Abstract
Background
Adaptive thermogenesis, defined as the decrease in the energy expenditure components beyond what can be predicted by changes in body mass stores, has been studied as a possible barrier to weight loss and weight maintenance. Intermittent energy restriction (IER), using energy balance refeeds, has been pointed out as a viable strategy to reduce adaptive thermogenesis and improve weight loss efficiency (greater weight loss per unit of energy deficit), as an alternative to a continuous energy restriction (CER). Following a randomized clinical trial design, the BREAK Study aims to compare the effects of IER versus CER on body composition and in adaptive thermogenesis, and understand whether participants will successfully maintain their weight loss after 12 months.
Methods
Seventy-four women with obesity and inactive (20–45 y) will be randomized to 16 weeks of CER or IER (8x2 weeks of energy restriction interspersed with 7x1 week in energy balance). Both groups will start with 2 weeks in energy balance before energy restriction, followed by 16 weeks in energy restriction, then 8 weeks in energy balance and finally a 12-month weight maintenance phase. Primary outcomes are changes in fat-mass and adaptive thermogenesis after weight loss and weight maintenance. Secondary outcomes include weight loss, fat-free mass preservation, alterations in energy expenditure components, and changes in hormones (thyroid function, insulin, leptin, and cortisol).
Discussion
We anticipate that The BREAK Study will allow us to better understand adaptive thermogenesis during weight loss and weight maintenance, in women with obesity. These findings will enable evidence-based decisions for obesity treatment.
Trial registration
ClinicalTrials.gov: NCT05184361.
1. Introduction
Despite extensive research into lifestyle interventions for weight loss (WL) [1], one of the major challenges for treating obesity is WL maintenance [2], since weight regain rates are high [3]. Lack of long-term adherence to dietary and/or exercise recommendations has been pointed out as the major reason [4], however the existence of compensatory mechanisms in response to a prolonged negative energy balance (EB) may also play a role [5].
A decrease in resting energy expenditure (REE) is expected during a WL intervention due to fat-mass (FM) and fat-free mass (FFM) losses. However, some authors [6–8] showed that these reductions tend to be greater than predicted by WL, a phenomenon called “adaptive thermogenesis” (AT). Then, AT can function as a barrier to WL and contribute to weight regain [9, 10]. Still, some studies reported contrasting findings, as they did not find a significant value for AT even after a considerable WL [11–14]. This can be explained by a large heterogeneity in the methods used to quantify AT, a high variability in study designs, between subjects, and the wide magnitude of WL [15].
Furthermore, its relevance on long-term weight management (WM) has been recently questioned [16], as AT seems to be attenuated or even non-existent [15] after periods of (two to five weeks [16–19]) of weight stabilization.
Continuous energy restriction (CER), meaning a constant daily energy restriction (ER) according to individual’s energy requirements, is the most common nutritional strategy for WL [20, 21]. However, some concerns have been pointed out to this strategy, since the behavioral, metabolic and endocrine adaptive responses it causes can compromise therapeutic adherence, undermining WL and WM [21–23]. These adaptive responses include an increased drive to eat, a reduced physical activity (PA) or energy cost of PA, a reduced energy expenditure (EE), and hormonal effects that facilitate the accumulation of adipose tissue and loss of lean tissues [24]. ER is associated with a reduction in thyroid hormones secretion (T3 and T4), variations in appetite-regulating hormones (decreased leptin, peptide YY, and increased ghrelin), variations in steroid hormones (increased cortisol), reduced insulin and testosterone, that all together influence EE, satiety and body composition [25, 26]. Simultaneously, some behavioral adaptations as a response to negative EB may occur, such as increasing sedentary behavior and/or decreasing light PA, more consistently observed in diet-only induced WL [26, 27].
Intermittent energy restriction (IER), which consists in interspersing periods of ER with periods of EB (the “refeed” or “diet breaks”), has been suggested as an alternative to CER [22]. The inclusion of periods of neutral EB is expected to attenuate adaptive responses to ER and WL in some regulatory hormones, which play a role in WL, satiety and REE [22, 24].
According to literature, there are 8 randomized clinical trials (RCT) comparing CER with IER in people with overweight/obesity, that achieved EB in the refeeding phases [6, 22, 23, 28–32]. Of these, only three resulted in a greater WL with IER [6, 23, 30]. Variability in study design in these 8 RCT may have contributed to the different findings, favoring IER or not in comparison with CER. The study design differed in the duration of the intervention, the pattern of intermittency (days of ER vs. EB), the severity of calorie restriction, the food provision, the exercise recommendations, the adherence to the intervention, the sample size, and the baseline characteristics of the study population (sex and BMI).
Campbell et al. [23] investigated body composition changes in lean resistance trained individuals following a ~25% ER for 7 weeks in conjunction with 4-days/week resistance training, randomized to either IER or CER. IER cycled 5 days of ER with 2 days of EB using carbohydrate refeeds. The authors found that a 2-day carbohydrate refeed preserved FFM, dry FFM and REE during ER, compared to CER in resistance trained individuals. Davoodi et al. [30] compared IER and CER in 74 women with overweight and obesity for a six-week period. IER consisted of 3 cycles of 2 weeks (6-week total), and each cycle included 11 days of ER followed by 3 days of EB. IER was associated with a greater improvement in anthropometric measures, showed a better adherence to the dietary plan and a higher REE by the end of ER period, compared to CER. Byrne at al. [6] examined whether IER improved WL efficiency compared to CER in 51 men with obesity, for a 16-week ER period. IER group cycled 14 days of ER followed by 14 days of EB (30 weeks in total), with an ER of 33% in both IER and CER groups. The authors found a greater WL and fat loss with IER, and reported AT only for the CER group (~209 kJ/d) after a weight loss of ~8,4% of the initial weight, despite a greater weight loss in the IER group ~12,9%. As only men were included in the Byrne et al. study [6], there is a need to study the effects of a similar intervention in women. Furthermore, considering that the compensatory metabolic responses following ER and WL need a period of 7-to-14-days post-WL to be reversed [6, 25], and in order to maximize the ER/EB days´ ratio, another IER pattern should be considered. To our knowledge, this will be the first RCT comparing CER with IER (cycling 14 days of ER followed by 7-day EB periods), in women with obesity.
