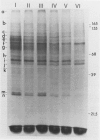
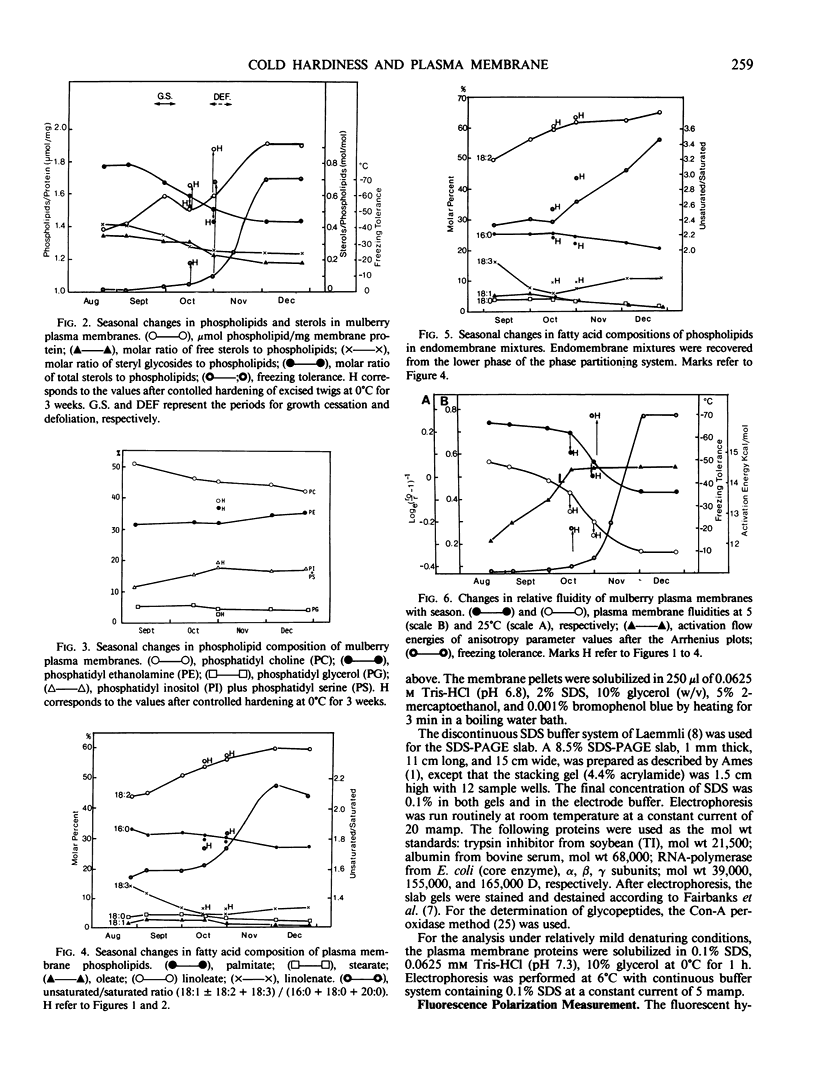
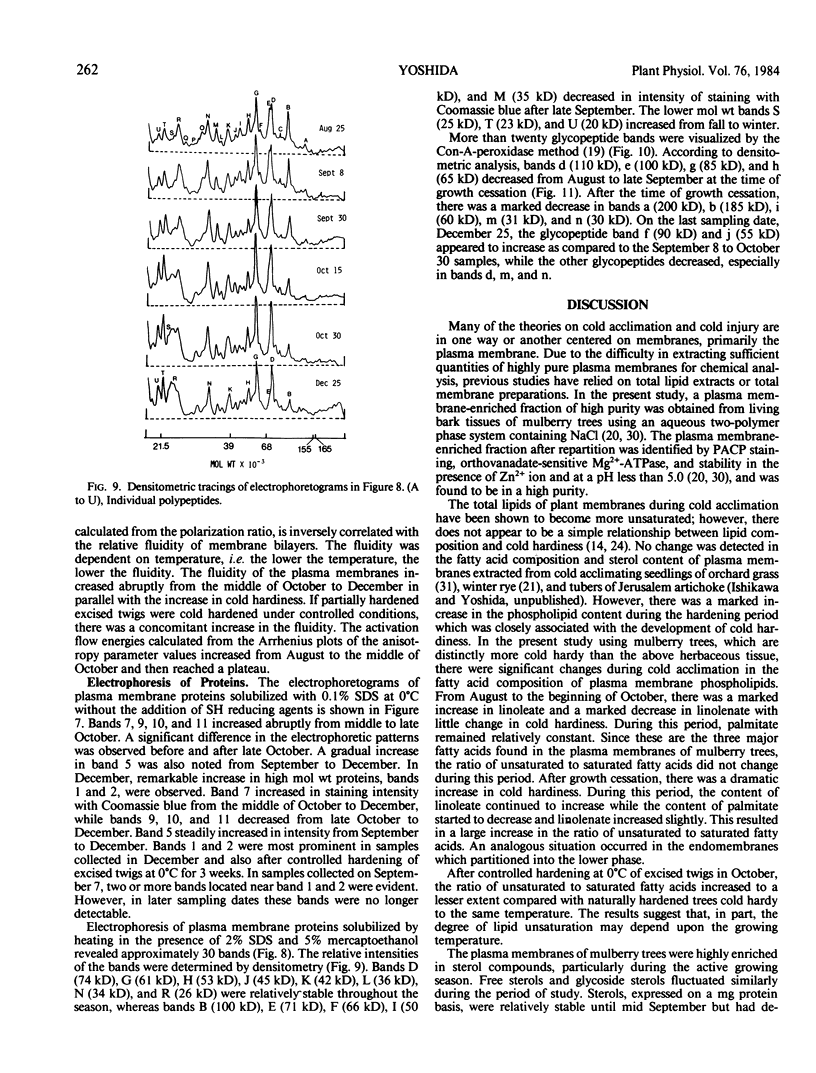
Abstract
The lipid and protein composition of the plasma membrane isolated from mulberry (Morus bombycis Koidz.) bark cells was analyzed throughout the cold acclimation period under natural and controlled environment conditions. There was a significant increase in phospholipids and unsaturation of their fatty acids during cold acclimation. The ratio of sterols to phospholipids decreased with hardiness, primarily due to the large increase in phospholipids. The fluidity of the plasma membrane, as determined by fluorescent polarization technique, increased with hardiness. Electrophoresis of plasma membrane proteins including glycoproteins revealed change in banding pattern during the early fall to winter period. Some of the protein changes could be related to growth cessation and defoliation. However, minor changes in proteins also occurred during the most active period of hardening. Changes in glycoproteins were coincident both with changes in growth stages and with the development of cold hardiness.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Lipid dynamics and lipid-protein interactions in rat enterocyte basolateral and microvillus membranes. Biochemistry. 1980 Jun 10;19(12):2763–2769. doi: 10.1021/bi00553a035. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., Rheaume B., Pomeroy K., Lepage M. Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology. 1968 Nov-Dec;5(3):202–225. doi: 10.1016/s0011-2240(68)80164-6. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Involvement of Plasma Membrane Alterations in Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1984 Jul;75(3):818–826. doi: 10.1104/pp.75.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Sarinana F. O. The staining of sciatic nerve glycoproteins on polyacrylamide gels with concanavalin A-peroxidase. Anal Biochem. 1975 Nov;69(1):320–322. doi: 10.1016/0003-2697(75)90597-7. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Changes in Microsomal Enzymes and Phospholipid during Dehardening in Stem Bark of Black Locust. Plant Physiol. 1976 May;57(5):710–715. doi: 10.1104/pp.57.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Studies on Freezing Injury of Plant Cells: I. Relation between Thermotropic Properties of Isolated Plasma Membrane Vesicles and Freezing Injury. Plant Physiol. 1984 May;75(1):38–42. doi: 10.1104/pp.75.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass (Dactylis glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984 May;75(1):31–37. doi: 10.1104/pp.75.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]