Abstract
PURPOSE
Standard therapy for myelofibrosis comprises Janus kinase inhibitors (JAKis), yet spleen response rates of 30%-40%, high discontinuation rates, and a lack of disease modification highlight an unmet need. Pelabresib (CPI-0610) is an investigational, selective oral bromodomain and extraterminal domain inhibitor (BETi).
METHODS
MANIFEST (ClinicalTrails.gov identifier: NCT02158858), a global, open-label, nonrandomized, multicohort, phase II study, includes a cohort of JAKi-naïve patients with myelofibrosis treated with pelabresib and ruxolitinib. The primary end point is a spleen volume reduction of ≥ 35% (SVR35) at 24 weeks.
RESULTS
Eighty-four patients received ≥ 1 dose of pelabresib and ruxolitinib. The median age was 68 (range, 37-85) years; 24% of patients were intermediate-1 risk, 61% were intermediate-2 risk, and 16% were high risk as per the Dynamic International Prognostic Scoring System; 66% (55 of 84) of patients had a hemoglobin level of < 10 g/dL at baseline. At 24 weeks, 68% (57 of 84) achieved SVR35, and 56% (46 of 82) achieved a total symptom score reduction of ≥ 50% (TSS50). Additional benefits at week 24 included 36% (29 of 84) of patients with improved hemoglobin levels (mean, 1.3 g/dL; median, 0.8 g/dL), 28% (16 of 57) with ≥ 1 grade improvement in fibrosis, and 29.5% (13 of 44) with > 25% reduction in JAK2V617F-mutant allele fraction, which was associated with SVR35 response (P = .018, Fisher's exact test). At 48 weeks, 60% (47 of 79) of patients had SVR35 response. Grade 3 or 4 toxicities seen in ≥ 10% patients were thrombocytopenia (12%) and anemia (35%), leading to treatment discontinuation in three patients. 95% (80 of 84) of the study participants continued combination therapy beyond 24 weeks.
CONCLUSION
The rational combination of the BETi pelabresib and ruxolitinib in JAKi-naïve patients with myelofibrosis was well tolerated and showed durable improvements in spleen and symptom burden, with associated biomarker findings of potential disease-modifying activity.
BACKGROUND
Myelofibrosis, a chronic, potentially life-threatening hematologic neoplasm, is characterized by clonal myeloproliferation, ineffective erythropoiesis, bone marrow (BM) stromal changes, extramedullary hematopoiesis, and aberrant cytokine expression.1 Patients typically present with splenomegaly, systemic symptoms, anemia, and BM fibrosis.
CONTEXT
Key Objective
To assess the efficacy and safety of the combination of pelabresib and ruxolitinib in Janus kinase inhibitor treatment-naïve patients with myelofibrosis.
Knowledge Generated
In Arm 3 of the MANIFEST phase II study, the combination of pelabresib and ruxolitinib demonstrated clinically meaningful durable improvements in splenomegaly and symptoms, was associated with biomarker findings indicating potential disease modification, and demonstrated a generally favorable safety profile without clinically relevant added or limiting toxicity.
Relevance (C.F. Craddock)
Combined pelabresib and ruxolitinib is well tolerated and has the potential to improve the standard of care for Janus kinase inhibitor treatment-naïve patients with myelofibrosis and warrants further investigation in prospective trials.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
Janus kinase (JAK) inhibitors (JAKis) ruxolitinib, fedratinib, and pacritinib have been approved by the Food and Drug Administration for patients with myelofibrosis on the basis of demonstrated splenic responses and symptom improvement in phase III clinical studies.2-6 Although ruxolitinib is the current standard of care, progressive disease and toxicity frequently lead to ruxolitinib discontinuation (median time: approximately 3 years),7 which is associated with poor survival.8-10
Pelabresib (CPI-0610) is an investigational, oral, small-molecule bromodomain and extraterminal domain (BET) inhibitor (BETi). BET proteins regulate transcription of specific genes integrating an array of oncogenic signals.11 BET inhibition could modify critical components of myelofibrosis pathobiology, including megakaryocyte differentiation and proliferation,12,13 and reduce proinflammatory cytokine expression via the nuclear factor kappa B (NF-κB) signaling pathway.14,15 In murine myelofibrosis models, BET inhibition reduced proinflammatory cytokine levels, spleen volume, and BM fibrosis.16 These benefits increased when BET inhibition was combined with the JAKi ruxolitinib, suggesting synergism by cooperated downregulation of JAK-driven oncogenic activity and BET-driven proinflammatory signaling.16 In a phase I study in patients with lymphoma, pelabresib demonstrated a wide therapeutic index, with a maximum tolerated dose approximately four times greater than the lowest active dose and an acceptable safety profile.17,18 In advanced myelofibrosis, pelabresib monotherapy demonstrated splenic responses and symptom improvement.19
We present results from JAKi treatment-naïve patients with myelofibrosis enrolled in the MANIFEST phase II study who were treated with pelabresib plus ruxolitinib.
METHODS
Study Design
MANIFEST, a global, open-label, nonrandomized, multicohort, phase II study (ClinicalTrials.gov identifier: NCT02158858), includes a cohort of JAKi treatment-naïve patients treated with pelabresib combined with ruxolitinib (Arm 3); enrollment for this cohort is complete (Data Supplement [Fig S1], online only). Pelabresib was administered in 21-day cycles at an initial dose of 125 mg once daily for 14 days, followed by a 7-day break, combined with continuous ruxolitinib (twice a day).20 The maximum dose permitted for this cohort was 175 mg once daily (additional dosing details are provided in the Data Supplement).
Patient Population
Eligible patients were JAKi and BETi treatment-naïve adults with confirmed diagnoses of primary myelofibrosis or postessential thrombocythemia or postpolycythemia vera myelofibrosis. Patients had to have a spleen volume of ≥ 450 cm3 determined by magnetic resonance imaging or computed tomography (CT), intermediate-2– or high-risk disease according to the Dynamic International Prognostic Scoring System (DIPSS) categories, and ≥ 2 measurable symptoms (score ≥ 3) or a total score of ≥ 10 using the Myelofibrosis Symptom Assessment Form version 4.0. Following a protocol amendment , patients with intermediate-1 DIPSS risk were excluded from the trial; this amendment ensured that the patient population reflected historical studies2 and was independent of interim assessment of efficacy or adverse events. Patients were required to have a platelet count of ≥ 100 × 109/L, an absolute neutrophil count of ≥ 1 × 109/L, a peripheral blood blast count of < 10%, and an Eastern Cooperative Oncology Group performance status of ≤ 2. Patients evaluable for review of BM samples were defined as those with available baseline and ≥ 1 postbaseline assessment; those discontinuing without postbaseline assessment were defined as not improved/not stabilized.
End Points and Assessments
The primary end point was defined as ≥ 35% reduction in spleen volume (SVR35) from baseline to 24 weeks, measured by imaging. The secondary end point was ≥ 50% reduction in total symptom score from baseline to 24 weeks (TSS50) measured by Myelofibrosis Symptom Assessment Form v4.0 (Critical Path Institute, Tucson, AZ). BM biopsies were collected at baseline and every 24 weeks thereafter during treatment. Exploratory end points included BM fibrosis improvement according to blinded central hematopathologist review (two blinded pathologists and one blinded adjudicator) following European consensus criteria for reticulin fibrosis grading and improvement in anemia and transfusion requirements (per Gale criteria21). Safety analyses used National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Mean hemoglobin increase was assessed on the basis of the rolling average of at least a 1.5 g/dL increase from baseline over any 12-week period without red blood cell transfusions, and the absolute increase was assessed at week 24 (excluding assessments within 2 weeks after a red blood cell transfusion).
Eighty-six myelofibrosis-linked cytokines were measured by enzyme-linked immunoassay from plasma obtained at baseline and after 2, 6, 12, and 24 weeks of treatment (InflammationMAP, CustomMAP, Simoa only for IL-6, IFN-g, and TNF-a; RBM, Austin, TX). Mutational analyses from peripheral whole blood samples were performed as an exploratory end point by targeted sequencing using the amplicon-based Rapid Heme Panel assay.22
Trial Oversight
The study was approved by the institutional review board or independent ethics committee at each participating site, conducted in accordance with the Declaration of Helsinki and overseen by a safety review committee. Written informed consent was obtained from each patient, their guardian, or their legal representative before study entry. Data were collected by study investigators and analyzed by the study sponsor.