Pending this approach as a potential opportunity for obesity’s treatment, this paper describes the protocol for a RCT, targeting to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, comparing to a CER. The primary aim of the trial is to assess whether IER will determine a greater FM loss and a reduced AT during WL and after 12-month maintenance, avoiding weight regain, compared to CER, in women with obesity and inactive. Secondary outcomes include WL, retention of FFM, alterations in EE components, and AT plasma-derived indices (thyroid function, insulin, leptin and cortisol).
2. Methods
2.1. Study design
The BREAK study is a RCT (allocation ratio of 1:1) that will be performed in adult women with obesity, randomly divided in 2 parallel groups: 1) CER and 2) IER. This study will include a three-phase intervention:
2 weeks of neutral EB;
Active WL phase, where both groups will undergo 16 weeks of ER: CER—16 weeks of continuous ER; IER—2 weeks of ER interspersed with 1 week in EB, leading to a total of 23 weeks. IER length is 7-week longer than CER due to the 7x1-week of neutral EB, to maintain the same magnitude of ER in both interventions;
8 weeks in neutral EB.
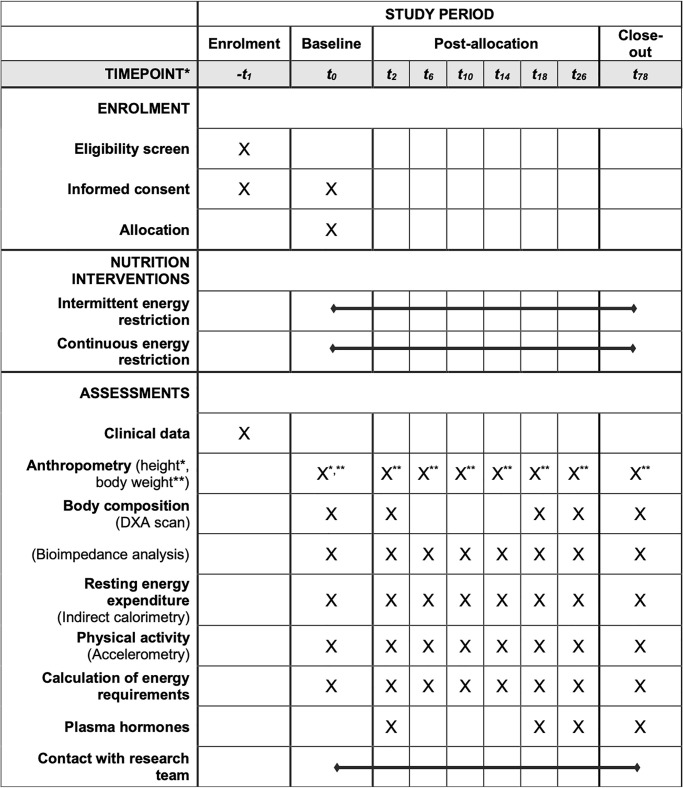
Participants will also be evaluated 12 months after the 3rd phase, to determine WL maintenance success. SPIRIT diagram is presented in Fig 1.
Fig 1. SPIRIT diagram.
*Time-points of the protocol: -t1, enrolment; t0, baseline; t2, 2 weeks after baseline, start of energy restriction; t6, 4 weeks in energy restriction; t10, 8 weeks in energy restriction; t14, 12 weeks in energy restriction; t18, 16 weeks in energy restriction; t26, 8 weeks in neutral energy balance; t78, 52 weeks in weight maintenance.
The study was approved by the Ethics Committee of the Faculty of Nutrition and Food Sciences, University of Porto (Approval Number 31/2021/CEFCNAUP/2021) and will be conducted in accordance to the declaration of Helsinki for human studies from the World Medical Association [33]. It has been registered at www.clinicaltrials.gov (NCT05184361) prior to participants’ recruitment.
All participants will be informed about the possible risks of this investigation before giving informed consent for enrollment in the study. Participants´ privacy and confidentiality will be ensured, during and after investigation, in accordance with the legislation in force.
2.2. Sample recruitment and selection
A total of 74 women with the identified criteria (Table 1) will be selected.
Table 1. Eligibility criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
The disclosure of the clinical trial occurred after its registration in https://clinicaltrials.gov/, and was publicized in the media and social networks. Evaluations and consultations will be taken place at the Exercise and Health Laboratory, CIPER, Faculty of Human Kinetics, Lisbon, Portugal.
2.3. Screening process
Screening process will be phased, to identify and recruit eligible participants and give all the necessary information for an informed consent. During this process, procedures will be detailed, motivations accessed, and expectations anticipated (Table 2).
Table 2. Screening process overview.
| Screening step 1—email and/or phone evaluation |
|
| Screening step 2—online one-to-one interview |
|
2.4. Randomization
Participants will be randomized to one of the two arms of the study through a simple automatic randomization scheme generated by computer, controlled by the researcher responsible for the data treatment (which is not the main investigator). Randomization will be performed before the 1st visit/evaluation. Participants will be informed of their assigned group during the 1st visit/evaluation.
2.5. Calculation of energy stores
Considering the principle of energy conservation [5, 35, 36], the rate of change in body energy storage (ES) is equal to the difference between the rates of energy intake (EI) and EE, expressed as energy per unit of time [35].
EB equation is the following:
When the EI surpasses the EE, changes in ES will be positive, leading to a positive EB. On the other hand, a negative EB will be created when the EI is lower than the EE.
