Abstract
Cancer metastasis is a multistep process that requires cancer cells to leave the primary site, survive in the blood stream, and finally colonize at a distant organ. It is the major cause of cancer morbidity and mortality. The organ-specific colonization requires close interaction and communication between cancer cells and host organs. Noncoding RNAs represent the majority of the transcriptome, with long noncoding RNAs (lncRNAs) making up a significant proportion. It has been suggested that lncRNAs play a key role in all stages of tumorigenesis and metastasis. This review will provide an overview of how lncRNAs are involved in cancer cell colonization in specific organ sites and the underlying mechanisms as well as therapeutic strategies.
Keywords: angiogenesis, cancer, cell invasion, colonize, hypoxia, long noncoding RNAs, metastasis, oligonucleotide therapeutics, organ-specific, small molecule inhibitor, tumor microenvironment
1 ∣. LncRNAs AS NOVEL PLAYERS IN CANCER METASTASIS
Long noncoding RNAs (lncRNAs) are generally defined as RNA transcripts longer than 200 nucleotides in length, with no protein-coding potential. Through the development of high-throughput technologies such as next-generation sequencing, followed by the subsequent interrogation of lncRNAs, the potential function of lncRNAs in disease pathogenesis has gradually been recognized by researchers. LncRNAs are found in both the cytosol and the nucleus. Depending on its location, a lncRNA can regulate gene expression either in trans or in cis.1-4 The dysregulation of lncRNAs has been increasingly linked to human cancer progression.5 Many lncRNAs are expressed at a low level with considerably high tissue or cell-type specificity, which makes them potential targets for cancer treatment. Metastasis is the most common cause of cancer death. Current evidence indicates that lncRNAs play a pivotal role in the metastatic cascade through multiple mechanisms (Table 1).
TABLE 1.
Summary of lncRNAs that promotes cancer metastasis
| LncRNA name | Alias | Genbank accession number | Function | References |
|---|---|---|---|---|
| CCAT2 | LINC00873 | NR_109834.1 | Metastasis | 11 |
| HOTAIR | HOXAS | NR_003716.3 | Oncogene | 12-16,32 |
| PCAT29 | DRAIC | NR_126437.1 | Metastasis | 18 |
| LncRNA-ATB | AL589182.3 | ENST00000493038 | Metastasis | 21 |
| LncRNA-HIT | 9530018H14RIK | … | EMT | 22 |
| MALAT1 | HCN | NR_002819.4 | EMT, angiogenesis | 23,31,33,34,57-59 |
| XIST | LINC00001 | NR_001564.2 | EMT | 24 |
| SPRY4-IT1 | SPRIGHTLY | NR_131221.1 | EMT | 25 |
| ANCR | DANCR | NR_024031.2 | Metastasis | 26 |
| LncRNA-LET | AK055007 | … | Hypoxia | 27 |
| BCAR4 | HCG1814062 | NR_024029.1 | Metastasis | 28 |
| MAYA | MNX1-AS1 | … | Metastasis | 30 |
| CASC15 | CANT | NR_015410.2 | Metastasis | 36 |
| Lnc-BM | AK055647 | … | Vascular cooption | 37 |
| TRERNA1 | treRNA | NR_051976.1 | EMT | 46 |
| ZEB1-AS1 | … | NR_024284.1 | Oncogene | 47 |
| GAPLINC | LINC01540 | NR_110428.1 | Cell invasion | 48 |
| HOTTIP | HOXA-AS6 | NR_037843.3 | Metastasis | 50 |
| CHRF | AK048451 | … | Metastasis | 51 |
| LncRNA-Hh | … | … | Metastasis | 52 |
| PVT1 | LINC00079 | NR_003367.3 | Angiogenesis | 54 |
| JHDM1D-AS1 | KDM7A-DT | NR_024451.1 | Angiogenesis | 56 |
| MVIH | … | … | Angiogenesis | 60 |
| BLACAT2 | LINC00958 | NR_038904.1 | Lymphangiogenesis | 63 |
| Lnc-EGFR | … | ENST00000554286.1 | Immunosuppression | 69 |
| UCA1 | LINC00178 | NR_015379.3 | Microenvironment | 67 |
| LINC00092 | NCRNA00092 | NR_024129.2 | Glycolysis | 74 |
| AK058003 | … | … | Hypoxia | 79 |
| LINK-A | LINC01139 | NR_015407.1 | Hypoxia | 80 |
| LncHIFCAR | MIR31HG | NR_027054 | Hypoxia | 81 |
Organ-specific metastasis is a nonrandom process and is dependent on intricate interactions between cancer cells and host organs.6 Each organ has its unique environment, which places diverse challenges for cancer cells to colonize. Some cancers predominately spread to one organ, such as uveal melanoma metastasis to the liver or prostate cancer metastasis to bone, whereas some cancers can colonize multiple organs, such as with breast cancer metastasis to bone, lungs, brain, and liver and lung cancer metastasis to the adrenal glands, liver, brain, and bone.7 Beyond lymph node spread, the liver, lungs, bone, and brain are the major organs colonized by different types of cancer.7 Here, we summarize lncRNAs' role in determining the organ tropism of metastasis.
