Abstract
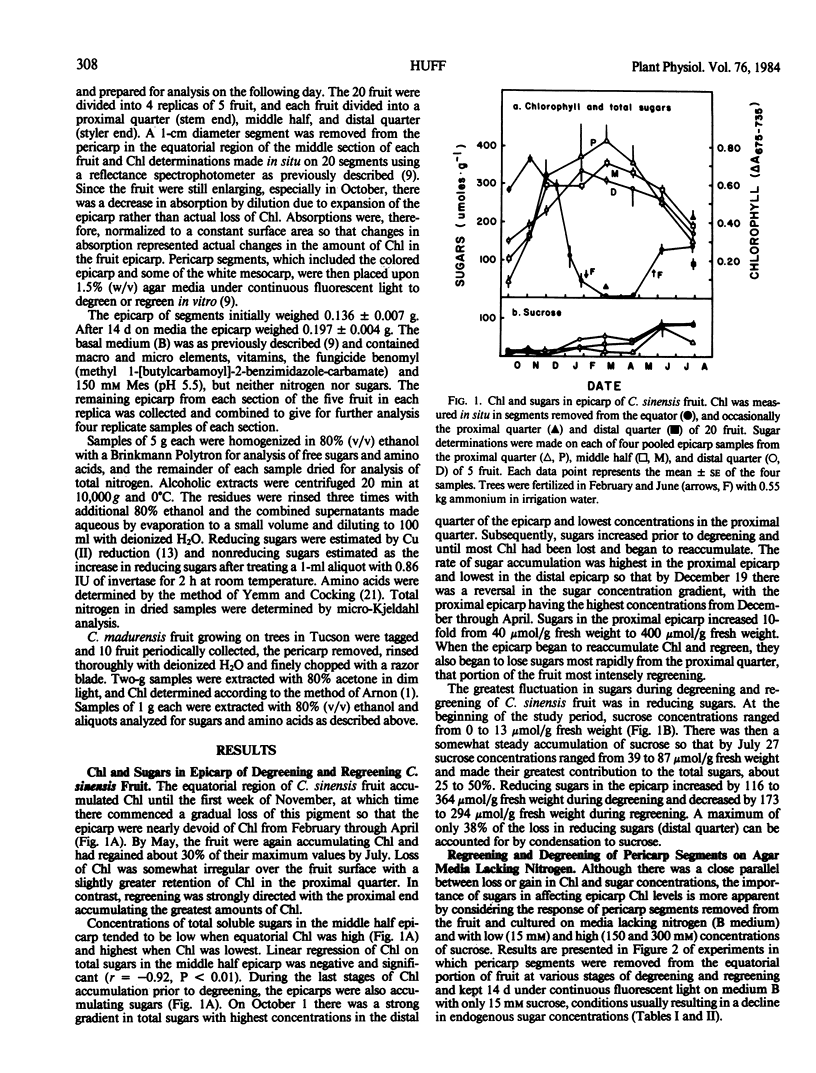
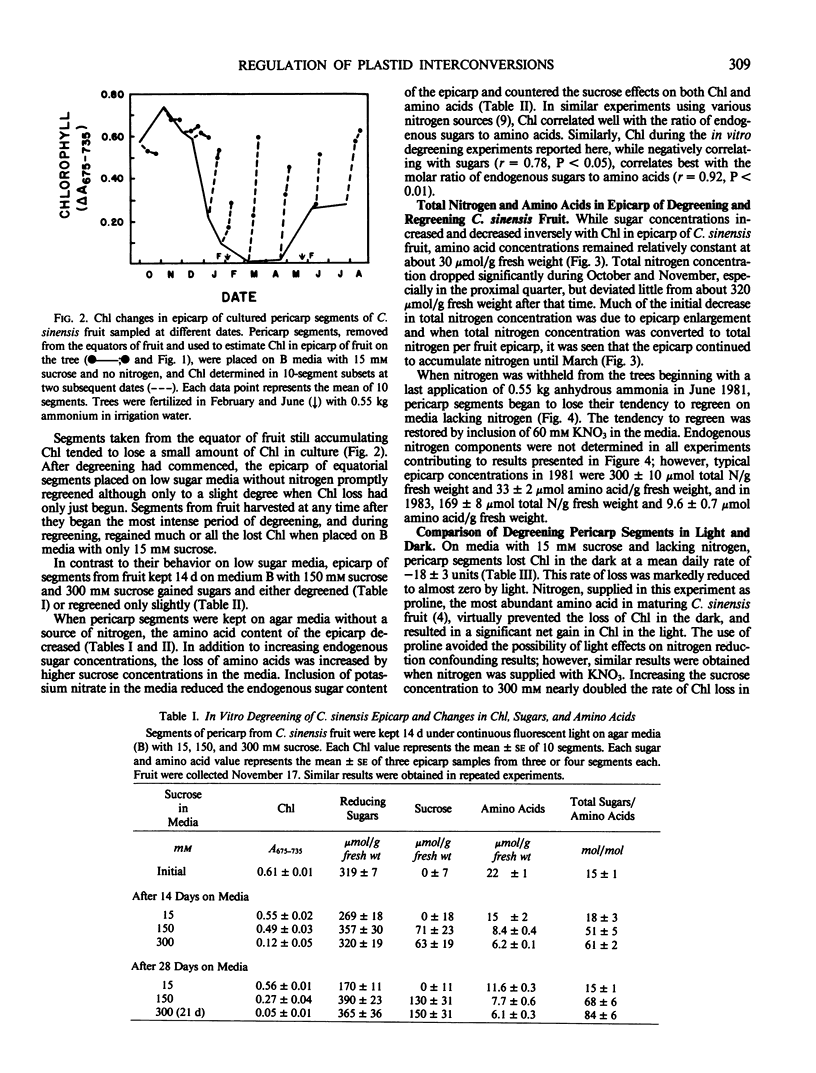
Seasonal transformations between chloroplasts and chromoplasts, as measured by changes in chlorophyll content, in the epicarp of degreening and regreening Citrus sinensis (L.) Osbeck cv Valencia fruit closely parallelled the accumulation and later loss of soluble sugars. At any stage of development, reversing the relative soluble sugar content in the epicarp by culturing pericarp segments on agar media with low (15 millimolar) or high (150 millimolar) sucrose concentrations reversed the direction of change in chlorophyll content. Fruit of C. madurensis Lour., which mature year around and do not regreen, also accumulated soluble sugars in the pericarp as degreening was initiated.
