Abstract
Organic carbon (OC) association with soil minerals stabilizes OC on timescales reflecting the strength of mineral–C interactions. We applied ramped thermal oxidation to subsoil B horizons with different mineral–C associations to separate OC according to increasing temperature of oxidation, i.e. thermal activation energy. Generally, OC released at lower temperatures was richer in bioavailable forms like polysaccharides, while OC released at higher temperatures was more aromatic. Organic carbon associated with pedogenic oxides was released at lower temperatures and had a narrow range of 14C content. By contrast, N-rich compounds were released at higher temperatures from samples with 2 : 1 clays and short-range ordered (SRO) amorphous minerals. Temperatures of release overlapped for SRO minerals and crystalline oxides, although the mean age of OC released was older for the SRO. In soils with more mixed mineralogy, the added presence of older OC released at temperatures greater than 450°C from clays resulted in a broader distribution of OC ages within the sample, especially for soils rich in 2 : 1 layer expandable clays such as smectite. While pedogenic setting affects mineral stability and absolute OC age, mineralogy controls the structure of OC age distribution within a sample, which may provide insight into model structures and OC dynamics under changing conditions.
This article is part of the Theo Murphy meeting issue ‘Radiocarbon in the Anthropocene’.
Keywords: radiocarbon, soil organic matter, mineral-associated organic matter, py-GC/MS, soil minerals
1. Introduction
Soils hold the largest terrestrial reservoir of organic carbon (OC) (1500 petagrams in the top 100 cm, [1]), and understanding the persistence of OC in soil is key to predicting soil feedbacks to changing climate. Importantly, the role of soil minerals has been established as a key mechanism for stabilizing soil organic matter (SOM) [2–4] in both top and subsoils. However, studies on SOM stabilization in the subsoil are more limited [5,6] despite the predominance of mineral–OM interactions [3,7], less saturation of mineral surfaces [8,9] and evidence of the reduced influence of surface plant-derived C [10,11].
The reactive mechanisms linking mineral surfaces and SOM are diverse and include processes such as sorption, complexation, ligand exchange and chelation, as well as stronger interactions with minerals having permanent surface charge such as 2 : 1 clays [3,4]. The strength of these interactions helps to determine how long OC persists. Total SOM storage has been linked to (pedogenic) Fe and Al oxy-(hydr)oxides concentrations [12,13], even in mixed mineralogy and clay-rich soils [14–17]. Soils rich in reactive short-range order (SRO) non-crystalline Al, Fe and Mn oxides strongly complex large quantities of SOM via dissociated functional groups, forming strong inner-sphere bonds [2,18,19]. Further weathering of these Fe and Al oxy-(hydr)oxides to secondary minerals produces lower-entropy crystalline oxides (CO), such as haematite and goethite, that are significantly less reactive and store less SOM.
SOM associations with silicate layer clay minerals depend strongly on the forms of clay present. For example, high-surface area 2 : 1 clays can adsorb large molecules through cation bridging (e.g. Ca2+) between negative charges on clay surfaces and negatively charged SOM functional groups (particularly carboxyl groups) [20]. Aromatic SOM can form hydrophobic microsites which may hinder decomposition and stimulate further accumulation of SOM through OM–OM interactions [21]. In addition, positively charged edges of 2 : 1 clay minerals may directly complex OM through rapid ligand exchange [22,23]. 1 : 1 layer silicate clays have much lower surface area and primarily form complexes at edge sites [24]. They can, however, be coated with more reactive Fe and Al oxides, as is typically observed in Oxisols [25,26]. Thus, multiple stabilization pathways can exist on and between individual minerals, e.g. edge charges and isomorphic substitutions in 2 : 1 clays, leading to a distribution of bond strengths and SOM resiliency [4].
Reactive soil mineral surfaces and OC input to soil are spatially heterogeneous. Notably, physical occlusion mechanisms can play a role in long-term persistence by isolating SOM from decomposers and extracellular enzymes [27,28]. Stable microaggregates formed by a mix of pedogenic (oxy)hydroxides, clay minerals and SOM present an efficient barrier for many microorganisms leading to the preservation of otherwise readily available SOM. Thus, the age of OC associated with the same minerals may differ depending on the stability of the structure in which these minerals are embedded.
In addition to mineral stabilization, the persistence of OM in soil has been attributed to chemical ‘recalcitrance’, or the difficulty with which soil microbes break bonds within an organic molecule, e.g. differentiating low molecular weight fresh sugars from more stable biopolymers such as lignin or poorly recognizable charred aromatic OM. The influence of chemical complexity along with the potential for one or more mineral stabilization mechanisms to select for organic molecules with specific properties likely results in quantifiable differences in the distribution of activation energies that control the time until a given organic molecule is made available for decomposition and its C released from soil back to the atmosphere [29,30].
The OC in SOM is known to have a range of ages that are related to soil mineralogy. Recent advances in multi-pool SOM models allow the calculation of the probabilistic 14C distribution for bulk SOM sampled at a given point in time [31]. This provides a method for comparison of models with 14C observations directly against either time series of samples from the same site or the age distribution of a single sample determined using laboratory physical or chemical fractionation. Thermal fractionation, which measures SOM decomposition into CO2 under increasing energy inputs (i.e. heat) can now be used to isolate the 14C signal of quantitatively and qualitatively distinct parts of SOM that differ in thermal stability, molecular structure and bonding mechanisms [32,33]. By using the temperature of thermal decomposition as a proxy for activation energy, the 14C distributions of OC can be compared with other fractionation approaches [34], time series [35] or model-predicted distributions [31]. Thus, thermal analysis results do not reflect just resistance of organic molecules to withstand thermally induced decomposition, but aggregate the strength of mineral sorption and complexes alongside thermal stability and chemical complexity. For example, Feng & Simpson [36] showed that the same molecule can have different thermal activation energies depending on the associated mineral matrix.
Given the wide range of potential physical and chemical interactions, SOM associated with various soil minerals differs greatly in quality, quantity and mean age, represented by the time OC has persisted in the soil. In order to predict SOM dynamics and parameterize SOM turnover in models, it is crucial to accurately represent different timescales of SOM stabilization and their patterns across soil types. Most models of SOM dynamics [37–39] require at least two ‘pools’ of SOM with different characteristic rates of decomposition to represent changes in SOM dynamics following e.g. land use change [40–43]. These decomposition rates are often linked to characteristics like soil clay content that provide a proxy for different timescales of mineral–OC interactions.
Experimentally, 14C analyses provide one of the only tools to quantify the age and rate of cycling of soil OC. However, relatively few studies have linked 14C to mineral stabilization mechanisms directly [17,44,45]. While 14C measurements of bulk SOM are increasingly common, mean values insufficiently describe SOM dynamics. This is evidenced by the rapid incorporation of recent bomb 14C into SOM with mean 14C ages of hundreds to thousands of years, or the ability of various chemical and physical fractionation methods to separate bulk SOM into fractions with different mean ages (e.g. [46–49].
In this study, we explore the potential of thermal analysis to improve the description of 14C-derived age distributions of mineral-associated organic matter (MOM) for geochemically and pedogenetically distinct soils with a wide range of mineral stabilization mechanisms. To address this, we proposed the following questions and hypotheses:
-
1.
How does mineralogy control MOM age structure and thermal stability? We hypothesize that variation in bonding mechanisms between SOM and minerals in different soils corresponds to variation in activation energy required to mineralize SOM and this will be reflected in the mean age of the OC oxidized at different temperatures. We further predict that the oldest OC would be associated with minerals with the strongest stabilization pathways—i.e. soils rich in amorphous short-range ordered (SRO) minerals require the highest amount of activation energy (most stable organo-mineral bonds and older C) followed by soils rich in expandable 2 : 1 clay minerals and pedogenic oxides. Mineralizing SOM in soils rich in end-member minerals such as non-expandable 1 : 1 clays, quartz and highly crystalline pedogenic oxides should require only a low amount of activation energy (least stable organo-mineral bonds and youngest C). Similarly, soils rich in primary minerals where soil development has not yet created strong organo-mineral bonds with secondary minerals will show comparatively low activation energy and younger C.
-
2.
How does mineralogy control MOM chemistry? We hypothesize that the relationship between thermal stability and mean age is driven primarily by the ability of soil minerals to stabilize organic molecules, and only secondarily by their biochemical molecular structure. As such, soils with the strongest mineral-related stabilization mechanisms will also have the ability to stabilize potentially bioavailable organic molecules, whereas soils with weak mineral-related stabilization mechanisms can be dominated by molecules that show inherent resistance to decomposition.
To test these hypotheses, we selected mineral fractions from soil B horizons, where mineral–organic interactions should dominate SOM dynamics. We chose soil B horizons because mineral–OM interactions likely dominate there, and because of recent studies suggesting that a portion of B horizon OM is cycling on timescales of decades [50] and is vulnerable to destabilization with soil warming [51]. To maximize the variation of expected mineral–organic matter interactions, we selected soils with previously quantified mineralogy that developed in different soil environments on a range of parent materials. These samples included previously investigated soils from a chronosequence study [52], and soils with similar age and climate but different parent materials [16,53], as well as soils with unique mineral–OM stabilization mechanisms, e.g. a Spodosol [54,55]. We subjected these samples to thermal fractionation, trapping the CO2 oxidized in different temperature ranges to determine patterns in quantity, 14C, and 13C of released C. The same samples also underwent Rock-Eval pyrolysis, an assessment of SOM evolution and maturity, and pyrolysis gas chromatography and mass spectrometry (py-GC/MS) to characterize the chemical composition of the MOM in each soil. By linking chemistry and 14C thermal analysis to distinct thermal fractions of MOM, we provide new insights on characterizing MOM composition and move beyond bulk 14C ages to better link soil minerals to the age structure of SOM.
2. Methods
(a) . Sample selection and site description
Samples were selected from well-characterized B horizons of soils representing a variety of mineralogies, including those with single mineral classes and combined mineralogies to test endpoints and mixtures (table 1). The influence of fresh plant inputs was minimized by removing free particulate organic matter (FPOM) using density separation and analysing only MOM. No soils contained carbonates, ensuring that all C released during thermal oxidation is derived from MOM. For simplicity, each sample is named to express its dominant mineral(s). Soil characteristics and relevant citations are presented in table 1. For example, ‘Quartz’ is from the quartz-rich Bh horizon of a Podzol developed on aeolian dune sands since the last ice age [54]. All other soils analysed were developed on igneous parent materials of varying geochemical composition to exclude 14C-free OC inherited from sedimentary mineral–OC associations. A previous publication demonstrated the potential for inherited OC from sedimentary parent material to influence the thermal analysis [34,59].
Table 1.