1.1 ∣. LncRNAs in liver metastasis
The liver is the main site of distant metastasis and a major cause of death in gastrointestinal cancers such as colorectal cancer (CRC), pancreatic cancer, and tumors of the gallbladder, as well as breast cancer, sarcoma, and melanoma. Several groups8-10 compared the expression profile of lncRNAs between CRC patients with and without liver metastasis. Thousands of differentially expressed lncRNAs were identified as related to CRC liver metastasis, suggesting that aberrantly expressed lncRNAs may play an essential role in the metastasis of CRC and could be used as novel prognostic biomarkers for liver metastasis. A novel gene transcript, colon cancer-associated transcript 2 (CCAT2) was recently cloned and found to be highly expressed in CRC.11 Enhanced expression of CCAT2 resulted in a higher incidence of liver metastasis. Another lncRNA, HOTAIR, not only plays key roles in development but also promotes cancer progression. Overexpression of HOTAIR has been associated with widespread gene expression changes and aggressive metastatic phenotypes in many cancers, such as breast cancer,12 gastric cancer,13,14 lung cancer,15 pancreatic cancer,16 and CRC.17 High HOTAIR expression, correlated tightly with the presence of liver metastasis, has been demonstrated in gastric cancer13,14 and CRC17 through different mechanisms. LncRNA PCAT29,18 which is directly induced by androgen receptors, negatively regulates prostate cancer proliferation and migration. Overexpression of lncRNA PCAT29 significantly decreases the growth of tumors and liver metastasis.
1.2 ∣. LncRNAs in lung metastasis
As the second most common site for metastasis colonization, almost any type of cancer has the ability to metastasize to the lungs. However, breast cancer, prostate cancer, gastrointestinal tumors, sarcoma, and lung cancer itself are the most common cancer types that can colonize to the lungs. Lung capillaries are lined with endothelial cells that are surrounded by a basement membrane and adjacent alveolar cells.19 To metastasize, cancer cells from the primary sites need to cross structural obstacles and only a small proportion of cancer cells survive and seed in distant organs.20 Epithelial mesenchymal transition (EMT) is considered as a critical step in the initiation of metastasis, and the transforming growth factor beta (TGF-β) signaling pathway is one of the most studied pathways that stimulates EMT. It can either act as a tumor suppressor by inducing growth arrest and promoting apoptosis, or act as an oncogene in advanced cancers by induction of EMT, which contributes to tumor invasion and metastasis. Several lncRNAs including lncRNA-ATB,21 lncRNA-HIT,22 MALAT1,23 and XIST24 act downstream of TGF-β to promote lung dissemination. In addition to influencing function downstream of cytokines, lncRNA SPRY4-IT1,25 ANCR,26 hypoxia signaling induced lncRNA-LET,27 BCAR4,28 and HOTAIR12 have been shown to promote metastatic colonization through reprogramming the chromatin state.
1.3 ∣. LncRNAs in bone metastasis
Bone is the third most frequent site of metastasis, behind liver and lung. About 60%-85% of patients with metastatic breast and prostate cancer harbor bone metastasis.29 Cancer is generally incurable once it has spread to bones. Currently, most lncRNAs related studies focus on osteosarcoma, which is the most common primary malignant bone tumor. LncRNAs MAYA30 and MALAT131 have been shown to be involved in cancer cell colonization to bones. LncRNA MAYA,30 which acts as a scaffold, brings together LLGL2 and NSUN6 to methylate and inactivate the Hippo/MST1 pathways and is confirmed a promising therapeutic target for breast cancer bone metastasis. MALAT131 is upregulated in osteoblasts when coculturing with PC3 cells, and targeting the Sost/Wnt/MALAT1 pathway may open up new avenues of therapeutic intervention in treating prostate cancer metastasis to bones.
1.4 ∣. LncRNAs in brain metastasis
About 20%-40% of advanced stage cancers metastasize to the brain, and brain metastasis has a particularly poor prognosis with high morbidity and mortality. It frequently occurs secondary to poorly controlled lung adenocarcinoma, breast cancer, and melanoma. HOTAIR and MALAT1 are the first two lncRNAs involved in cancer brain metastasis. Nakagawa et al. examined the expression of HOTAIR in 77 nonsmall cell lung cancers (NSCLC), adjacent normal lung tissues, and brain metastases and found high expression of HOTAIR in metastatic brain tissues compared to primary cancer tissues.32 MALAT133,34 expression is also significantly higher in lung tumor tissues with brain metastasis than those without brain metastasis, and a high level of MALAT1 is correlated with shorter disease-free survival of patients with NSCLC. Alterations that increase DNA copy number are relatively frequent in human cancers.35 CASC1536 is located on chromosome 6p22.3, which is actively transcribed in metastatic melanoma cells. The upregulation of CASC15 in metastatic melanoma is correlated with the frequent copy number gains at the CASC15 locus, and is associated with progression to brain metastasis. Lnc-BM37 is the most recently discovered long noncoding RNA involved in breast cancer brain metastasis. To colonize the brain, metastatic cells have to cross densely packed blood vessels that constitute the blood-brain barrier. Secretion of cytokine chemokine (C-C motif) ligand 2 (CCL2) by mammary tumors recruits inflammatory monocytes to facilitate breast cancer lung38 and brain39 metastasis. The JAK2 binding lncRNA lnc-BM induces secretion of CCL2 through JAK2/STAT3 pathway activation, which wires up breast cancer cells and the brain microenvironment.