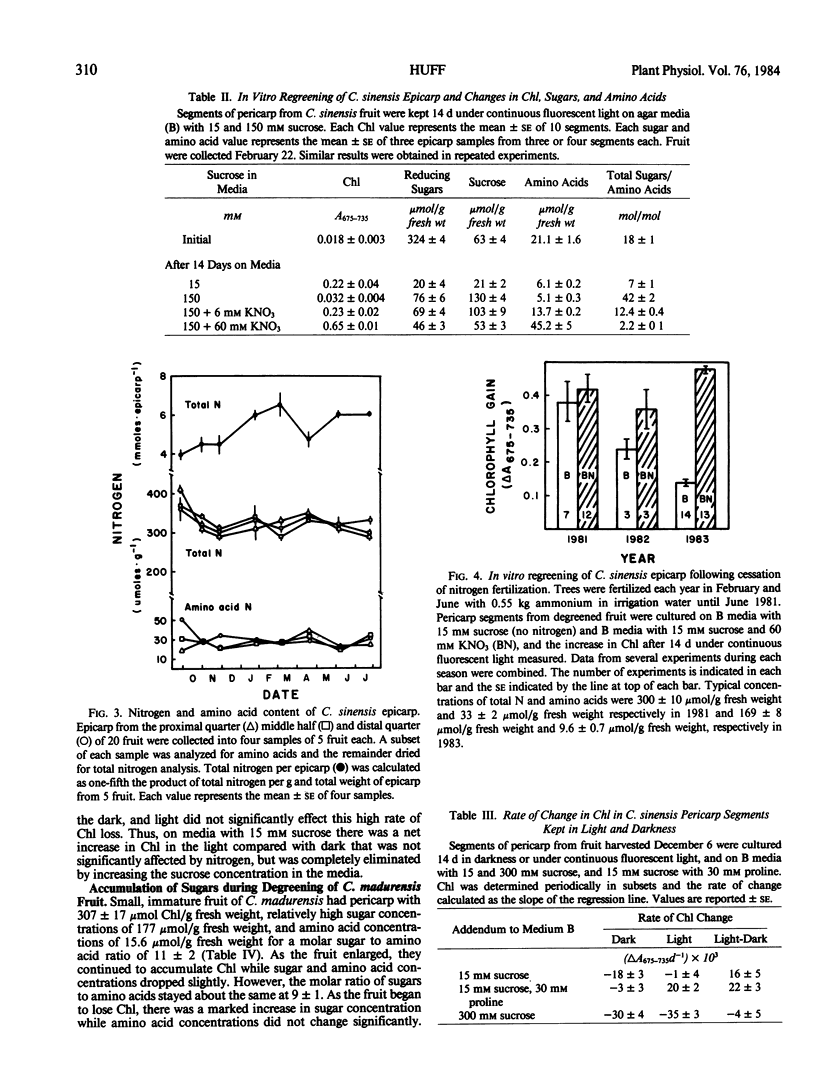
The epicarp of C. sinensis fruit accumulated nitrogen, but total nitrogen concentrations and amino acid concentrations changed little, during degreening and regreening of C. sinensis fruit. Cessation of nitrogen fertilization reduced the tendency of pericarp segments to regreen in vitro during subsequent years, but regreening tendency was restored by inclusion of KNO3 in the media.
It is concluded that chloroplasts become chromoplasts and citrus fruit degreen partially in response to the accumulation of sugars in the epicarp and that the reverse transformation accompanying regreening of certain citrus species occurs when accumulated sugars disappear. Change in nitrogen flux to the fruit is probably not a factor in regulating seasonal transformations, but an abundance of nitrogen in the epicarp diminishes the effects of high sugar concentrations in inducing transformation of chloroplasts to chromoplasts, thereby retarding degreening and promoting regreening.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. D., Parker E. R. WEEKLY ABSORPTION OF NITRATE BY YOUNG, BEARING ORANGE TREES GROWING OUT OF DOORS IN SOLUTION CULTURES. Plant Physiol. 1942 Jul;17(3):366–376. doi: 10.1104/pp.17.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrum M. A., Schwartzbach S. D. Nutritional Regulation of Organelle Biogenesis in Euglena: REPRESSION OF CHLOROPHYLL AND NADP-GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE SYNTHESIS. Plant Physiol. 1980 Feb;65(2):382–386. doi: 10.1104/pp.65.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais J. P., De Wit J. L., Quicke G. V. A critical examination of the Nelson-Somogyi method for the determination of reducing sugars. Anal Biochem. 1966 Jun;15(3):373–381. doi: 10.1016/0003-2697(66)90098-4. [DOI] [PubMed] [Google Scholar]


