Abstract
The psychrotroph Rhodococcus sp. strain Q15 was examined for its ability to degrade individual n-alkanes and diesel fuel at low temperatures, and its alkane catabolic pathway was investigated by biochemical and genetic techniques. At 0 and 5°C, Q15 mineralized the short-chain alkanes dodecane and hexadecane to a greater extent than that observed for the long-chain alkanes octacosane and dotriacontane. Q15 utilized a broad range of aliphatics (C10 to C21 alkanes, branched alkanes, and a substituted cyclohexane) present in diesel fuel at 5°C. Mineralization of hexadecane at 5°C was significantly greater in both hydrocarbon-contaminated and pristine soil microcosms seeded with Q15 cells than in uninoculated control soil microcosms. The detection of hexadecane and dodecane metabolic intermediates (1-hexadecanol and 2-hexadecanol and 1-dodecanol and 2-dodecanone, respectively) by solid-phase microextraction–gas chromatography-mass spectrometry and the utilization of potential metabolic intermediates indicated that Q15 oxidizes alkanes by both the terminal oxidation pathway and the subterminal oxidation pathway. Genetic characterization by PCR and nucleotide sequence analysis indicated that Q15 possesses an aliphatic aldehyde dehydrogenase gene highly homologous to the Rhodococcus erythropolis thcA gene. Rhodococcus sp. strain Q15 possessed two large plasmids of approximately 90 and 115 kb (shown to mediate Cd resistance) which were not required for alkane mineralization, although the 90-kb plasmid enhanced mineralization of some alkanes and growth on diesel oil at both 5 and 25°C.
In many temperate and cold climates, the bioremediation of sites contaminated with aliphatic hydrocarbons, resulting from the spillage or leakage of diesel fuels or kerosenes, is possible, although it is hampered by low ambient temperatures for much of the year. In many Arctic sites, the rates of biodegradation are thought to be too low to rapidly remove hydrocarbon contaminants, and consequently, the contaminants remain in cold Arctic ecosystems for long periods following contamination (3, 12). At low temperatures, the viscosity of oil increases, reducing the degree of spreading of the oil in soil and aquatic matrices. Conversely, low temperatures retard the volatilization of short-chain alkanes (<C10), which can increase their solubility in the aqueous phase and, consequently, increase their microbial toxicity, which may delay the onset of biodegradation (3, 12). The degradation of long-chain alkanes, many of which are solid at temperatures of <10°C, is hindered by their limited bioavailability. Nevertheless, biodegradation of many of the components of petroleum hydrocarbons has been observed at low temperatures in a variety of soil, water, and marine systems from temperate, sub-Arctic, Arctic, Antarctic, and alpine sites (3, 4, 12, 15, 16, 31). The observed biodegradation is a result of the activity of indigenous psychrophilic and psychrotrophic microorganisms, which are characterized by low-temperature growth ranges of ≤0 to 15 to 20°C and ≤0 to 30 to 35°C, respectively.
To optimize the biodegradative activity of psychrophiles and psychrotrophs in contaminated sites, it is essential to develop a basic understanding of their physiology and ecology as well as the genetics and biochemistry of their catabolic pathways. These latter traits of cold-adapted bacteria are relatively poorly understood compared with those of mesophilic bacteria, which are metabolically inactive at temperatures of ≤8 to 10°C. For example, only the genetics of the alk pathway of the mesophile Pseudomonas oleovorans (28), which oxidizes short-chain alkanes (C5 to C12), has been thoroughly characterized. The microbial catabolic pathways for the degradation of n-alkanes from a wide variety of bacteria, fungi, and yeasts have been biochemically characterized. These include the common hydroxylation pathways, such as the terminal oxidation pathway, the terminal oxidation pathway followed by ω oxidation, and the less common subterminal oxidation pathway (30). In the terminal oxidation pathway, the initial oxidation step is catalyzed by a monooxygenase to give the corresponding primary alcohol, which is further oxidized to the corresponding aldehyde by alcohol dehydrogenases and then to the corresponding fatty acid by aldehyde dehydrogenases. In the subterminal pathway, the alkane is oxidized by a monooxygenase to the corresponding secondary alcohol, then to the ketone, and eventually to a fatty acid. Dioxygenase systems also exist in some microorganisms, where the n-alkane is initially oxidized to the corresponding hydroperoxide and then is transformed to the corresponding primary alcohol (30) or to the corresponding aldehyde, as originally postulated by Finnerty (6) and recently demonstrated by Sakai et al. (23) in Acinetobacter sp. strain M-1.
In an initial investigation, a number of psychrotrophic bacteria were isolated from environmental samples, obtained from both contaminated and noncontaminated sites across Canada, and shown to mineralize a variety of petroleum hydrocarbon components (toluene, naphthalene, and alkanes) at 5°C (32). One psychrotrophic bacterium, originally isolated from Lake Ontario and tentatively identified as Rhodococcus sp. strain Q15, readily mineralized both shorter-chain 14C-labelled alkanes (dodecane and hexadecane) and longer-chain alkanes (octacosane and dotriacontane) at 23°C. In the present study, we further characterized this bacterium and studied its ability to mineralize alkanes and diesel oil at low temperatures. The genetic and biochemical bases of its alkane catabolic pathway were also examined.
MATERIALS AND METHODS
Isolation and characterization of Rhodococcus sp. strain Q15.
The psychrotrophic strain used in this study was previously isolated from the Bay of Quinte, Ontario, Canada (10), and tentatively identified as a Rhodococcus sp. (32). The strain, designated Q15, was further characterized in the present study by standard morphological, physiological, and biochemical techniques (13) and was identified by substrate utilization (Biolog GP microplate identification system; Biolog, Hayward, Calif.), cell wall fatty acid analysis (Microcheck Inc., Northfield Falls, Vt.), and 16S ribosomal DNA (rDNA) sequencing (essentially as described by Denis-Larose et al. [5]). Strain Q15 and other psychrotrophic and mesophilic alkane-degradative strains used in this study were grown on Trypticase soy agar (TSA) at 24 or 37°C (psychrotrophic and mesophilic strains, respectively) and maintained at 4°C. Resistance to cadmium was determined by monitoring the growth of Q15 in Trypticase soy broth (TSB) supplemented with various concentrations of Cd in the form of Cd(NO3)2 · 5H2O.