2 ∣. MOLECULAR MECHANISM OF LncRNAs IN CANCER METASTASIS
The metastatic cascade is a coordinated sequence of cell-biological events, which includes local cell invasion that enables cancer cells to escape from the primary site, develop new blood vessels (angiogenesis), migrate and invade through microenvironments, conduct intravasation and extravasation of blood and lymphatic vessels, survive in circulation, and finally colonize distant organs.40 Increasing evidence has revealed the essential role of lncRNAs at every step of the metastasis cascade (Figure 1). A clear understanding of how lncRNAs regulate multiple mechanisms in metastasis may lead to novel therapeutic intervention for patients with cancer.
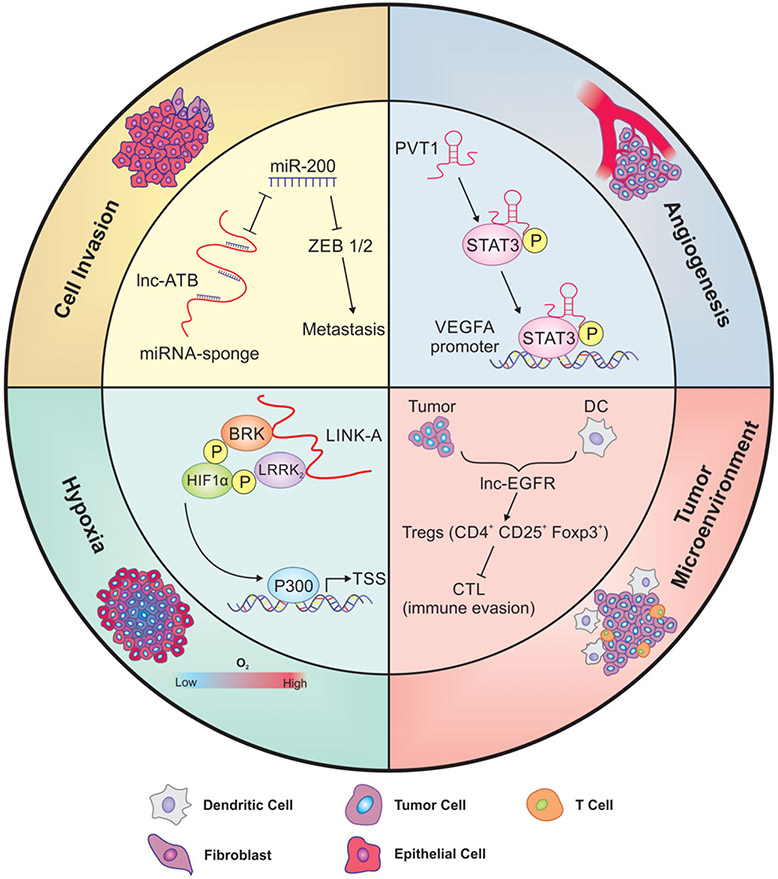
FIGURE 1.
The role of lncRNAs in metastatic processes. Metastasis is characterized by a series of cell-biological events, and lncRNAs have been shown to play an indispensable role in every step of metastasis. To colonize distant organs, cancer cells must have the capacity to move, adapt, and survive in the new environment. Cell invasion: the acquisition of EMT features leads to increased migratory ability and invasiveness, which results in tumor cell dissemination. Lnc-ATB functions as a miRNA sponge, competitively binds to miR-200, and reduces the effect of miRNA on its targets ZEB1/2, thus promoting metastasis. Hypoxia: as a tumor outgrows the oxygen supply, it either co-opts preexisting vessels of host organs or induces angiogenesis. LINK-A facilitates the recruitment of BRK and LRRK2 to phosphorylate and stabilize HIF-1α. Stabilized HIF-1α upregulates downstream genes to promote cancer cell survival in low-oxygen conditions. Angiogenesis: in the meantime, the generation of new blood vessels to obtain adequate supply of oxygen and nutrients becomes an indispensable step in the process of cancer outgrowth. PVT1 directly binds to STAT3 in the nucleus and increases its protein stability which further activates VEGFA expression to stimulate angiogenesis. Tumor microenvironment: the communication between cancer cells and their microenvironment is also critical for sustained tumor growth in distant organs. In response to evolving environmental conditions, stromal cells such as immune cells, CAFs, and ECM components change over the process of cancer progression to provide favorable surroundings and support tumor growth. Treg cells have been found to promote tumor metastasis through suppressing innate and adaptive antitumor immunity. Lnc-EGFR regulates Treg differentiation and interferes with cytotoxic T cell function which leads to immune escape
2.1 ∣. LncRNAs in cell invasion
To disseminate at distant organ sites, cancer cells must detach from the primary tumor using extracellular proteases to break down the extracellular matrix (ECM) and invade adjacent parenchyma. Metastasis then occurs when invasive cancer cells intrude into the blood and lymphatic vessels, travel via the blood stream, penetrate the endothelium, and finally settle at a distant organ and build a second tumor.41 Epithelial-mesenchymal transition (EMT) is one centrally important process that enables stationary epithelia cells to gain migratory ability and invade.42,43 EMT is executed by activation of a series of transcription factors (EMT-TFs), mainly of the ZEB, SNAIL, and TWIST families.44 Many groups have reported lncRNAs as master regulators of invasion. Here, we summarized the most thoroughly studied lncRNAs involved in regulating EMT-TFs to promote metastasis. Several lncRNAs, including lncRNA-ATB21 and HOTAIR,45 function as miRNA sponges to modulate ZEB and SNAIL levels in cancer. Some lncRNAs have also been involved in the epigenetic regulation of EMT-TFs expression, such as TRERNA1's46 function as an enhancer of SNAI1 and ZEB1-AS1's47 recruitment of p300 to the ZEB1 promoter. LncRNAs also function through RNA/protein interactions to regulate metastasis. For example, GAPLINC48 stimulates the expression of SNAI2 via binding to PSF and NONO protein. In addition to regulating SNAIL and ZEB, Hu et al.49 screened potential lncRNAs involved in TWIST-induced EMT processes and found that more than 99 lncRNAs were regulated. The detailed mechanisms of how lncRNAs associated with TWIST/EMT signaling pathways were further confirmed by other groups. TWIST binds to lncRNA HOTTIP, which recruits and directs WDR5 to the HOX cluster and induces HOXA9 expression.50 High levels of HOXA9 are correlated with an aggressive cellular phenotype in prostate cancer. Other than directly binding to TWIST, LncRNA CHRF has been shown to regulate the TWIST/EMT signaling pathway by acting as an endogenous sponge of miR-489.51 CHRF knockdown inhibits TWIST expression and further suppresses EMT progression in CRC.51 Another LncRNA-Hh52 has been shown to be involved in regulation of cancer stem-like properties in TWIST-induced EMT processes.