Statistical Analysis
This JAKi treatment-naïve cohort was planned to enroll 81 patients. On the basis of a Simon's two-stage design to allow the possibility of early stopping for futility, this yields a one-sided type I error rate of 0.05 and a power of 80% when the true splenic response rate was 45% versus 30% under the null hypothesis. In stage 1, 27 patients were enrolled; as > nine responses occurred in these patients, 57 additional patients were enrolled in stage 2. Week 24 spleen volume was imputed for six patients (four responders and two nonresponders) from scans at week 31 (n = 2) and weeks 32, 34, 37, and 39 (all n = 1); ability to perform radiographic assessment was affected by the COVID-19 pandemic. Patients who discontinued without week 24 assessment for any reason were included as nonresponders. The primary end point was analyzed on the basis of the intent-to-treat population. Spleen volume and symptoms were assessed using a 95% exact binomial confidence interval, whereas biomarker assessments included one-/two-sample tests, proportional tests, Fisher's exact tests, and linear mixed effect model for longitudinal analysis.
RESULTS
Patients
As of September 10, 2021, 84 JAKi treatment-naïve patients were enrolled and received pelabresib in combination with ruxolitinib (Fig 1). Of these, 53 remained on study treatment at the time of data cutoff. The median follow-up for time on treatment was 94 weeks (95% CI, 91 to 97), with 95%, 77%, and 62% of patients having completed ≥ 24, ≥ 48, and ≥ 60 weeks of study treatment, respectively. The median follow-up time for time on treatment was 94, 73, and 53 weeks for DIPSS intermediate-1–, intermediate-2–, and high-risk patients, respectively. All patients reached week 24 or discontinued earlier (primary end point analysis).
FIG 1.
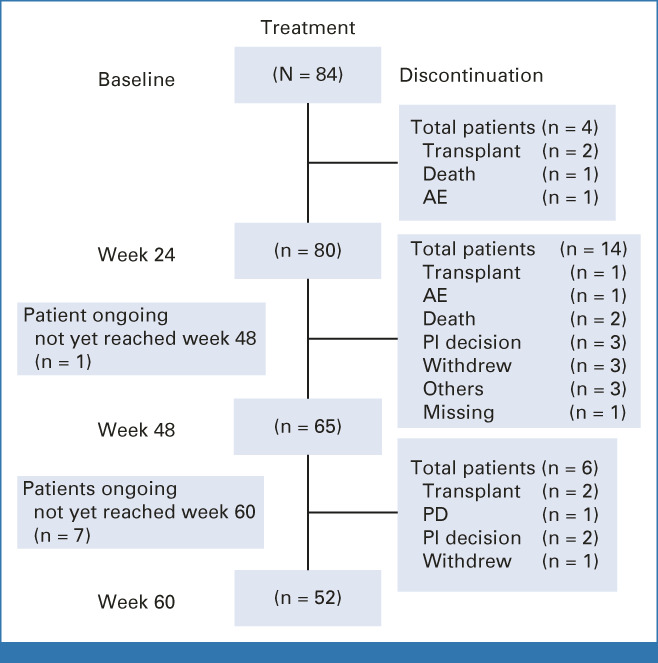
Patient disposition. AE, adverse event; PD, progressive disease; PI, principal investigator.
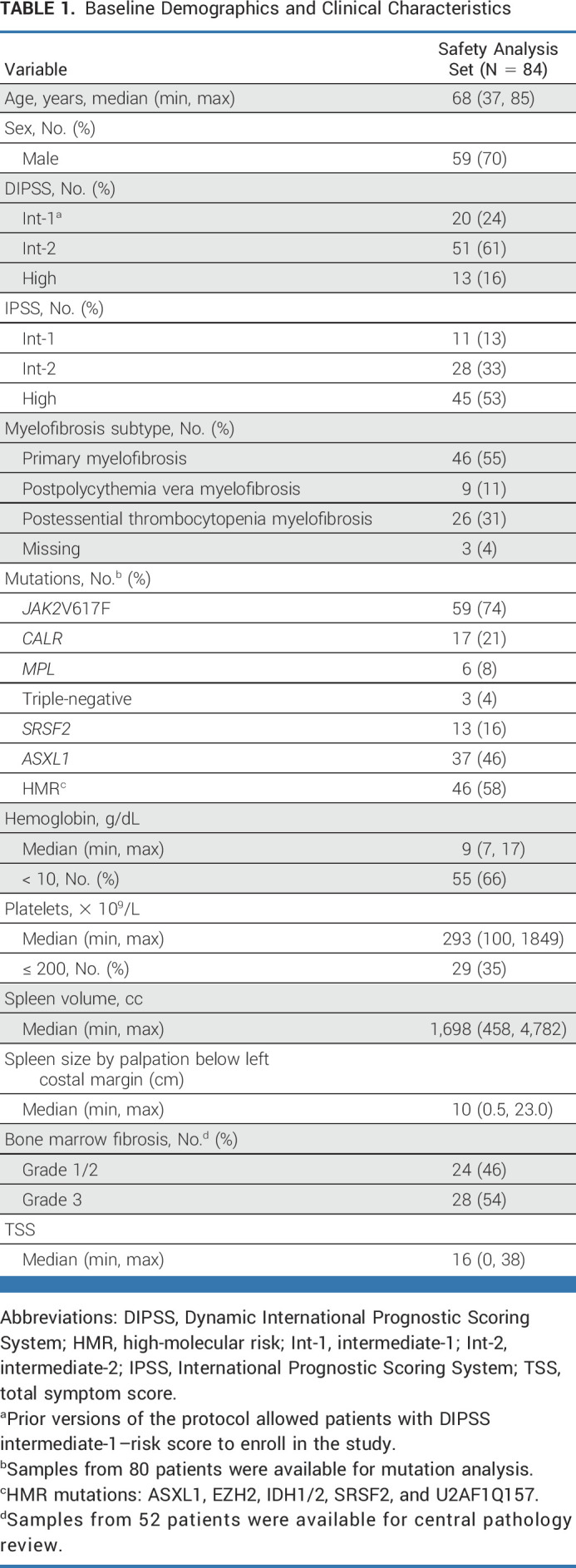
Patients were predominantly male (70%), had a median age of 68 years, and had primary myelofibrosis, with 24%, 61%, and 16% at intermediate-1, intermediate-2, or high risk, respectively, per DIPSS (Table 1). The median time since diagnosis was approximately 8.5 months. The most frequent mutations at baseline were JAK2V617F (74%), ASXL1 (46%), CALR (21%), and MPL (8%).
TABLE 1.
Baseline Demographics and Clinical Characteristics
Efficacy
Primary end point.
