Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned coprimary or secondary analyses are not yet available. Clinical trial updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We present the final analysis of the phase III noninferiority, randomized ShortHER trial comparing 9 weeks versus 1 year of adjuvant trastuzumab with chemotherapy in patients with human epidermal growth factor receptor 2–positive (HER2+) early breast cancer (BC). Women with HER2+ BC were randomly assigned to anthracycline-taxane combinations plus 1-year trastuzumab (arm A, long) or 9-week trastuzumab (arm B, short). Here, we report the second coprimary end point overall survival (OS), updated disease-free survival (DFS), and outcomes according to hormone receptor status, age, and nodal status. At a median follow-up of 9 years, 10-year DFS is 77% versus 78% in the long versus short arm, respectively. Ten-year OS is 89% versus 88% in the long versus short arm, respectively. 10-year DFS rates in the long versus short arm according to nodal status are N0 81% versus 85%; N1-3 77% versus 79%; and N4+ 63% versus 53%. Ten-year OS rates in long versus short arm according to nodal status are N0 89% versus 95%%; N1-3 92% versus 89%; and N4+ 84% versus 64%. The updated analysis of the ShortHER trial shows that 1-year trastuzumab is the standard treatment for patients with HER2+ early BC as noninferiority cannot be claimed. However, numerically, the differences for the patients at low or intermediate risk (N0/N1-3) is negligible, while patients with N4+ have a clear benefit with 1-year trastuzumab.
INTRODUCTION
The multicenter, investigator-driven, phase III randomized noninferiority ShortHER study compared 9 weeks (short arm) versus 1 year (long arm) of adjuvant trastuzumab combined with chemotherapy in patients with human epidermal growth factor receptor 2–positive (HER2+) early breast cancer (BC). The first primary end point was the event-driven analysis of disease-free survival (DFS). The HR was 1.13 (90% CI, 0.89 to 1.42) and the noninferiority could not be claimed as the upper border of CI crossed the upper limit of 1.29 chosen as the noninferiority margin.1
In the present paper, we report the final analysis, including the coprimary end point of overall survival (OS), of the ShortHER trial. The planned event-driven analysis for DFS of this study has been previously published.1
METHODS
Study Design
The ShortHER trial was a multicenter, investigator-driven, phase III randomized noninferiority study conducted in Italy within the frame of a clinical research program launched by AIFA (Agenzia Italiana del Farmaco [Italian Medicines Agency]) to improve the efficiency of the National Health System. The trial was approved by local ethical committees, and conducted in compliance with the principles of Good Clinical Practice and the Declaration of Helsinki. An independent data monitoring committee monitored the study. Patients provided written informed consent before enrollment.
Participants
Women age 18-75 years with surgically resected, HER2-positive BC were eligible. Women had to have node positivity, or in case of node negativity, at least one additional risk factor: pT size >2 cm, grade 3, lymphovascular invasion, Ki-67 >20%, age younger than 35 years, or hormone receptor–negative (estrogen receptor and progesterone receptor <10%).
Procedures
Eligible patients were stratified according to nodal status and hormone receptor status, and randomly assigned through a web-based system. Chemotherapy in arm A (long) consisted of AC (doxorubicin 60 mg/sqm + cyclophosphamide 600 mg/sqm) or EC (epidoxorubicin 90 mg/sqm + cyclophosphamide 600 mg/sqm) administered once every 3 weeks for four courses followed by paclitaxel 175 mg/sqm or docetaxel 100 mg/sqm once every 3 weeks for four courses. Trastuzumab was administered once every 3 weeks for 18 doses, starting with the first taxane dose (8 mg/kg loading dose at first cycle, and 6 mg/kg thereafter). Chemotherapy in arm B (short) consisted of docetaxel 100 mg/sqm once every 3 weeks for three courses followed by FEC (fluorouracil 600 mg/sqm, epidoxorubicin 60 mg/sqm, and cyclophosphamide 600 mg/sqm) administered once every 3 weeks for three courses. Trastuzumab was administered once per week for 9 weeks, starting concomitantly with docetaxel (4 mg/kg loading dose at first week, and 2 mg/kg thereafter). When indicated, radiation therapy and hormonal therapy according to local standard were carried out at the end of chemotherapy.
The primary end point was DFS with OS as a coprimary end point.
Statistical Analyses
This study is designed to assess whether a shorter trastuzumab administration is noninferior to the long one in respect with DFS. An HR <1.29 was set as a noninferiority margin. After amendment because of low recruitment, the sample size was reduced to 1,252 patients with 198 events with a power of 0.56. The Bayesian analysis was planned at the beginning of the study.2
RESULTS
Study design and patient characteristics have been published previously.1 In brief, 1,254 patients were randomly assigned to arms A (long, 627) and B (short, 627; Table 1).
