Abstract
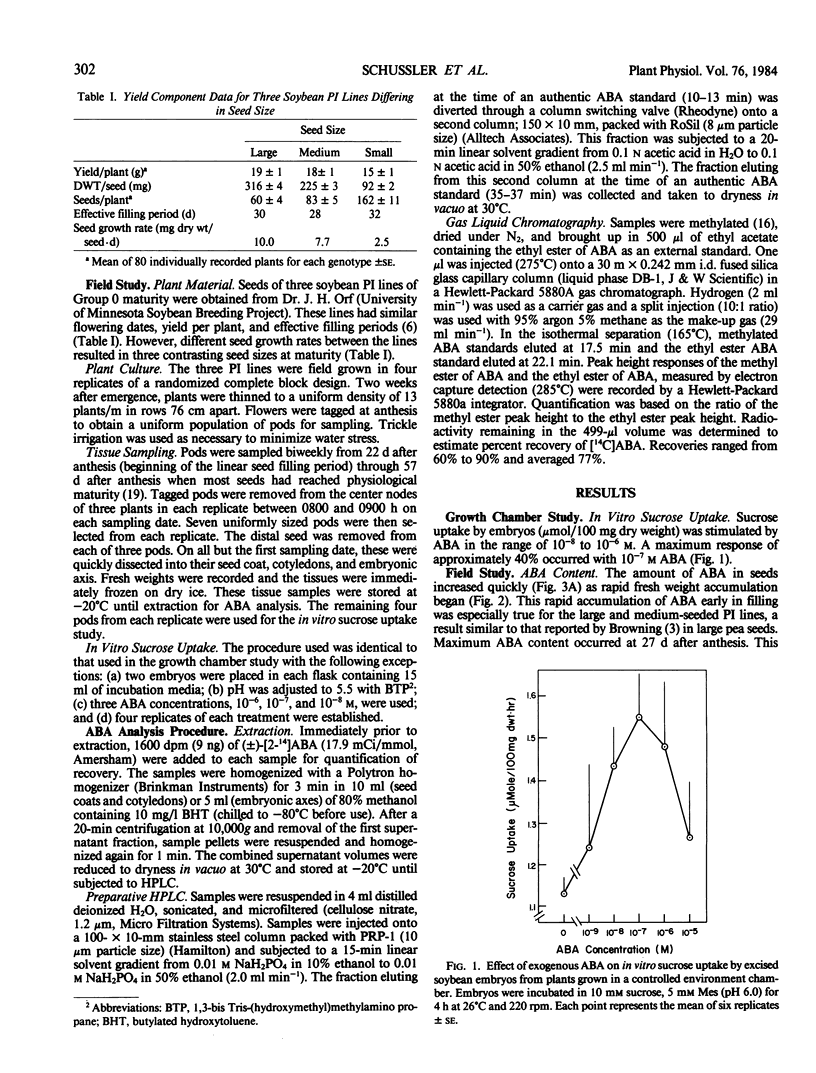
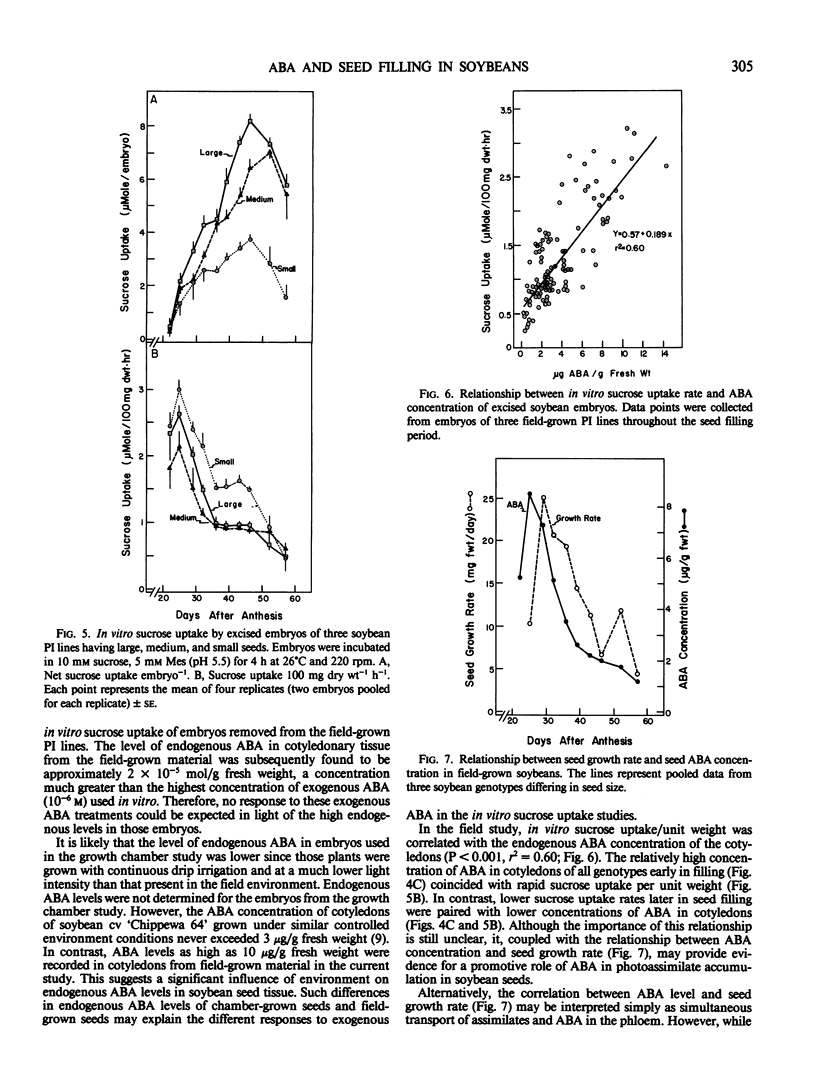
The effect of exogenous abscisic acid (ABA) on the rate of sucrose uptake by soybean (Glycine max L. Merr.) embryos was evaluated in an in vitro system. In addition, the concentrations of endogenous ABA in seeds of three soybean Plant Introduction (PI) lines, differing in seed size, were commpared to their seed growth rates. ABA (10−7 molar) stimulated in vitro sucrose uptake in soybean (cv `Clay') embryos removed from plants grown in a controlled environment chamber, but not in embryos removed from field-grown plants of the three PI lines. However, the concentration of ABA in seeds of the three field-grown PI lines correlated well with their in situ seed growth rates and in vitro [14C] sucrose uptake rates.
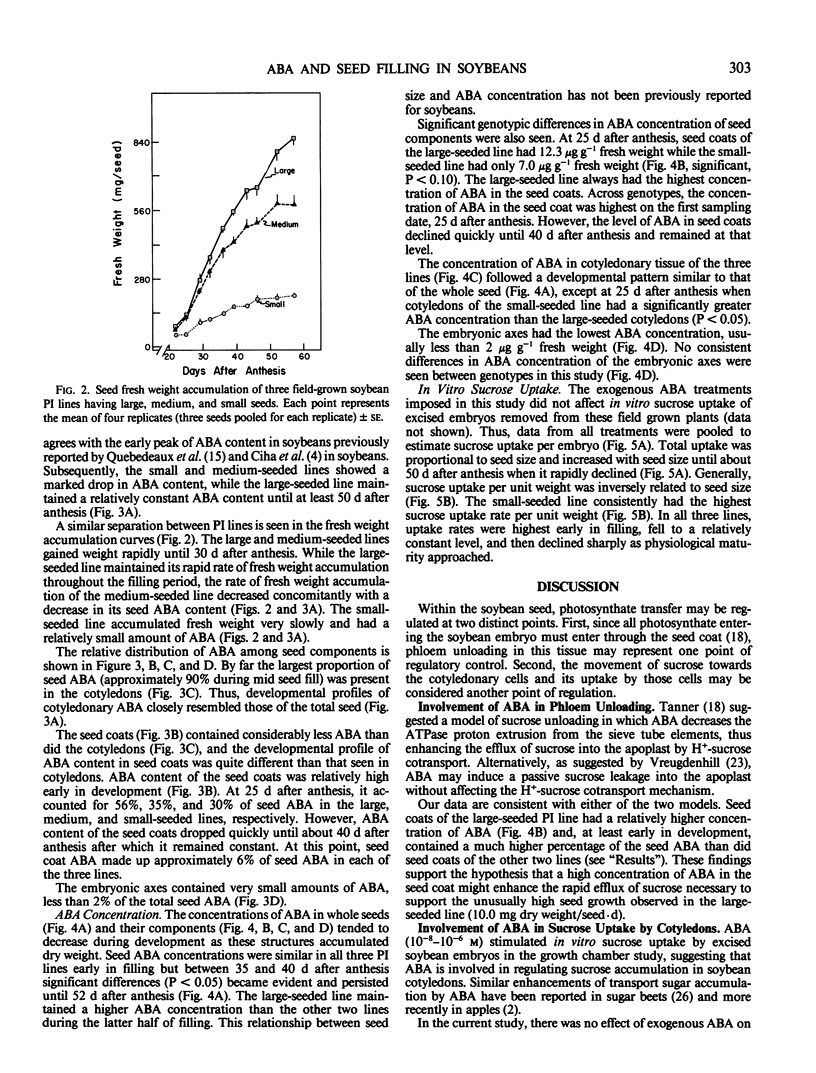
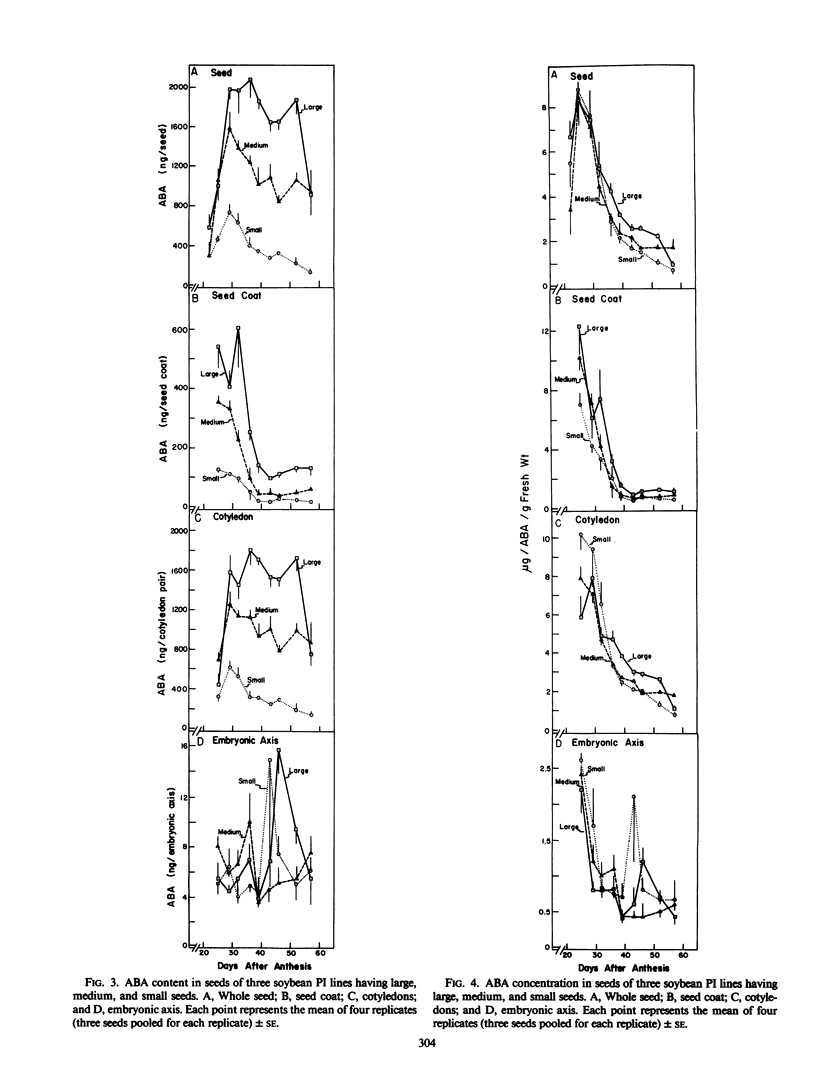