Properties of selected soils, in order of lowest to highest degree of weathering. B horizons were selected to isolate unsaturated mineral phases. ‘Fe dith’ is dithionite extractable iron, and ‘Fe ox’ and‘Al ox’ are oxalate extractable iron and aluminium, respectively.
| depth of B | MOM Fm | MOM Fm | depth-corrected | MOM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| group | sample ID | location | horizon | 14C | agea | Fmb | δ13C | organic C | pH | Fe dith | Fe ox | Al ox | clay | kaolin | smectite | chlorite | feldspar | quartz | GPPc | citation |
| cm | year BP | 45 cm | ‰ | % | g kg−1 | g kg−1 | g kg−1 | % | % of clay-sized fractiond |
g C m−2 y−1 | ||||||||||
| quartz sand dominated | Quartz | Michigan, USA | 56–76 | 0.907 | 800 | 0.90 | −25.32 | 1.0 | 4.0 | 1.50 | 0.18 | 0 | 0 | 0 | 0 | 0 | 5 | 89 | 1589 | [54,55] |
| primary mineral dominated | Int. PM + SRO | Sierra Nevada Range, USA | 20–30 | 0.892 | 900 | 0.88 | −23.21 | 1.1 | 6.0 | 16.03 | 6.92 | 38.58 | 6 | 6 | 36 | 2 | 31 | 2 | 1508 | [17,53] |
| primary mineral dominated | Felsic PM + Mixed Clay | Sierra Nevada Range, USA | 20–30 | 0.987 | 100 | 0.91 | −23.75 | 0.4 | 6.1 | 4.30 | 2.60 | 4.4 | 6 | 3 | 1 | 5 | 40 | 23 | 1279 | [17,53] |
| primary mineral dominated | Mafic PM + CO | Sierra Nevada Range, USA | 20–30 | 0.938 | 500 | 0.87 | −22.01 | 0.8 | 6.0 | 9.57 | 3.47 | 14.75 | 11 | 12 | 0 | 0 | 41 | 0 | 1431 | [17,53] |
| amorphous mineral dominated | SRO | Hawaii, USA | 38–54 | 0.674 | 3200 | 0.68 | −26.69 | 5.9 | 4.8 | 24.20 | 8.82 | 29.4 | 4 | 1 | 0 | 0 | 2 | 6 | 2315 | [2,10,52] |
| clay dominated | 2 : 1 Clay + CO | South Africa | 11–31 | 0.873 | 1100 | 0.79 | −12.3 | 1.3 | 7.7 | 2.90 | 0.12 | 2 | 49 | 5 | 93 | 0 | 0 | 2 | 1088 | [16] |
| clay dominated | Mixed Clay + Quartz | South Africa | 15–29 | 1.009 | Modern | 1.00 | −13.8 | 0.5 | 6.8 | 0.16 | 0.07 | 0.7 | 47 | 53 | 24 | 15 | 0 | 44 | 1528 | [16] |
| clay dominated | 1 : 1 Clay + Quartz | South Africa | 41–62 | 0.921 | 650 | 0.94 | −14.9 | 0.2 | 5.6 | 0.36 | 0.03 | 0 | 17 | 79 | 1 | 21 | 0 | 75 | 1528 | [16] |
aMOM age calculated using a one-pool model as described by Khomo et al. [56].
bEstimated 14C at 45 cm depth calculated via mass-preserving quadratic spline method [57] using R package ‘mpspline2’ (see electronic supplementary material, figure S1).
cData from Zhang et al. [58].
dData for soil ‘SRO’ and ‘CO + 1 : 1 Clay’ from less than 2 mm fraction.
(i). Amorphous mineral-dominated soils
We selected a volcanic soil rich in amorphous minerals, with a high OC content. Site ‘SRO’ is dominated by reactive secondary SRO minerals. The ‘SRO’ soil was collected from site Laupahoehoe of the LSAG parent material weathering chronosequence in Hawaii (montane rainforests, MAT = 16°C, MAP = ∼2500 mm, parent material mixture of volcanic tephra and lava) [2,52,60]. ‘SRO’ is classified as an Andisol (Aquic Hydrudand) developed on a 20 000-year-old lava flow. The soil is composed of the weathering products of olivine, pyroxene and plagioclase that are dominated by amorphous meta-stable SRO minerals like ferrihydrite, nanogoethite and poorly crystalline forms of oxides and aluminosilicates including allophane [2].
(ii). Clay-dominated soils
Soils that represent an array of clay mineral compositions were selected from previous studies in Kruger National Park in South Africa [16,56,61,62], from sites with similar climate conditions and savannah vegetation. These soils have very low SRO or primary mineral content, and the main stabilization mechanisms are associated with crystalline clay minerals (table 1). Sites ‘1 : 1 Clay + Quartz’ and ‘Mixed Clay + Quartz’ were collected from a catena toposequence developed on granitic parent material and represent the crest and toeslope positions, respectively, with a midslope seep and leaching zone in between. ‘1 : 1 Clay + Quartz’ (crest position) is classified as a lower productivity Entisol (Ustorthent) with a clay fraction (17% texture) dominated by non-expandable 1 : 1 clays with some non-expandable 2 : 1 chlorite (table 1). ‘Mixed Clay + Quartz’ (toeslope position) is classified as a higher productivity Alfisol (Natrusalf) with higher clay content (47% texture) and higher amounts of expandable 2 : 1 clays including smectite. ‘2 : 1 Clay + CO’ is classified as a Vertisol (Haplustert), developed on basalt parent material. It is a high-clay (43% texture), low-oxide soil with relatively high SOM stocks. It is dominated by smectite (93% of the clay-sized fraction), thus representing an expandable 2 : 1 clay-dominated soil.
(iii). Primary mineral-dominated soils
To contrast soils rich in secondary minerals with those in early development stages, we included soils with varying parent material from a cooler climate transect site in the Sierra Nevada mountains in California, USA [17,53]. These samples have significant concentrations of the primary mineral feldspar and a large variation in mineral reactivity due to differences in the geochemistry of soil parent material. ‘Felsic PM + Mixed Clay’ is classified as an Inceptisol (Dystroxerept) developed on granite parent material and contains low levels of SRO and clay minerals. ‘Int. PM + SRO’ is classified as an Andisol (Haploxerand) developed on andesite, an intermediate igneous parent material. It contains high levels of SRO minerals and a small amount of smectite. ‘Mafic PM + CO’ is classified as an Inceptisol (Haploxerept) developed on mafic basalt parent material containing intermediate levels of SRO and comparably low clay content (11% by texture).
(iv). Quartz sand-dominated soils
As a measure of OC stabilization and turnover in soils nearly devoid of reactive minerals (including clays), we selected soil developed on post-glacial dune sands located in northern Michigan, USA. ‘Quartz’ is classified as a Spodosol (Entic Haplorthod) where the only mineral-derived stabilization mechanism is (oxy)hydroxides precipitated on sand particles. Although classified as a Spodosol, the concentrations of extractable metals from this mineral B horizon are low (table 1, [55]).
(b) . Thermal analysis
(i). Thermal fractionation
For our analysis, we selected only the MOM fraction in the B horizons of the investigated soils after density separation (1.9 g cm−2 sodium polytungstate (SPT) solution) for thermal analysis to remove low-density particulate OM. Note that samples ‘1 : 1 Clay + Quartz’, ‘2 : 1 Clay + CO’ and ‘Mixed Clay + Quartz’ were not density fractionated, but were determined to have low (less than 5% of soil OC) FPOM concentrations in prior work [16]. Furthermore, since substantial amounts of OC were lost from ‘Quartz’ via dissolution in SPT an unfractionated bulk sample was also analysed for comparison (electronic supplementary material, figures S2–S5).
Methods for thermal fractionation are described in detail by Stoner et al. [34] and elsewhere [32,33]. First, temperature ranges of CO2 collection (approx. 200 to approx. 500°C) are selected by producing an initial profile of OC release (thermogram) which is deconvolved and transformed to a probability distribution of activation energy (Ea) based on the time-temperature relationship of sample collection (below, [33]). Component peaks underlying the thermogram can then be represented as Gaussian distributions, and thermal ‘fractions’ of OC can be isolated by trapping CO2 released in specific temperature ranges(s). Using this methodology, a total of five thermal fractions were collected for each sample. Briefly, a sample is heated at a constant rate (12°C min−1) from 40°C to 900°C under carrier gas flow composed of 75% N2 and 25% O2 (650 ml min−1 total). Any C released from the sample is fully oxidized to CO2 by a platinum catalyst held at 800°C. The produced CO2 is then quantified by a non-dispersive infrared (NDIR) detector. Carrier gas and CO2 then flow through a manifold consisting of parallel glass U-traps submerged in liquid nitrogen (LN2) under vacuum (10−2 mBar) and CO2 is cryogenically frozen and removed from the carrier gas. Once the sample reaches a desired upper-limit temperature, the first trap is sealed to trap CO2 produced at this temperature interval before a second parallel trap is opened and the next aliquot of CO2 is collected. The process is repeated for each subsequent temperature range.
The CO2 trapped at LN2 temperatures (−190°C) is then purified and quantified on a vacuum line using an isopropanol and dry ice trap to remove water and additional LN2 traps. A small CO2 subsample is collected for 13C measurement with a syringe and transferred to an He-flushed vial. The remainder is frozen into borosilicate glass tubes containing Ag and CuO wire and flame-sealed. Sample tubes are baked at 525°C for 1 h, during which the Ag and CuO remove additional contaminant gases (mostly N oxides that form from the reaction of N2 and O2 in carrier gases at high temperatures that freeze in the LN2 trap), necessary to avoid interferences with sample graphitization before 14C analyses (see §2b(iv)).
(ii). Activation energy
Activation energy (Ea) distributions were calculated using the ‘rampedpyrox’ Python package [33,63]. For this, thermograms are transformed using a time-temperature model to density distributions of Ea. In this study, Ea distributions and means (μE) are used to describe the average bond strength of OC released, and standard deviations (σE) to describe the heterogeneity of the bonding environment, where greater σE indicates more diverse types and strengths of bonds. While these values should not be compared with Ea metrics determined via other methods, they can be used to compare samples measured under the same conditions.
(iii). Analytical pyrolysis (py-GC/MS)
The molecular structure of SOM was studied by analytical pyrolysis (pyrolysis gas chromatography-mass spectrometry: py-GC/MS) using a double-shot micro-furnace pyrolyzer (model 2020i; Frontier Laboratories Ltd., Fukushima, Japan) attached to a GC/MS system (Agilent 6890N/5973MSD, Agilent Technologies, Santa Clara, CA, USA). First, a general overview of the sample pyrolyzate was obtained by direct pyrolysis of the samples at 400°C [64]. Then, a sequential multishot pyrolysis approach was performed using the same five temperatures as those used for the thermal fractionation described above.