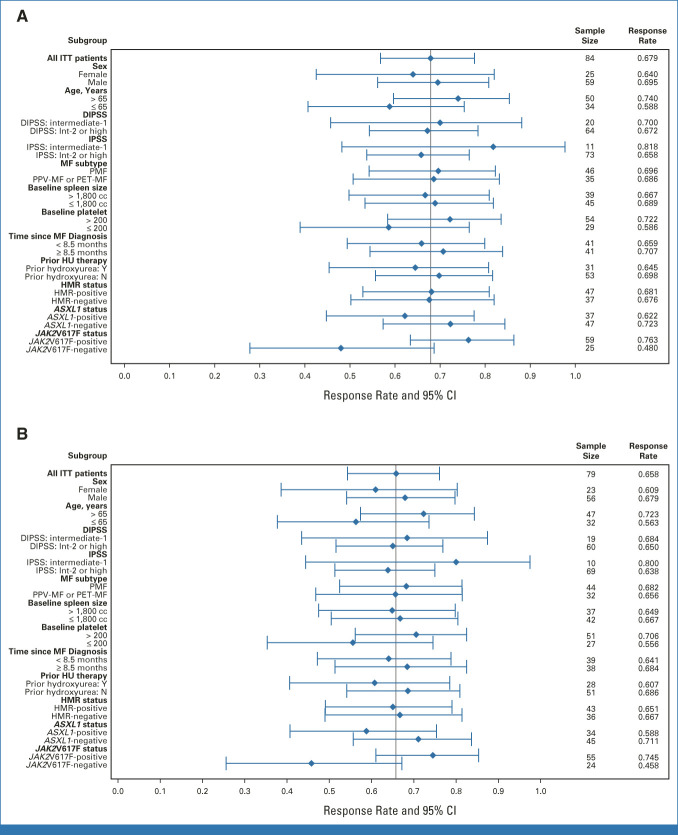
At week 24, SVR35 responses were observed in 57 of 84 patients (68%; 95% CI, 57 to 78; Fig 2A and Data Supplement [Fig S2]; best SVR35 response at any time: 80% [67 of 84]). The median SVR was −50% (range, −84% to 28%). Responses were consistent across all subgroups analyzed, including patient groups stratified by DIPSS and International Prognostic Scoring System (IPSS) risk category (Fig 3 and Data Supplement [Table S1]). SVR35 responses at week 24 were observed in 70% (14 of 20) and 67% (43 of 64) of patients with DIPSS intermediate-1– and intermediate-2–/high-risk disease, respectively, and 82% (9 of 11) and 66% (48 of 73) of those with intermediate-1– and intermediate-2–/high-risk disease, respectively, according to IPSS. No change in SVR35 was observed with imputation for missing values because of delays caused by the COVID-19 pandemic. At week 48, there were 47 (59.5%) SVR35 responders of 79 evaluable patients; the median SVR was –54.6% (range, –85.5% to 24.0%; Fig 2B). Kaplan-Meier estimates of patients with SVR35 response at any time point (n = 67) showed that 93.5% (95% CI, 87.4 to 99.7) of responders sustained their response 36 weeks after onset.
FIG 2.
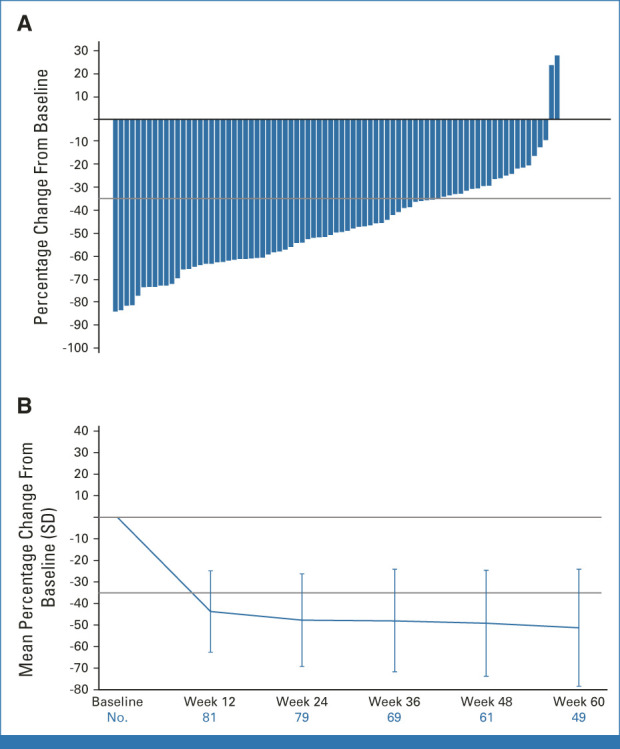
Change in spleen volume by local review after treatment with pelabresib and ruxolitinib. (A) Waterfall plot of percentage change from baseline spleen volume at week 24 by local review. (B) Mean percentage change from baseline spleen volume over time. Patients were evaluable for assessment of spleen volume reduction at week 24 if they had a week 24 assessment by the data cutoff or discontinued without a week 24 assessment at any time. Five patients who discontinued before week 24 spleen assessment were considered nonresponders. Spleen volume median percentage change includes patients with available spleen volume assessment for the corresponding time points. Five patients were nonevaluable at week 48 assessment as they had not yet reached week 48, and 18 patients who discontinued before week 48 were included as nonresponders. For SVR35 at week 24, 95% CIs were 57 to 78. SD, standard deviation; SVR35, ≥ 35% reduction in spleen volume from baseline.
FIG 3.
Subgroup analysis of SVR35 (ITT population). Subgroup analysis of SVR35 at (A) week 24 and (B) week 48. DIPSS, Dynamic International Prognostic Scoring System; HMR, high-molecular risk mutation; HU, hydroxyurea; Int-2, intermediate-2; IPSS, International Prognostic Scoring System; ITT, intent-to-treat; MF, myelofibrosis; N, no; PET, postessential thrombocythemia; PMF, primary MF; PPV, postpolycythemia vera; SVR35; ≥ 35% reduction in spleen volume from baseline; Y, yes; yrs, years.
Secondary end points.
Of 84 patients, 82 were evaluable for symptom scores. Two were excluded: one had a missing baseline value and one had a baseline TSS of 0 (both are ongoing with 52 and 96 weeks of treatment). TSS50 was observed in 46 of 82 (56%; 95% CI, 45 to 67) patients at week 24 (Fig 4A and Data Supplement [Fig S3]; best TSS50 response at any time: 83% [68 of 82]). The median change in TSS was −59% (range, −100% to 225%). At 48 weeks, 43% (34 of 79) had a TSS50 response; the median change in TSS was −54.8% (range, −100% to 307.1%), indicating sustained symptom responses over a 48-week treatment period (Fig 4B).
FIG 4.
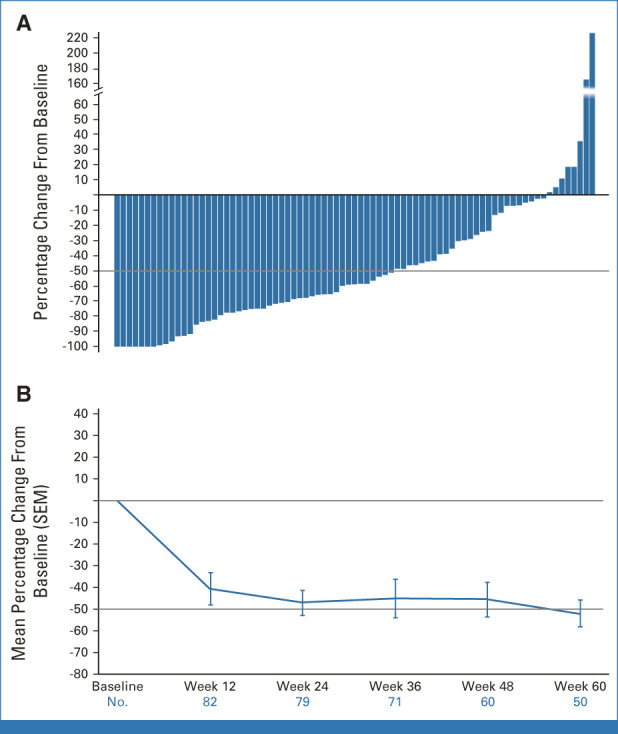
Change in total symptom score after treatment with pelabresib and ruxolitinib. (A) Waterfall plot of percentage change in TSS from baseline to week 24. (B) TSS mean percentage change over time. Patients were evaluable for change in total symptom score at week 24 if they had a week 24 TSS assessment by the data cutoff date or discontinued without a week 24 assessment at any time. Two patients with ongoing treatment were nonevaluable for TSS50 at week 24: one because of a missing baseline assessment and one because of a baseline TSS = 0. Long-term data are still maturing. For TSS50 at week 24, 95% CIs were 45 to 67. SEM, standard error of the mean; TSS, total symptom score; TSS50, ≥ 50% reduction in TSS from baseline.