EB can be calculated from the change in body energy stores from the beginning to the end of the WL intervention. Therefore, using the established energy densities of 1.0 kcal.g-1 for FFM and 9.5 kcal.g-1 for FM, the following equation will be used to quantify the average rate of changed body energy stored or lost in kilocalories per day:
Where ΔFFM and ΔFM represent the change in grams of FFM and FM, respectively, from the beginning to end of the intervention and Δt is the time length of the intervention in days [37–39].
2.6. Nutritional intervention and estimation of energy requirements
Nutritional intervention will comprise a personalized dietary plan prescribed for each participant, considering their daily energy requirements (DER) through each phase of the intervention. The DER will be estimated by multiplying the REE measured (mREE) by a PA level (PAL) [40], in phase 1, and using REE and accelerometry data [41], in phase 2 and 3. DER will be calculated in every visit/evaluation performed during the trial (8 in total). The main investigator will be responsible for all appointments, follow-ups and diet plans, including calculation of DER.
The energy distribution by macronutrients will be 35% from protein, 35% from carbohydrate and 30% from fat in all study-phases. A Mediterranean-style diet will be prescribed for both groups, aiming for improving diet quality, and using portion control to achieve energy requirements during EB, ER and WM. The Mediterranean-style diet includes the following recommendations: high intake of vegetables including leafy green vegetables, fruits, wholegrain cereals, nuts and pulses, legumes, and extra virgin (cold pressed) olive oil; moderate intake of fish, seafood, eggs, poultry, and dairy products; low intake of red meat (less than twice a week) and red wine should be consumed in moderation [42]. Processed foods, sweets, cookies, chips, high-fat cheeses, sausages and unhealthy foods are to be avoided. The dietary plan will be adjusted when necessary, according to participant´s feedback.
2.6.1. Neutral energy balance for weight stabilization
For weight stabilization, a personalized dietary plan meeting 100% of their DER will be prescribed for each participant. This dietary plan will take into consideration calculations of DER at every visit/evaluation, and will be adjusted when needed.
Participants will be provided with a digital scale on the 1st visit, to track their weight daily at home [43, 44], and will be instructed to weight themselves after a 10h-overnight fast, wearing only underwear, and weekly share with the main investigator their overnight fasting body weight. During EB, if they identify a weight gain of more than 1 kg, participants will have clear instructions on how to adjust their dietary plan, in order to reduce and stabilize weight [6].
2.6.2. Energy restriction for weight loss
For both groups, an ER of 33% of one’s DER will be created [6, 32] to achieve WL. Participants will have their personalized dietary plan adjusted every 4 weeks during the ER phase, according to REE measurements and PA monitoring, to assure the same energy deficit throughout this phase.
During this phase, participants will continue to track their weight daily at home [43, 44], and weekly share with the main investigator their overnight fasting body weight.
2.6.3. Energy intake and diet compliance
Compliance to the dietary plan will be monitored once a week by phone, and participants will be asked to send, via social media platforms, daily photo food records during the intervention [45].
2.6.4. Adherence to diet
Participants from both groups will undergo an ER meeting 33% of their DER.
Adherence will be assessed through the following equation proposed by Racette et al [46]:
Where the EI will be calculated by the “intake-balance method” [47], through changes in FM and FFM, together with total daily EE (TDEE). The following equation was used:
Where EE represents the total daily EE measured by accelerometry, and EB calculated through changes in FM and FFM. The degree of ER during the WL phase will also be calculated through this equation. It will be also assessed if participants from IER group not only accomplished the prescribed ER but also if underwent the 1-week periods of neutral EB.
2.7. Physical activity
To enroll this study, as stated in inclusion criteria, participants needed to be considered inactive (less then 150 min/week of moderate PA or 75 min/week of vigorous PA [34]). This PA level should be maintained throughout the WL and WM phase.
2.8. Retention of the participants
All efforts will be made before, during and after the intervention to retain participants in the study, in order to manage costs, equipment, human resources and data colleting time.
2.8.1. Strategies to engage participants, avoid low attendance and dropouts
During the intervention, and throughout the 8 visits to the laboratory for assessment and consultations, participants will be reminded of the importance of adhering to the recommendations, as well as attending appointments. Some flexibility will be taken into consideration, as long as it does not interfere with the study plan/goals. A 1-week adjustment will be considered as an on-time assessment, maintaining the previously defined length of each study phase.
Between the scheduled visits and consultations, additional contacts will be held by phone, following up results and adherence to the dietary plan.
After the intervention phase is over (7th visit), and the 12-month WM phase starts, the research team will remain available to follow-up the participants, with at least one phone/video consultation per month.
2.8.2. Rewards for participation
Participants will not receive any type of financial incentive. Nevertheless, they will benefit from a free nutritional intervention for WL and WM, which includes 8 nutrition appointments and weekly remote follow-up by a certified clinical dietitian for 78 weeks (CER group), or 85 weeks (IER group).
3. Measurements
Body weight, body composition and resting energy expenditure (REE) will be collected at 8 different moments, and DXA scans at 5 different moments, as described in Fig 2. Participants will be informed not to take diuretics in the previous 7 days, have alcoholic drinks/coffee in the previous 24 hours, and perform vigorous PA 24-hours prior to the laboratory visits, and to urinate 30 minutes before evaluations [48, 49]. All evaluations will be performed with a minimum 10-hour overnight fast, barefoot and wearing underwear and a disposable vest, to preserve participants comfort.
Fig 2. Schematic description of the study design.
Arrows indicate points for measurements. A—body weight, body composition using bioimpedance analysis (BIA), resting energy expenditure (REE), physical activity (PA) using accelerometry, and calculation of daily energy requirements (DER). B—body composition using Dual-Energy X-ray Absorptiometry (DXA). C—determination of plasma hormones (cortisol, insulin, leptin, thyroid free T3 and T4). *—phone/video contact with research team.