2.2 ∣. LncRNAs in angiogenesis
Angiogenesis, the development of new blood vessels from preexisting blood vessels, is required for advanced tumor growth and metastasis and is an essential step in the control of tumor progression. Blood vessels provide not only nutrients to support tumor growth but also a route for tumor cells to escape and disseminate to distant organs. Anti-angiogenic therapies that target VEGF or its receptors have become a mainstay of cancer therapy.53 LncRNA PVT1 is amplified in many types of cancer.54 Other than regulating MYC stability, PVT1 is shown to increase capillary formation ability in endothelial cells. It forms complexes with STAT3 and stimulates angiogenesis via activation of the STAT3/VEGFA axis.55 The upregulation of PVT1 in gastric cancer is positively correlated with VEGFA, and high levels of PVT1 and VEGFA indicate poor prognosis.55 JHDM1D-AS1, which is a long noncoding antisense transcript of JHDM1D-AS1, is induced under nutrient starvation.56 Overexpression of JHDM1D-AS1 accelerates pancreatic tumor growth via upregulation of several proangiogenic factors such as HGF and FGF1, and proinflammatory factors such as MMP3 and MMP9 without affecting JHDM1D expression. MALAT1 is found to be enriched and conserved in endothelial cells.57 Loss of MALAT1 inhibited endothelial cell proliferation and reduced neonatal retina vascularization. The role of MALAT1 in promoting angiogenesis is further confirmed by two other groups in neuroblastoma58 and gastric cancer.59 LncRNA MVIH is another noncoding RNA, which is associated with microvascular invasion and tumor node metastasis in hepatocellular carcinoma.60 Yuan et al. reported that MVIH activates tumor-inducing angiogenesis by inhibiting the secretion of phosphoglycerate kinase 1 (PGK1), which is involved in the angiogenic process as a disulfide reductase.61
Bladder cancer is frequently associated with regional lymph node metastasis. Lymphangiogenesis, which is the process of forming lymphatic vessels, is correlated with lymph node metastasis and metastasis-free survival in bladder cancer.62 LncRNA BLACAT2 is identified from high-grade muscle-invasive bladder cancer.63 Patients with high BLACAT2 have shorter overall and metastasis-free survival, emphasizing the role of lncRNAs in bladder cancer progression. Interestingly, BLACAT2 level also correlates with intratumoral and peritumoral lymphatic vessel density through epigenetic upregulation of VEGF-C levels in bladder cancer. Targeting BLACAT2, therefore, may be a potential target of bladder cancer with lymphangiogenesis and lymphatic metastasis.63
Evidence suggests that some cancers can grow and metastasize without angiogenesis by using preexisting vessels of the host organ.53 Vascular co-option also explains why some tumors do not respond to anti-angiogenic agents. The lungs, liver, and brain, which are vessel-rich organs, have been described as common sites for hosting nonangiogenic tumors.64 Lnc-BM-mediated breast cancer brain metastasis promotes cancer cell adherence and stretching on vascular capillaries.37 Knockdown of Lnc-BM facilitates FasL-induced apoptosis via inhibition of ICAM1. This data suggests that lncRNAs also play important roles in promoting nonangiogenic tumor growth and metastasis.37
2.3 ∣. LncRNAs in tumor microenvironment
In cancer metastasis, only a small number of cells that detach from the primary site can make it to the distant organs. Once they leave their favorable surroundings, bidirectional communication between cancer cells and microenvironments is critical for cancer cells to survive in the new environment. Cancer cells are surrounded by stromal cells, which include inflammatory/immune cells, cancer-associated fibroblasts (CAFs), endothelia cells (vascular), and ECM components (collagen, laminin, and proteoglycan complex).65 The relationship between lncRNAs and endothelial cells will be discussed separately in the section “lncRNA in angiogenesis”.