Briefly, soil samples (15–25 mg, depending on OC content) were placed in stainless-steel capsules (Frontier Laboratories Ltd. Eco-Cup LF) and introduced in a pre-heated pyrolysis micro-furnace at the starting temperature for 1 min. For the direct pyrolysis, the furnace starting temperature was set at 400°C and the pyrolysis was performed once in single shot mode. For sequential pyrolysis analysis, the furnace was set at the lowest temperature for each fraction, before a sample was introduced into the pre-heated furnace and thermal desorption was performed for 1 min. The resulting gases were directly injected into the GC/MS system. The sample in processing was immediately moved to a cold area of the pyrolyzer while the furnace temperature increased to the next temperature and the sample was reintroduced to the pyrolyzer hot area for 1 min. Following this procedure, the treatment was repeated for each temperature increment using the same sample. Thus, in total and using aliquots, six chromatograms per sample were analysed; one for the direct pyrolysis at 400°C and five for the sequential analysis, corresponding to each of the selected temperatures.
The GC was equipped with a low polar-fused silica (5% phenyl-methylpolysiloxane) capillary column (Agilent J&W HP-5 ms UI), of 30 m × 250 µm × 0.25 µm film thickness. The carrier gas was He with constant flow at 1 ml min−1. The oven temperature was held at 50°C for 1 min, increased to 100°C at 30°C min−1, from 100°C to 300°C at 10°C min−1 and stabilized at 300°C for 10 min. Mass spectra were acquired at 70 eV ionizing energy. The compound assignment was achieved via single-ion monitoring for various homologous series, low-resolution mass spectrometry and by comparison with published and stored (NIST and Wiley libraries) data. The relative abundance of each pyrolysis product was calculated as a percentage of the chromatographic area of all identified compounds.
(iv). C isotope analysis
In order to quantify the 14C in each sample, collected CO2 (see 2b(i)) was graphitized following the method of Steinhof et al. [65] and measured on a MICADAS AMS system (Ion Plus, Switzerland). Data were corrected for blank C contribution as described by Stoner et al. [34], and are expressed as Fraction Modern 14C (Fm) [66]. For the reader, we have also expressed Fm as an equivalent mean OC age by fitting the 14C data for the year of sample collection to a one-pool model as described by Khomo et al. [56].
Analysis of δ13C was performed on an aliquot collected via syringe during purification (§2b(i)) using a modified gasbench inlet to a continuous flow isotope ratio mass spectrometer (IRMS) [67].
(v). Rock-Eval
To assess the relative degree of decomposition and thermal lability versus stability of MOM and implied biogeochemical stability in soil [68], we applied Rock-Eval 6 pyrolysis analysis. In addition to commonly reported OI and HI values representing the ratio of H:C and O:C in SOM [69], we calculated I- and R-indices (‘immature’ and ‘refractory’, respectively) designed for SOM comparison [70].
Briefly, approximately 60 mg of powder-ground sample was added to a Rock-Eval 6 Turbo (Vinci Technologies, France, analysed by GEO-Data mbH, Garbsen, Germany) and underwent two consecutive heating phases, first in a pyrolysis oven (200–650°C; thermal ramping rate of 25°C min−1; under N2 atmosphere) then in a combustion oven (300–850°C; thermal ramping rate of 20°C min−1; under laboratory air atmosphere). At the beginning of pyrolysis, samples underwent an isothermal step at 300°C for 180 s, during which the free hydrocarbons (HC) were vaporized (S1 peak), before proceeding to higher temperatures as described above. The pyrolysis effluents (mostly HC) were detected and quantified with flame ionization detection, while CO and CO2 were quantified by infrared detection during both the pyrolysis and oxidation stages.
Rock-Eval 6 indices I (immature) and R (refractory) that better describe SOM were developed by Sebag et al. [70]. These I and R indices were calculated by comparing the relative areas of the pyrograms. Briefly, the I-index is equal to
where F1, F2 and F3 are the relative areas of the deconvolution of Gaussian curves composing the S2 pyrogram. I-index values of SOM range from +0.64 in organic horizons to −1.32 in Bh horizons, although interquartile range is 0.11–0.34 for B horizons [70]. The R-index is defined as the proportion of the S2 pyrogram integrated after 400°C (a range of increasing refractory nature from 0.0 to 1.0). Note that low R-index values hereby indicate a low degree of thermal stability of SOM. By contrast, low I-index values indicate a high degree of SOM maturity and decomposition.
(vi). Caveats concerning pyrolysis versus oxidation
Thermal analysis techniques commonly measure soil characteristics with or without the presence of oxygen as an oxidizing agent, depending on the theory and goals of the research [71]. In this study, we employ both oxidative (thermal fractionation) and pyrolytic (py-GC/MS, Rock-Eval 6) methods to describe MOM characteristics with regard to increasing Ea. The primary advantage of oxidation in our study is in minimizing artefacts due to charring, or the conversion of OC released at low temperatures to high-stability molecules rather than being released as a COX gas, potentially misrepresenting SOM thermal stability. Although the mechanisms of decomposition can vary between methods, previous studies have observed no significant difference in the Ea or the 14C measured in thermal fractions collected under ramped pyrolysis and oxidation [35,72]. Thus, we are confident that comparisons can be drawn between OC released along Ea gradients between both methods.
3. Results
(a) . Thermograms
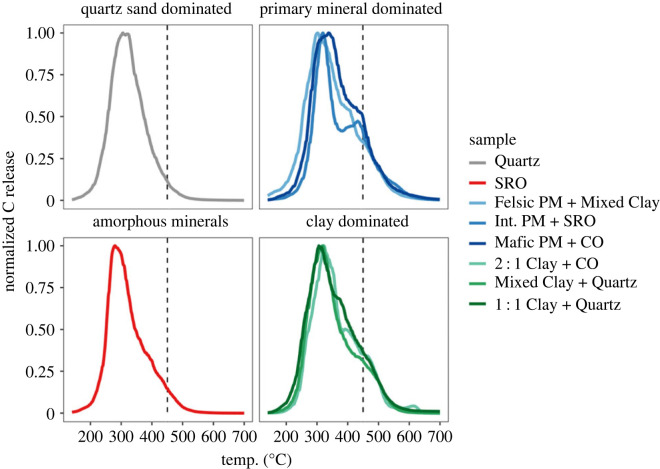
Ramped thermal oxidation yielded distinct trends and differences between soils according to their dominant minerals (figure 1). All samples released 99% of total OC below 600°C, confirming the absence of calcium carbonate. All samples showed peak OC release around 320°C except ‘SRO’ with a peak at 285°C. The release of OC from the low-reactivity sample ‘Quartz’ and the short-range order amorphous mineral-rich sample ‘SRO’ followed an approximately normal distribution, with most OC released close to the temperature of peak release. Primary mineral- and clay-dominated sample thermograms had ‘shoulders’ after the main peak, indicating a smaller portion of SOM released at higher temperatures than the bulk of SOM in a sample. ‘Int. PM + SRO’ showed a second smaller, but distinct peak at 420°C.
Figure 1.
Thermograms of OC release as a function of temperature under oxidative conditions, heated at 12°C min−1. All thermograms are normalized by setting the peak of C release to a value of 1 in order to better compare soils with varying OC contents. Thermograms are grouped by dominant mineralogy (amorphous minerals, primary minerals, clays). The vertical dashed line indicates 450°C, above which OC is quantified by the index T450–550 to describe the high-temperature OC which may be strongly bound to clay minerals (see text).
High-temperature releases (450–550°C) likely contain OC that may be strongly associated with clay minerals, which is described by a thermal index (T450–550, figure 1 and table 2, [73]). In this temperature range (T450–550) there was significantly less (t-test, p < 0.001) high-temperature OC release in ‘Quartz’, and ‘SRO’ (2.8–4.2% total C, table 2) than the other samples (11.3–15.9% total C).
Table 2.
Activation energy distribution data for all samples (sorted from lowest to highest Ea), and slope of Fraction modern 14C (Fm) as a function of increasing Ea (kJ mol−1; figure 2). T450–550 is the percentage of total OC released between 450 and 550°C. ‘E peak’ is the Ea value at which the greatest magnitude of C release is observed.
| dominant mineralogy | sample ID | E mean (μE) (kJ mol−1) | E peak (kJ mol−1) | E std (σE) (kJ mol−1) | youngest fraction (years) | oldest fraction (years) | slope Fm/Ea (kJ mol−1) | C release at T450–550 (%) |
|---|---|---|---|---|---|---|---|---|
| amorphous minerals | SRO | 138.8 | 126.3 | 14.3 | 3582 | 4046 | −0.0009 | 4.2 |
| quartz sand | Quartz | 139.4 | 135.1 | 13.4 | 845 | 1268 | −0.0012 | 2.8 |
| primary minerals | Mafic PM + CO | 144.6 | 136.0 | 18.0 | 368 | 1404 | −0.0022 | 11.3 |
| clay | 1 : 1 Clay + Quartz | 146.1 | 134.2 | 17.5 | 584 | 1623 | −0.0023 | 11.7 |
| clay | Mixed Clay + Quartz | 146.2 | 136.0 | 17.4 | 211 | 919 | −0.0023 | 12.1 |
| clay | 2 : 1 Clay + CO | 148.9 | 140.4 | 17.2 | 522 | 1713 | −0.0027 | 12.9 |
| primary minerals | Felsic PM + Mixed Clay | 150.4 | 142.1 | 18.8 | 773 | 1478 | −0.0014 | 12.9 |
| primary minerals | Int. PM + SRO | 152.1 | 136.0 | 19.1 | 766 | 1676 | −0.0018 | 15.9 |
Figure 2.
Fm 14C content for thermal fractions, represented as the proportion of total OC on the x-axis. Positive or negative height of bars indicates difference from the mean value (dashed horizontal lines). Fractions are ordered from low to high temperature. Note that the y-axis is absolute Fm value, and y-axis ranges are consistent between plots to show relative differences from mean values.
(b) . Activation energy
Similar grouping trends were observed in Ea, as ‘SRO’ and ‘Quartz’ yielded significantly lower mean activation energies (μE) than other samples (138.8–139.4 kJ mol−1; t-test, p < 0.01; table 2). Standard deviation of Ea distribution (σE), a proxy measure for the diversity of OC bonding strengths, was also much lower in these samples (13.0–14.3 kJ mol−1; t-test, p < 0.01; table 2). Here, the three primary mineral-dominated soils had the highest σE (‘Mafic PM + CO’ < ‘Felsic PM + Mixed Clay’ < ‘Int. PM + SRO’), suggesting the most heterogeneous bonds. Samples containing expandable 2 : 1 minerals and variable abundances of SRO and CO (‘2 : 1 Clay + CO’, ‘Felsic PM + Mixed Clay’, ‘Int. PM + SRO’) had significantly higher μE (148.9–152.1 kJ mol−1) and high σE (17.2–19.1 kJ mol−1).
(c) . Radiocarbon
Bulk soil mean 14C data (table 1) ranged from a low of 0.674 fraction modern (Fm) (one-pool model mean age of approx. 3200 years) measured for ‘SRO’ to a high of 1.009 Fm (most OC fixed in the last approx. 100 years) measured for ‘Mixed Clay + Quartz’. Most of the other soils had Fm 14C values between 0.85 and 0.99, indicating bulk mean ages of about 100–1500 years.