TABLE 1.
Patient Baseline Characteristics
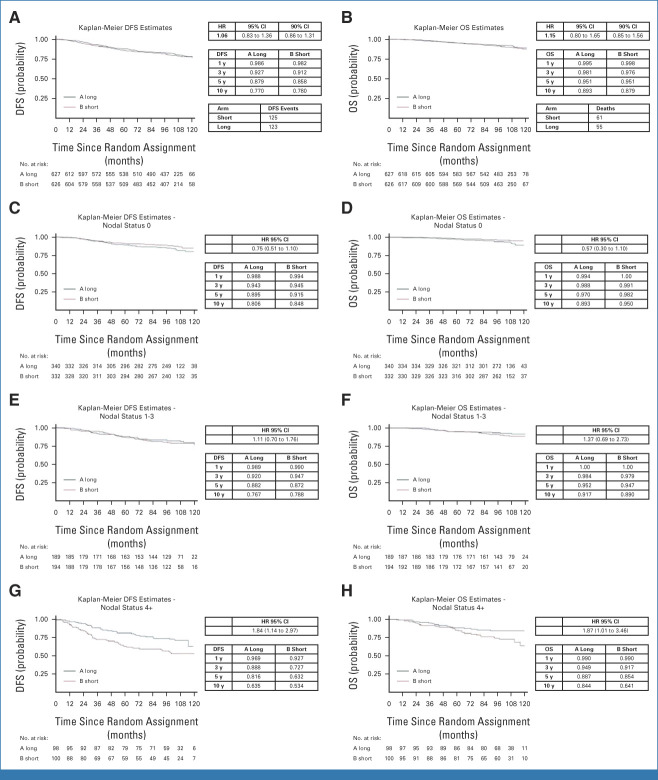
In this report for the coprimary end point of OS, the median follow-up is 9 years, with 248 DFS events and 116 deaths. The DFS and OS curves by treatment arm are shown in Figures 1A and 1B, respectively. The 10-year DFS is 77% in the long arm and 78% in the short arm (HR, 1.06; 90% CI, 0.86 to 1.31); the 10-year OS is 89% in the long arm and 88% in the short arm (HR, 1.15[90% CI, 0.85 to 1.56]).
FIG 1.
Kaplan-Meier curves. (A) DFS in the overall population. (B) OS in the overall population. (C) DFS in node-negative patients (N0). (D) OS in node-negative patients. (E) DFS in the N1 cohort. (F) OS in the N1 cohort. (G) DFS in patients with N ≥4 (N4+). (H) OS in patients with N ≥4 (N4+). DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
According to the Bayesian analysis, which is based on observed data at 10 years, assuming a noninformative previous distribution, the posterior probability that the short arm is not inferior to the long one is 93.2, as shown in Appendix Figure A1 (online only).
Subgroup analysis according to age, stage, nodal status, and hormone receptor status is shown in Figure 2.
FIG 2.
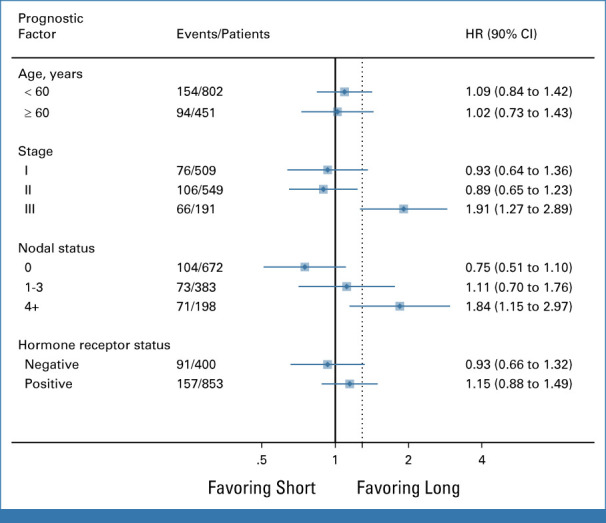
Subgroup analysis by age (<60 years v ≥60 years), stage (I v II v III), nodal status (N0 v N1 v N ≥4), and hormone receptor status (negative v positive). HR, hazard ratio.
According to nodal status, the 10-year DFS and OS rates are N0 patients 81% and 89% in the long, 85% and 95% in the short arm, respectively; N1-3 patients 77% and 92% in the long, 79% and 89% in the short arm, respectively; and N4+ patients 63% and 84% in the long, 53% and 64% in the short arm, respectively (Figs 1C, 1E, 1G for DFS and Figs 1D, 1F, 1H for OS).