Pelabresib combined with ruxolitinib led to stabilization or improvements in hemoglobin levels (absolute change from baseline between −1 and + ≥ 1.5 g/dL) in 55% (46 of 84) of patients at week 24 (Data Supplement [Table S2]). Hemoglobin levels improved in 36% (29 of 84) of patients (mean, 1.3 g/dL, median, 0.8 g/dL), whereas 30% (25 of 84) of patients experienced a worsening (decrease of ≥ 1 g/dL from baseline). Over time, steady improvements in mean hemoglobin levels were observed in patients with baseline levels < 10 g/dL, and an initial decrease was observed in patients with baseline levels ≥ 10 g/dL, with a return to or above baseline levels thereafter (Fig 5). Twenty-four percent (20 of 84) of patients achieved a mean hemoglobin increase of ≥ 1.5 g/dL from baseline over any 12-week period without red blood cell transfusions. Ruxolitinib dose levels, spleen, and symptom responses in this group were similar to those of the overall study population.
FIG 5.
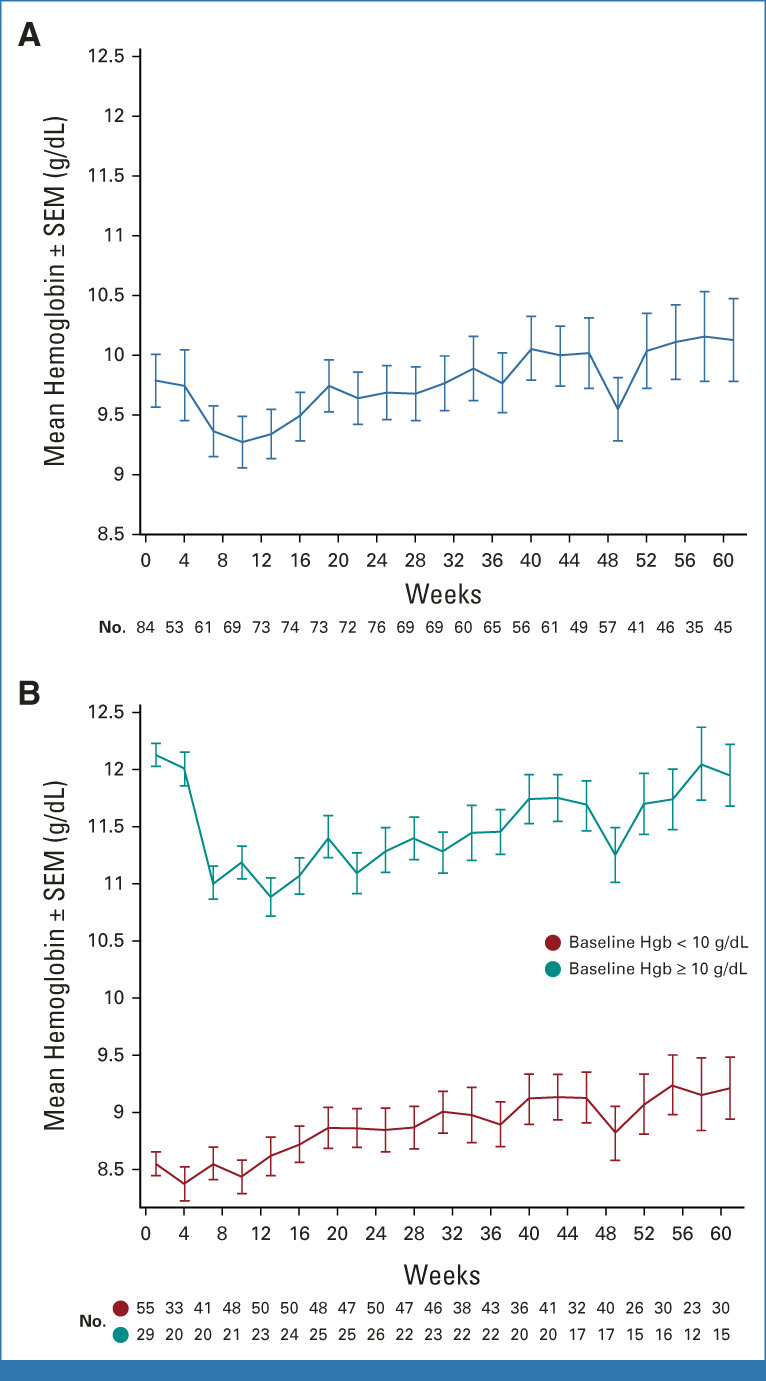
Mean hemoglobin levels over time. (A) Mean hemoglobin levels over time in all patients and (B) in patients on the basis of baseline levels of < 10 g/dL or ≥ 10 g/dL. Hemoglobin values within 2 weeks after transfusions are excluded. Hgb, hemoglobin; SEM, standard error of the mean.
Exploratory end points.
Per blinded central pathology review of BM samples, 28% (16 of 57) of evaluable patients had ≥ 1 grade improvement in reticulin fibrosis at week 24, including 7% (4 of 57) with improvements of two grades; 44% (25 of 57) had no change. Of 24 patients with Grade 1 or 2 reticulin fibrosis at baseline, four (17%) experienced worsening (two patients each with Grade 1 or Grade 2; Data Supplement [Table S3]). No patients with Grade 1 reticulin fibrosis improved to Grade 0; 4 of 7 remained at Grade 1; and 2 of 7 worsened; data for one patient are missing. No significant associations were observed between reticulin fibrosis improvement and clinical end points.
Plasma cytokines were clustered into six groups on the basis of increased expression at baseline and reduced expression during treatment (Data Supplement [Fig S4]). At baseline, the majority of analyzed cytokines showed increased levels (up to 10-fold) compared with healthy donors. In 51 patients with available data, rapid and durable (2-24 weeks) downregulation was observed in a cluster of 19 cytokines (including CD40L, RANTES, TNFα, IL-6, CRP, and IL-18; median of −46% across cytokines and visits) and a second cluster of 24 cytokines (including TARC, ICAM-1, MMP-10, IP-10, and IL-8; median of −23% across cytokines and visits). These clusters were enriched in cytokines previously shown to be NF-κB targets, inflammation-related, and/or elevated in myelofibrosis.23-27 Hepcidin, ferritin (FRTN), EPO, and IFN-g were clustered together because of their upregulation at baseline and during treatment (median increase of 122% across cytokines and visits). Similar changes in plasma cytokines were observed in relapsed/refractory patients with myelofibrosis treated with pelabresib monotherapy19 and with pelabresib add-on treatment in patients with suboptimal response to ruxolitinib.28
At baseline, the JAK2V617F variant was identified in 59 of 80 assayed patients with a mean mutant allele fraction (MAF) of 62.9%. In 44 patients with available blood samples, 29.5% achieved a reduction > 25.0% (allelic reduction was represented as the percentage change in MAF at week 24 compared with baseline). The mean reduction at week 24 was 13% (P < .0001; Data Supplement [Fig S5]). Longitudinal analysis across baseline and weeks 24, 48, and 72 showed significant MAF reduction over time (P = .0028), which correlated with spleen volume reduction (P = .0044). No association of transfusion-dependent status, sex, and age with reduction in MAF has been observed. Compared with patients with primary myelofibrosis, the MAF reduction in patients with postpolycythemia vera myelofibrosis was similar, whereas that of patients with postessential thrombocythemia myelofibrosis was greater (P = .0036)—likely because of the different distribution of MAF across subtypes.
MAF reductions were also observed for CALR and MPL at weeks 24 and 48, not reaching statistical significance with the relatively small sample size. No reduction in MAF was observed for ASXL1 at week 24 or 48.
Exposure
The starting dose of ruxolitinib was 5 mg twice a day in 4 (5%) patients, 10 mg twice a day in 32 (38%) patients, 15 mg twice a day in 44 (52%) patients, and 20 mg twice a day in 4 (5%) patients. The median ruxolitinib dose for the first 24 weeks and at 36 and 48 weeks was 10 mg twice a day (min, max: 5, 25 mg). The median pelabresib dose for the first 24 weeks and at 36 and 48 weeks was 125 mg once daily. The ruxolitinib dose was increased (to 15 or 20 mg twice a day) for four patients (5%) because of not achieving an SVR35 at week 12.