Despite the large range in mean MOM 14C, the difference in 14C between the thermal fractions and respective MOM values varied consistently, with the highest Fm 14C (youngest C) released at the lowest temperatures, and the lowest Fm 14C (oldest C) released at highest temperatures (figure 2 and table 2). For ‘SRO’ and ‘Quartz’, the samples with narrow thermograms with peak OC release at relatively low temperatures and thus the presumed weakest thermal stabilization mechanisms, the range in 14C age across thermal fractions was equivalent to a mean fraction age difference (oldest to youngest) of 420–460 years (table 2). By contrast, the mean 14C age difference between the oldest and youngest thermal fractions in samples with mixed mineralogies was larger, equivalent to a mean age difference of 700–1 200 years between OC released at the lowest and highest temperatures. In general, 14C content of thermal fractions decreased with increased Ea (figure 2 and table 2). However, there were distinct differences in the rate of decrease. ‘SRO’, ‘Quartz’ and ‘Felsic PM + Mixed Clay’ (in order) decreased in Fm with Ea much less rapidly (−0.0009 to −0.0014 Fm per kJ mol−1) than all other samples (−0.0018 to −0.0027 Fm per kJ mol−1).
(d) . py-GC/MS
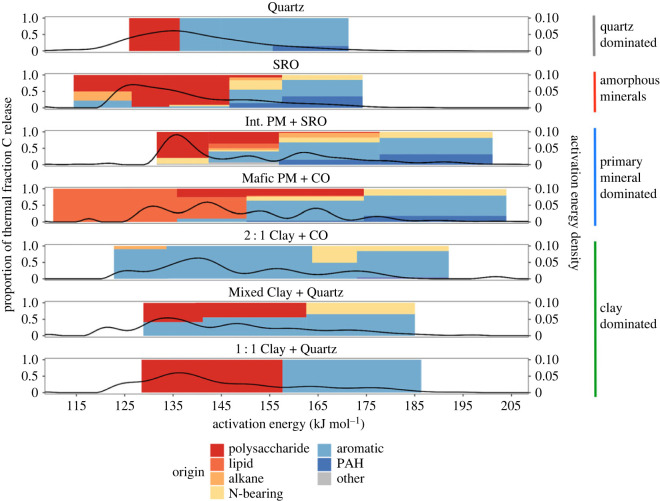
Pyrolysis gas chromatography and mass spectrometry (py-GC/MS) revealed divergent trends in SOM quality and composition across mineralogies and temperatures (figure 3, electronic supplementary material, figures S4–S12). Of the 222 compounds detected across all samples, 74 were aromatic in nature, and 48 were polycyclic aromatic hydrocarbons (PAH). No lignin biomarkers (hydroxyphenyl, guaiacyl or syringyl subunits) were identified in any sample. Notably, the highest variety of organic compounds was detected in soil samples that contain various forms of SRO and CO. From those, the presence of SRO minerals had the strongest effect on chemical diversity, with 127 unique components detected in ‘SRO’, 85 in ‘Int. PM + SRO’ and 58 in ‘Mafic PM + CO’. Other samples contained fewer distinct compounds: in order, 14 in ‘2 : 1 Clay + CO’, 10 in ‘Quartz’ and 7 in both ‘1 : 1 Clay + Quartz’ and ‘Mixed Clay + Quartz’. Note that the sample ‘Felsic PM + Mixed Clay’ was not analysed due to late addition to the study and COVID-19 laboratory staff access restrictions.
Figure 3.
py-GC/MS profiles with calculated Ea probability distribution (PDF) functions calculated from oxidative thermograms overlain (black lines) to show the relative amount of OC associated with a given set of chemistry range of activation energies. PAH refers to ‘polycyclic aromatic hydrocarbons'. Right-hand axis represents density of OC released at a given temperature. Coloured bars on the right side correspond to sample groupings. ‘Felsic PM + Mixed Clay’ was not analysed.
With the exception of the ‘SRO’ sample, aromatic molecules were the most prominent group in all soils. Nitrogen-bearing compounds were detected in the ‘SRO’ soil, and soils with expandable 2 : 1 clay minerals. These compounds were generally released in thermal fractions of approximately 150 kJ mol−1 or more, but the two SRO soils (Int. PM + SRO and SRO) released N-containing OM with lower Ea than non-SRO soils (132–150 kJ mol−1). Polysaccharides, bioavailable compounds of potentially microbial origin, were detected in all samples except for ‘2 : 1 Clay + CO’. No soil released polysaccharides above approximately 178 kJ mol−1. Alkane/alkene chains were detected at low temperatures in ‘2 : 1 Clay + CO’ and ‘SRO’, while ‘Int. PM + SRO’ and ‘Mafic PM + CO’ (primary mineral-dominated soils) released alkanes with Ea > 175 kJ mol−1. The only strong lipid signals were detected in ‘Mafic PM + CO’ and a small amount in ‘Int. PM + SRO’, all below Ea of 178 kJ mol−1.
(e) . Rock-Eval soil organic matter indices
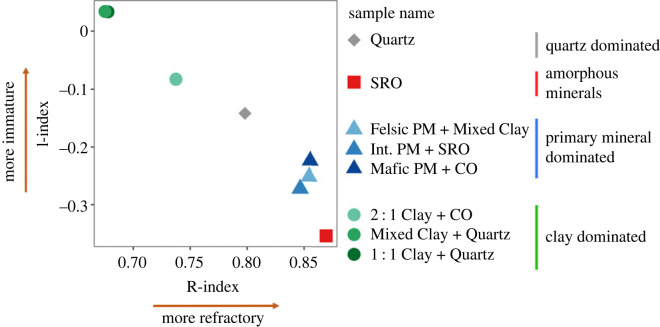
Rock-Eval SOM indices for all samples were in the expected range for B horizons [58] and followed the general trend of decreasing I-index values associated with increasing R-index values, suggesting a higher degree of SOM decomposition and more refractory SOM, respectively. Clay-dominated soils (‘2 : 1 Clay + CO’, ‘Mixed Clay + Quartz’, ‘1 : 1 Clay + Quartz’) showed the least thermally stable (low R-index) C, that was at the same time least decomposed (high I-index). Among the clay-dominated samples, ‘2 : 1 Clay + CO’, with high content of expandable clays, had thermally stable SOM (high R-index) that was more mature (low I-index). Interestingly, primary mineral-dominated soils (‘Int. PM, + SRO’, ‘Felsic PM + Mixed Clay’, ‘Mafic PM + CO’) had more stable SOM (high R-index) that was at the same time more decomposed (low I-index) than the clay-dominated soils. Overall, the highest R-index values were observed for the amorphous mineral-dominated soil ‘SRO’. Sample ‘Quartz’ was nearly centred for both indices and distinct from all other groups of samples.
4. Discussion
(a) . Mineral and organic controls on soil organic matter thermal stability, age distribution and chemistry
Our data confirm that the abundance, biochemical characteristics and mean 14C values of MOM can differ greatly depending on mineralogy and degree of mineral weathering. However, despite a variety of soil settings in our samples, we find distinct relationships between mineral reactivity, patterns of 14C distribution, and the type and number of chemical species in MOM. Following the hypothesis that greater activation energy (Ea) corresponds to stability in soil, ‘labile’ forms of OC should be oxidized at lower temperature, decompose more easily, and be younger than the mean MOM 14C age, while high Ea OC should be older and more aromatic in nature [22]. This was generally observed across all samples (figure 3), with the additional observation that the low Ea OC was associated with pedogenic oxide minerals (SRO and CO), while high Ea OC was associated with clay minerals, especially 2 : 1 clays. Thus, fractionating MOM along a continuous Ea gradient allows us to describe which minerals tend to be associated with faster cycling (younger) or slower cycling (older) OC. This helps define SOM model pool structures and may have broad implications for assessing patterns of OC persistence across soil systems.
MOM forms through numerous types of interactions [4]. These include strong interactions between reactive functional groups in dissolved OM with reactive mineral surfaces, through weaker interactions between hydrophobic moieties in solution with surface-sorbed OM (e.g. hydrophobic seclusion [21,74]), or through the incorporation of local microbial necromass and exudates [75–78]. The thermogram and age structure of C oxidized thus reflects the overall stability of an organic moiety in a specific setting. Below, we briefly discuss the different types of mineral–OM interactions analysed (e.g. SRO, CO, clays and primary minerals) and patterns of OC chemistry and relative age distributions associated with each. As most soils, even ones predominantly consisting of clay minerals, can have substantial amounts of OC associated with pedogenic oxides and oxyhydroxides, we also discuss patterns observed in mixed mineralogy soils.
(i). Amorphous mineral (soil organic matter) rich samples
Reactive SRO minerals display the capacity to store large amounts of OC over long time spans and can protect a diverse range of compounds from mineralization by creating energetic or physical barriers that deter microbial decomposers [2,79,80]. Despite the majority (main thermogram peak) of the MOM in ‘SRO’ being oxidized at relatively low Ea in mostly bioavailable forms, it contained very old OC (Fm 0.63–0.67, figures 2 and 3, table 1). The narrow Ea range and 14C distribution of MOM in ‘SRO’ (table 2) indicate homogeneous stabilization mechanisms, such as OM–OM bonds or co-precipitation of metals and OM [81,82], that do not provide strong protection under thermally oxidizing laboratory conditions. However, as indicated by the overall age of the bulk MOM and high pyrolytic Rock-Eval R-index (figure 4), the thermal oxidation method may bypass physical protection mechanisms that can be responsible for greater mean OC ages. This interpretation is supported by the presence of more diverse organic compounds in soils with expandable clays as well as amorphous SRO minerals, e.g. ‘Int. PM + SRO’ (figure 3).
Figure 4.
Results of Rock-Eval 6 SOM across samples calculated to assess the degree of decomposition using I-index (immaturity) and the thermal stability using the R-index (refractory) [70].
(ii). Quartz sample
In Podzol B horizons, pedogenic Al and Fe oxides are the primary method of OC stabilization. The ‘Quartz’ sample in this study was composed of wind-blown quartz sand (100% sand by texture, [54]), with only small amounts of extractable Fe (table 1). Limited stabilization pathways resulted in the narrow thermogram and 14C distribution (figures 1 and 2). The simplistic chemistry reflects stronger, aromatic accumulation in hydrophobic fractions via ligand exchange (higher temperatures), and weaker, bioavailable polysaccharides in the hydrophilic fractions (lower temperatures) [83,84], although the high sand content may have favoured carbohydrate accumulation [85]. We can attribute the long transit times (845–1268 years, table 2) in this subsoil to low OC and nutrient concentrations limiting microbial decomposition [86,87].