Across genotypes, the concentration of ABA in seeds peaked at 8.5 micrograms per gram fresh weight, corresponding to the time of most rapid seed growth rate, and declined to 1.2 micrograms per gram at physiological maturity. Seeds of the large-seeded genotype maintained an ABA concentration at least 50% greater than that of the small-seeded genotype throughout the latter half of seed filling. A higher concentration of ABA was found in seed coats and cotyledons than in embryonic axes. Seed coats of the large-seeded genotype always had a higher concentration of ABA than seed coats of the small-seeded line. It is suggested that this higher concentration of ABA in seed coats of the large-seeded genotype stimulates sucrose unloading into the seed coat apoplast and that ABA in cotyledons may enhance sucrose uptake by the cotyledons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coombe B. G., Hale C. R. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiol. 1973 Apr;51(4):629–634. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F. C. Abscisic Acid Accumulation in Developing Seeds of Phaseolus vulgaris L. Plant Physiol. 1979 Mar;63(3):552–556. doi: 10.1104/pp.63.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Sweetser P. B., Rowell J. C. Abscisic Acid Levels in Soybean Reproductive Structures during Development. Plant Physiol. 1976 Sep;58(3):363–366. doi: 10.1104/pp.58.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Abscisic Acid Translocation and Metabolism in Soybeans following Depodding and Petiole Girdling Treatments. Plant Physiol. 1981 Apr;67(4):774–779. doi: 10.1104/pp.67.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Kinetics of C-photosynthate uptake by developing soybean fruit. Plant Physiol. 1980 May;65(5):975–979. doi: 10.1104/pp.65.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Morphology and ultrastructure of maternal seed tissues of soybean in relation to the import of photosynthate. Plant Physiol. 1981 May;67(5):1016–1025. doi: 10.1104/pp.67.5.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]