Mineralization of alkanes at low temperatures by Rhodococcus sp. strain Q15.
The mineralization of dodecane by Rhodococcus sp. strain Q15 and that by the mesophilic alkane-degradative strain P. oleovorans ATCC 29347 were compared at 10, 20, and 30°C by a serum bottle assay with mineral salts medium (MSM) as previously described (32). The ability of Rhodococcus sp. strain Q15 to mineralize the shorter-chain alkanes, dodecane (C12) and hexadecane (C16), and longer-chain alkanes, octacosane (C28) and dotriacontane (C32), was also examined at 0 and 5°C. All assays which included uninoculated controls were performed in duplicate. The 14C-labelled organic substrates (Sigma, St. Louis, Mo.) used in the mineralization assays were [1-14C]dodecane (specific activity, 3.7 mCi/mmol), [1-14C]hexadecane (specific activity, 2.2 mCi/mmol), [14,15-14C]octacosane (specific activity, 11.7 mCi/mmol), and [16,17-14C]dotriacontane (specific activity, 8.8 mCi/mmol).
Utilization of diesel oil by Rhodococcus sp. strain Q15 at 5°C.
To determine the range of alkanes utilized by Q15, the growth of the organism on diesel fuel, which consists mostly of linear and branched alkanes, was examined. Erlenmeyer flasks containing MSM (50 ml) supplemented with 0.1% (vol/vol) diesel fuel and 0, 5, or 10 ppm of yeast extract (YE) were inoculated with Q15 to an initial optical density at 600 nm (OD600) of 0.025. The flasks were sealed tightly with screw caps, and the cultures were incubated at 5°C for 14 or 28 days, or at 24°C for 4 days, with shaking (150 rpm) (i.e., until heavy growth was observed). An uninoculated control flask was incubated in parallel to monitor abiotic losses of the diesel substrate. Following the incubation periods, the diesel oil was extracted from the growth medium and analyzed by gas chromatography-mass spectrometry (GC-MS) essentially as described by Geerdink et al. (7). An extraction standard (docosane) was added to each flask (final concentration, 10 ppm), the pH of the growth medium was adjusted to pH 13 with 10 N NaOH, and the suspension was extracted three times with 10 ml of diethyl ether in a separatory funnel. The diethyl ether phase was collected from the flasks, the volume of each extract was adjusted to 30 ml, and the extract was dehydrated with 1 to 2 g of Na2SO4. Twenty microliters of an injection standard (1,3,5-trichlorobenzene; 1.7 g/liter) was added to each extract (0.5 ml) to a final concentration of 40 ppm. The samples were analyzed by GC-MS in a model 5890 GC (Hewlett-Packard, Avondale, Pa.) connected to a model 5970 quadrupole MS (EI70 eV) (Hewlett-Packard). A DB-5 capillary column (30 m by 0.25 mm by 0.25 μm; J & W Scientific, Folsom, Calif.) was used, with He as the carrier gas and a temperature program consisting of an initial oven temperature of 55°C for 3 min increased at a rate of 4°C/min to 245°C for 5 min. The injector and detector were maintained at 250 and 280°C, respectively. To determine the extent of degradation of various hydrocarbon components of diesel fuel, the areas of individual peaks (from duplicate samples) were quantified by integration, normalized to the phytane peak from each sample, and expressed as the percentage degraded relative to the amount of the corresponding peak remaining in the appropriate abiotic control samples. Phytane was not degraded by this organism.
Determination of the alkane catabolic pathway of Rhodococcus sp. strain Q15 by SPME–GC-MS and substrate utilization.
To elucidate the alkane degradation pathway of Rhodococcus sp. strain Q15, two analyses were performed (i) to identify potential metabolic intermediates resulting from the growth of Q15 on dodecane or hexadecane and (ii) to examine the organism’s ability to grow on terminal and subterminal pathway intermediates. For the identification of metabolic intermediates, Q15 cells were grown at 24°C with shaking (150 rpm) in MSM (50 ml) supplemented with 25 ppm of YE and 50 ppm of dodecane or hexadecane. The presence of metabolic intermediates in the culture broth was monitored daily by collecting 1 ml of the growth medium in a 1.0-ml glass vial. Bacterial cells were removed by centrifugation in a microcentrifuge (12,000 × g for 2 min), and the supernatant was collected and stored at −20°C. Metabolic intermediates were extracted from the 1-ml samples by solid-phase microextraction (SPME) (2, 21) by immersing an SPME fiber, coated with a 75-μm-thick film of polyacrylate (Supelco, Bellefonte, Pa.) to adsorb hydrophobic organic compounds, in the sample for 20 min. The SPME fiber was inserted into the GC injection port and held for 10 min to thermally desorb analytes from the SPME fiber into the GC injector for analysis by GC-MS. A Varian GC-MS equipped with a Saturn 4D ion trap detector and integrated with a split or splitless injector and a DB-5 column (15-m by 0.25-mm by 0.25-μm film) with He as the carrier gas was used for GC-MS analysis. The temperature program had an initial oven temperature of 90°C for 2 min, which was then increased at a rate of 7°C/min to 270°C. The injector, with a front pressure of 11 lb/in2, was set at 270°C in the splitless mode for 5 min and then changed to the split mode of 1/10 to the column. The MS had a mass range of 30 to 400 atomic mass units at a scan rate of 0.5 s per scan. The acquisition time was set for 25 min. Various metabolic intermediates were identified by matching the retention times and mass spectra with authentic standards which had been extracted from aqueous medium by SPME and analyzed by GC-MS, as described above. To ensure that detected metabolic intermediates were a result of Q15 biotic activity, sterile controls were supplemented with dodecane or hexadecane and run in parallel with the inoculated media and were analyzed by SPME–GC-MS.