(iii). Clay mineral-rich samples
Compared to clay-poor samples ‘SRO’ and ‘Quartz’, clay minerals, even in low abundance, increase the range of activation energies and ages (table 2) detected within a sample as observed most strongly in ‘2 : 1 Clay + CO’, the most clay-rich soil analysed (47% clay by texture, 93% of which is smectite, table 1). Clay content, as well as total N content, has been found to correlate with thermal stability, with direct mineral–OC associations attributed to C release in the temperature range of 450–550°C ([73,88,89]; figure 1). The 200–300°C temperature range has previously been attributed to bioavailable SOM components, as the amount of C released at low Ea decreased the most after soil incubation [90,91]. In our samples, most OC in clay-dominated soils is released between 200 and 400°C, and is thus likely composed of OC released from organo–organo bonds and organo-mineral bonds with co-occurring (oxy)hydroxides [92–94] that may precipitate on clay surfaces [29,55], with older, clay edge-bound OC releasing at higher temperatures [73,95]. Notably, among the clay-dominated and primary mineral-dominated soils, we did not observe strong effects of clay type (1 : 1 versus 2 : 1) on thermal stability (figure 1) despite evidence that different clays sorb very different SOM ([3,89,96,97]; figure 3). The predominance of polysaccharides released at low temperature, and aromatics and N-bearing SOM released at high temperature, respectively, suggests two distinct pools of SOM with different pathways of clay-mineral stabilization: (i) younger, bioavailable polysaccharides associated with greater microbial activity (root exudation and greater microbial enzyme activity [96,98]) on clays [99] and (ii) dominantly aromatic OC bound to clays via hydrophobic exclusion, cation bridging and hydrogen [3,4].
(iv). Organic matter quality patterns
Polysaccharides make up the majority of the main peak of OC release in most soils (figure 3) and showed mean ages of approximately 200 up to approximately 1 000 years (albeit approx. 3 400 years for ‘SRO’) (table 2 and electronic supplementary material, table S1). However, N-bearing compounds are a critical MOM pool that can persist on minerals for centuries, despite N limitation in most soils (figure 2, [100–104]). Generally, proteins and amino sugars are strongly amphiphilic [105] and bear one of the few SOM functional groups (–NHX) that may be positively charged, thus forming strong bonds with negatively charged mineral surfaces, e.g. oxides and 2 : 1 clay minerals [106]. Even more importantly, they may act as the first ‘wetting’ layer upon which subsequent OM can bind, although potentially more weakly [106–109]. Indeed, we observed that only soils containing smectite (‘2 : 1 Clay + CO’, ‘Mixed Clay + Quartz’, ‘Int. PM + SRO’ and ‘Mafic PM + CO’) and SRO minerals (‘SRO’ and ‘Int. PM + SRO’) yielded N-containing C, released at high Ea and in the oldest thermal fraction within each sample (figure 3). Following a multi-layer model of MOM accumulation, the outer layers of MOM may be oxidized at lower Ea, giving way to N-bearing and aromatic OC bound to mineral surfaces. However, the exact processes of MOM oxidation that can disrupt OM–OM as well as OM-mineral bonds are still unknown [109]. In the case of allophane soils (SRO), it is not clear if these N-bearing compounds are preserved preferentially or included in the diverse MOM in sample ‘SRO’ that has at the same time a low immaturity index and high 14C age (figures 2 and 4, table 2). We argue that if this OC is of microbial origin, as evidenced by the lack of lignin biomarkers (figure 3), it indicates active cycling of rather old C, highlighting the importance of considering pathways for pre-aged OC to enter MOM when interpreting these 14C values.
In summary, soils that were able to provide better protection for SOM (older 14C age and higher mean Ea) were also able to stabilize bioavailable OC species against decomposition (figure 3). While in general, our hypothesis held that greater Ea was associated with older and more aromatic OC within a given soil, the overall bulk 14C did not follow the expected patterns. The overall oldest OC was in the ‘SRO’ soil, yet it released OC with lower Ea than OC associated with clay in other soils, which was younger (figures 1 and 2). Furthermore, the high R-index (figure 4, electronic supplementary material, table S2) under pyrolytic Rock-Eval analysis, during which allophane may degrade to more crystalline minerals at temperatures greater than 600°C [110], likely provides a better indicator of the apparent long-term stability of MOM in ‘SRO’ than oxidative thermograms alone. Thus, the pedogenic setting of samples plays the dominant role in determining the absolute age of OC in B horizons, while mineralogy controls how OC age is distributed around the mean 14C value.
(b) . Mineral-associated organic matter as a heterogeneous 14C pool
(i). Implications for pool model structure
To explain observations that include the response of SOM over time to changes in vegetation inputs, or to fit time series of 14C over the last decades has required models to represent SOM using pools with different timescales of turnover, ranging from years to millennia. Thermal analysis approaches help determine which mineral stabilization mechanisms may give rise to different model structures. For example, in subsoils with mixed mineralogy (‘2 : 1 Clay + CO’, ‘Mixed Clay + Quartz’, ‘1 : 1 Clay + Quartz’, ‘Int. PM + SRO’, ‘Felsic PM + Mixed Clay’, ‘Mafic PM + CO’, table 1) our results suggest that faster cycling SOM is more bioavailable and potentially associated with more crystalline metal oxides and/or weaker OM–OM bonds, while older MOM is associated either with amorphous SRO (reflecting potentially occlusion or physical isolation) or expandable 2 : 1 clay minerals that have a larger reactive surface area and strong ionic stabilization of OC (figures 2 and 3). By contrast, SOM turnover in soils dominated by a single mineral (e.g. ‘Quartz’, ‘SRO’) could potentially be modelled as a single pool. Indeed, thermal fractionation indicates that the range of 14C ages within a given thermogram feature can be relatively narrow (figure 2), indicating that mixed mineral soils with clay can be modelled as two homogeneous pools with different mean C ages. These pools also reflect different chemical composition, with low-temperature OC reflecting microbial polysaccharides while OC released at higher temperatures consists of slowly cycling aromatic and N-bearing OC (figure 3).
(ii). Linking age distributions and model structures
Isolating fractions of varying thermal stability and Ea in combination with describing the chemical quality of MOM from distinct thermal fractions proved to be a useful tool for separating SOM according to the type of mineral with which it is associated and its relative age in distinct soils. While the directional changes of processes in complex soil systems are not well understood and are difficult to predict [89], thermal fractionation may have the potential to identify the proportion of the MOM in subsoils that is more weakly stabilized and thus may be more vulnerable to change on multi-decadal to multi-centennial timescales. We found that MOM contains molecules with diverse chemistry, Ea, and age dependent on mineral composition (figures 2 and 3). More research is needed to understand the evolution of soil age/depth distributions using gradients that can track changes in minerals and OC age through time across soil of varying development trajectories. Such studies are also needed to understand the potential vulnerability of different mineral–OC associations to climatic or vegetation change.
(iii). Controls on absolute organic carbon age
While thermal oxidation clearly provided a distribution of 14C ages within a given sample, we were not able to predict the mean 14C age of a sample from mineralogy or OM chemistry alone. For example, polysaccharides released at low Ea (<160 kJ mol−1) make up the majority of OC release in most soils (figure 3) but had mean ages ranging from approximately 200 up to approximately 1000 years (and approx. 3400 years for ‘SRO’, table 2 and electronic supplementary material, table S1). The mean age of C can reflect a number of processes. For example, the pre-ageing of OC in roots or plant stems, or in recycling of older C released from minerals by microbes can provide ‘fresh’ substrates that were originally fixed from the atmosphere hundreds of years previously. Another mechanism for pre-ageing of C inputs at depth reflects rates of vertical transport from regions of high C input to lower C availability. For example, the 14C content of SOM arriving in subsoil (e.g. Spodosol B horizons) reflects the mean time required for OC to arrive in that horizon and is not necessarily a good measure for the in situ stability or decomposition rate of B horizon SOM [111]. Thus, the age of OC in a given pool may not reflect its present vulnerability to destabilization or decomposition.
Future climates may de-stabilize OC through enhanced microbial activity [51,112], enhanced weathering [7,80,113,114] and changing redox conditions [80,115,116]. Combining the effects of environmental, site-specific conditions with an improved understanding of the dynamics of soil C stabilization dynamics and mineral stability may better constrain transit times of SOM and greatly improve the predictive power of soil C turnover models. For example, a large fraction of OC in our mixed mineralogy soils is associated with pedogenic oxides but situated in greatly different bioclimatic settings. Future studies could test the degree to which the age and age distribution of associated OM relates to the stability of minerals or the conditions that control C input and turnover by studying gradients of redox cycling or changes in pH associated with root exudates or microbial enzyme activities. Moving forward, MOM must be considered a large, dynamic pool of OC that is not insensitive to changing environmental conditions.
5. Conclusion and future work
A direct comparison of diverse MOM using a variety of thermal oxidation and pyrolysis methods successfully highlighted the distinct effects of soil minerals on MOM persistence. Soils with singular mineral stabilization pathways and without clay minerals had narrower 14C and energy distributions and, excluding soils rich in amorphous SRO minerals, stored generally less chemically diverse compounds with younger 14C ages. Soils containing a larger variety of minerals offered multiple stabilization mechanisms leading to a much wider range of activation energies, SOM ages, and generally greater diversity of organic compounds. Through the combination of several thermal stability analyses and characterization of MOM molecular structure, we were able to identify distinct timescales for MOM turnover and the biochemical structure of MOM associated with different types of soil minerals. MOM in soils containing 2 : 1 clays had overall higher thermal stability and released older OC at higher temperatures than soils dominated by non-expandable 1 : 1 clay minerals or highly crystalline minerals. In all soils, the majority of MOM appears to be associated with (pedogenic) (oxy)hydroxides and amorphous SRO minerals or weakly stabilizing OM–OM bonds. These minerals were able to store abundant and diverse OC compounds, including potentially bioavailable and more accessible SOM. Thus, our results support previous studies showing the role of oxides as dominant drivers of total SOM storage, but also show a smaller but significant role of clays in strongly stabilizing relatively old and chemically distinct C. Thermal oxidation demonstrated that it can successfully distinguish OC associated with different minerals and provide predictable timescales and chemical characteristics. We conclude that while the directional changes of processes in complex soil systems are not well understood and remain difficult to predict, thermal fractionation may have the potential to identify SOM that is more weakly stabilized and more likely to be vulnerable to future change on multi-decadal to multi-centennial timescales. The mineralogical control on the age structure of MOM has important implications for how soil SOM models need to account for differences in soil mineralogy, and how specific portions of SOM may have distinct reactions to changing environmental conditions that affect plant C input, microbial C turnover and mineral OC stabilization to varying degrees and at varying timescales.
Acknowledgements
We gratefully acknowledge Dr Axel Steinhof and the Jena 14C laboratory, and Anke Jurisch at Geodata GmbH for Rock-Eval analysis. We would also like to thank our funding sources and Royal Society journal reviewers. Thanks are due to Dr Kate Heckman and USGS for providing additional sample materials, and to those researchers who procured the soil characteristic data used in this analysis.