3.1 Body composition
3.1.1. Anthropometry
Anthropometry will consider the procedures and recommendations described in ISAK guidelines [50]. Weight and height will be determined using a digital scale with a stadiometer Seca 704s, with 0,1 kg and 0,1 cm intervals (Seca, Hamburg, Germany). Body mass index (BMI) will be calculated using the formula [weight (kg)/height2(m2)] and the cut-off points of the World Health Organization (WHO) will be used [51].
3.1.2. Dual-Energy X-ray Absorptiometry
In order to assess body composition stores–Fat Mass (FM) and Fat-Free Mass (FFM), a whole-body dual energy X-ray absorptiometry (DXA) scan (Hologic Explorer-W, Waltham, USA) will be used. All the assessments will be performed by the same investigator. Total abdominal fat, which includes intra-abdominal fat plus subcutaneous fat, will be distinguished by identifying a specific region of interest (ROI) within the analysis program. Specific DXA ROIs for abdominal regional fat will be defined as follows: from ROI 1, the upper edge of the second lumbar vertebra (approximately 10 cm above the L4 to L5) to above the iliac crest and laterally encompasses the entire breadth of the abdomen, thus determining total abdominal fat mass [52].
3.1.3. Bioimpedance analysis (BIA)
Bioimpedance analysis will be performed using BIA-101 BIVA PRO (Akern srl, Florence, Italy). Before the test, subjects will be instructed to lie in a supine position with their arms and legs abducted at a 45 angle for 10 min [53]. Four electrodes will be placed on the dorsal surfaces of the right foot and ankle, as well as the right wrist and hand.
This equipment will measure the resistance (Rz) and reactance (Xc) using a 250 mA alternating current at 50 kHz ± 1%. Calibration will happen every morning according to be manufacturer instructions. Bioelectrical phase angle will be calculated as the tangent arc of Xc/R x 180º/ π. Vectorial analysis of bioimpedance will use BIVA method, normalizing R and Xc for height in meters. FM and FFM percentage will be determined using Bodygram® software (AkernSrl., Florence, Italy) [54].
3.2. Resting energy expenditure
REE will be determined using indirect calorimetry COSMED Fitmate (Cosmed, Rome, Italy) using a face mask. Fitmate is a metabolic analyzer designed for measurement of oxygen consumption and EE during rest and exercise. It uses a turbine flowmeter for measuring ventilation and a galvanic fuel cell oxygen sensor for analyzing the fraction of oxygen in expired gases. REE is calculated from oxygen consumption, at a fixed respiratory quotient of 0.85, and estimated grams of urinary nitrogen using a modified Weir equation [55, 56].
The metabolic analyzer will be calibrated in the morning before testing, using a pumping gas with 3 L calibration syringe through the flow meter, according to the manufacturer’s recommendations. The test will be performed early morning (8 a.m. to 11 a.m.), after a minimum 10h-overnight fast. Individuals will be advised to reduce PA the most until the test. All measurements will happen in a thermic neutral environment (approximately 22ºC and humidity between 40–50%) [53], a quiet and dimly lit room, in semi-recumbent position [55]. During the test, participants will be asked to relax and keep immobile, without doing any activities, such as fidgeting, reading, listening to music, talking, nor falling asleep [57]. Rest duration will be 15 minutes, followed by a 30-minute test duration, ignoring the first 10 minutes [58]. Fitmate steady state is achieved when the CV in VO2 is <10% during the 30-minute measurement.
3.2.1. Adaptive thermogenesis
The REE will be predicted (pREE) through linear regression analysis, with the baseline measured REE as a dependent variable, and FM (kg) and FFM (kg) as independent variables. The predictive equation will be used to assess pREE at each time point, using the FM and FFM values of the respective time.
Adaptive thermogenesis (AT) will be assessed by the following equation:
where negative values will indicate a lower-than-expected REE due to body composition changes [59].
3.3. Free-living physical activity and total energy expenditure
PA will be determined using ActiGraph wGT3X-BT accelerometer (ActiGraph, Pensacola FL, USA), which expresses minutes per day spent in different activities. Accelerometers will be placed on the right hip close to the iliac crest, and activated when participants go to the laboratory visit, being used during 7 days. The devices must be used while participants are awake, and will only be asked to be removed during water activities, such as shower and swimming. Participants will be asked to register the time and reason each time they take off the accelerometer. The activation of the devices, download and processing will be held with Actilife software (v.6.9.1).
The cutoff values used for defining PA intensity and the average time at each intensity level (sedentary or light, moderate, hard and very hard PA intensities) will be as follows: sedentary or light: <2689 counts per minute-1 (<3.00 METs); moderate: 2690–6166 counts per minute-1 (3.00–5.99 METs); hard: 6167–9642 counts per minute-1 (6.00–8.99 METs); very hard: >9642 counts per minute-1 (>8.99 METs) [60].
Among adults, at least 3–5 days of monitoring are required to estimate usual PA [61], therefore participants will be included if they show a minimum of three valid days of accelerometer data. A valid-day will be defined as having 600 or more minutes (≥ 10 h) of monitor wearing, corresponding to the minimum daily use of the accelerometer.
TDEE will be calculated as the sum of REE, thermic effect of food (TEF) and PA energy expenditure (PAEE) [62]. PAEE data will be collected through accelerometry using Crouter and colleagues’ equations [61, 63, 64].
3.4. Plasma hormonal determination
Plasma cortisol, insulin, leptin and thyroid free-T3 and T4 will be determined for AT analysis in 4 moments (Fig 1). Measurements of plasma thyroid levels (free-T3 and T4) and cortisol will be determined by immunoassay with chemiluminescence detection (Advia Centaur, Siemens). Insulin assessment will be performed in an automated analyser with chemiluminescence detection (Advia Centaur, Siemens), and leptin plasma levels by enzyme immunoassay (ELISA). Reference values for these parameters will be considered.