Tumor-associated macrophages (TAMs) are a class of immune cells in the tumor microenvironment and are important regulators of tumorigenesis and metastasis. Ye et al.66 recently showed that macrophage depolarization from the M2 phenotype by lncRNA cox-2 prevents metastasis of hepatocellular carcinoma. Overexpression of lncRNA cox-266 or inhibition of UCA167 suppresses cancer metastasis, together highlighting the role of lncRNAs in reeducating TAMs. Regulatory T cells (Tregs) are another type of immune cells that play a pivotal role in tumor immune escape.68 Lnc-EGFR is found to be highly expressed in Tregs.69 Enforced expression of lnc-EGFR stimulates Treg differentiation and interferes with cytotoxic T cell function and promotes hepatocellular carcinoma growth.69 As such, reversing the polarization of TAMs or inhibition of immunosuppressive cells by regulating lncRNAs levels might have beneficial anti-metastatic effects.
CAFs are different from normal fibroblasts and are responsible for synthesis, deposition, and remodeling of the ECM in the tumor microenvironment. Once accumulated in the tumor, they can be activated by TGF-β, platelet-derived growth factor, FGF2, and secreted proteases.70 Activated fibroblasts are also producers of cytokines, chemokines, metabolites, enzymes, and ECM.71 Several recent studies have reported that CAFs-secreted TGF-β promotes cancer metastasis via regulation of lncRNA expression.72,73 CXCL14 high CAFs upregulated LINC00092, which directly binds to PFKBP2 and promotes metastasis of ovarian cancer by altering glycolysis.74 These findings suggest that modulation of CAF-derived signals by targeting lncRNAs could serve as a novel therapeutic strategy.
2.4 ∣. LncRNAs in hypoxia
Low oxygen tension, also called hypoxia, is a key microenvironment factor that arises as a result of oxygen supply and demand imbalance. Tumor cells quickly exhaust their oxygen supply because of intensive proliferating and expanding. In the meantime, blood vessels in tumors are often highly abnormal, distended capillaries with leaky walls and sluggish flow75 which result in insufficient oxygen supply. Hypoxia activates a number of complex signaling pathways, and hypoxia-inducible factor (HIF) signaling is the major pathway involved. Under normoxic conditions, HIF transcription factors are degraded rapidly through proteasomal activity.76 Hypoxia activates HIF signaling by stabilizing HIF-α subunits. Over the last few years, accumulating evidence has indicated that some lncRNAs are regulated by hypoxia.77,78 Our discussion here only summarizes the role of hypoxia-related lncRNAs in cancer metastasis. lncRNA-AK058003 is induced upon hypoxia treatment compared to normoxic conditions.79 AK058003 is also found to be frequently upregulated in gastric cancer and promotes cancer migration and invasion via hypoxia/lncRNA-AK058003/SNCG pathway.79 However, how AK058003 regulates SNCG expression is still not clear. Several lncRNAs such as LINK-A80 and lncRNALET27 have been shown to modulate HIF-1 signaling through lncRNA-protein interaction. LINK-A regulates the phosphorylation and protein stabilization of HIF-1α via recruitment of two protein kinases, BRK and LRRK2.80 Most importantly, the LINK-A transgenic mouse model demonstrates increased breast cancer lung metastasis (unpublished data), reinforcing the role of lncRNA in hypoxia-involved cancer metastasis. Other than regulating HIF-1α stability or localization, HIF-1α coactivator LncHIFCAR can directly interact with HIF-1α, enhance the recruitment of coactivator p300, and facilitate the activation of the HIF-1 transcriptional network.81 Inhibition of LncHIFCAR significantly impaired oral cancer cell invasion ability and compromised distant colonization in xenograft models.
Taken together, we have summarized the most studied lncRNAs in regulating the metastasis cascade which covers cell invasion, angiogenesis, hypoxia, and tumor microenvironment. More research is needed to understand all the steps of metastasis, especially how lncRNAs determine organ-specific colonization, to develop new treatments.