The growth of Q15 on potential metabolic intermediates of dodecane and hexadecane was studied by monitoring respiration. Serum bottles (100 ml) containing 20 ml of MSM and a 5-ml test tube containing 1 ml of 0.1 N KOH to trap CO2 were supplemented with 100 ppm of the primary substrate (hexadecane or dodecane) or their various metabolic intermediates (1-hexadecanol, 2-hexadecanol, 2-hexadecanone, hexadecanedioic acid, 1-dodecanol, 2-dodecanol, 1-dodecanal, 2-dodecanone, and docanedioc acid) as the sole carbon and energy source and were inoculated with Q15 to a final OD600 of 0.025 and sealed with Teflon septa and crimp caps. The serum bottles were incubated at 24°C in a G24 incubator-shaker (New Brunswick Scientific, Edison, N.J.) at 150 rpm. CO2 was periodically recovered from the KOH trap, precipitated, and quantified as described by Anderson (1) and was reported as micromoles of CO2 evolved (essentially as described by Whyte et al. [33]). Uninoculated controls and bottles inoculated with Q15 but not supplemented with a carbon or energy source were monitored for background CO2 evolution. All analyses were performed in triplicate.
PCR and plasmid analyses of Rhodococcus sp. strain Q15.
In an initial investigation (32), PCR and Southern analyses indicated that Q15 may possess a gene with low homology to P. oleovorans alkB, encoding alkane hydroxylase, which is responsible for the hydroxylation of short-chain alkanes (C5 to C12) in the first step of the alkane degradation pathway of P. oleovorans (28). In the present study, Q15 was examined by PCR analysis essentially as described by Whyte et al. (32) for the presence of other recently described bacterial genes potentially involved in alkane catabolism, including the Acinetobacter calcoaceticus rubredoxin and rubredoxin reductase genes, which are required by that bacterium for growth on dodecane and hexadecane (8), and for the aldehyde dehydrogenase gene (thcA) of Rhodococcus erythropolis NI86/21, which transforms C3 to C10 aliphatic aldehydes to the corresponding aliphatic acids (20). To verify PCR amplification, the putative thcA PCR fragment obtained from Rhodococcus sp. strain Q15 and the “alkB” PCR fragment previously obtained from Rhodococcus sp. strain Q15 (32) were purified and sequenced and the nucleotide sequences obtained were analyzed as previously described (33).
Rhodococcus sp. strain Q15 was screened for the presence of large catabolic plasmids, similar to the alkane catabolic plasmid OCT found in P. oleovorans, using a modified alkaline lysis method (5). Southern analyses of the plasmid and chromosomal bands found in Rhodococcus sp. strain Q15, using DNA probes specific for alkB from Rhodococcus sp. strain Q15 (32) and thcA from Rhodococcus sp. strain Q15, were performed essentially as described by Whyte et al. (33), employing high-stringency hybridization and washing conditions at 65°C. Plasmid curing of Q15 was performed by growing the organism at 35.5°C (the maximum growth temperature of Q15) in Luria-Bertani medium with shaking (150 rpm) for 3 days. After being subcultured eight times, isolated colonies, obtained on Luria-Bertani agar plates, were screened for the presence of the large plasmids as described above. Southern analysis was performed on plasmid preparations from the parental strain and strains lacking one or both plasmids, using a DNA probe constructed from an ∼4-kb EcoRI fragment obtained from the Q15 90-kb plasmid by standard molecular biology techniques (24). Plasmid-cured strains were tested for their ability to mineralize C12, C16, and C28; growth in MSM supplemented with 0.1% diesel fuel; and resistance to cadmium (100 ppm), as described above.
Mineralization of hexadecane in soils bioaugmented with Rhodococcus sp. strain Q15.
Hexadecane mineralization in contaminated and pristine soils was monitored in soil microcosms to determine if bioaugmentation with Rhodococcus sp. strain Q15 could enhance the biodegradation of alkanes at low temperatures. The contaminated soil (a silty clay soil with 30% water content and ∼5,000 mg of total petroleum hydrocarbons/kg) was collected from a crude-oil-contaminated site at Ville St. Pierre, Quebec, Canada. For comparison, a nonimpacted soil (pristine) with similar physiochemical characteristics was collected near a water treatment plant in Stanstead, Quebec, Canada. Soil aliquots (20 g) were placed in 100-ml serum bottles. For bioaugmentation, a cellular suspension of Q15 was prepared by growing Q15 in TSB overnight at 23°C. The cells were harvested by centrifugation at 5,000 × g for 10 min at 4°C, and the pellet was washed twice with cold 0.1% sodium tetrapyrophosphate and then resuspended in the wash buffer to an OD600 of 10. Appropriate volumes (∼100 μl) of the Q15 cellular suspension were added to the soil microcosms to give final concentrations of ∼108 cells/g (wet weight) of soil and were mixed with a sterile spatula. The actual number of cells added to each soil microcosm was determined by viable plate counts on TSA. A small test tube containing 1 ml of 0.5 M KOH (a CO2 trap) was placed inside each serum bottle, and 20-μl aliquots of 14C-labelled hexadecane were added to the soil microcosms, resulting in final concentrations of 100 ppm of hexadecane and ∼100,000 dpm. The serum bottles were sealed with rubber stoppers and aluminum crimps and incubated at 5°C, and the amount of radioactivity trapped in the KOH trap was determined (9) at regular intervals. To monitor mineralization of hexadecane by the indigenous microbial population, nonbioaugmented soil microcosms were examined as described above. All soil microcosms were set up in triplicate.
Nucleotide sequence accession number.
The Q15 16S rDNA sequence has been submitted to GenBank and assigned accession no. AF046885.
RESULTS
Characterization of Rhodococcus sp. strain Q15.
The alkane biodegradative strain was a gram-positive, oxidase-negative, catalase-positive rod and produced creamy white circular colonies on TSA which became mucoid following extended growth at 5°C. The isolate possessed a growth temperature range of 0 to ∼35°C with an optimum growth temperature of ∼30°C, indicating that the bacterium should be classified as a psychrotroph. The psychrotroph did not reduce nitrate to nitrite but was urease positive. The strain could grow in TSB supplemented with up to 200 mg of Cd/liter. Preliminary identification of Q15 by the Biolog GP microplate identification system and by fatty acid composition indicated that the isolate belonged to the genus Rhodococcus. 16S rDNA sequence analysis of Q15 showed 99.5% identity to the DNA sequence of Nocardia calcarea (EMBL accession no. X80618), which is a synonym for R. erythropolis, a species belonging to Rhodococcus rDNA group IV (the largest Rhodococcus cluster) (22), and 99.4% identity to the DNA sequence of an undescribed Rhodococcus isolate, DN22 (EMBL accession no. X89240). On the basis of these data, the psychrotrophic strain is referred to as Rhodococcus sp. strain Q15.
