Outcome improvement for T-cell acute lymphoblastic leukemia and Induction failure in a contemporary treatment era.
Abstract
PURPOSE
Historically, patients with T-cell acute lymphoblastic leukemia (T-ALL) who fail to achieve remission at the end of induction (EOI) have had poor long-term survival. The goal of this study was to examine the efficacy of contemporary therapy, including allogeneic hematopoietic stem cell transplantation (HSCT) in first remission (CR1).
METHODS
Induction failure (IF) was defined as the persistence of at least 5% bone marrow (BM) lymphoblasts and/or extramedullary disease after 4-6 weeks of induction chemotherapy. Disease features and clinical outcomes were reported in 325 of 6,167 (5%) patients age 21 years and younger treated in 14 cooperative study groups between 2000 and 2018.
RESULTS
With a median follow-up period of 6.4 years (range, 0.3-17.9 years), the 10-year overall survival (OS) was 54.7% (SE = 2.9), which is significantly higher than the 27.6% (SE = 2.9) observed in the historical cohort from 1985 to 2000. There was no significant impact of sex, age, white blood cell count, central nervous system disease status, T-cell maturity, or BM disease burden at EOI on OS. Postinduction complete remission (CR) was achieved in 93% of patients with 10-year OS of 59.6% (SE = 3.1%) and disease-free survival (DFS) of 56.3% (SE = 3.1%). Among the patients who achieved CR, 72% underwent HSCT and their 10-year DFS (with a 190-day landmark) was significantly better than nontransplanted patients (63.8% [SE = 3.6] v 45.5% [SE = 7.1]; P = .005), with OS of 66.2% (SE = 3.6) versus 50.8% (SE = 6.8); P = .10, respectively.
CONCLUSION
Outcomes for patients age 21 years and younger with T-ALL and IF have improved in the contemporary treatment era with a DFS benefit among those undergoing HSCT in CR1. However, outcomes still lag considerably behind those who achieve remission at EOI, warranting investigation of new treatment approaches.
INTRODUCTION
T-cell acute lymphoblastic leukemia (T-ALL) comprises about 10% of acute lymphoblastic leukemia (ALL) in young children and 25%-30% in adolescents and young adults with a historically worse prognosis than B-cell acute lymphoblastic leukemia (B-ALL).1,2 Outcomes have improved in recent trials using risk-adapted intensive therapy; however, resistant and recurrent disease remain a challenge, not least in young adults.3-24 CNS involvement at diagnosis is more common in T-ALL25 and the kinetics of bone marrow (BM) disease response in T-ALL is slower than B-ALL with a higher proportion showing prednisone poor response (34.7% v 6.3% B-ALL), induction failure (IF; 8% v 1.5%),26-28 and persistence of high minimal residual disease (MRD) levels at the end of consolidation (EOC) therapy (≥5 × 10–4 in 20.9% v 5.9%) in AIEOP-BFM trials.6,29
CONTEXT
Key Objective
What are the outcomes for children with T-cell acute lymphoblastic leukemia (T-ALL) who fail induction therapy (≥5% marrow blasts) in a contemporary treatment era?
Knowledge Generated
The majority of children with T-ALL induction failure (IF) achieve a complete remission (CR) with post-induction chemotherapy and their 10-year overall survival rates have nearly doubled over the past 20 years and now approach 60%. Among children who achieve a CR, disease-free survival was superior with hematopoietic stem cell transplantation in first remission compared with chemotherapy alone in this retrospective analysis from 14 treating consortia.
Relevance (S. Bhatia)
While hematopoietic cell transplantation are a therapeutic option for T-ALL IF patients who subsequently attain a CR with conventional chemotherapy, these patients should be candidates for new T-cell targeted therapy including cellular approaches.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH, FASCO.
Patients with T-ALL and IF had a very poor outcome (10-year overall survival [OS], 28%) in a previous intergroup Ponte di Legno (PDL) study.28 As some studies have shown higher cure rates with allogeneic hematopoietic stem cell transplant (HSCT),30,31 this treatment approach has been pursued in first remission (CR1) in many groups. To determine if greater application of CR1 HSCT and the use of nelarabine may have improved outcomes in this high-risk subgroup, we, as intergroup PDL, analyzed a cohort of IF T-ALL cases diagnosed between 2000 and 2018, who failed to achieve complete remission (CR) at the end of induction (EOI) therapy. Our primary aim was to assess long-term outcome with contemporary therapy, including the role of HSCT.
METHODS
Study Design and Patients
Data from 14 cooperative study groups (Appendix Table A1, online only) in Europe, North America, and Asia were collected on patients registered on clinical trials conducted from 2000 to 2018 (included). All the clinical trials from which data were used in this analysis had received approval from the relevant institutional review boards or ethics committees, and written informed consent had been obtained from patients or guardians.