3.5. Statistics
Statistical analysis will be performed using SPSS statistics software version 29.0, 2022 (SPSS Inc., an IBM Company, Chicago IL, USA), with a statistical significance set at p<0.05 (2-tailed).
Descriptive statistics will be calculated (mean, standard deviation, and range). Linear mixed models will be used to assess outcomes for the impact of group, time and group*time interaction, including the randomized group (control vs intervention group) and time (baseline at the 2-week EB, at the start and every 4-week of ER intervention phase, at the start and at the end of the 8-week post-diet EB phase, and at the end of WM phase) as fixed effects. When necessary, adjustments for confounding variables (covariates) will be considered. The covariance matrix for repeated measures within subjects over time will be modelled as unstructured or, if necessary, compound symmetry. The normality of model residual distributions will be examined graphically and with the Kolmogorov-Smirnov test. Difference-in-differences between IER and CER throughout time will be assessed by performing contrasts [Difference-in-differences (DiD)], calculated as the difference between changes for IER (Difference_IER = T2 -T1) and changes for CER (Difference_CER = Time2 –Time1): DiD = (Difference_IER)–(Difference_CER).
All analysis will be intention-to-treat, including data from all the participants who will assign in this study. Sensitivity analyses will be carried out for some variables of interest, by using single imputation of missing data to predict missing outcomes from demographics and baseline measures.
3.5.1. Power sample calculation
For sample and power calculations, this study is powered based on changes in total body fat assessed by DXA. Considering a type I error of 5% and a power of 95% to detect differences at the dependent variable, with a statistical significance and an effect size of 0.93 (differences in FM (kg) were considered following Byrne et al. results [6]), a total of 26 participants per group will be needed (using GPower software version 3.1.9.6). Considering a 30% drop-out rate [6] throughout the study, we will recruit 74 participants (37 in each group).
3.5.2. Trial status
Recruitment for this clinical trial started on January 13th, 2022, and is expected to end on June 30th, 2024.
4. Discussion
This RCT aims primarily to evaluate the effects of an IER, interspersing 14 days of ER with 7 days of EB, on body composition (body weight, FM and FFM), and more specifically on AT, during WL and WM phase. It also aims to understand whether participants from both groups (IER and CER) will successfully maintain their WL 12 months after completion of the intervention.
Secondary objectives of this study are the following: (i) to compare the effects of IER and CER on WL, FM loss, and preservation of FFM and REE during the intervention phase; (ii) to determine which group is more successful in the 12-month WM phase and in improving body composition profile (higher FM loss with best preservation of FFM); (iii) to analyze if AT is maintained during WM phase; (iv) to analyze the impact of AT in WM phase, regarding the hormonal adaptation (plasma hormones: cortisol, insulin, leptin, thyroid free T3 and T4).
A 5% WL has been pointed out to be enough to improve metabolic parameters (glycemic measures and triglycerides, blood pressure, HDL and LDL cholesterol) and is currently a standard goal in WL interventions [65]. Nevertheless, a greater reduction of 10% or more can lead to maximal health benefits, reducing many of the comorbidities associated with obesity [66, 67]. In fact, according to Wing and Hill [68], successful WL maintainers should be defined as “individuals who have intentionally lost at least 10% of their body weight and kept if off at least 1 year”. With The BREAK Study we expect to find a clinically significant weight loss [69] of 10% in both groups, considering the length of the ER intervention, and the magnitude of ER [70].
According to Byrne and colleagues [6, 25, 71], adaptive responses to ER and WL can be reversed by a 7-to-14-day period of EB after, in adults with overweight or obesity. In “MATADOR” Study, a IER using a 2-week EB periods improved WL efficiency compared with CER, being AT only found in CER group, despite the lower WL and FM loss [6]. In review by Peos and colleagues [21], it was stated that “refeeds” with at least 7 days attenuate the adaptive responses to longer-term periods of ER, potentially by attenuating the reductions in EE. Furthermore, these authors also mentioned that adopting refeed periods in IER may provide a mental break from extended periods of ER, leading to a higher long-term adherence to the dietary plan compared to CER. Therefore, we also anticipate a major weight and FM loss in IER group, comparing to CER, with a greater retention of FFM, reducing therefore AT [6, 23, 30], due to the inclusion of 7-day breaks to restore EB every 2-weeks of ER, which can minimize the compensatory mechanisms associated with ER and WL, such as changes in appetite-related hormones [24] and some behavioral compensations such as increases in sedentary behavior [26].
During WL phase, we expect to find a reduction in all EE components, as usually seen during ER, namely REE and non-resting EE (spontaneous PA (SPA), non-exercise PA (NEPA), and exercise PA (EPA)) [72].
WL usually causes a reduction in thyroid hormones (T3 and T4), insulin and leptin, increasing appetite [7, 73], and causes an increase in cortisol, which reduces EE [22]. We expect to find a lower reduction in insulin, leptin, thyroid T3 and T4, as well as a lower increase in cortisol in IER, comparing to CER. The minimization of these compensatory adaptations can be explained by the 1-week EB every 2-weeks of ER. After ER intervention, we anticipate a successful WL maintenance in both groups, possibly greater in the IER group, as well as a lower AT.
The BREAK Study focuses not only in reducing energy intake through portion control, but also in improving nutritional quality of the diet, by increasing fruit and vegetable intake, and decreasing processed and energy-dense foods. Participants will be educated and encouraged to daily monitor their behavior, weight, and eating pattern, leading to self-efficacy for diet and WM, which are determinants of WL maintenance [66]. Although maintaining high levels of PA has been pointed out as a determinant of WL maintenance [74], the BREAK Study is a diet-only [75] intervention. Therefore, no PA recommendations will be given to participants throughout the WL and WM phases.