DISCUSSION
After the impressive results of the pivotal trials showing that 1-year trastuzumab was able to reduce the risk of relapse and death by 34% and 33%,3 respectively, the new challenges were how to improve over these results and how to reduce the biological and financial burden associated with 1-year trastuzumab. For high-risk patients, new anti-HER2 therapies have been successful to further improve prognosis.4-6 For patients at lower risk, two different strategies have been pursued: de-escalated chemotherapy7-10 or shorter duration of trastuzumab. In particular, the shorter trastuzumab duration has been investigated through phase III randomized trials on the basis of a noninferiority design. The PHARE11 and the PERSEPHONE trials12 have compared 6-month trastuzumab to standard 1-year duration. The SOLD trial,13 similarly to our ShortHER, has compared 9 weeks of trastuzumab to 1-year duration. With the exception of the PERSEPHONE study,12 all these trials1,11,13,14 have failed the primary end point of noninferiority, although, numerically, the differences between the 1-year arm and the experimental arms were minimal or very limited. Recently, these trials have been meta-analyzed and a noninferiority has been demonstrated for 6-month trastuzumab.15 Despite these data, shorter trastuzumab administration is not recommended for any risk category of patients with HER2+ early BC.
In the present paper, we report, at a median follow-up of 9 years, the second coprimary end point OS and updated DFS, for the entire patient population and for patients at different risk according to the nodal status. At 10 years, the OS is 89% with 1-year trastuzumab and 88% with 9-week trastuzumab; the DFS is 77% with 1-year trastuzumab and 78% with 9-week trastuzumab. According to the risk categories on the basis of the nodal status, DFS and OS are very similar for N0 and N1-3 patients, while there is a clear advantage in favor of 1-year trastuzumab for 4+ positive nodes.
Importantly, it should be acknowledged that the ShortHER trial has limited power (56%) to detect clinically meaningful difference, as a result of limited number of accrual (half of the number of patients that was initially established).
Do these data still have an impact on the management of patients with HER2+ early BC today when guidelines recommend once per week paclitaxel plus 1-year trastuzumab for low-risk patients, non–anthracycline-based regimens plus trastuzumab for 1 year for intermediate-risk patients, and non–anthracycline-based regimens plus 1-year dual blockade with trastuzumab and pertuzumab for node-positive disease or neoadjuvant chemotherapy plus dual blockade (with the opportunity to switch to trastuzumab emtansine) for high-risk patients?
The answer is probably no if all the patients with HER2+ disease can receive 1-year trastuzumab. Unfortunately, this is not the case. In Western countries, a proportion of patients has to stop trastuzumab before 1 year for a variety of reasons (up to 15% of the patients in pivotal trials) or cannot start the treatment for financial reasons.16 The figure is even more dismal if we look worldwide: according to a recently published survey on essential anticancer drugs aimed to finalize the WHO Essential Medicine List, trastuzumab is available for only 15% of the patients living in low-middle–income countries, while access to the drug would imply catastrophic expenses for 68% of the patients.17
In conclusion, at a median follow-up of 9 years, 9 weeks of trastuzumab can guarantee 10-year DFS and OS rates of 78% and 88%, respectively, which, statistically, cannot be claimed as noninferior to the 77% DFS and 89% OS of 1-year trastuzumab. Nevertheless, these long-term data can reassure clinicians in case of early discontinuation of adjuvant trastuzumab (for any reason) in patients at low risk. Importantly, these updated results might also have a role in facilitating access to a less-expensive treatment to the thousands of patients worldwide who cannot afford the cost of 1-year trastuzumab.
APPENDIX
FIG A1.
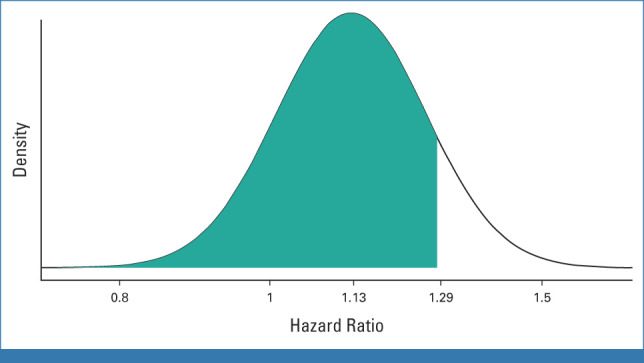
Bayesian analysis of posterior distribution of treatment on DFS. DFS, disease-free survival.