Data accessibility
Data and code will be maintained in this repository: https://github.com/ShaneStoner/Mineralogy14C. It is also available with a DOI via Zenodo: https://zenodo.org/record/7998659 [117].
The data are provided in electronic supplementary material [118].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
S.S.: conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; S.E.T.: conceptualization, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing; J.A.G.-P: formal analysis, investigation, writing—original draft; M.S.: conceptualization, investigation, methodology, supervision, writing—original draft; C.A.S.: investigation, methodology, supervision, visualization, writing—original draft; A.M.H.: supervision, writing—original draft; O.C.: data curation, resources, writing—original draft; S.D.: conceptualization, investigation, methodology, supervision, visualization, writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Open access funding provided by the Max Planck Society.
Open Access funding enabled and organized by Projekt DEAL. This research has been supported by the Max Planck Institute for Biogeochemistry and the European Research Council (Horizon 2020 Research and Innovation Programme, Grant Agreement no. 695101).
References
- 1.Scharlemann JPW, Tanner EVJ, Hiederer R, Kapos V. 2014. Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Manag. 5, 81-91. ( 10.4155/cmt.13.77) [DOI] [Google Scholar]
- 2.Chorover J, Amistadi MK, Chadwick OA. 2004. Surface charge evolution of mineral-organic complexes during pedogenesis in Hawaiian basalt. Geochim. Cosmochim. Acta 68, 4859-4876. ( 10.1016/j.gca.2004.06.005) [DOI] [Google Scholar]
- 3.von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. 2006. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review. Eur. J. Soil Sci. 57, 426-445. ( 10.1111/j.1365-2389.2006.00809.x) [DOI] [Google Scholar]
- 4.Kleber M, Bourg IC, Coward EK, Hansel CM, Myneni SCB, Nunan N. 2021. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2, 402-421. ( 10.1038/s43017-021-00162-y) [DOI] [Google Scholar]
- 5.Jobbagy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423. ( 10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2) [DOI] [Google Scholar]
- 6.Mathieu JA, Hatté C, Balesdent J, Parent É. 2015. Deep soil carbon dynamics are driven more by soil type than by climate: a worldwide meta-analysis of radiocarbon profiles. Glob. Chang. Biol. 21, 4278-4292. ( 10.1111/gcb.13012) [DOI] [PubMed] [Google Scholar]
- 7.Lacroix EM, Masue-Slowey Y, Dlott GA, Keiluweit M, Chadwick OA, Fendorf S. 2022. Mineral protection and resource limitations combine to explain profile-scale soil carbon persistence. J. Geophys. Res. Biogeosci. 127, 1-14. ( 10.1029/2021JG006674)35251875 [DOI] [Google Scholar]
- 8.Chabbi A, Kögel-Knabner I, Rumpel C. 2009. Stabilised carbon in subsoil horizons is located in spatially distinct parts of the soil profile. Soil Biol. Biochem. 41, 256-261. ( 10.1016/j.soilbio.2008.10.033) [DOI] [Google Scholar]
- 9.Georgiou K et al. 2022. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 13, 3797. ( 10.1038/s41467-022-31540-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikutta R, Schaumann GE, Gildemeister D, Bonneville S, Kramer MG, Chorover J, Chadwick OA, Guggenberger G. 2009. Biogeochemistry of mineral-organic associations across a long-term mineralogical soil gradient (0.3–4100 kyr), Hawaiian Islands. Geochim. Cosmochim. Acta 73, 2034-2060. ( 10.1016/j.gca.2008.12.028) [DOI] [Google Scholar]
- 11.Roth VN et al. 2019. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat. Geosci. 12, 755-761. ( 10.1038/s41561-019-0417-4) [DOI] [Google Scholar]
- 12.Wiseman CLS, Püttmann W. 2005. Soil organic carbon and its sorptive preservation in central Germany. Eur. J. Soil Sci. 56, 65-76. ( 10.1111/j.1351-0754.2004.00655.x) [DOI] [Google Scholar]
- 13.Rasmussen C et al. 2018. Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137, 297-306. ( 10.1007/s10533-018-0424-3) [DOI] [Google Scholar]
- 14.Kaiser K, Guggenberger G, Haumaier L, Zech W. 1997. Dissolved organic matter sorption on subsoils and minerals studied by 13C-NMR and DRIFT spectroscopy. Eur. J. Soil Sci. 48, 301-310. ( 10.1111/j.1365-2389.1997.tb00550.x) [DOI] [Google Scholar]
- 15.Sposito G, Skipper NT, Sutton R, Park SH, Soper AK, Greathouse JA. 1999. Surface geochemistry of the clay minerals. Proc. Natl Acad. Sci. USA 96, 3358-3364. ( 10.1073/pnas.96.7.3358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khomo L, Trumbore SE, Bern CR, Chadwick OA. 2017. Timescales of carbon turnover in soils with mixed crystalline mineralogies. Soil 3, 17-30. ( 10.5194/soil-3-17-2017) [DOI] [Google Scholar]
- 17.Rasmussen C, Throckmorton H, Liles G, Heckman K, Meding S, Horwath WR. 2018. Controls on soil organic carbon partitioning and stabilization in the California Sierra Nevada. Soil Syst. 2, 1-18. ( 10.3390/soilsystems2030041)31276103 [DOI] [Google Scholar]
- 18.Masiello CA, Chadwick OA, Southon J, Torn MS, Harden JW. 2004. Weathering controls on mechanisms of carbon storage in grassland soils. Global Biogeochem. Cycles 18, 1-9. ( 10.1029/2004GB002219) [DOI] [Google Scholar]
- 19.González-Pérez JA, Arbelo CD, González-Vila FJ, Rodríguez AR, Almendros G, Armas CM, Polvillo O. 2007. Molecular features of organic matter in diagnostic horizons from andosols as seen by analytical pyrolysis. J. Anal. Appl. Pyrolysis. 80, 369-382. ( 10.1016/j.jaap.2007.04.008) [DOI] [Google Scholar]
- 20.Kramer MG, Sanderman J, Chadwick OA, Chorover J, Vitousek PM. 2012. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Chang. Biol. 18, 2594-2605. ( 10.1111/j.1365-2486.2012.02681.x) [DOI] [Google Scholar]
- 21.Spaccini R, Piccolo A, Conte P, Haberhauer G, Gerzabek MH. 2002. Increased soil organic carbon sequestration through hydrophobic protection by humic substances. Soil Biol. Biochem. 34, 1839-1851. ( 10.1016/S0038-0717(02)00197-9) [DOI] [Google Scholar]
- 22.Ni J, Pignatello JJ. 2018. Charge-assisted hydrogen bonding as a cohesive force in soil organic matter: water solubility enhancement by addition of simple carboxylic acids. Environ. Sci. Process. Impacts 20, 1225-1233. ( 10.1039/C8EM00255J) [DOI] [PubMed] [Google Scholar]
- 23.Schwarzenbach RP, Gschwend P, Imboden DM. 2012. Environmental organic chemistry, 3rd edn. Hoboken, NJ: Wiley-Interscience. [Google Scholar]
- 24.Tombácz E, Libor Z, Illés E, Majzik A, Klumpp E. 2004. The role of reactive surface sites and complexation by humic acids in the interaction of clay mineral and iron oxide particles. Org. Geochem. 35, 257-267. ( 10.1016/j.orggeochem.2003.11.002) [DOI] [Google Scholar]
- 25.Balbino LC, Bruand A, Cousin I, Brossard M, Quétin P, Grimaldi M. 2004. Change in the hydraulic properties of a Brazilian clay Ferralsol on clearing for pasture. Geoderma 120,297-307. ( 10.1016/j.geoderma.2003.08.017) [DOI] [Google Scholar]
- 26.Oades JM. 1993. The role of biology in the formation, stabilization and degradation of soil structure. In Soil structure/soil biota interrelationships (eds Brussaard L, Kooistra MJ), pp. 377-400. Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- 27.Jastrow JD, Amonette JE, Bailey VL. 2007. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 80, 5-23. ( 10.1007/s10584-006-9178-3) [DOI] [Google Scholar]
- 28.Kravchenko AN, Guber AK, Razavi BS, Koestel J, Quigley MY, Robertson GP, Kuzyakov Y. 2019. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 10, 1-10. ( 10.1038/s41467-019-11057-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikutta R, Kleber M, Torn MS, Jahn R. 2006. Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77, 25-56. ( 10.1007/s10533-005-0712-6) [DOI] [Google Scholar]
- 30.Sierra CA, Hoyt AM, He Y, Trumbore SE. 2018. Soil organic matter persistence as a stochastic process: age and transit time distributions of carbon in soils. Glob. Biogeochem. Cycles 32,1574-1588. ( 10.1029/2018GB005950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanca I, Trumbore SE, Macario K, Sierra CA. 2022. Probability distributions of radiocarbon in open linear compartmental systems at steady-state. J. Geophys. Res. Biogeosci. 127, 1-23. ( 10.1029/2021JG006673)35251875 [DOI] [Google Scholar]
- 32.Rosenheim BE, Galy V. 2012. Direct measurement of riverine particulate organic carbon age structure. Geophys. Res. Lett. 39, 1-6. ( 10.1029/2012GL052883) [DOI] [Google Scholar]
- 33.Hemingway JD, Rothman DH, Rosengard SZ, Galy VV. 2017. Technical note: an inverse method to relate organic carbon reactivity to isotope composition from serial oxidation. Biogeosciences 14, 5099-5114. ( 10.5194/bg-14-5099-2017) [DOI] [Google Scholar]
- 34.Stoner SW, Schrumpf M, Hoyt A, Sierra CA, Doetterl S, Galy V, Trumbore SE. 2023. How well does ramped thermal oxidation quantify the age distribution of soil carbon? Assessing thermal stability of physically and chemically fractionated soil organic matter. Biogeosciences 20, 3151-3163. ( 10.5194/bg-20-3151-2023) [DOI] [Google Scholar]
- 35.Sanderman J, Grandy SA. 2020. Ramped thermal analysis for isolating biologically meaningful soil organic matter fractions with distinct residence times. Soil 6, 131-144. ( 10.5194/soil-6-131-2020) [DOI] [Google Scholar]
- 36.Feng X, Simpson MJ. 2008. Temperature responses of individual soil organic matter components. J. Geophys. Res. Biogeosci. 113, 1-14. ( 10.1029/2008JG000743) [DOI] [Google Scholar]
