, and Govers LL. 2018. Review: host-pathogen dynamics of seagrass diseases under future global change. Mar. Pollut. Bull 134: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday J, Raeburn L, and Hart MW. 2008. Emerging infectious disease in sea stars: castrating ciliate parasites in Patiria miniata. Dis. Aquat. Organ 81: 173–176. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Kai S, and Yamashita H. 2012. Mass stranding of crown-of-thorns starfish. Coral Reefs 31: 821. [Google Scholar]
- Thorpe JP, and Spencer EL. 2000. A mass stranding of the asteriod Asterias rubens on the Isle of Man. J. Mar. Biol. Assoc. U.K 80: 749–750. [Google Scholar]
- Tiffany WJ 1978. Mass mortality of Luidia senegalensis (Lamarck, 1816) on Captiva Island, Florida, with a note on its occurrence in Florida Gulf coastal waters. Fla. Sci 41: 63–64. [Google Scholar]
- Tinoco AB, Semmens DC, Patching EC, Gunner EF, Egertova M, and Elphick MR. 2018. Characterization of NGFFYamide signaling in starfish reveals roles in regulation of feeding behavior and locomotory systems. Front. Endocrinol 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy AM, Pielmeier ML, Yoshioka RM, Heron SF, and Harvell CD. 2019. Increases and decreases in marine disease reports in an era of global change. Proc. R. Soc. B Biol. Sci 286: 20191718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiger S, Konar B, Doroff A, and McCaslin L. 2016. Sea otters versus sea stars as major clam predators: evidence from foraging pits and shell litter. Mar. Ecol. Prog. Ser 560: 73–86. [Google Scholar]
- Trotter JA, and Koob TJ. 1989. Collagen and proteoglycan in a sea urchin ligament with mutable mechanical properties. Cell Tissue Res. 258: 527–539. [DOI] [PubMed] [Google Scholar]
- Uthicke S, Shaffelke B, and Byrne M. 2009. A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol. Monogr 79: 3–24. [Google Scholar]
- Van Veldhuizen HD, and Oakes VJ. 1981. Behavioral responses of seven species of asteroids to the asteroid predator, Solaster dawsoni. Oecologia 48: 214–220. [DOI] [PubMed] [Google Scholar]
- Van Volkom K, Harris LG, and Dijkstra JA. 2021. Not all prey are created equal: invasive ascidian diet mediates sea star wasting in Henricia sanguinolenta. J. Exp. Mar. Biol. Ecol 544: 151610. [Google Scholar]
- Vevers HG 1951. The biology of Asterias rubens L. II. Parasitization of the gonads by the ciliate Orchitophrya stellarum Cepede. J. Mar. Biol. Assoc. U.K 29: 619–624. [Google Scholar]
- Vicknair K, and Estes JA. 2012. Interactions among sea otters, sea stars, and suspension-feeding invertebrates in the western Aleutian archipelago. Mar. Biol 159: 2641–2649. [Google Scholar]
- Wahltinez SJ, Stacy NI, Lahner LL, and Newton AL. 2019. Coelomic fluid evaluation in clinically normal ochre sea stars Pisaster ochraceus: cell counts, cytology, and biochemistry reference intervals. J. Aquat. Anim. Health 31: 239–243. [DOI] [PubMed] [Google Scholar]
- Wahltinez SJ, Newton AL, Harms CA, Lahner LL, and Stacy NI. 2020. Coelomic fluid evaluation in Pisaster ochraceus affected by sea star wasting syndrome: evidence of osmodysregulation, calcium homeostasis derangement, and coelomocyte responses. Front. Vet. Sci 7: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chang Y, Yu J, Li C, and Xu G. 2007. Acute peristome edema disease in juvenile and adult sea cucumbers Apostichopus japonicus (Selenka) reared in North China. J. Invertebr. Pathol 96: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wares JP, and Schiebelhut LM. 2016. What doesn’t kill them makes them stronger: an association between elongation factor 1-alpha overdominance in the sea star Pisaster ochraceus and “sea star wasting disease.” PeerJ 4: e1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M, and Jeffs A. 2019. Impacts of sea star predation on mussel bed restoration. Restor. Ecol 27: 189–197. [Google Scholar]
- Wilkie IC 1979. The juxtaligamental cells of Ophiocomina nigra (Abildgaard) (Echinodermata: Ophiuroidea) and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res. 197: 515–530. [DOI] [PubMed] [Google Scholar]
- Wilkie IC 2002. Is muscle involved in the mechanical adaptability of echinoderm mutable collagenous tissue? J. Exp. Biol 205: 159–165. [DOI] [PubMed] [Google Scholar]
- Wilkie IC, Carnevali C, and Bonasoro F. 1992. The compass depressors of Paracentrotus lividus (Echinodermata, Echinoida): ultrastructural and mechanical aspects of their variable tensility and contractility. Zoomorphology 112: 143–153. [Google Scholar]
- Wobber DR 1975. Agonism in asteroids. Biol. Bull 148: 483–496. [DOI] [PubMed] [Google Scholar]
- Work TM, Weatherby TM, DeRito CM, Besemer RM, and Hewson I. 2021. Sea star wasting disease pathology in Pisaster ochraceus shows a basal-to-surface process affecting color phenotypes differently. Dis. Aquat. Org 145: 21–33. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tang P, Xu Y, Fang W, Wang X, Fang Z, and Xiao Y. 2016. Lutimaribacter marinistellae sp. nov., isolated from a starfish. Int. J. Syst. Evol. Microbiol 66: 3675–3680. [DOI] [PubMed] [Google Scholar]