3 ∣. TARGETING LncRNAs AGAINST CANCER METASTASIS
LncRNAs are emerging as contributors to tumorigenesis and metastasis, which makes them potential targets for drug development. Currently, available strategies include (1) oligonucleotide therapeutics, including double-stranded RNA function through RNA interference, single-stranded antisense oligonucleotides (ASO)-mediated activation of RNase H cleavage, and aptamers and morpholinos.82 All ASO need extensive chemical modification in order to improve stability while decreasing potential off-target effects in vivo.83,84 Some groups also work on optimizing nucleic acid delivery efficiency to increase cellular entry and escape degradation.84,85 Preclinical studies using xenografted human cell lines28,86 and patient-derived xenograft models87 have demonstrated the therapeutic potential of targeting lncRNAs. Given the clinical success in LNA microRNA inhibitors,88 we believe that oligonucleotide therapeutics may prove useful for cancer metastasis in the near future. (2) Small molecules: with the development of modern analytical techniques such as cryoelectron microscopy, nuclear magnetic resonance spectroscopy, small-angle X-ray scattering, and selective 2′-hydroxyl acylation analyzed by primer extension,89 we can now better predict the secondary and tertiary structure of lncRNAs even at a single-nucleotide resolution. Small molecule inhibitors that disrupt RNA/protein or RNA/DNA interactions or target specific structural elements could provide exciting possibilities for modulating lncRNA levels and translate into clinical scenarios.
4 ∣. CONCLUSION REMARKS AND PERSPECTIVE
Metastasis is a complex process that involves many diverse molecular mechanisms. Significant progress in our understanding of cancer metastasis at the molecular and cellular level, as well as our understanding of signaling pathways, provides us with new insights on finding targets for intervention. LncRNAs are emerging as new players in tumorigenesis and metastasis. Fully understanding their expression and biological functions will boost our knowledge of the roles of lncRNAs in metastasis and warrant the development of lncRNA-related therapeutic strategies. Despite a rapid growth in the number of known lncRNAs and the fact that many lncRNAs have proven to be potential candidates for targeted therapy, our understanding of their function remains limited. Animal models are promising tools for exploring the phenotypic impact of lncRNAs. Recent advances in the CRISPR/Cas9 DNA editing system90 and CRISPR-Cas13 RNA editing system91 expedite the study of lncRNA functions in vitro and in vivo. The use of patient-derived xenograft models and genetically engineered mouse models will provide us with new tools to investigate lncRNAs' role in cancer metastasis before translating these developments into clinical scenarios. Unlike protein-coding genes, lncRNAs are still poorly defined and only a small fraction of lncRNAs has been fully characterized. How to clearly separate the role of coding genes from noncoding genes when manipulating genomic locus in vivo requires more rigorous investigation.92 New experimental approaches and technologies could be an addition to the lncRNA field and also improve our understanding of lncRNAs' role in cancer metastasis.
ACKNOWLEDGMENTS
We apologize to colleagues whose work was not able to be included in this review due to space limitations.
Funding information
Cancer Prevention and Research Institute of Texas, Grant/Award Number: R1218, RP150094, RP180259; Congressionally Directed Medical Research Programs, Grant/Award Number: BC151465; National Cancer Institute, Grant/Award Number: R00CA166527R01 CA218025-01R01 CA218036-01; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: R00DK094981; Department of Defense Breakthrough award, Grant/Award Number: BC151465; Cancer Prevention Research Institute of Texas First-time Faculty Recruitment award, Grant/Award Number: R1218; National Cancer Institute R01 award, Grant/Award Numbers: 1 R01 CA218036-01, 1 R01 CA218025-01; National Institutes of Health Pathway to Independence award, Grant/Award Numbers: R00CA166527, R00DK094981
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Rashid F, Shah A, Shan G. Long non-coding RNAs in the cytoplasm. Genomics Proteomics Bioinformatics. 2016;14:73–80. 10.1016/j.gpb.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu B, Shan G. Functions of long noncoding RNAs in the nucleus. Nucleus. 2016;7:155–166. 10.1080/19491034.2016.1179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772. 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Yan X, Hu Z, Feng Y, et al. Comprehensive genomic characterization of long non-coding RNAs across human cancers. Cancer Cell. 2015;28: 529–540. 10.1016/j.ccell.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153–162. 10.1007/s10911-007-9047-3. [DOI] [PubMed] [Google Scholar]
- 7.Obenauf AC, Massague J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1:76–91. 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, Sun Q, Cheng X, et al. Genome-wide analysis of long noncoding RNA (lncRNA) expression in colorectal cancer tissues from patients with liver metasasis. Cancer Med. 2016;5:1629–1639. 10.1002/cam4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye LC, Ren L, Qiu JJ, et al. Aberrant expression of long noncoding RNAs in colorectal cancer with liver metastasis. Tumour Biol. 2015;36: 8747–8754. 10.1007/s13277-015-3627-4. [DOI] [PubMed] [Google Scholar]
- 10.Kong H, Wu W, Zhu M, et al. Long non-coding RNAs: novel prognostic biomarkers for liver metastases in patients with early stage colorectal cancer. Oncotarget. 2016;7:50428–50436. 10.18632/oncotarget.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461. 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZZ, Shen ZY, Shen YY, et al. HOTAIR long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(c)-binding protein (PCBP) 1. Mol Cancer Ther. 2015;14:1162–1170. 10.1158/1535-7163.MCT-14-0695. [DOI] [PubMed] [Google Scholar]