Mineralization of alkanes at low temperatures by Rhodococcus sp. strain Q15.
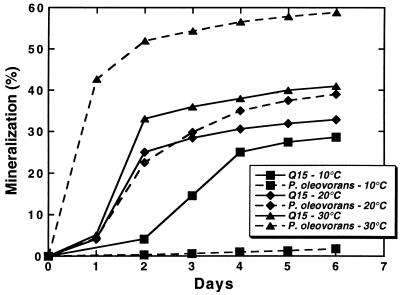
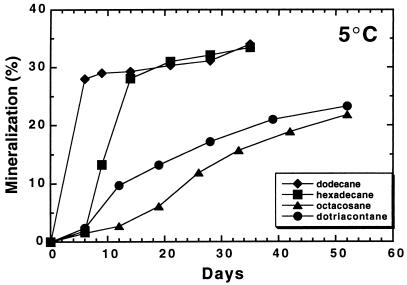
The ability of Q15 to mineralize dodecane was compared with that of the mesophile P. oleovorans at 10, 20, and 30°C (Fig. 1). As shown by the rates and extents of dodecane mineralization, both organisms readily mineralized dodecane at 20 and 30°C. However, at 10°C, only negligible amounts of dodecane were mineralized by the mesophile, while the psychrotroph readily mineralized this alkane at the lower temperature, demonstrating the intrinsic advantage psychrotrophic microorganisms possess for degrading contaminants at lower temperatures. At 5°C, Rhodococcus sp. strain Q15 mineralized the shorter-chain alkanes dodecane (C12) and hexadecane (C16) to a greater extent than that observed for the longer-chain alkanes octacosane (C28) and dotriacontane (C32) (Fig. 2). After 102 days of incubation at 0°C, Q15 mineralized C12 (8%), C16 (6.1%), C28 (1.6%), and C32 (4.3%), but at markedly lower rates and to a lesser extent than was observed at 5°C, as expected. The organism was unable to mineralize 14C-labelled toluene or naphthalene. Cumulative background levels of 14CO2 in the uninoculated serum bottles for all of the tested substrates were less than 1% at the end of the incubation period.
FIG. 1.
Mineralization of [1-14C]dodecane by the psychrotroph Rhodococcus sp. strain Q15 and the mesophile P. oleovorans at 10, 20, and 30°C. Mineralization by Rhodococcus sp. strain Q15 and P. oleovorans was determined in 20 ml of MSM supplemented with 50 mg of YE/liter, as described in Materials and Methods. Each point represents the mean from duplicate cultures.
FIG. 2.
Mineralization of [1-14C]dodecane, [1-14C]hexadecane, [14,15-14C]octacosane, or [16,17-14C]dotriacontane by Rhodococcus sp. strain Q15 at 5°C, determined as described in the legend to Fig. 1. Each point represents the mean from duplicate cultures.
Utilization of diesel oil by Rhodococcus sp. strain Q15.
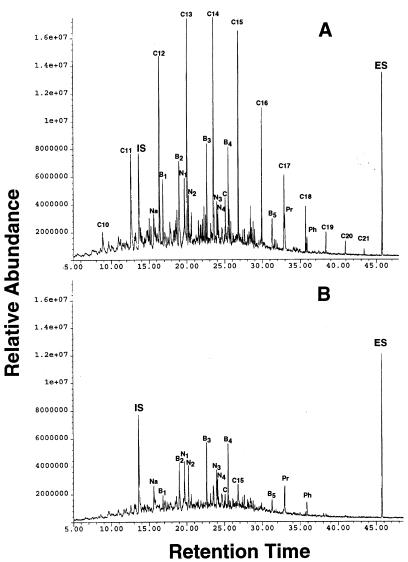
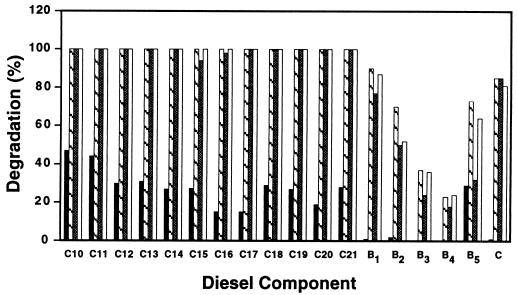
After 28 days of growth at 5°C in MSM containing 0.1% diesel fuel, Rhodococcus sp. strain Q15 degraded almost all the n-alkanes (C10 to C21) and, to a lesser degree, many of the branched alkanes found in diesel fuel (Fig. 3). The remaining peaks were naphthalene and naphthalene derivatives as well as some branched alkanes, including phytane and pristane, which did not appear to be degraded by the organism. During shorter incubation periods, the ability of Q15 to grow on and catabolize diesel fuel components was markedly enhanced by supplementing MSM with a small amount of YE (10 ppm) (Fig. 4). After 2 weeks of growth at 5°C, the alkane peaks were degraded to a lesser extent (15 to 53%), while branched alkanes were not utilized in MSM containing only diesel fuel (Fig. 4). In comparison, Q15 readily degraded the various diesel fuel components after 14 days at 5°C in MSM containing 10 ppm of YE, similar to the degradation observed following 4 weeks of incubation at 5°C in MSM alone. Four weeks of incubation at 5°C in MSM supplemented with YE did not markedly increase overall degradation in comparison with that in MSM alone. Supplementation of the growth medium with YE also noticeably enhanced diesel fuel utilization at 24°C after 4 days (data not shown). Overall, this bacterium is capable of utilizing a broad range of alkanes and aliphatic components present in diesel fuel at low and moderate temperatures, and although a constituent(s) present in YE increased the rate of diesel fuel degradation by Q15, it was not found to be essential.
FIG. 3.