Safety
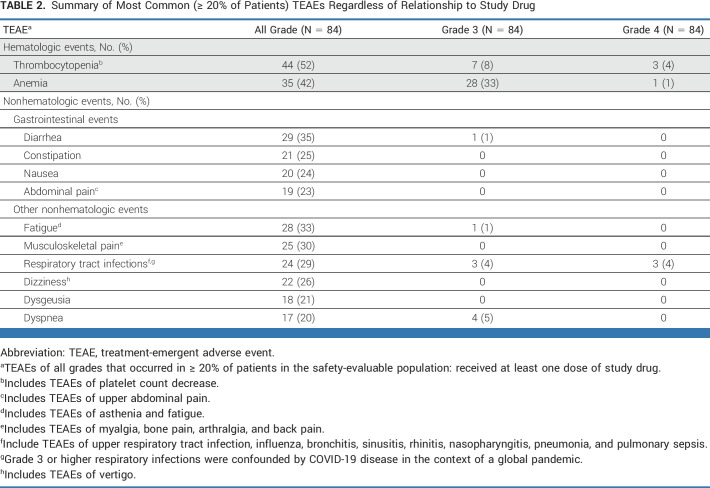
Ninety-six percent of patients (81 of 84) experienced ≥one treatment-emergent adverse event (TEAE), and 63% of patients (53 of 84) experienced ≥one TEAE of Grade 3 or higher. Thrombocytopenia (52%) and anemia (42%) were the most frequent hematologic TEAEs (Table 2). Of these, 12% and 34% were Grade 3/4 thrombocytopenia and anemia, respectively. These cytopenias were manageable and generally reversible with dose modifications or interruptions, with a low discontinuation rate: three (4%) patients discontinued pelabresib and ruxolitinib, two because of Grade 3 thrombocytopenia and one because of Grade 3 anemia.
TABLE 2.
Summary of Most Common (≥ 20% of Patients) TEAEs Regardless of Relationship to Study Drug
The most frequent (≥25% of patients) nonhematologic TEAEs (Table 2) were diarrhea (35%), fatigue (33%), musculoskeletal pain (30%), respiratory tract infection (29%), and constipation (25%). These events were Grade 1 or 2, with the exception of Grade 3 diarrhea (1%), Grade 3 fatigue (1%), Grade 3 dyspnea (5%), and Grade 3 (4%) and Grade 4 (4%) respiratory tract infections; all were managed with appropriate supportive care or dose modification. No events of tuberculosis or hepatitis B reactivation were observed. Herpes zoster was reported in nine (10.7%), Herpes simplex in three (3.6%), and Varicella zoster in three (3.6%) patients; however, these were mostly lower grade and nonserious events, with only one patient experiencing Grade 3 Herpes zoster. One patient each had pulmonary nocardiosis, bacterial endocarditis, cytomegalovirus infection, and Achromobacter infection.
Overall, 37% of patients had pelabresib dose reduction, and 36% had ruxolitinib dose reduction because of TEAEs; 19% required dose reduction of both agents because of TEAEs (additional details on dose reductions, interruptions, and discontinuations are provided in the Data Supplement). Five deaths occurred during study treatment or within 30 days after the last pelabresib dose: four were assessed by the investigator as unrelated to pelabresib and are described in the Data Supplement. One patient died from multiorgan failure because of sepsis secondary to pneumonia deemed as related to pelabresib.
Transformation to acute myeloid leukemia was observed in two (2%) patients on the basis of blast count in BM.
DISCUSSION
In MANIFEST Arm 3, pelabresib combined with ruxolitinib was well tolerated and showed durable spleen and symptom responses, with potential disease-modifying activity as indicated by biomarker findings, in JAKi treatment-naïve patients with myelofibrosis.
The SVR35 response rate at week 24 was 68%; 94% of patients with an SVR35 response at any time point (n = 67) sustained their response 36 weeks after its onset. Symptom improvement was also reported by a substantial proportion of patients (56%). Pivotal studies of JAKi monotherapy have previously demonstrated SVR35 response rates of 29%-42%2-5 and TSS50 rates of 34%-46% at week 24.2,3,5 In general, the MANIFEST patient population was comparable with populations in other studies of JAKis, such as COMFORT-I2; 77% of patients had IPSS intermediate-2– or high-risk disease, and 56% had high-molecular risk mutations.5,9,29 Although baseline hemoglobin levels were lower, time from diagnosis to treatment was shorter, and rate of pretreatment with hydroxyurea was lower compared with historic controls, these parameters did not significantly affect the achievement of SVR35 (Fig 3). A high proportion of patients (46%) harbored adverse prognostic ASXL1 mutations30; however, this did not affect SVR35, TSS50, duration of response, or treatment duration (data not shown), indicating potential for this combination to improve outcomes across subgroups, including those harboring high-risk molecular profiles. The median time since diagnosis of approximately 8.5 months is shorter than that reported in historical studies of JAKi monotherapy; longer time from diagnosis to start of therapy has been associated with poor response.31
Pelabresib combined with ruxolitinib showed high clinical activity without clinically relevant added or limiting toxicity. Discontinuation of ruxolitinib in the real world is frequent, with 1-, 2-, and 3-year rates reported to be 49%, 71%, and 86% (n = 51), respectively, because of loss of therapeutic effect, lack of response, and drug-induced cytopenia.10,29 In the JUMP phase IIIb, expanded-access study, 43% of patients discontinued ruxolitinib within 2 years.32 In this study, 37% (31 of 84) of patients discontinued treatment within 2 years, whereas treatment for 50% (42 of 84) of patients was still ongoing without reaching a 2-year follow-up despite adverse baseline characteristics. Hematologic adverse events, most commonly anemia and thrombocytopenia, were manageable, and two patients discontinued treatment because of Grade 3 thrombocytopenia. Herpes zoster and bacterial infections were mostly low grade and occurred at similar rates to those previously observed with ruxolitinib monotherapy, which is known to have immunosuppressive activity.33
Results of a matching-adjusted indirect comparison analysis to adjust for baseline characteristics and enable a cross-trial comparison focusing on Arm 3 of the MANIFEST trial versus phase III ruxolitinib (COMFORT-I and-II), fedratinib (JAKARTA), and momelotinib (SIMPLIFY-1) trials provide further evidence to support a potentially higher efficacy rate of pelabresib combined with ruxolitinib versus JAKi monotherapy.34
Clinical and preclinical studies have demonstrated that BET proteins are key coactivators of NF-κB–controlled gene expression, including inflammatory target genes such as IL-8.16,18,35 Pelabresib combined with ruxolitinib resulted in early and sustained reduction in levels of cytokines linked to inflammation and NF-κB. We previously reported36 that pelabresib, with or without ruxolitinib, downregulates several cytokines (eg, DKK1, CD27, and TIMP3) that have not previously been demonstrated to be regulated by JAKis37,38 and that the decrease with pelabresib and ruxolitinib treatment was higher than that observed after ruxolitinib monotherapy.2 Centrally reviewed BM samples showed improved (≥ 1 grade) or stable BM fibrosis in 31% and 48% of patients, respectively (Data Supplement [Table S3]). Mutational analysis showed reductions in the mean JAK2V617F MAF, which were associated with SVR35 response. The observed effects on JAK2V617F MAF appear to be comparable with ruxolitinib monotherapy effects in the COMFORT-I study39; however, limitations of cross-trial comparisons and differences in mutation assay methodology constrain comparative analyses.
To our knowledge, the MANIFEST trial in JAKi treatment-naïve patients is the first study with a rational combination of BETi pelabresib and ruxolitinib that showed clinically meaningful durable improvements in splenomegaly and symptoms, was associated with biomarker findings indicating potential disease modification, and demonstrated a generally favorable safety profile. This combination has the potential to improve the standard of care for treatment-naïve patients with myelofibrosis and warrants further investigation. A randomized, double-blind, phase III study (MANIFEST-2) compares safety and efficacy of pelabresib in combination with ruxolitinib with those of ruxolitinib monotherapy in JAKi treatment-naïve patients (ClinicalTrails.gov identifier: NCT04603495).