One of the strengths of The BREAK Study is the 2-week baseline EB for determining energy requirements, stabilizing weight, and adapting participants to the prescribed dietary plan. Another strength is the determination of REE and PA monitoring every 4 weeks of ER, allowing adjustment of the dietary plan to ensure the energy deficit of 33% during the 16-week ER phase. Monitoring AT-related hormone changes during WL and WM is also a strong point of this study, enabling a better understand of AT in both groups.
However, some limitations should be addressed, such as: i) the nutritional intervention occurs in a free-living scenario, with no food being delivered to participants, preventing a valid assurance that participants are in fact consuming the prescribed energy. To minimize this adherence limitation, participants are instructed to weekly share with the main investigator their overnight fasting body weight and photo records of their meals; ii) a large interindividual variability in PAEE is expected and may not be accurately detected by accelerometry; iii) the eventual more than expected drop out of the study, due to its length, possibly weakening the statistical power of the analysis.
We anticipate that The BREAK Study will allow us to better understand AT during WL and WM interventions in women with obesity. Moreover, we expect to find a successful alternative to CER, enabling more tailored nutritional interventions, according to individuals needs and lifestyle. This study will also allow participants to lose weight and FM, while attenuating AT, improving their metabolic health, and encouraging them to adhere to a healthy lifestyle and acquire nutritional knowledge that will facilitate WM in the long-term. Finally, the findings of this trial will enable evidence-based decisions for the treatment of obesity.
Supporting information
(DOC)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors express their gratitude to all the participants involved in this study.
Data Availability
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding Statement
Funding: F.M.C. was supported by Farmodiética S.A. https://www.farmodietica.com/en/home/ C.L.N. was supported with a PhD scholarship from the Portuguese Foundation for Science and Technology (SFRH/BD/143725/2019). https://www.fct.pt/en/ V.H.T. was supported by the Foundation for Science and Technology, through the FCT/UIDB/00617/2020 (CIAFEL) and LA/P/0064/2020 (ITR) Projects. https://www.fct.pt/en/ The funders did not and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Twells LK, Harris Walsh K, Blackmore A, Adey T, Donnan J, Peddle J, et al. Nonsurgical weight loss interventions: A systematic review of systematic reviews and meta-analyses. Obesity Reviews. 2021;22(11):e13320. doi: 10.1111/obr.13320 [DOI] [PubMed] [Google Scholar]
- 2.Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. doi: 10.2147/DMSO.S89836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulloo AG, Montani JP. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: an overview. Obes Rev. 2015;16 Suppl 1:1–6. [DOI] [PubMed] [Google Scholar]
- 4.Paixão C, Dias CM, Jorge R, Carraça EV, Yannakoulia M, de Zwaan M, et al. Successful weight loss maintenance: A systematic review of weight control registries. Obes Rev. 2020;21(5):e13003. doi: 10.1111/obr.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JO, Wyatt HR, Peters JC. The Importance of Energy Balance. Eur Endocrinol. 2013;9(2):111–5. doi: 10.17925/EE.2013.09.02.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne NM, Sainsbury A, King NA, Hills AP, Wood RE. Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obes (Lond). 2018;42(2):129–38. doi: 10.1038/ijo.2017.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller MJ, Bosy-Westphal A. Adaptive thermogenesis with weight loss in humans. Obesity (Silver Spring). 2013;21(2):218–28. doi: 10.1002/oby.20027 [DOI] [PubMed] [Google Scholar]
- 8.Dulloo AG. Physiology of weight regain: Lessons from the classic Minnesota Starvation Experiment on human body composition regulation. Obes Rev. 2021;22 Suppl 2:e13189. doi: 10.1111/obr.13189 [DOI] [PubMed] [Google Scholar]
- 9.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24(8):1612–9. doi: 10.1002/oby.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond). 2013;37(10):1371–7. doi: 10.1038/ijo.2013.1 [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Arbelaez D, Crujeiras AB, Castro AI, Martinez-Olmos MA, Canton A, Ordoñez-Mayan L, et al. Resting metabolic rate of obese patients under very low calorie ketogenic diet. Nutr Metab (Lond). 2018;15:18. doi: 10.1186/s12986-018-0249-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins M, Gibbons C, Caudwell P, Hellstrom PM, Naslund E, King NA, et al. The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. Eur J Clin Nutr. 2014;68(5):581–6. doi: 10.1038/ejcn.2013.277 [DOI] [PubMed] [Google Scholar]
- 13.Marlatt KL, Redman LM, Burton JH, Martin CK, Ravussin E. Persistence of weight loss and acquired behaviors 2 y after stopping a 2-y calorie restriction intervention. Am J Clin Nutr. 2017;105(4):928–35. doi: 10.3945/ajcn.116.146837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Glüer CC, Müller MJ. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr. 2014;99(4):779–91. doi: 10.3945/ajcn.113.071829 [DOI] [PubMed] [Google Scholar]