Pier F. Conte
Consulting or Advisory Role: Daiichi Sankyo/Lilly, Reveal Genomics, Gilead Sciences
Speakers' Bureau: Roche/Genentech, Novartis, AstraZeneca, Lilly, BMS
Research Funding: Merck KGaA (Inst)
Patents, Royalties, Other Intellectual Property: HER2Dx patent
Expert Testimony: AstraZeneca
Federico Piacentini
Expert Testimony: Gilead Sciences
Travel, Accommodations, Expenses: Novartis, Lilly, AstraZeneca/Daiichi Sankyo, GENTILI
Elisa Anselmi
Travel, Accommodations, Expenses: MSD Oncology
Alessio Schirone
Honoraria: Novartis, Daiichi Sankyo/Astra Zeneca, Lilly
Consulting or Advisory Role: Novartis, Daiichi Sankyo/Astra Zeneca, Pfizer
Antonino Musolino
Consulting or Advisory Role: Lilly, Eisai Europe, Seagen, Daiichi Sankyo/Astra Zeneca
Research Funding: Lilly, Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
Ornella Garrone
Honoraria: Eisai, Novartis, Pfizer, Lilly
Consulting or Advisory Role: EISAI, Seagen, AstraZeneca/Daiichi Sankyo, MSD, Gilead Sciences
Travel, Accommodations, Expenses: Daiichi Sankyo/Astra Zeneca, Ipsen
Anna Turletti
Honoraria: Novartis
Travel, Accommodations, Expenses: Lilly, gentili
Federica Miglietta
Consulting or Advisory Role: MSD, Astrazeneca
Speakers' Bureau: Pfizer, Gilead, Seagen, Novartis
Travel, Accommodations, Expenses: Novartis, Gilead
Maria Vittoria Dieci
Consulting or Advisory Role: Lilly, Novartis, Exact Sciences, Pfizer, Seagen, MSD, Gilead Sciences, Daiichi Sankyo, Roche
Patents, Royalties, Other Intellectual Property: Patent pending HER2DX licensed to University of Padova
Valentina Guarneri
Consulting or Advisory Role: Lilly, Novartis, MSD, Gilead Sciences, Eisai, Merck Serono, Olema Oncology, Exact Sciences
Speakers' Bureau: Novartis, Lilly, GlaxoSmithKline, Amgen, AstraZeneca
Research Funding: AstraZeneca (Inst), Roche (Inst), Novartis (Inst), Merck (Inst), BMS (Inst), Lilly (Inst), Merck KGaA (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Nerviano Medical Sciences (Inst), GlaxoSmithKline (Inst), Synthon (Inst)
Expert Testimony: Lilly
Travel, Accommodations, Expenses: Gilead/Forty Seven
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2017 ASCO Annual Meeting, Chicago, IL, June 4, 2017; and in part at the 2023 ASCO Annual Meeting, Chicago, IL, June 3, 2023.
SUPPORT
Supported by research grant from Agenzia Italiana del Farmaco (grant number FARM62MC97).
CLINICAL TRIAL INFORMATION
EUDRACT number: 2007-004326-25; NCI ClinicalTrials.gov identifier: NCT00629278
DATA SHARING STATEMENT
The data sets that support the findings of this study are not publicly available to protect patient privacy. The data will be available on reasonable request from the corresponding author: P.F.C., pierfranco.conte@unipd.it.