ACKNOWLEDGMENT
All the authors fulfilled International Committee of Medical Journal Editors authorship criteria. The authors thank the study participants and trial staff, specifically April Chiu, Dong Chen, Curtis A. Hanson, and Horatio Olteanu from the Division of Hematopathology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, and the following study investigators: Wael Harb, Jeanne Marie Palmer, Candido Rivera, Mark Heaney, Prithviraj Bose, Laura Michaelis, Brady Stein, Gary Schiller, Stephen Oh, Shireen Sirhan, Elena Liew, Lynda Foltz, Brian Leber, Jan Van Droogenbroeck, Mark Drummond, Steven Knapper, Mary McMullin, Nauman Butt, Jonathan Lambert, Anna Godfrey, Eric Jourdan, Vincent Ribrag, Nathalie Cambier, Suzanne Tavitian, Florian Heidel, Uwe Platzbecker, Lino Teichmann, Christof Scheid, Alessandra Iurlo, Roberto Massimo Lemoli, Simona Tomassetti, Vittorio Rosti, Peter Te Boekhorst, Gwendolyn Van Gorkom, Ilona Seferynska, Witold Prejzner, and Tomasz Sacha. The authors thank Adrian Senderowicz, Patrick Trojer, Jennifer Mertz, and Debbie Johnson for the development and initiation of the MANIFEST study.
John Mascarenhas
Consulting or Advisory Role: Incyte, Geron, Novartis, CTI BioPharma Corp, Kartos Therapeutics, Constellation Pharmaceuticals, Sierra Oncology, AbbVie, Roche/Genentech, PharmaEssentia, Karyopharm Therapeutics, Galecto
Research Funding: Incyte (Inst), PharmaEssentia (Inst), Merck (Inst), Geron (Inst), AbbVie (Inst), Kartos Therapeutics (Inst), Roche/Genentech (Inst), CTI BioPharma Corp (Inst), Novartis (Inst), Celgene/Bristol Myers Squibb (Inst)
Marina Kremyanskaya
Honoraria: Constellation Pharmaceuticals, Protagonist Therapeutics, Incyte, AbbVie, CTI BioPharma Corp
Consulting or Advisory Role: Protagonist Therapeutics, Constellation Pharmaceuticals, Incyte, AbbVie, CTI BioPharma Corp
Andrea Patriarca
Consulting or Advisory Role: Sanofi, SOBI
Speakers' Bureau: Novartis Italy, Incyte
Francesca Palandri
Honoraria: Novartis, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, CTI
Consulting or Advisory Role: Novartis, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, CTI
Timothy Devos
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Incyte
Francesco Passamonti
Consulting or Advisory Role: ROCHE, AMOMED, Novartis, Celgene/Bristol Myers Squibb, Sandoz, Sierra Oncology, AbbVie, Karyopharma, Sumitomo Dainippon Pharma Oncology
Speakers' Bureau: Novartis, AOP Orphan Pharmaceuticals, Celgene/Bristol Myers Squibb, AbbVie
Raajit K. Rampal
Honoraria: Protagonist Therapeutics
Consulting or Advisory Role: Incyte, Zentalis, Constellation Pharmaceuticals, Bristol Myers Squibb/Celgene, Novartis, Promedior, CTI BioPharma Corp, Blueprint Medicines, Stemline Therapeutics, Galecto, PharmaEssentia, AbbVie, Sierra Oncology, Disc Medicine, Sumitomo Dainippon, SERVIER, Karyopharm Therapeutics
Research Funding: Zentalis, Ryvu Therapeutics, Constellation Pharmaceuticals, Stemline Therapeutics
Travel, Accommodations, Expenses: Incyte, Sierra Oncology
Adam J. Mead
Stock and Other Ownership Interests: Alethiomics
Honoraria: Novartis, Celgene/Bristol Myers Squibb, AbbVie, CTI, Karyopharm Therapeutics, Constellation Pharmaceuticals
Research Funding: Celgene/Bristol Myers Squibb, Novartis, Galecto, Alethiomics
Patents, Royalties, Other Intellectual Property: AJM is co-founder and equity holder in Alethiomics Ltd, a spin out company from the University of Oxford. AJM has licensed a patent to Alethiomics
Gabriela Hobbs
Consulting or Advisory Role: Incyte, AbbVie, Novartis, Blueprint Medicines, Keros Therapeutics, Pfizer, GlaxoSmithKline, Pharmaxis
Research Funding: Incyte
Joseph M. Scandura
Stock and Other Ownership Interests: Scholar Rock, Protagonist Therapeutics, Cellectis
Consulting or Advisory Role: AbbVie, MorphoSys, CTI BioPharma Corp, SDP Oncology, PharmaEssentia
Moshe Talpaz
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Kyowa Kirin International, Imago Pharma, SDP Oncology, Sierra Oncology, GlaxoSmithKline
Research Funding: Bristol Myers Squibb/Celgene, Arcus Biosciences
Nikki Granacher
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Alexion Pharmaceuticals, Alexion Pharmaceuticals
Travel, Accommodations, Expenses: AbbVie, Incyte
Tim C. P. Somervaille
Honoraria: Novartis, Celgene/Bristol Myers Squibb
Consulting or Advisory Role: Novartis, AbbVie (Inst), Oryzon Genomics (Inst)
Research Funding: Imago Biosciences, Cellcentric
Ronald Hoffman
Consulting or Advisory Role: Protagonist Therapeutics, Novartis
Research Funding: Novartis (Inst), Scholar Rock (Inst), Scholar Rock (Inst), Turning Point Therapeutics (Inst)
Marielle J. Wondergem
Other Relationship: Novartis (Inst)
Mohamed E. Salama
Employment: Sonic Healthcare USA
Leadership: Techcyte Inc, Sonic Healthcare USA
Stock and Other Ownership Interests: Techcyte Inc
Gozde Colak
Employment: Constellation Pharmaceuticals
Stock and Other Ownership Interests: MorphoSys
Jike Cui
Employment: Constellation Pharmaceuticals
Stock and Other Ownership Interests: MorphoSys
Research Funding: MorphoSys
Patents, Royalties, Other Intellectual Property: Coauthored one patent at Constellation Pharmaceutical
Jean-Jacques Kiladjian
Consulting or Advisory Role: Novartis, Incyte, BMS, AOP Orphan Pharmaceuticals, AbbVie, PharmaEssentia
Alessandro M. Vannucchi
Consulting or Advisory Role: Novartis, AbbVie, Roche, Incyte, Celgene, Roche
Speakers' Bureau: Novartis, AOP Orphan Pharmaceuticals, Incyte, Roche, Celgene, Blueprint Medicines, AbbVie
Srdan Verstovsek
Consulting or Advisory Role: Constellation Pharmaceuticals, Sierra Oncology, Incyte, Novartis, Celgene
Research Funding: Incyte (Inst), Celgene (Inst), Protagonist Therapeutics (Inst), Sierra Oncology (Inst), PharmaEssentia (Inst), Telios (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), Geron (Inst), Galecto (Inst), Kartos Therapeutics (Inst)
Claire Harrison
Honoraria: Novartis, CTI BioPharma Corp, Geron, Janssen, AbbVie
Consulting or Advisory Role: Promedior, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, Novartis, CTI, Gilead Sciences, Shire, Roche, Janssen, Geron, Galecto, Constellation Pharmaceuticals, Keros Therapeutics
Speakers' Bureau: Novartis, CTI BioPharma Corp, Geron, Sierra Oncology, Bristol Myers Squibb, AbbVie
Research Funding: Novartis (Inst), Constellation Pharmaceuticals (Inst), Bristol Myers Squibb (Inst)
Vikas Gupta
Honoraria: Novartis, Sierra Oncology, Bristol Myers Squibb/Celgene, AbbVie, Constellation Pharmaceuticals, Pfizer, Takeda
Consulting or Advisory Role: Novartis, Sierra Oncology, Roche, AbbVie, Bristol Myers Squibb/Celgene
Research Funding: Novartis (Inst)
No other potential conflicts of interest were reported.
See accompanying Article, p. 5044
PRIOR PRESENTATION
Presented in part at the 60th ASH Annual Meeting and Exposition, San Diego, CA, December 1-4, 2018; ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019; 61st ASH Annual Meeting and Exposition, Orlando, FL, December 7-10, 2019; 25th Annual Congress of EHA, virtual, June 11-21, 2020; 62nd ASH Annual Meeting and Exposition, virtual, December 5-8, 2020; 26th Annual Congress of EHA, virtual, June 9-17, 2021; 63rd ASH Annual Meeting and Exposition, Atlanta, GA, December 11-14, 2021; and 27th Annual Congress of EHA, Vienna, Austria, June 9-17, 2022.