- 14.Endo H, Shiroki T, Nakagawa T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang Y, Wang X, Nguyen HT, et al. Induction of long intergenic noncoding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol. 2013;6:35. 10.1186/1756-8722-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71: 6320–6326. 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 18.Malik R, Patel L, Prensner JR, et al. The lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol Cancer Res. 2014;12:1081–1087. 10.1158/1541-7786.MCR-14-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 21.Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Richards EJ, Zhang G, Li ZP, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) beta: LncRNA-hit-mediated TGFbeta-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290:6857–6867. 10.1074/jbc.M114.610915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Y, Shen B, Tan M, et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531–1541. 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Wan L, Liu Z, et al. Long non-coding RNA XIST promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018; 418:185–195. 10.1016/j.canlet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Liu XH, Lu KH, et al. EZH2-mediated epigenetic suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell proliferation and metastasis by affecting the epithelial-mesenchymal transition. Cell Death Dis. 2014;5:e1298. 10.1038/cddis.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Hou P, Fan D, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71. 10.1038/cdd.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F, Huo XS, Yuan SX, et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell. 2013;49:1083–1096. 10.1016/j.molcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Xing Z, Lin A, Li C, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159:1110–1125. 10.1016/j.cell.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Wang S, Xing Z, et al. A ROR1-HER3-lncRNA signalling axis modulates the hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–119. 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebastian A, Hum NR, Hudson BD, Loots GG. Cancer-osteoblast interaction reduces Sost expression in osteoblasts and up-regulates lncRNA MALAT1 in prostate cancer. Microarrays (Basel). 2015;4:503–519. 10.3390/microarrays4040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T, Endo H, Yokoyama M, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436:319–324. 10.1016/j.bbrc.2013.05.101. [DOI] [PubMed] [Google Scholar]
- 33.Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121:101–108. 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 35.Myllykangas S, Himberg J, Böhling T, Nagy B, Hollmén J, Knuutila S. DNA copy number amplification profiling of human neoplasms. Oncogene. 2006;25:7324–7332. 10.1038/sj.onc.1209717. [DOI] [PubMed] [Google Scholar]
- 36.Lessard L, Liu M, Marzese DM, et al. The CASC15 long Intergenic noncoding RNA locus is involved in melanoma progression and phenotype switching. J Invest Dermatol. 2015;135:2464–2474. 10.1038/jid.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Liang K, Hu Q, et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest. 2017;127:4498–4515. 10.1172/JCI91553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafri MA, Al-Qahtani MH, Shay JW. Role of miRNAs in human cancer metastasis: implications for therapeutic intervention. Semin Cancer Biol. 2017;44:117–131. 10.1016/j.semcancer.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Leber MF, Efferth T. Molecular principles of cancer invasion and metastasis (review). Int J Oncol. 2009;34:881–895. [DOI] [PubMed] [Google Scholar]
- 42.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Tillo E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–3456. 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu YW, Sun M, Xia R, et al. LincHOTAIR epigenetically silences miR34a by binding to PRC2 to promote the epithelial-to-mesenchymal transition in human gastric cancer. Cell Death Dis. 2015;6:e1802. 10.1038/cddis.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Hu Y, Liu X, et al. LncRNA TRERNA1 function as an enhancer of SNAI1 promotes gastric cancer metastasis by regulating epithelial-Mesenchymal transition. Mol Ther Nucleic Acids. 2017;8:291–299. 10.1016/j.omtn.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Lin J. Long noncoding RNA ZEB1-AS1 acts as an oncogene in osteosarcoma by epigenetically activating ZEB1. Am J Transl Res. 2016;8:4095–4105. [PMC free article] [PubMed] [Google Scholar]
- 48.Yang P, Chen T, Xu Z, Zhu H, Wang J, He Z. Long noncoding RNA GAPLINC promotes invasion in colorectal cancer by targeting SNAI2 through binding with PSF and NONO. Oncotarget. 2016;7:42183–42194. 10.18632/oncotarget.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu P, Yang J, Hou Y, et al. LncRNA expression signatures of twist-induced epithelial-to-mesenchymal transition in MCF10A cells. Cell Signal. 2014;26:83–93. 10.1016/j.cellsig.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Malek R, Gajula RP, Williams RD, et al. TWIST1-WDR5-Hottip regulates Hoxa9 chromatin to facilitate prostate cancer metastasis. Cancer Res. 2017;77:3181–3193. 10.1158/0008-5472.CAN-16-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao Y, Han T, Zhang T, Ma C, Sun C. LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal cancer via TWIST1/EMT signaling pathway. Oncotarget. 2017;8:36410–36422. 10.18632/oncotarget.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou M, Hou Y, Yang G, et al. LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells. 2016;34:55–66. 10.1002/stem.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayson GC, Hicklin DJ, Ellis LM. Antiangiogenic therapy–evolving view based on clinical trial results. Nat Rev Clin Oncol. 2012;9:297–303. 10.1038/nrclinonc.2012.8. [DOI] [PubMed] [Google Scholar]