GC profiles of diesel oil extracted from the aqueous phase of MSM medium after 28 days of incubation at 5°C with and without inoculation with Rhodococcus sp. strain Q15. (A) Abiotic control (uninoculated); (B) inoculated with Q15. IS, injection standard; ES, extraction standard; C10 to C21, n-alkanes (numbers designate the number of C atoms); B1 to B5, branched alkanes; C, substituted cyclohexane; Na, naphthalene; N1 to N4, substituted naphthalenes; Pr, pristane; Ph, phytane. The alkane, naphthalene, phytane, and pristane peaks were identified by comparison of their retention times and mass spectra with authentic standards. The branched alkanes, substituted naphthalenes, and substituted cyclohexane were tentatively identified by mass spectra database comparisons following GC-MS analysis.
FIG. 4.
Degradation of specific diesel fuel components by Rhodococcus sp. strain Q15 at 5°C in MSM containing 0.1% (vol/vol) diesel fuel after 14 days of growth in MSM alone (solid bars) or supplemented with 10 ppm of YE (hatched bars) and after 28 days of growth in MSM alone (shaded bars) or supplemented with 10 ppm of YE (open bars). Abbreviations are described in the legend to Fig. 3 and correspond to the peaks shown in Fig. 3. Each point represents the mean from duplicate cultures.
Determination of the alkane catabolic pathway of Rhodococcus sp. strain Q15.
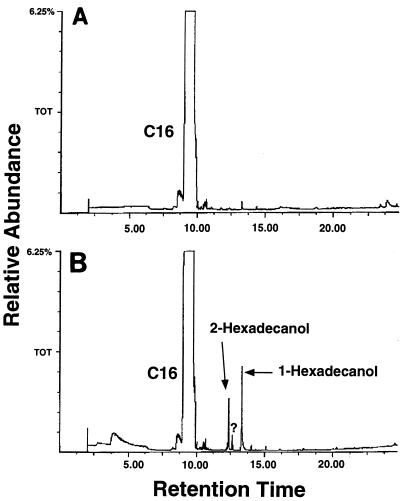
Metabolic intermediates were extracted from Q15 culture broth by SPME and identified by GC-MS. After 24 h of incubation on hexadecane, both 1-hexadecanol and 2-hexadecanol were identified by comparison of their retention times and mass spectra with authentic standards (Fig. 5). After 48 h, both the 1-hexadecanol and 2-hexadecanol peaks had disappeared but traces of hexadecanoic acid could be detected (data not shown). After 24 h of incubation on dodecane, 1-dodecanol and 2-dodecanone were detected and identified by GC-MS. A third peak was observed that had a retention time and a mass spectrum similar to those of 2-dodecanol or 1-dodecanal (which had identical retention times and very similar mass spectra), but this peak’s exact identity was not resolved by mass spectral analysis. After 96 h of incubation, the 1-dodecanol peak had almost disappeared while the 2-dodecanone peak had increased in size. Peaks corresponding to dodecane or hexadecane metabolic intermediates were not seen in parallel sterile controls.
FIG. 5.
GC profile of intermediate metabolites of hexadecane degradation by Rhodococcus sp. strain Q15. Q15 was grown in MSM supplemented with 50 ppm of hexadecane and 50 ppm of YE at 23°C. Metabolic intermediates were extracted from the culture supernatant by SPME and analyzed by GC-MS. (A) Intermediates extracted at time zero; (B) intermediates extracted after 24 h of incubation.
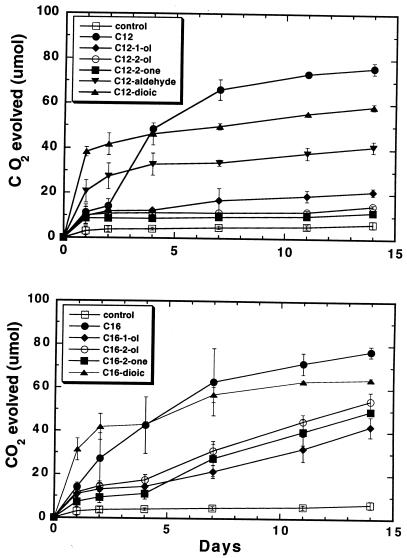
The growth of Q15 on potential dodecane or hexadecane metabolic intermediates as the sole carbon and energy sources at 24°C was also monitored by CO2 respiration to confirm the SPME–GC-MS results. The highest levels of CO2 respiration were observed during the growth of Q15 on the primary substrates dodecane and hexadecane, followed by the ω-oxidation pathway intermediates dodecanedioic acid and hexadecanedioic acid (Fig. 6). Less CO2 was produced from both the hexadecane subterminal pathway metabolites 2-hexadecanol and 2-hexadecanone and the hexadecane terminal pathway metabolite 1-hexadecanol. Significant levels of CO2 were produced from the dodecane terminal pathway intermediates 1-dodecanal and, to a lesser extent, 1-dodecanol (Fig. 6). Respiration during growth on the subterminal dodecane intermediates 2-dodecanol and 2-dodecanone was only slightly greater than that observed in the control medium containing no carbon source.
FIG. 6.
Utilization of dodecane and hexadecane and their potential metabolic intermediates by Rhodococcus sp. strain Q15 at 23°C. Q15 was grown in MSM supplemented with 100 ppm of the indicated n-alkane or metabolic intermediate as the sole carbon and energy source. Bacterial growth was monitored by CO2 respiration. Each point represents the mean number of micromoles of CO2 evolved from triplicate samples, with the error bars representing the standard deviations of the means. The control values represent the background CO2 respired from MSM inoculated with Rhodococcus sp. strain Q15 but lacking a carbon source.
PCR and plasmid analyses of Q15.