SUPPORT
Supported by Constellation Pharmaceuticals Inc, a MorphoSys Company (Boston, MA). Medical writing support for this publication was provided by Torsten Gerike, Mark Winderlich, Pietro Taverna (MorphoSys Inc), and Ramya Kollipara (LiNK Medical). T.C.P.S. was supported by Cancer Research UK grant No. C5759/A20971.
CLINICAL TRIAL INFORMATION
V.G. and C.H. contributed equally to this article.
DATA SHARING STATEMENT
Data sharing requests by qualified researchers pertaining to Arm 3 of the MANIFEST study will be considered only for noncommercial use on a case-by-case basis (to be approved by MorphoSys; Delphine.ElMehdi@morphosys.com), starting 12 months from acceptance of the manuscript and until 36 months thereafter; approval may be subject to a data access agreement.
AUTHOR CONTRIBUTIONS
Conception and design: John Mascarenhas, Marina Kremyanskaya, Andrea Patriarca, Francesco Passamonti, Ronald Hoffman, Mohamed E. Salama, Gozde Colak, Jike Cui, Claire Harrison
Provision of study materials or patients: Andrea Patriarca, Francesca Palandri, Timothy Devos, Francesco Passamonti, Gabriella Hobbs, Joseph M. Scandura, Moshe Talpaz, Ronald Hoffman, Marielle J. Wondergem, Alessandro M. Vannucchi, Claire Harrison, Vikas Gupta
Collection and assembly of data: John Mascarenhas, Andrea Patriarca, Francesca Palandri, Francesco Passamonti, Raajit K. Rampal, Gabriella Hobbs, Moshe Talpaz, Tim C. P. Somervaille, Ronald Hoffman, Gozde Colak, Jike Cui, Alessandro M. Vannucchi, Natalia Curto-García
Data analysis and interpretation: John Mascarenhas, Marina Kremyanskaya, Andrea Patriarca, Timothy Devos, Francesco Passamonti, Adam J. Mead, Joseph M. Scandura, Nikki Granacher, Tim C. P. Somervaille, Ronald Hoffman, Marielle J. Wondergem, Mohamed E. Salama, Gozde Colak, Jike Cui, Jean-Jacques Kiladjian, Alessandro M. Vannucchi, Srdan Verstovsek, Claire Harrison, Vikas Gupta
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
MANIFEST: Pelabresib in Combination With Ruxolitinib for Janus Kinase Inhibitor Treatment-Naïve Myelofibrosis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
John Mascarenhas
Consulting or Advisory Role: Incyte, Geron, Novartis, CTI BioPharma Corp, Kartos Therapeutics, Constellation Pharmaceuticals, Sierra Oncology, AbbVie, Roche/Genentech, PharmaEssentia, Karyopharm Therapeutics, Galecto
Research Funding: Incyte (Inst), PharmaEssentia (Inst), Merck (Inst), Geron (Inst), AbbVie (Inst), Kartos Therapeutics (Inst), Roche/Genentech (Inst), CTI BioPharma Corp (Inst), Novartis (Inst), Celgene/Bristol Myers Squibb (Inst)
Marina Kremyanskaya
Honoraria: Constellation Pharmaceuticals, Protagonist Therapeutics, Incyte, AbbVie, CTI BioPharma Corp
Consulting or Advisory Role: Protagonist Therapeutics, Constellation Pharmaceuticals, Incyte, AbbVie, CTI BioPharma Corp
Andrea Patriarca
Consulting or Advisory Role: Sanofi, SOBI
Speakers' Bureau: Novartis Italy, Incyte
Francesca Palandri
Honoraria: Novartis, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, CTI
Consulting or Advisory Role: Novartis, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, CTI
Timothy Devos
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Incyte
Francesco Passamonti
Consulting or Advisory Role: ROCHE, AMOMED, Novartis, Celgene/Bristol Myers Squibb, Sandoz, Sierra Oncology, AbbVie, Karyopharma, Sumitomo Dainippon Pharma Oncology
Speakers' Bureau: Novartis, AOP Orphan Pharmaceuticals, Celgene/Bristol Myers Squibb, AbbVie
Raajit K. Rampal
Honoraria: Protagonist Therapeutics
Consulting or Advisory Role: Incyte, Zentalis, Constellation Pharmaceuticals, Bristol Myers Squibb/Celgene, Novartis, Promedior, CTI BioPharma Corp, Blueprint Medicines, Stemline Therapeutics, Galecto, PharmaEssentia, AbbVie, Sierra Oncology, Disc Medicine, Sumitomo Dainippon, SERVIER, Karyopharm Therapeutics
Research Funding: Zentalis, Ryvu Therapeutics, Constellation Pharmaceuticals, Stemline Therapeutics
Travel, Accommodations, Expenses: Incyte, Sierra Oncology
Adam J. Mead
Stock and Other Ownership Interests: Alethiomics
Honoraria: Novartis, Celgene/Bristol Myers Squibb, AbbVie, CTI, Karyopharm Therapeutics, Constellation Pharmaceuticals
Research Funding: Celgene/Bristol Myers Squibb, Novartis, Galecto, Alethiomics
Patents, Royalties, Other Intellectual Property: AJM is co-founder and equity holder in Alethiomics Ltd, a spin out company from the University of Oxford. AJM has licensed a patent to Alethiomics
Gabriela Hobbs
Consulting or Advisory Role: Incyte, AbbVie, Novartis, Blueprint Medicines, Keros Therapeutics, Pfizer, GlaxoSmithKline, Pharmaxis
Research Funding: Incyte
Joseph M. Scandura
Stock and Other Ownership Interests: Scholar Rock, Protagonist Therapeutics, Cellectis
Consulting or Advisory Role: AbbVie, MorphoSys, CTI BioPharma Corp, SDP Oncology, PharmaEssentia
Moshe Talpaz
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Kyowa Kirin International, Imago Pharma, SDP Oncology, Sierra Oncology, GlaxoSmithKline
Research Funding: Bristol Myers Squibb/Celgene, Arcus Biosciences
Nikki Granacher
Consulting or Advisory Role: Novartis, Bristol Myers Squibb/Celgene, Alexion Pharmaceuticals, Alexion Pharmaceuticals
Travel, Accommodations, Expenses: AbbVie, Incyte
Tim C. P. Somervaille
Honoraria: Novartis, Celgene/Bristol Myers Squibb
Consulting or Advisory Role: Novartis, AbbVie (Inst), Oryzon Genomics (Inst)
Research Funding: Imago Biosciences, Cellcentric
Ronald Hoffman
Consulting or Advisory Role: Protagonist Therapeutics, Novartis
Research Funding: Novartis (Inst), Scholar Rock (Inst), Scholar Rock (Inst), Turning Point Therapeutics (Inst)
Marielle J. Wondergem
Other Relationship: Novartis (Inst)
Mohamed E. Salama
Employment: Sonic Healthcare USA
Leadership: Techcyte Inc, Sonic Healthcare USA
Stock and Other Ownership Interests: Techcyte Inc
Gozde Colak
Employment: Constellation Pharmaceuticals
Stock and Other Ownership Interests: MorphoSys
Jike Cui
Employment: Constellation Pharmaceuticals
Stock and Other Ownership Interests: MorphoSys
Research Funding: MorphoSys
Patents, Royalties, Other Intellectual Property: Coauthored one patent at Constellation Pharmaceutical
Jean-Jacques Kiladjian
Consulting or Advisory Role: Novartis, Incyte, BMS, AOP Orphan Pharmaceuticals, AbbVie, PharmaEssentia
Alessandro M. Vannucchi
Consulting or Advisory Role: Novartis, AbbVie, Roche, Incyte, Celgene, Roche
Speakers' Bureau: Novartis, AOP Orphan Pharmaceuticals, Incyte, Roche, Celgene, Blueprint Medicines, AbbVie
Srdan Verstovsek
Consulting or Advisory Role: Constellation Pharmaceuticals, Sierra Oncology, Incyte, Novartis, Celgene
Research Funding: Incyte (Inst), Celgene (Inst), Protagonist Therapeutics (Inst), Sierra Oncology (Inst), PharmaEssentia (Inst), Telios (Inst), Constellation Pharmaceuticals (Inst), AbbVie (Inst), Geron (Inst), Galecto (Inst), Kartos Therapeutics (Inst)
Claire Harrison
Honoraria: Novartis, CTI BioPharma Corp, Geron, Janssen, AbbVie
Consulting or Advisory Role: Promedior, Celgene, AOP Orphan Pharmaceuticals, Sierra Oncology, Novartis, CTI, Gilead Sciences, Shire, Roche, Janssen, Geron, Galecto, Constellation Pharmaceuticals, Keros Therapeutics
Speakers' Bureau: Novartis, CTI BioPharma Corp, Geron, Sierra Oncology, Bristol Myers Squibb, AbbVie
Research Funding: Novartis (Inst), Constellation Pharmaceuticals (Inst), Bristol Myers Squibb (Inst)
Vikas Gupta
Honoraria: Novartis, Sierra Oncology, Bristol Myers Squibb/Celgene, AbbVie, Constellation Pharmaceuticals, Pfizer, Takeda
Consulting or Advisory Role: Novartis, Sierra Oncology, Roche, AbbVie, Bristol Myers Squibb/Celgene