AUTHOR CONTRIBUTIONS
Conception and design: Pier F. Conte, Valentina Guarneri
Administrative support: Pier F. Conte
Provision of study materials or patients: Giancarlo Bisagni, Federico Piacentini, Samanta Sarti, Elisa Anselmi, Michele Aieta, Vittorio Gebbia, Antonino Musolino, Ornella Garrone, Valentina Guarneri
Collection and assembly of data: Pier F. Conte, Giancarlo Bisagni, Federico Piacentini, Samanta Sarti, Santino Minichillo, Elisa Anselmi, Michele Aieta, Vittorio Gebbia, Alessio Schirone, Antonino Musolino, Ornella Garrone, Alessandra Beano, Anita Rimanti, Francesco Giotta, Anna Turletti, Federica Miglietta, Maria Vittoria Dieci, Roberto Vicini
Data analysis and interpretation: Pier F. Conte, Michele Aieta, Antonino Musolino, Roberto Vicini, Sara Balduzzi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nine-Week Versus One-Year Trastuzumab for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: 10-Year Update of the ShortHER Phase III Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pier F. Conte
Consulting or Advisory Role: Daiichi Sankyo/Lilly, Reveal Genomics, Gilead Sciences
Speakers' Bureau: Roche/Genentech, Novartis, AstraZeneca, Lilly, BMS
Research Funding: Merck KGaA (Inst)
Patents, Royalties, Other Intellectual Property: HER2Dx patent
Expert Testimony: AstraZeneca
Federico Piacentini
Expert Testimony: Gilead Sciences
Travel, Accommodations, Expenses: Novartis, Lilly, AstraZeneca/Daiichi Sankyo, GENTILI
Elisa Anselmi
Travel, Accommodations, Expenses: MSD Oncology
Alessio Schirone
Honoraria: Novartis, Daiichi Sankyo/Astra Zeneca, Lilly
Consulting or Advisory Role: Novartis, Daiichi Sankyo/Astra Zeneca, Pfizer
Antonino Musolino
Consulting or Advisory Role: Lilly, Eisai Europe, Seagen, Daiichi Sankyo/Astra Zeneca
Research Funding: Lilly, Roche (Inst)
Travel, Accommodations, Expenses: Pfizer
Ornella Garrone
Honoraria: Eisai, Novartis, Pfizer, Lilly
Consulting or Advisory Role: EISAI, Seagen, AstraZeneca/Daiichi Sankyo, MSD, Gilead Sciences
Travel, Accommodations, Expenses: Daiichi Sankyo/Astra Zeneca, Ipsen
Anna Turletti
Honoraria: Novartis
Travel, Accommodations, Expenses: Lilly, gentili
Federica Miglietta
Consulting or Advisory Role: MSD, Astrazeneca
Speakers' Bureau: Pfizer, Gilead, Seagen, Novartis
Travel, Accommodations, Expenses: Novartis, Gilead
Maria Vittoria Dieci
Consulting or Advisory Role: Lilly, Novartis, Exact Sciences, Pfizer, Seagen, MSD, Gilead Sciences, Daiichi Sankyo, Roche
Patents, Royalties, Other Intellectual Property: Patent pending HER2DX licensed to University of Padova
Valentina Guarneri
Consulting or Advisory Role: Lilly, Novartis, MSD, Gilead Sciences, Eisai, Merck Serono, Olema Oncology, Exact Sciences
Speakers' Bureau: Novartis, Lilly, GlaxoSmithKline, Amgen, AstraZeneca
Research Funding: AstraZeneca (Inst), Roche (Inst), Novartis (Inst), Merck (Inst), BMS (Inst), Lilly (Inst), Merck KGaA (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Nerviano Medical Sciences (Inst), GlaxoSmithKline (Inst), Synthon (Inst)
Expert Testimony: Lilly
Travel, Accommodations, Expenses: Gilead/Forty Seven
No other potential conflicts of interest were reported.
REFERENCES
- 1.Conte P, Frassoldati A, Bisagni G, et al. : Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study‡. Ann Oncol 29:2328-2333, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Spiegelhalter DJ, Abrams KR, Myles JP: Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Chichester, United Kingdom, John Wiley & Sons, Ltd, 2004 [Google Scholar]
- 3.Bradley R, Braybrooke J, Gray R, et al. : Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol 22:1139-1150, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan A, Delaloge S, Holmes FA, et al. : Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:367-377, 2016 [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Huang C-S, Mano MS, et al. : Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617-628, 2019 [DOI] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Procter M, de Azambuja E, et al. : Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolaney SM, Barry WT, Dang CT, et al. : Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134-141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. : Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:1630-1640, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Schneeweiss A, Chia S, Hickish T, et al. : Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278-2284, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273-1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pivot X, Romieu G, Debled M, et al. : 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 393:2591-2598, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Earl HM, Hiller L, Vallier A-L, et al. : 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393:2599-2612, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joensuu H, Fraser J, Wildiers H, et al. : Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer. JAMA Oncol 4:1199, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavroudis D, Saloustros E, Malamos N, et al. : Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 26:1333-1340, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Earl H, Hiller L, Dunn J, et al. : Individual patient data meta-analysis of 5 non-inferiority RCTs of reduced duration single agent adjuvant trastuzumab in the treatment of HER2 positive early breast cancer. Ann Oncol 32:S1283-S1346, 2021. (suppl 5) [Google Scholar]
- 16.Ehsan AN, Wu CA, Minasian A, et al. : Financial toxicity among patients with breast cancer worldwide. JAMA Netw Open 6:e2255388, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fundytus A, Sengar M, Lombe D, et al. : Access to cancer medicines deemed essential by oncologists in 82 countries: An international, cross-sectional survey. Lancet Oncol 22:1367-1377, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets that support the findings of this study are not publicly available to protect patient privacy. The data will be available on reasonable request from the corresponding author: P.F.C., pierfranco.conte@unipd.it.