- 37.Jenkinson DS, Harkness DD, Vance ED, Adams DE, Harrison AF. 1992. Calculating net primary production and annual input of organic matter to soil from the amount and radiocarbon content of soil organic matter. Soil Biol. Biochem. 24, 295-308. ( 10.1016/0038-0717(92)90189-5) [DOI] [Google Scholar]
- 38.Baisden WT, Canessa S. 2013. Using 50 years of soil radiocarbon data to identify optimal approaches for estimating soil carbon residence times. Nucl. Instrum. Methods Phys. Res. B 294, 588-592. ( 10.1016/j.nimb.2012.06.021) [DOI] [Google Scholar]
- 39.Sierra CA, Müller M, Trumbore SE. 2014. Modeling radiocarbon dynamics in soils: SoilR version 1.1. Geosci. Model Dev. 7, 1919-1931. ( 10.5194/gmd-7-1919-2014) [DOI] [Google Scholar]
- 40.Harrison KG, Broecker WS, Bonani G. 1993. The effect of changing land use on soil radiocarbon. Science 262, 725-726. ( 10.1126/science.262.5134.725) [DOI] [PubMed] [Google Scholar]
- 41.Römkens PFAM, Hassink J, van der Pflicht J. 1998. Soil organic 14c dynamics: effects of pasture installation on Arable Land. Radiocarbon 40, 1023-1031. ( 10.1017/S0033822200018993) [DOI] [Google Scholar]
- 42.Sanderman J, Creamer C, Baisden WT, Farrell M, Fallon S. 2017. Greater soil carbon stocks and faster turnover rates with increasing agricultural productivity. Soil 3, 1-16. ( 10.5194/soil-3-1-2017) [DOI] [Google Scholar]
- 43.Stoner SW, Hoyt AM, Trumbore SE, Sierra CA, Schrumpf M, Doetterl S, Troy Baisden W, Schipper LA. 2021. Soil organic matter turnover rates increase to match increased inputs in grazed grasslands. Biogeochemistry 156, 145-160. ( 10.1007/s10533-021-00838-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heckman K, Throckmorton H, Horwath W, Swanston C, Rasmussen C. 2018. Variation in the molecular structure and radiocarbon abundance of mineral-associated organic matter across a lithosequence of forest soils. Soil Syst. 2, 36. ( 10.3390/soilsystems2020036) [DOI] [Google Scholar]
- 45.Hemingway JD, Rothman DH, Grant KE, Rosengard SZ, Eglinton TI, Derry LA, Galy V. 2019. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570, 228-231. ( 10.1038/s41586-019-1280-6) [DOI] [PubMed] [Google Scholar]
- 46.Sollins P, Kramer MG, Swanston C, Lajtha K, Filley T, Aufdenkampe AK, Wagai R, Bowden RD. 2009. Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96, 209-231. ( 10.1007/s10533-009-9359-z) [DOI] [Google Scholar]
- 47.Schrumpf M, Kaiser K. 2015. Large differences in estimates of soil organic carbon turnover in density fractions by using single and repeated radiocarbon inventories. Geoderma 239–240, 168-178. [cited 2014 Nov 3]. [Google Scholar]
- 48.Heckman K et al. 2022. Beyond bulk: density fractions explain heterogeneity in global soil carbon abundance and persistence. Glob. Chang. Biol. 28, 1178-1196. ( 10.1111/gcb.16023) [DOI] [PubMed] [Google Scholar]
- 49.Schrumpf M, Kaiser K, Mayer A, Hempel G, Trumbore SE. 2021. Age distribution, extractability, and stability of mineral-bound organic carbon in central European soils. Biogeosciences 18, 1241-1257. ( 10.5194/bg-18-1241-2021) [DOI] [Google Scholar]
- 50.Hopkins FM, Torn MS, Trumbore SE. 2012. Warming accelerates decomposition of decades-old carbon in forest soils. Proc. Natl Acad. Sci. USA 109, E1753-E1761. ( 10.1073/pnas.1120603109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hicks Pries CE, Castanha C, Porras R, Phillips C, Torn MS. 2018. The whole-soil carbon flux in response to warming. Science 359, 1420-1423. ( 10.1126/science.aao0457) [DOI] [PubMed] [Google Scholar]
- 52.Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM. 1997. Mineral control of soil organic carbon and storage and turnover. Nature 389, 3601-3603. ( 10.1038/38260) [DOI] [Google Scholar]
- 53.Beem-Miller JP, Rasmussen C, Hoyt AM, Schrumpf M, Guggenberger G, Trumbore SE. 2022. Soil minerals mediate climatic control of soil C cycling on annual to centennial timescales. EGUsphere 2022, 1-27. ( 10.5194/egusphere-2022-1083) [DOI] [Google Scholar]
- 54.Schaetzl RJ. 2002. A spodosol-entisol transition in Northern Michigan. Soil Sci. Soc. Am. J. 66, 1272-1284. ( 10.2136/sssaj2002.1272) [DOI] [Google Scholar]
- 55.Heckman K, Lawrence CR, Harden JW. 2018. A sequential selective dissolution method to quantify storage and stability of organic carbon associated with Al and Fe hydroxide phases. Geoderma 312, 24-35. ( 10.1016/j.geoderma.2017.09.043) [DOI] [Google Scholar]
- 56.Khomo L, Chadwick OA, Rogers K, Trumbore SE. 2012. Short and long-term controls of SOM dynamics in a South African Landscape.14, 7193. [Google Scholar]
- 57.Bishop TFA, McBratney AB, Laslett GM. 1999. Modelling soil attribute depth functions with equal-area quadratic smoothing splines. Geoderma 91, 27-45. ( 10.1016/S0016-7061(99)00003-8) [DOI] [Google Scholar]
- 58.Zhang Y, Xiao X, Wu X, Zhou S, Zhang G, Qin Y, Dong J.. 2017. A global moderate resolution dataset of gross primary production of vegetation for 2000–2016. Sci. Data 4, 170165. ( 10.1038/sdata.2017.165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant KE, Hilton RG, Galy VV. 2023. Global patterns of radiocarbon depletion in subsoil linked to rock-derived organic carbon. Geochem. Perspect. Lett. 25, 36-40. ( 10.7185/geochemlet.2312) [DOI] [Google Scholar]
- 60.Vitousek PM, Farrington H. 1997. Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37, 63-75. ( 10.1023/A:1005757218475) [DOI] [Google Scholar]
- 61.Khomo L, Hartshorn AS, Rogers KH, Chadwick OA. 2011. Impact of rainfall and topography on the distribution of clays and major cations in granitic catenas of southern Africa. CATENA 87, 119-128. ( 10.1016/j.catena.2011.05.017) [DOI] [Google Scholar]
- 62.Khomo L, Bern CR, Hartshorn AS, Rogers KH, Chadwick OA. 2013. Chemical transfers along slowly eroding catenas developed on granitic cratons in southern Africa. Geoderma 202–203, 192-202. ( 10.1016/j.geoderma.2013.03.023) [DOI] [Google Scholar]
- 63.Hemingway JD. 2017. Rampedpyrox: open-source tools for thermoanalytical data analysis, 2016. (http://pypi.python.org/pypi/rampedpyrox) [Google Scholar]
- 64.Jiménez-Morillo NT, Almendros G, De la Rosa JM, Jordán A, Zavala LM, Granged AJP, González-Pérez JA. 2020. Effect of a wildfire and of post-fire restoration actions in the organic matter structure in soil fractions. Sci. Total Environ. 728, 138715. ( 10.1016/j.scitotenv.2020.138715) [DOI] [PubMed] [Google Scholar]
- 65.Steinhof A, Altenburg M, Machts H. 2017. Sample preparation at the Jena 14C laboratory. Radiocarbon 59, 815-830. ( 10.1017/RDC.2017.50) [DOI] [Google Scholar]
- 66.Schuur EAG, Trumbore SE, Druffel ERM. 2016. Radiocarbon and Climate Change: Mechanisms, Applications and Laboratory Techniques. Cham, Switzerland: Springer.
- 67.Wendeberg M, Richter JM, Rothe M, Brand WA. 2013. Jena Reference Air Set (JRAS): a multi-point scale anchor for isotope measurements of CO2 in air. Atmos. Meas. Tech. 6, 817-822. ( 10.5194/amt-6-817-2013) [DOI] [Google Scholar]
- 68.Saenger A, Cécillon L, Poulenard J, Bureau F, De Daniéli S, Gonzalez JM, Brun JJ. 2015. Surveying the carbon pools of mountain soils: a comparison of physical fractionation and Rock-Eval pyrolysis. Geoderma 241–242, 279-88. ( 10.1016/j.geoderma.2014.12.001) [DOI] [Google Scholar]
- 69.Tissot BP, du Petrole ENS, Welte DH. 1978. Petroleum formation and occurrence. A new approach to oil and gas exploration. [Book in German]. Berlin; Heidelberg: Springer. [Google Scholar]
- 70.Sebag D et al. 2016. Dynamics of soil organic matter based on new Rock-Eval indices. Geoderma 284, 185-203. ( 10.1016/j.geoderma.2016.08.025) [DOI] [Google Scholar]
- 71.Plante AF, Fernández JM, Leifeld J. 2009. Application of thermal analysis techniques in soil science. Geoderma 153, 1-10. ( 10.1016/j.geoderma.2009.08.016) [DOI] [Google Scholar]
- 72.Grant KE, Galy VV, Chadwick OA, Derry LA. 2019. Thermal oxidation of carbon in organic matter rich volcanic soils: insights into SOC age differentiation and mineral stabilization. Biogeochemistry 144, 291-304. ( 10.1007/s10533-019-00586-1) [DOI] [Google Scholar]
- 73.Kučerík J, Tokarski D, Demyan MS, Merbach I, Siewert C. 2018. Linking soil organic matter thermal stability with contents of clay, bound water, organic carbon and nitrogen. Geoderma 316, 38-46. ( 10.1016/j.geoderma.2017.12.001) [DOI] [Google Scholar]
- 74.Vogel C, Mueller CW, Höschen C, Buegger F, Heister K, Schulz S, Schloter M, Kögel-Knabner I. et al. 2014. Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat. Commun. 5, 1-7. ( 10.1038/ncomms3947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanderman J, Maddern T, Baldock J. 2014. Similar composition but differential stability of mineral retained organic matter across four classes of clay minerals. Biogeochemistry 121, 409-424. ( 10.1007/s10533-014-0009-8) [DOI] [Google Scholar]
- 76.Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ. 2015. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 8, 776-779. ( 10.1038/ngeo2520) [DOI] [Google Scholar]
- 77.Liang C, Schimel JP, Jastrow JD. 2017. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105. ( 10.1038/nmicrobiol.2017.105) [DOI] [PubMed] [Google Scholar]
- 78.Lavallee JM, Soong JL, Cotrufo MF. 2020. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 26, 261-273. ( 10.1111/gcb.14859) [DOI] [PubMed] [Google Scholar]