Research Funding: Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tefferi A: Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol 96:145-162, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Verstovsek S, Mesa RA, Gotlib J, et al. : A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366:799-807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesa RA, Kiladjian JJ, Catalano JV, et al. : SIMPLIFY-1: A phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol 35:3844-3850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison C, Kiladjian JJ, Al-Ali HK, et al. : JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366:787-798, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Pardanani A, Harrison C, Cortes JE, et al. : Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncol 1:643-651, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Mascarenhas J, Hoffman R, Talpaz M, et al. : Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: A randomized clinical trial. JAMA Oncol 4:652-659, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verstovsek S, Mesa RA, Gotlib J, et al. : Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: Results of a median 3-year follow-up of COMFORT-I. Haematologica 100:479-488, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascarenhas J, Mehra M, He J, et al. : Patient characteristics and outcomes after ruxolitinib discontinuation in patients with myelofibrosis. J Med Econ 23:721-727, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Newberry KJ, Patel K, Masarova L, et al. : Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood 130:1125-1131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palandri F, Breccia M, Bonifacio M, et al. : Life after ruxolitinib: Reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer 126:1243-1252, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Cheung KL, Kim C, Zhou MM: The functions of BET proteins in gene transcription of biology and diseases. Front Mol Biosci 8:728-777, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratton MS, Haldar SM, McKinsey TA: BRD4 inhibition for the treatment of pathological organ fibrosis. F1000Res 6:F1000 Faculty Rev-1015, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding N, Hah N, Yu RT, et al. : BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci U S A 112:15713-15718, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceribelli M, Kelly PN, Shaffer AL, et al. : Blockade of oncogenic IκB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A 111:11365-11370, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tefferi A, Vaidya R, Caramazza D, et al. : Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: A comprehensive cytokine profiling study. J Clin Oncol 29:1356-1363, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kleppe M, Koche R, Zou L, et al. : Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell 33:785-787, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum K, Abramson J, Maris M, et al. : A phase I study of CPI-0610, a bromodomain and extra terminal protein (BET) inhibitor in patients with relapsed or refractory lymphoma. Ann Oncol 97 (suppl 3):iii7-9, 2018
- 18.Blum KA, Supko JG, Maris M, et al. : A phase I study of pelabresib (CPI-0610), a small-molecule inhibitor of BET proteins, in patients with relapsed or refractory lymphoma. Cancer Research Communications 2:795-805, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremyanskaya M, Mascarenhas J, Palandri F, et al. : Pelabresib (CPI-0610) monotherapy in patients with myelofibrosis—Update of clinical and translational data from the ongoing MANIFEST trial. Blood 138, 2021. (suppl 1; abstr 141) [Google Scholar]
- 20.Kanagasabai T, Venkatesan T, Natarajan U, et al. : Regulation of cell cycle by MDM2 in prostate cancer cells through Aurora Kinase-B and p21WAF1(/CIP1) mediated pathways. Cell Signal 66:109435, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Gale RP, Barosi G, Barbui T, et al. : What are RBC-transfusion-dependence and -independence? Leuk Res 35:8-11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluk MJ, Lindsley RC, Aster JC, et al. : Validation and implementation of a custom next-generation sequencing clinical assay for hematologic malignancies. J Mol Diagn 18:507-515, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher DAC, Miner CA, Engle EK, et al. : Cytokine production in myelofibrosis exhibits differential responsiveness to JAK-STAT, MAP kinase, and NFκB signaling. Leukemia 33:1978-1995, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tefferi A, Vainchenker W: Myeloproliferative neoplasms: Molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol 29:573-582, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Muth M, Engelhardt BM, Kroger N, et al. : Thrombospondin-1 (TSP-1) in primary myelofibrosis (PMF)—A megakaryocyte-derived biomarker which largely discriminates PMF from essential thrombocythemia. Ann Hematol 90:33-40, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Zhang L, Joo D, et al. : NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Study of CPI-0610 in myelofibrosis (MF) (MANIFEST-2) NCT04603495 (2022). https://clinicaltrials.gov/ct2/show/NCT04603495
- 28.Mascarenhas J, Kremyanskaya M, Patriarca A, et al. : S198: BET inhibitor pelabresib (CPI-0610) combined with ruxolitinib in patients with myelofibrosis—JAK inhibitor-naïve or with supoptimal response to ruxolitinib—preliminary data from the MANIFEST study. HemaSphere 6:99-100, 2022 [Google Scholar]
- 29.Abdelrahman RA, Begna KH, Al-Kali A, et al. : Revised assessment of response and long-term discontinuation rates among 111 patients with myelofibrosis treated with momelotinib or ruxolitinib. Leukemia 29:498-500, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Liu W, Wang M, et al. : Prognostic value of ASXL1 mutations in patients with primary myelofibrosis and its relationship with clinical features: A meta-analysis. Ann Hematol 100:465-479, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palandri F, Palumbo GA, Bonifacio M, et al. : Baseline factors associated with response to ruxolitinib: An independent study on 408 patients with myelofibrosis. Oncotarget 8:79073-79086, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ali HK, Griesshammer M, Foltz L, et al. : Primary analysis of JUMP, a phase 3b, expanded-access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br J Haematol 189:888-903, 2020 [DOI] [PubMed] [Google Scholar]
- 33.Elli EM, Barate C, Mendicino F, et al. : Mechanisms underlying the anti-inflammatory and immunosuppressive activity of ruxolitinib. Front Oncol 9:1186, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta V, Mascarenhas J, Kremyanskaya M, et al. : P1029: Matching-adjusted indirect comparison (MAIC) of pelabresib (CPI-0610) in combination with ruxolitinib vs ruxolitinib or fedratinib monotherapy in patients with intermediate or high-risk myelofibrosis. HemaSphere 6:919-920, 2022 [Google Scholar]
- 35.Kunsch C, Rosen CA: NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13:6137-6146, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zavidij O, Keller P, Cui J, et al. : EP1080: The BET inhibitor pelabresib decreases inflammatory cytokines, improves bone marrow fibrosis and function, and demonstrates clinical response irrespective of mutation status in myelofibrosis patients in the phase 2 MANIFEST trial. HemaSphere 5:515, 2021 [Google Scholar]
- 37.Verstovsek S, Kantarjian H, Mesa RA, et al. : Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 363:1117-1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talpaz M, Kiladjian JJ: Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia 35:1-17, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deininger M, Radich J, Burn TC, et al. : The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood 126:1551-1554, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing requests by qualified researchers pertaining to Arm 3 of the MANIFEST study will be considered only for noncommercial use on a case-by-case basis (to be approved by MorphoSys; Delphine.ElMehdi@morphosys.com), starting 12 months from acceptance of the manuscript and until 36 months thereafter; approval may be subject to a data access agreement.