PCR analysis indicated that Rhodococcus sp. strain Q15 was negative for the rubredoxin and rubredoxin reductase genes of A. calcoaceticus but was positive for thcA and, as shown previously, for alkB. However, subsequent DNA sequence analysis of the Rhodococcus sp. strain Q15 “alk” PCR fragment revealed that it had 46% identity to the nucleotide sequence of P. oleovorans alkB and the derived amino acid sequence of Q15 “AlkB” had very low similarity (∼10%) with that of P. oleovorans AlkB. Further comparison of the derived amino acid sequences of Q15 AlkB with data bank entries revealed homology with eubacterial metabolite transporter proteins (∼50% DNA sequence identity and ∼60% amino acid similarity), suggesting that the alkB primers probably amplified a Rhodococcus gene homologous to eubacterial metabolite transporter genes rather than an alkB-homologous gene. Sequence analysis of the Q15 thcA fragment, comprising 45% of the R. erythropolis thcA gene, revealed that it had 95% identity with the nucleotide sequence of R. erythropolis thcA. The derived amino acid sequence of Q15 ThcA had 98% similarity with the amino acid sequence of R. erythropolis ThcA and contained glutamic acid-catalytic and cysteine-catalytic consensus sequences and the glycine NAD+ coenzyme binding motif characteristic of aldehyde dehydrogenases (20).
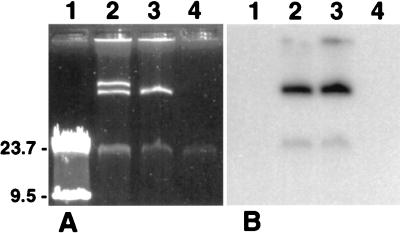
Plasmid analysis of Rhodococcus sp. strain Q15 revealed the presence of two large cryptic plasmids (Fig. 7) of approximately 90 and 115 kb, as determined by comparison with other previously sized plasmids in our laboratory (data not shown). A DNA probe constructed from the Q15 thcA PCR fragment hybridized to the chromosomal DNA band from Rhodococcus sp. strain Q15 but not to the large plasmids (data not shown). Following plasmid curing by high-temperature treatment, 31% (8 of 26) of the isolated Q15 colonies tested lacked the larger, 115-kb plasmid (Fig. 7). One of these isolates, designated Q15 H, was subsequently cured of the smaller plasmid by the same plasmid-curing protocol, resulting in a plasmidless strain designated Q15 NP, as shown by plasmid analysis and Southern hybridization with a 4-kb EcoRI restriction fragment obtained from the 90-kb plasmid (Fig. 7). Similar analysis with a 6-kb EcoRI restriction fragment obtained from the 115-kb plasmid showed that both Q15 H and Q15 NP lacked the larger plasmid (data not shown). Despite repeated attempts, it was not possible to obtain a strain of Q15 cured of only the smaller plasmid. At both 24 and 5°C, Q15 strains lacking the larger plasmid or both plasmids mineralized C12, C16, and C28, strongly indicating that alkane catabolism in this Rhodococcus sp. is not plasmid mediated. However, the rate and extent of C12 mineralization by Q15 NP was less than that observed with Q15 and Q15 H at both temperatures, as was C28 mineralization at 5°C. Compared with that of Q15 and Q15 H, growth of Q15 NP in MSM supplemented with 0.1% diesel fuel was also markedly reduced at 5 and 24°C. Both Q15 H and Q15 NP were unable to grow in the presence of 100 mg of Cd/liter, indicating that Cd resistance in Q15 is mediated by the larger, 115-kb plasmid.
FIG. 7.
Detection of plasmids in parental Rhodococcus sp. strain Q15 and the plasmid-cured strains Q15 H and Q15 NP by plasmid and Southern analyses. Total DNA (chromosomal and plasmid DNAs) from the three strains was isolated and examined by agarose gel electrophoresis. (A) Agarose gel electrophoresis (0.7%) showing the presence of the ∼90- and ∼115-kb plasmids in Q15, the ∼90-kb plasmid in Q15 H, and the lack of both plasmids in Q15 NP. (B) Southern hybridization analysis of chromosomal and plasmid DNAs shown in panel A transferred to a nylon membrane and probed with a DNA probe constructed from an ∼4-kb EcoRI fragment derived from the Q15 90-kb plasmid. Lanes 1, lambda (HindIII) ladder; lanes 2, Rhodococcus sp. strain Q15; lanes 3, Q15 H; lanes 4, Q15 NP.
Mineralization of hexadecane in soils bioaugmented with Rhodococcus sp. strain Q15.
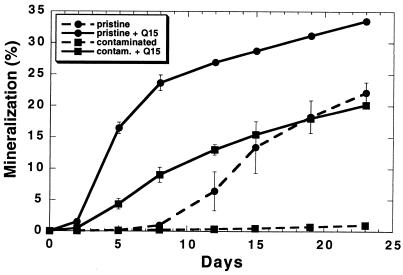
In both hydrocarbon-contaminated and pristine soil microcosms, mineralization of 14C-labelled hexadecane at 5°C was significantly greater in soil microcosms which were seeded with 108 Rhodococcus sp. strain Q15 cells/g (wet weight) of soil than in uninoculated control soil microcosms (Fig. 8). Bioaugmentation with 105 Q15 cells/g (wet weight) of soil did not enhance mineralization significantly in either the pristine or the contaminated soils (data not shown), indicating that a threshold inoculum level is required to effectively enhance biodegradation of alkanes in soil. Surprisingly, in the hydrocarbon-contaminated soil, very little indigenous microbial mineralization activity was observed.
FIG. 8.
The effect of bioaugmentation with Rhodococcus sp. strain Q15 (108 CFU/g of soil) on mineralization of [1-14C]hexadecane at 5°C in pristine and hydrocarbon-contaminated soil microcosms. Each point represents the mean cumulative mineralization (percent CO2) from triplicate soil microcosms, with the error bars representing standard deviations of the means. Contam., contaminated.
DISCUSSION
The ability of a psychrotrophic Rhodococcus sp. to assimilate aliphatic hydrocarbons was assessed and characterized at low temperatures. As demonstrated by the mineralization assays and by the utilization of diesel fuel, this organism clearly utilized a wide variety of hydrocarbons from 0 to 30°C, temperatures which parallel the active microbial temperature growth range of many temperate and cold environments. Psychrotrophic microorganisms may be better suited for in situ bioremediation of contaminated sites from these environments than either mesophiles, which have very low activities at ≤10°C, or psychrophiles, whose growth is inhibited at temperatures of ≥15 to 20°C (although in permanently cold habitats, psychrophiles may possess greater activities than psychrotrophs).