- 79.Thompson A, Chadwick OA, Boman S, Chorover J. 2006. Colloid mobilization during soil iron redox oscillations. Environ. Sci. Technol. 40, 5743-5749. ( 10.1021/es061203b) [DOI] [PubMed] [Google Scholar]
- 80.Thompson A, Chadwick OA, Rancourt DG, Chorover J. 2006. Iron-oxide crystallinity increases during soil redox oscillations. Geochim. Cosmochim. Acta 70, 1710-1727. ( 10.1016/j.gca.2005.12.005) [DOI] [Google Scholar]
- 81.Eusterhues K, Rennert T, Knicker H, Kögel-Knabner I, Totsche KU, Schwertmann U. 2011. Fractionation of organic matter due to reaction with ferrihydrite: coprecipitation versus adsorption. Environ. Sci. Technol. 45, 527-533. ( 10.1021/es1023898) [DOI] [PubMed] [Google Scholar]
- 82.Tamrat WZ, Rose J, Grauby O, Doelsch E, Levard C, Chaurand P, Basile-Doelsch I. 2019. Soil organo-mineral associations formed by co-precipitation of Fe, Si and Al in presence of organic ligands. Geochim. Cosmochim. Acta 260, 15-28. ( 10.1016/j.gca.2019.05.043) [DOI] [Google Scholar]
- 83.Skjemstad JO, Waters AG, Hanna JV, Oades JM. 1992. Genesis of podzols on coastal dunes in southern Queensland. IV. Nature of the organic fraction as seen by 13C nuclear magnetic resonance spectroscopy. Soil Res. 30, 667-681. ( 10.1071/SR9920667) [DOI] [Google Scholar]
- 84.Kaiser K, Guggenberger G. 2000. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 31, 711-725. ( 10.1016/S0146-6380(00)00046-2) [DOI] [Google Scholar]
- 85.Sorge C, Schnitzer M, Leinweber P, Schulten HR. 1994. Molecular-chemical characterization of organic matter in whole soil and particle-size fractions of a spodosol by pyrolysis-field ionization mass spectrometry. Soil Sci. 158, 189-203. ( 10.1097/00010694-199409000-00005) [DOI] [Google Scholar]
- 86.Buurman P, Jongmans AG. 2005. Podzolisation and soil organic matter dynamics. Geoderma 125, 71-83. ( 10.1016/j.geoderma.2004.07.006) [DOI] [Google Scholar]
- 87.Rumpel C, Eusterhues K, Kögel-Knabner I. 2010. Non-cellulosic neutral sugar contribution to mineral associated organic matter in top- and subsoil horizons of two acid forest soils. Soil Biol. Biochem. 42, 379-382. ( 10.1016/j.soilbio.2009.11.004) [DOI] [Google Scholar]
- 88.Langier-Kuźniarowa A. 2001. Thermal analysis of organo-clay complexes. In Organo-clay complexes and interactions (eds Yariv S, H Cross), pp. 273-344. New York, NY: Marcel Dekker. [Google Scholar]
- 89.Yariv S. 2003. Differential thermal analysis (DTA) in the study of thermal reactions of organo-clay complexes. In Natural and laboratory-simulated thermal geochemical processes (ed. Ikan R), pp. 253-296. Dordrecht: Springer. ( 10.1007/978-94-017-0111-2_8) [DOI] [Google Scholar]
- 90.Siewert C, Demyan MS, Kučerík J. 2012. Interrelations between soil respiration and its thermal stability. J. Therm. Anal. Calorim. 110, 413-419. ( 10.1007/s10973-011-2099-z) [DOI] [Google Scholar]
- 91.Kučerík J, Čtvrtníčková A, Siewert C. 2013. Practical application of thermogravimetry in soil science: Part 1. Thermal and biological stability of soils from contrasting regions. J. Therm. Anal. Calorim. 113, 1103-1111. ( 10.1007/s10973-012-2849-6) [DOI] [Google Scholar]
- 92.Schulten HR, Leinweber P. 1999. Thermal stability and composition of mineral-bound organic matter in density fractions of soil. Eur. J. Soil Sci. 50, 237-248. ( 10.1046/j.1365-2389.1999.00241.x) [DOI] [Google Scholar]
- 93.Dell'Abate MT, Benedetti A, Brookes PC. 2003. Hyphenated techniques of thermal analysis for characterisation of soil humic substances. J. Sep. Sci. 47, 367-371. [Google Scholar]
- 94.Plante AF, Pernes M, Chenu C. 2005. Changes in clay-associated organic matter quality in a C depletion sequence as measured by differential thermal analyses. Geoderma 129, 186-199. ( 10.1016/j.geoderma.2004.12.043) [DOI] [Google Scholar]
- 95.Siewert C. 2004. Rapid screening of soil properties using thermogravimetry. Soil Sci. Soc. Am. J. 68, 1656-1661. ( 10.2136/sssaj2004.1656) [DOI] [Google Scholar]
- 96.Wattel-Koekkoek EJW, Van Genuchten PPL, Buurman P, Van Lagen B. 2001. Amount and composition of clay-associated soil organic matter in a range of kaolinitic and smectitic soils. Geoderma 99, 27-49. ( 10.1016/S0016-7061(00)00062-8) [DOI] [Google Scholar]
- 97.Wattel-Koekkoek EJW, Buurman P, Van Der Plicht J, Wattel E, Van Breemen N. 2003. Mean residence time of soil organic matter associated with kaolinite and smectite. Eur. J. Soil Sci. 54, 269-278. ( 10.1046/j.1365-2389.2003.00512.x) [DOI] [Google Scholar]
- 98.Schimel J, Becerra CA, Blankinship J. 2017. Estimating decay dynamics for enzyme activities in soils from different ecosystems. Soil Biol. Biochem. 114, 5-11. ( 10.1016/j.soilbio.2017.06.023) [DOI] [Google Scholar]
- 99.Liang C, Amelung W, Lehmann J, Kästner M. 2019. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 25, 3578-3590. ( 10.1111/gcb.14781) [DOI] [PubMed] [Google Scholar]
- 100.Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P. 2008. Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 171, 61-82. [cited 2012 Oct 30]. ( 10.1002/jpln.200700048) [DOI] [Google Scholar]
- 101.Bingham AH, Cotrufo MF. 2016. Organic nitrogen storage in mineral soil: implications for policy and management. Sci. Total Environ. 551–552, 116-126. ( 10.1016/j.scitotenv.2016.02.020) [DOI] [PubMed] [Google Scholar]
- 102.Jones DL, Magthab EA, Gleeson DB, Hill PW, Sánchez-Rodríguez AR, Roberts P, Ge T, Murphy DV. 2018. Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol. Biochem. 117, 72-82. ( 10.1016/j.soilbio.2017.10.024) [DOI] [Google Scholar]
- 103.Meyer N, Welp G, Rodionov A, Borchard N, Martius C, Amelung W. 2018. Nitrogen and phosphorus supply controls soil organic carbon mineralization in tropical topsoil and subsoil. Soil Biol. Biochem. 119, 152-161. ( 10.1016/j.soilbio.2018.01.024) [DOI] [Google Scholar]
- 104.Peixoto L, Elsgaard L, Rasmussen J, Olesen JE. 2021. Nitrogen and phosphorus co-limit mineralization of labile carbon in deep subsoil. Eur. J. Soil Sci. 72, 1879-1884. ( 10.1111/ejss.13083) [DOI] [Google Scholar]
- 105.Horbett TA, Brash JL. 1995. Proteins at interfaces II: fundamentals and applications. Washington, DC: ACS Publications. [Google Scholar]
- 106.Kleber M, Sollins P, Sutton R. 2007. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85, 9-24. ( 10.1007/s10533-007-9103-5) [DOI] [Google Scholar]
- 107.Sollins P, Swanston C, Kleber M, Filley T, Kramer M, Crow S, Caldwell BA, Lajtha K, Bowden R. 2006. Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol. Biochem. 38, 3313-3324. [cited 2013 Jun 3]. ( 10.1016/j.soilbio.2006.04.014) [DOI] [Google Scholar]
- 108.Kopittke PM, Hernandez-Soriano MC, Dalal RC, Finn D, Menzies NW, Hoeschen C, Mueller CW. 2018. Nitrogen-rich microbial products provide new organo-mineral associations for the stabilization of soil organic matter. Glob. Chang. Biol. 24, 1762-1770. ( 10.1111/gcb.14009) [DOI] [PubMed] [Google Scholar]
- 109.Gao J, Mikutta R, Jansen B, Guggenberger G, Vogel C, Kalbitz K. 2020. The multilayer model of soil mineral–organic interfaces—a review. J. Plant Nutr. Soil Sci. 183, 27-41. ( 10.1002/jpln.201900530) [DOI] [Google Scholar]
- 110.Wilson MA, Wada K, Wada SI, Kakuto Y. 1988. Thermal transformations of synthetic allophane and imogolite as revealed by nuclear magnetic resonance. Clay Miner. 23, 175-190. ( 10.1180/claymin.1988.023.2.05) [DOI] [Google Scholar]
- 111.Koarashi J, Hockaday WC, Masiello CA, Trumbore SE. 2012. Dynamics of decadally cycling carbon in subsurface soils. J. Geophys. Res. Biogeosci. 117, 1-13. ( 10.1029/2012JG002034) [DOI] [Google Scholar]
- 112.Crowther TW et al. 2016. Quantifying global soil carbon losses in response to warming. Nature 540, 104-108. ( 10.1038/nature20150) [DOI] [PubMed] [Google Scholar]
- 113.Lawrence C, Harden J, Maher K. 2014. Modeling the influence of organic acids on soil weathering. Geochim. Cosmochim. Acta 139, 487-507. ( 10.1016/j.gca.2014.05.003) [DOI] [Google Scholar]
- 114.Chamley H. 1989. Clay formation through weathering. In Clay sedimentology, pp. 21-50. Berlin, Germany: Springer Berlin Heidelberg. [Google Scholar]
- 115.Reddy KR, Feijtel TC, Patrick WH. 1986. Effect of soil redox conditions on microbial oxidation of organic matter. In The role of organic matter in modern agriculture (eds Chen Y, Avnimelech Y), pp. 117-156. Dordrecht: Martinus Nijhoff Publishers. ( 10.1007/978-94-009-4426-8_6) [DOI] [Google Scholar]
- 116.Chen C, Hall SJ, Coward E, Thompson A. 2020. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 11, 1-13. ( 10.1038/s41467-020-16071-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stoner S. 2023. Code for: ShaneStoner/BGS\_ThermalFractionation: Publication, v1.0 (publication). Zenodo. ( 10.5281/zenodo.7998659) [DOI]
- 118.Stoner S, Trumbore SE, González-Pérez JA, Schrumpf M, Sierra CA, Hoyt AM, Chadwick O, Doetterl S. 2023. Relating mineral–organic matter stabilization mechanisms to carbon quality and age distributions using ramped thermal analysis. Figshare. ( 10.6084/m9.figshare.c.6845635) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stoner S. 2023. Code for: ShaneStoner/BGS\_ThermalFractionation: Publication, v1.0 (publication). Zenodo. ( 10.5281/zenodo.7998659) [DOI]
Data Availability Statement
Data and code will be maintained in this repository: https://github.com/ShaneStoner/Mineralogy14C. It is also available with a DOI via Zenodo: https://zenodo.org/record/7998659 [117].
The data are provided in electronic supplementary material [118].