- 54.Tseng YY, Moriarity BS, Gong W, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J, du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018; 37:4094–4109. 10.1038/s41388-018-0250-z. [DOI] [PubMed] [Google Scholar]
- 56.Kondo A, Nonaka A, Shimamura T, et al. Long noncoding RNA JHDM1D-AS1 promotes tumor growth by regulating angiogenesis in response to nutrient starvation. Mol Cell Biol. 2017;37:1–16. 10.1128/MCB.00125-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014; 114:1389–1397. 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 58.Tee AE, Liu B, Song R, et al. The long noncoding RNA MALAT1 promotes tumor-driven angiogenesis by up-regulating pro-angiogenic gene expression. Oncotarget. 2016;7:8663–8675. 10.18632/oncotarget.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Wu Z, Yuan J, et al. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. 10.1016/j.canlet.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 60.Yuan SX, Yang F, Yang Y, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients' poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 61.Lay AJ, Jiang XM, Kisker O, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature. 2000;408: 869–873. 10.1038/35048596. [DOI] [PubMed] [Google Scholar]
- 62.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922–928. 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He W, Zhong G, Jiang N, et al. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J Clin Invest. 2018;128:861–875. 10.1172/JCI96218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnem T, Reynolds AR, Kuczynski EA, et al. Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer. 2018;18:323–336. 10.1038/nrc.2018.14. [DOI] [PubMed] [Google Scholar]
- 65.Gangadhara S, Barrett-Lee P, Nicholson RI, Hiscox S. Pro-metastatic tumor-stroma interactions in breast cancer. Future Oncol. 2012;8: 1427–1442. 10.2217/fon.12.134. [DOI] [PubMed] [Google Scholar]
- 66.Ye Y, Xu Y, Lai Y, et al. Long non-coding RNA cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. J Cell Biochem. 2018;119:2951–2963. 10.1002/jcb.26509. [DOI] [PubMed] [Google Scholar]
- 67.Chen S, Shao C, Xu M, et al. Macrophage infiltration promotes invasiveness of breast cancer cells via activating long non-coding RNA UCA1. Int J Clin Exp Pathol. 2015;8:9052–9061. [PMC free article] [PubMed] [Google Scholar]
- 68.Halvorsen EC, Mahmoud SM, Bennewith KL. Emerging roles of regulatory T cells in tumour progression and metastasis. Cancer Metastasis Rev. 2014;33:1025–1041. 10.1007/s10555-014-9529-x. [DOI] [PubMed] [Google Scholar]
- 69.Jiang R, Tang J, Chen Y, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6: 392–401. 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 71.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11:1–14. 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang J, Lu Q, Shen B, et al. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep. 2015;5:11924. 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Y, Jia HH, Xu YQ, et al. Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-ss1 secretion. Mol Cancer. 2018;17:5. 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao L, Ji G, le X, et al. Long noncoding RNA LINC00092 acts in cancer-associated fibroblasts to drive glycolysis and progression of ovarian cancer. Cancer Res. 2017;77:1369–1382. 10.1158/0008-5472.CAN-16-1615. [DOI] [PubMed] [Google Scholar]
- 75.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 76.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 77.Shih JW, Kung HJ. Long non-coding RNA and tumor hypoxia: new players ushered toward an old arena. J Biomed Sci. 2017;24:53. 10.1186/s12929-017-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang YN, Zhang K, Hu ZM, et al. Hypoxia-regulated lncRNAs in cancer. Gene. 2016;575:1–8. 10.1016/j.gene.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Liu X, Zhang H, et al. Hypoxia-inducible lncRNA-AK058003 promotes gastric cancer metastasis by targeting gamma-synuclein. Neoplasia. 2014;16:1094–1106. 10.1016/j.neo.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin A, Li C, Xing Z, et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–224. 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shih JW, Chiang WF, Wu ATH, et al. Long noncoding RNA LncHIF-CAR/MIR31HG is a HIF-1alpha co-activator driving oral cancer progression. Nat Commun. 2017;8:15874. 10.1038/ncomms15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long noncoding RNAs in cancer. Trends Mol Med. 2018;24:257–277. 10.1016/j.molmed.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35:238–248. 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chery J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 2016;4:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. 10.1186/s13073-017-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutschner T, Hammerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–1189. 10.1158/0008-5472.Can-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leucci E, Vendramin R, Spinazzi M, et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature. 2016;531:518–522. 10.1038/nature17161. [DOI] [PubMed] [Google Scholar]
- 88.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 89.Connelly CM, Moon MH, Schneekloth JS. The emerging role of RNA as a therapeutic target for small molecules. Cell Chem Biol. 2016;23: 1077–1090. 10.1016/j.chembiol.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu S, Li W, Liu J, et al. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat Biotechnol. 2016;34:1279–1286. 10.1038/nbt.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cox DBT, Gootenberg JS, Abudayyeh OO, et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–1027. 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bassett AR, Akhtar A, Barlow DP, et al. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e03058. 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]