The mineralization data suggested that Q15 degrades shorter-chain alkanes (C12 and C16) more readily than the longer-chain alkanes (C28 and C32), which is a common feature of many other alkane-degradative microorganisms (28). The mineralization of the longer-chain alkanes was reduced to a greater extent than that of the shorter-chain alkanes, particularly dodecane, as the temperature decreased. As dodecane is the only one of the alkanes tested that remains a liquid at 0°C, while octacosane and dotriacontane form relatively large crystals at 0 and 5°C, the decreased bioavailability of the longer-chain alkanes at lower temperatures may be responsible for their increased recalcitrance. The lack of growth of A. calcoaceticus S30 on metabolic intermediates of octadecane (11) and of Rhodococcus sp. strain S1 on anthracene crystals (27) was attributed to their decreased bioavailabilities. Enhanced long-chain alkane recalcitrance at low temperatures may have important implications for in situ bioremediation attempts in cold climates. For example, successful bioremediation strategies could require the application of cold-active solubilizing agents to increase the bioavailability of long-chain alkanes.
Bioaugmentation of contaminated sites at cold temperatures may become a particularly viable in situ bioremediation strategy for such sites because the short summer seasons do not permit long acclimatization periods for hydrocarbon-degradative populations. In the present study, bioaugmentation of a contaminated and a pristine soil with Rhodococcus sp. strain Q15 not only decreased the lag time before C16 mineralization occurred but increased the rate and extent at which C16 mineralization occurred at 5°C, indicating that psychrotrophs (such as Q15) could eventually be used to enhance the degradation of contaminated environments subjected to low temperatures. However, bioaugmentation with this organism would probably require a simultaneous nutrient supplement for optimal in situ alkane-degradative activity, as demonstrated by the enhanced degradation of diesel fuel at 5°C when supplemented with YE. Components present in the YE, such as a growth factor(s) or vitamin(s), may have increased the rate of alkane consumption by contributing to a more rapid production of Q15 biomass. Bioaugmentation with a psychrotrophic actinomycete only slightly enhanced biodegradation in diesel-fuel-contaminated alpine soils at 10°C during the initial phases of treatment (15). Similarly, bioaugmentation with a psychrotrophic diesel-oil-degradative yeast, plus or minus biostimulation with an inorganic fertilizer, did not enhance degradation at 10°C in alpine soils to a significantly greater extent than did biostimulation alone (16).
The detection of potential metabolic intermediates of C12 or C16 catabolism from both the terminal and the subterminal pathways by SPME–GC-MS during the growth of Q15 on these alkanes, and the ability of Q15 to grow on many of their potential metabolic intermediates from both pathways, indicates that Q15 oxidizes alkanes by both the terminal and the subterminal oxidation pathways. Other rhodococci with multiple aliphatic catabolic pathways have been reported (29). For example, Rhodococcus rhodochrous PNKb1 possesses both the terminal and subterminal pathways for C2 to C8 alkanes while Rhodococcus sp. strain BPM 1613 catabolizes pristane by the terminal pathway and by terminal oxidation followed by ω oxidation. On the other hand, R. erythropolis ATCC 4277 appears to degrade C5 to C16 alkanes only by the subterminal pathway (14) while Rhodococcus salmonicolor utilizes the terminal oxidation pathway (29). Thus, it appears that different members of the genus Rhodococcus, recently recognized for their metabolic virtuosity in degrading many environmental contaminants, possess a variety of alkane-catabolic pathways.
Plasmid analysis of Rhodococcus sp. strain Q15 demonstrated that neither the smaller nor the larger plasmid found in Q15 is required for alkane mineralization by the organism, indicating that alkane degradation by Q15 is not plasmid encoded, unlike the P. oleovorans alk system. The alkane-degradative systems found in Acinetobacter spp. (8, 30) also appear to be located on the chromosome. However, slower rates of mineralization on alkanes and of growth on diesel fuel by the plasmidless strain, in comparison with those by the parental strain and a Q15 strain containing only the larger plasmid, indicate that the smaller Q15 plasmid may carry genes which have a positive impact on hydrocarbon catabolism, perhaps by decreasing the toxicity of hydrocarbons, increasing hydrocarbon bioavailability, and/or mediating uptake of aliphatic hydrocarbons.
Rhodococcus sp. strain Q15 possessed a thcA gene that was highly homologous to the thcA gene of R. erythropolis, which is located on the chromosome. Although thcA is widespread in the rhodococci, including other alkane-degradative actinomycetes analyzed in this study (data not shown), its role in alkane metabolism remains to be confirmed. The thcA gene was originally found to be induced in R. erythropolis NI86/21 during exposure to the herbicide thiocarbamate, where its gene product was most likely responsible for transforming aliphatic aldehydes (generated by N-dealkylation of thiocarbamate) to the corresponding carboxylic acids (20). However, thcA is not part of the operon encoding the P-450 cytochrome system genes that are responsible for the initial oxidation of thiocarbamate (20). The thcA gene is located within an unknown operon similar in gene organization to the P. oleovorans alk operon because it contains adjacent open reading frames, one of which most likely encodes an alcohol dehydrogenase (20). These observations, and the results of the present study, suggest that thcA may be part of an alkane-degradative operon or pathway in the genus Rhodococcus. The initial alkane oxidation may be mediated by an alkane-oxidizing cytochrome P-450 system, since Q15 does not contain an alkane monooxygenase similar to that of P. oleovorans. A variety of cytochrome P-450 enzymes have been implicated in alkane degradation, including octane degradation by R. rhodochrous (26), long-chain alkane degradation in A. calcoaceticus (17) and perhaps in Bacillus megaterium (18, 19), and a variety of yeast (25). We are currently exploring the exact role that thcA, and possibly P-450, plays in alkane catabolism in Rhodococcus sp. strain Q15.
ACKNOWLEDGMENTS
We thank Helen Bergeron, Chantale Beaulieu, Simen-Jan Slagman, and Helene Legasse for their technical assistance.