- 15.Nunes CL, Casanova N, Francisco R, Bosy-Westphal A, Hopkins M, Sardinha LB, et al. Does adaptive thermogenesis occur after weight loss in adults? A systematic review. British Journal of Nutrition. 2021:1–19. doi: 10.1017/S0007114521001094 [DOI] [PubMed] [Google Scholar]
- 16.Martins C, Gower B, Hill J, Hunter G. Metabolic adaptation is not a major barrier to weight-loss maintenance. The American journal of clinical nutrition. 2020;112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doucet E, St-Pierre S, Alméras N, Després JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr. 2001;85(6):715–23. doi: 10.1079/bjn2001348 [DOI] [PubMed] [Google Scholar]
- 18.Nymo S, Coutinho SR, Torgersen LH, Bomo OJ, Haugvaldstad I, Truby H, et al. Timeline of changes in adaptive physiological responses, at the level of energy expenditure, with progressive weight loss. Br J Nutr. 2018;120(2):141–9. doi: 10.1017/S0007114518000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karl JP, Roberts SB, Schaefer EJ, Gleason JA, Fuss P, Rasmussen H, et al. Effects of carbohydrate quantity and glycemic index on resting metabolic rate and body composition during weight loss. Obesity (Silver Spring). 2015;23(11):2190–8. doi: 10.1002/oby.21268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maroofi M, Nasrollahzadeh J. Effect of intermittent versus continuous calorie restriction on body weight and cardiometabolic risk markers in subjects with overweight or obesity and mild-to-moderate hypertriglyceridemia: a randomized trial. Lipids Health Dis. 2020;19(1):216. doi: 10.1186/s12944-020-01399-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peos JJ, Norton LE, Helms ER, Galpin AJ, Fournier P. Intermittent Dieting: Theoretical Considerations for the Athlete. Sports (Basel). 2019;7(1). doi: 10.3390/sports7010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peos JJ, Helms ER, Fournier PA, Sainsbury A. Continuous versus intermittent moderate energy restriction for increased fat mass loss and fat free mass retention in adult athletes: protocol for a randomised controlled trial-the ICECAP trial (Intermittent versus Continuous Energy restriction Compared in an Athlete Population). BMJ Open Sport Exerc Med. 2018;4(1):e000423. doi: 10.1136/bmjsem-2018-000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell BI, Aguilar D, Colenso-Semple LM, Hartke K, Fleming AR, Fox CD, et al. Intermittent Energy Restriction Attenuates the Loss of Fat Free Mass in Resistance Trained Individuals. A Randomized Controlled Trial. J Funct Morphol Kinesiol. 2020;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainsbury A, Wood R, Seimon R, Hills A, King N, Gibson A, et al. Rationale for novel intermittent dieting strategies to attenuate adaptive responses to energy restriction: Using energy balance to improve weight loss. Obesity Reviews. 2018;19:47–60. [DOI] [PubMed] [Google Scholar]
- 25.Byrne NM, Hills AP. Biology or Behavior: Which Is the Strongest Contributor to Weight Gain? Current Obesity Reports. 2013;2(1):65–76. [Google Scholar]
- 26.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39(8):1188–96. doi: 10.1038/ijo.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva AM, Júdice PB, Carraça EV, King N, Teixeira PJ, Sardinha LB. What is the effect of diet and/or exercise interventions on behavioural compensation in non-exercise physical activity and related energy expenditure of free-living adults? A systematic review. British Journal of Nutrition. 2018;119(12):1327–45. doi: 10.1017/S000711451800096X [DOI] [PubMed] [Google Scholar]
- 28.Wing RR, Jeffery RW. Prescribed "breaks" as a means to disrupt weight control efforts. Obes Res. 2003;11(2):287–91. doi: 10.1038/oby.2003.43 [DOI] [PubMed] [Google Scholar]
- 29.Arguin H, Dionne IJ, Senechal M, Bouchard DR, Carpentier AC, Ardilouze JL, et al. Short- and long-term effects of continuous versus intermittent restrictive diet approaches on body composition and the metabolic profile in overweight and obese postmenopausal women: a pilot study. Menopause. 2012;19(8):870–6. doi: 10.1097/gme.0b013e318250a287 [DOI] [PubMed] [Google Scholar]
- 30.Davoodi SH, Ajami M, Ayatollahi SA, Dowlatshahi K, Javedan G, Pazoki-Toroudi HR. Calorie shifting diet versus calorie restriction diet: a comparative clinical trial study. Int J Prev Med. 2014;5(4):447–56. [PMC free article] [PubMed] [Google Scholar]
- 31.Keogh JB, Pedersen E, Petersen KS, Clifton PM. Effects of intermittent compared to continuous energy restriction on short-term weight loss and long-term weight loss maintenance. Clin Obes. 2014;4(3):150–6. doi: 10.1111/cob.12052 [DOI] [PubMed] [Google Scholar]
- 32.Coutinho SR, Halset EH, Gåsbakk S, Rehfeld JF, Kulseng B, Truby H, et al. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin Nutr. 2018;37(3):815–23. doi: 10.1016/j.clnu.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 33.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–4. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 34.ACSM’s guidelines for exercise testing and prescription. Tenth edition. ed. Riebe D, Ehrman JK, Liguori G, Magal M, editors. Philadelphia: Wolters Kluwer; 2018.
- 35.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. The American journal of clinical nutrition. 2012;95(4):989–94. doi: 10.3945/ajcn.112.036350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126–32. doi: 10.1161/CIRCULATIONAHA.111.087213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jonge L, Bray GA, Smith SR, Ryan DH, de Souza RJ, Loria CM, et al. Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity (Silver Spring). 2012;20(12):2384–9. doi: 10.1038/oby.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, et al. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8(2):155–64. doi: 10.1177/1740774511398369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins C, Roekenes J, Gower BA, Hunter GR. Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutrition & Metabolism. 2021;18(1):60. doi: 10.1186/s12986-021-00587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndahimana D, Kim EK. Measurement Methods for Physical Activity and Energy Expenditure: a Review. Clin Nutr Res. 2017;6(2):68–80. doi: 10.7762/cnr.2017.6.2.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean Diet; a Literature Review. Nutrients. 2015;7(11):9139–53. doi: 10.3390/nu7115459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuorinen AL, Helander E, Pietilä J, Korhonen I. Frequency of Self-Weighing and Weight Change: Cohort Study With 10,000 Smart Scale Users. J Med Internet Res. 2021;23(6):e25529. doi: 10.2196/25529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinberg DM, Bennett GG, Askew S, Tate DF. Weighing every day matters: daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet. 2015;115(4):511–8. doi: 10.1016/j.jand.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burrows TL, Ho YY, Rollo ME, Collins CE. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front Endocrinol (Lausanne). 2019;10:850. doi: 10.3389/fendo.2019.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302(4):E441–8. doi: 10.1152/ajpendo.00290.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, et al. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol. 1996;270(3 Pt 2):R496–504. doi: 10.1152/ajpregu.1996.270.3.R496 [DOI] [PubMed] [Google Scholar]
- 48.Guedes D. Clinical procedures used for analysis of the body composition. Revista Brasileira de Cineantropometria & Desempenho Humano. 2013;15:113–29. [Google Scholar]
- 49.Fidilio E, Comas M, Giribés M, Cárdenas G, Vilallonga R, Palma F, et al. Evaluation of Resting Energy Expenditure in Subjects with Severe Obesity and Its Evolution After Bariatric Surgery. Obes Surg. 2021;31(10):4347–55. doi: 10.1007/s11695-021-05578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton K. Standards for Anthropometry Assessment. 2018. p. 68–137. [Google Scholar]
- 51.Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021. [PubMed]
- 52.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(7):978–83. doi: 10.1038/sj.ijo.0801982 [DOI] [PubMed] [Google Scholar]
- 53.Silva AM, Nunes CL, Matias CN, Jesus F, Francisco R, Cardoso M, et al. Champ4life Study Protocol: A One-Year Randomized Controlled Trial of a Lifestyle Intervention for Inactive Former Elite Athletes with Overweight/Obesity. Nutrients. 2020;12(2). doi: 10.3390/nu12020286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toselli S, Badicu G, Bragonzoni L, Spiga F, Mazzuca P, Campa F. Comparison of the Effect of Different Resistance Training Frequencies on Phase Angle and Handgrip Strength in Obese Women: a Randomized Controlled Trial. Int J Environ Res Public Health. 2020;17(4). doi: 10.3390/ijerph17041163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lange E, Głąbska D, Włodarek D. INDIRECT CALORIMETRY IN THE ASSESSMENT OF THE ENERGY REQUIREMENT IN OVERWEIGHT AND OBESE WOMEN. Advances in Science and Technology—Research Journal. 2013;20:91–7. [Google Scholar]
- 56.Nieman DC, Austin MD, Benezra L, Pearce S, McInnis T, Unick J, et al. Validation of Cosmed’s FitMate in measuring oxygen consumption and estimating resting metabolic rate. Res Sports Med. 2006;14(2):89–96. doi: 10.1080/15438620600651512 [DOI] [PubMed] [Google Scholar]
- 57.Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, Lee PS, et al. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet. 2015;115(9):1417–46.e2. doi: 10.1016/j.jand.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 58.Borges JH, Guerra-Júnior G, Gonçalves EM. Methods for data analysis of resting energy expenditure measured using indirect calorimetry. Nutrition. 2019;59:44–9. doi: 10.1016/j.nut.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 59.Nunes CL, Jesus F, Francisco R, Matias CN, Heo M, Heymsfield SB, et al. Adaptive thermogenesis after moderate weight loss: magnitude and methodological issues. Eur J Nutr. 2022;61(3):1405–16. doi: 10.1007/s00394-021-02742-6 [DOI] [PubMed] [Google Scholar]
- 60.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14(5):411–6. doi: 10.1016/j.jsams.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 61.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: best practices and research recommendations. Med Sci Sports Exerc. 2005;37(11 Suppl):S582–8. doi: 10.1249/01.mss.0000185292.71933.91 [DOI] [PubMed] [Google Scholar]
- 62.Hills AP, Mokhtar N, Byrne NM. Assessment of Physical Activity and Energy Expenditure: An Overview of Objective Measures. Frontiers in Nutrition. 2014;1. doi: 10.3389/fnut.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crouter SE, Kuffel E, Haas JD, Frongillo EA, Bassett DR Jr. Refined two-regression model for the ActiGraph accelerometer. Med Sci Sports Exerc. 2010;42(5):1029–37. doi: 10.1249/MSS.0b013e3181c37458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 65.Ryan DH, Yockey SR. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr Obes Rep. 2017;6(2):187–94. doi: 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varkevisser RDM, van Stralen MM, Kroeze W, Ket JCF, Steenhuis IHM. Determinants of weight loss maintenance: a systematic review. Obesity Reviews. 2019;20(2):171–211. doi: 10.1111/obr.12772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323 [DOI] [PubMed] [Google Scholar]
- 69.Ross R. Is setting a criterion for ‘clinically significant weight loss’ necessary? Obesity. 2016;24(4):791-. doi: 10.1002/oby.21437 [DOI] [PubMed] [Google Scholar]
- 70.Thomas DM, Martin CK, Lettieri S, Bredlau C, Kaiser K, Church T, et al. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes (Lond). 2013;37(12):1611–3. doi: 10.1038/ijo.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byrne NM, Weinsier RL, Hunter GR, Desmond R, Patterson MA, Darnell BE, et al. Influence of distribution of lean body mass on resting metabolic rate after weight loss and weight regain: comparison of responses in white and black women. Am J Clin Nutr. 2003;77(6):1368–73. doi: 10.1093/ajcn/77.6.1368 [DOI] [PubMed] [Google Scholar]
- 72.Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes Rev. 2012;13 Suppl 2:105–21. doi: 10.1111/j.1467-789X.2012.01041.x [DOI] [PubMed] [Google Scholar]
- 73.Muller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016;5(4):413–23. doi: 10.1007/s13679-016-0237-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flore G, Preti A, Carta MG, Deledda A, Fosci M, Nardi AE, et al. Weight Maintenance after Dietary Weight Loss: Systematic Review and Meta-Analysis on the Effectiveness of Behavioural Intensive Intervention. Nutrients. 2022;14(6):1259. doi: 10.3390/nu14061259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charlot K, Chapelot D, Siracusa J, Lavoué C, Colin P, Oustric P, et al. An augmented food strategy leads to complete energy compensation during a 15-day military training expedition in the cold. Physiol Rep. 2021;9(11):e14591. doi: 10.14814/phy2.14591 [DOI] [PMC free article] [PubMed] [Google Scholar]