REFERENCES
- 1.Anderson J P E. Soil respiration. In: Page A L, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. 2nd ed. Madison, Wis: American Society of Agronomy; 1982. pp. 836–841. [Google Scholar]
- 2.Arthur C L, Pawliszyn J. Solid-phase microextraction. Anal Chem. 1990;66:2145–2148. [Google Scholar]
- 3.Atlas R M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981;45:180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossert I, Bartha R. The fate of petroleum in soil ecosystems. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: Macmillan Publishing Co.; 1984. pp. 435–474. [Google Scholar]
- 5.Denis-Larose C, Labbé D, Bergeron H, Jones A M, Greer C W, Al-Hawari J, Grossman M J, Sankey B M, Lau P C K. Conservation of plasmid-encoded dibenzothiophene desulfurization genes in several rhodococci. Appl Environ Microbiol. 1997;63:2915–2919. doi: 10.1128/aem.63.7.2915-2919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnerty W R. The biochemistry of microbial alkane oxidation: new insights and perspectives. Trends Biochem Sci. 1977;2:73–75. [Google Scholar]
- 7.Geerdink M J, van Loosdrecht M C M, Luyben K C A M. Biodegradability of crude oil. Biodegradation. 1996;7:73–81. [Google Scholar]
- 8.Geissdorfer W, Frosch S C, Haspel G, Ehrt S, Hillen W. Two genes encoding proteins with similarities to rubredoxin and rubredoxin reductase are required for conversion of dodecane to lauric acid in Acinetobacter calcoaceticus ADP1. Microbiology. 1995;141:1425–1432. doi: 10.1099/13500872-141-6-1425. [DOI] [PubMed] [Google Scholar]
- 9.Greer C, Masson L, Comeau Y, Brousseau R, Samson R. Application of molecular biology techniques for isolating and monitoring pollutant-degrading bacteria. Water Pollut Res J Can. 1993;28:275–287. [Google Scholar]
- 10.Inniss W E, Mayfield C I. Growth rates of psychrotrophic sediment microorganisms. Water Res. 1978;12:231–236. [Google Scholar]
- 11.Lai B, Khanna S. Mineralization of [14C]octacosane by Acinetobacter calcoaceticus S30. Can J Microbiol. 1996;42:1225–1231. [Google Scholar]
- 12.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechevalier H A. Section 17: Nocardioforms. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams and Wilkins Co.; 1986. pp. 1458–1506. [Google Scholar]
- 14.Ludwig B, Akundi A, Kendall K. A long-chain secondary alcohol dehydrogenase from Rhodococcus erythropolis ATCC 4277. Appl Environ Microbiol. 1995;61:3729–3733. doi: 10.1128/aem.61.10.3729-3733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margesin R, Schinner F. Bioremediation of diesel-oil-contaminated alpine soils at low temperatures. Appl Microbiol Biotechnol. 1997;47:462–468. [Google Scholar]
- 16.Margesin R, Schinner F. Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl Environ Microbiol. 1997;63:2660–2664. doi: 10.1128/aem.63.7.2660-2664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller R, Asperger O, Kleber H P. Purification of cytochrome P-450 from n-hexadecane-grown Acinetobacter calcoaceticus. Biomed Biochim Acta. 1989;48:243–254. [PubMed] [Google Scholar]
- 18.Munroe A W, Lindsay J G. Bacterial cytochromes P-450. Mol Microbiol. 1996;20:1115–1125. doi: 10.1111/j.1365-2958.1996.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 19.Munroe A W, Lindsay J G, Coggins J R. Alkane metabolism by cytochrome P-450 BM-3. Biochem Soc Trans. 1993;21:412S. doi: 10.1042/bst021412s. [DOI] [PubMed] [Google Scholar]
- 20.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter D W, Pawliszyn J. Rapid determination of polyaromatic hydrocarbons and polychlorinated biphenyls in water using solid-phase microextraction and GC-MS. Environ Sci Technol. 1994;28:298–305. doi: 10.1021/es00051a017. [DOI] [PubMed] [Google Scholar]
- 22.Rainey F A, Burghardt J, Kroppenstedt R M, Klatte S, Stackebrandt E. Phylogenetic analysis of the genera Rhodococcus and Nocardia and evidence for the evolutionary origin of the genus Nocardia from within the radiation of Rhodococcus species. Microbiology. 1995;141:523–528. [Google Scholar]
- 23.Sakai Y, Maeng J H, Kubato S, Tani A, Tani Y, Kato N. A nonconventional dissimilation pathway for long chain n-alkanes in Acinetobacter sp. M-1 that starts with a dioxygenase reaction. J Ferment Bioeng. 1996;81:286–291. [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sariaslani F S. Microbial cytochromes P-450 and xenobiotic metabolism. Adv Appl Microbiol. 1991;36:133–178. doi: 10.1016/s0065-2164(08)70453-2. [DOI] [PubMed] [Google Scholar]
- 26.Sariaslani F S, Omer C A. Actinomycete cytochromes P-450 involved in oxidative metabolism: biochemistry and molecular biology. Crit Rev Plant Sci. 1992;11:1–16. [Google Scholar]
- 27.Tongpim S, Pickard M A. Growth of Rhodococcus S1 on anthracene. Can J Microbiol. 1996;42:289–294. doi: 10.1139/m96-042. [DOI] [PubMed] [Google Scholar]
- 28.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 29.Warhurst A M, Fewson C A. Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol. 1994;14:29–73. doi: 10.3109/07388559409079833. [DOI] [PubMed] [Google Scholar]
- 30.Watkinson R, Morgan P. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation. 1990;1:79–92. doi: 10.1007/BF00058828. [DOI] [PubMed] [Google Scholar]
- 31.Westlake D W S, Jobson A, Phillippe R, Cook F D. Biodegradability and crude oil composition. Can J Microbiol. 1974;20:915–928. doi: 10.1139/m74-141. [DOI] [PubMed] [Google Scholar]
- 32.Whyte L G, Greer C W, Inniss W E. Assessment of the biodegradation potential of psychrotrophic microorganisms. Can J Microbiol. 1996;42:99–106. doi: 10.1139/m96-016. [DOI] [PubMed] [Google Scholar]
- 33.Whyte L G, Bourbonnière L, Greer C W. Biodegradation of petroleum hydrocarbons by psychrotrophic Pseudomonas strains possessing both alkane (alk) and naphthalene (nah) catabolic pathways. Appl Environ Microbiol. 1997;63:3719–3723. doi: 10.1128/aem.63.9.3719-3723.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]