Each study group was asked to identify all patients age 21 years and younger with T-ALL who had IF defined as persistence of at least 5% BM lymphoblasts by morphology and/or persistence of extramedullary disease (EMD) at EOI, which was scheduled according to protocol, between days 28 and 43. Medullary IF was confirmed by MRD analysis (≥10–2) in 211 of the 220 patients with available data (96%), using a more contemporary MRD-based definition of treatment failure.32 A predefined set of data were collected for each patient: clinical, biologic, and genetic characteristics; treatment protocol, including treatment arm and HSCT; early treatment responses, including MRD level at EOI and EOC where available; and clinical outcomes, including the achievement of CR with postinduction treatment (defined as a blast percentage by morphology <5% and no EMD), relapse, second malignant neoplasm (SMN), and death. All data were centrally reviewed for consistency and completeness before analyses.
Follow-up extended through May 2021 with a median of 6.4 years (range, 0.3-17.9); in particular, 70% of patients without a first relapse or death in CR were followed for more than 5 years. Treatment strategies for patients with EOI failure differed among the study groups. Most common postinduction schedules consisted of protocol IB (consolidation), augmented IB, nelarabine followed by augmented IB, or intensive chemotherapy blocks.3,6,9,10,14,15,18-20,22,24 Frequently, there was a protocol indication to proceed to CR1 HSCT in patients who obtained CR with postinduction treatment.
Statistical Analysis
Baseline characteristics are reported as percentages. The main end points were OS and disease-free survival (DFS). OS was calculated from diagnosis to death of any cause or date of last contact, if alive. DFS was computed only for subjects who achieved CR with postinduction therapy and was defined as the time from diagnosis until relapse, death in CR, development of a SMN, or date of last contact, if disease-free. Date of diagnosis was used as time of origin since date of CR differed among study groups and was not uniformly available. The Kaplan-Meier estimator was used for OS and DFS, with associated SEs calculated by Greenwood and the log-rank test was used for comparisons.
We further analyzed the patients with T-ALL described in the historical cohort reported by Schrappe et al28 for assessment of OS and achievement of remission with postinduction treatment to be able to compare their outcome data with those of the more recent cohort reported here. To minimize potential bias in the comparison of outcome between patients treated with chemotherapy followed by transplantation and with intensive chemotherapy only, the Kaplan-Meier curves were adjusted to account for the waiting time to transplantation: the curves originated at a landmark (median time to transplantation) and did not include patients who experienced events or whose data were censored before that time; the curves were also adjusted to account for the delayed entry of patients into the transplantation group, when transplantation occurred after the landmark.33
To deal with the lack of proportional hazards, as seen by graphical check, between the two treatment cohorts (HSCT v no HSCT) and to model the profile of the hazard ratio (HR) in time, we applied a piecewise Poisson model on DFS (in intervals of 30 days).33 In the model, transplantation was treated as a time-dependent variable (a transplanted patient was included in the chemotherapy group until HSCT). The time since diagnosis was modeled by a flexible B-spline function (six degrees of freedom), whereas the time dependence of the treatment effect (ie, nonproportional hazards) was accommodated by including a term for interaction between treatment and time since transplantation (modeled as B-spline with one knot at 180 days). The model was adjusted for age, sex, white blood cell count, BM at the EOI, and period of diagnosis. Survival after different types of transplants (from date of HSCT) was also estimated and compared. Analyses were carried out using R and SAS 9.4 (SAS Institute, Cary, NC) software programs. P < .05 was considered statistically significant.
RESULTS
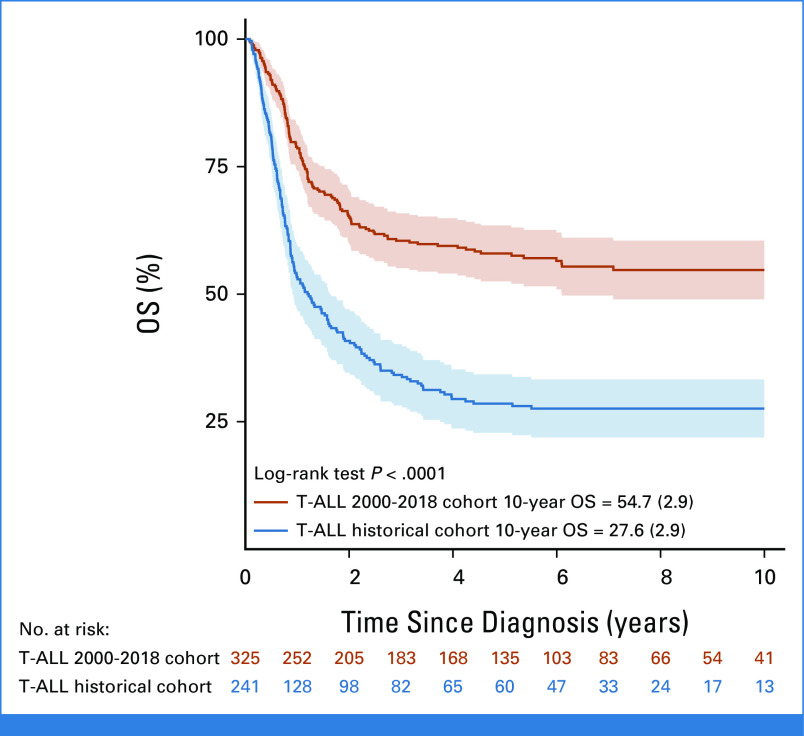
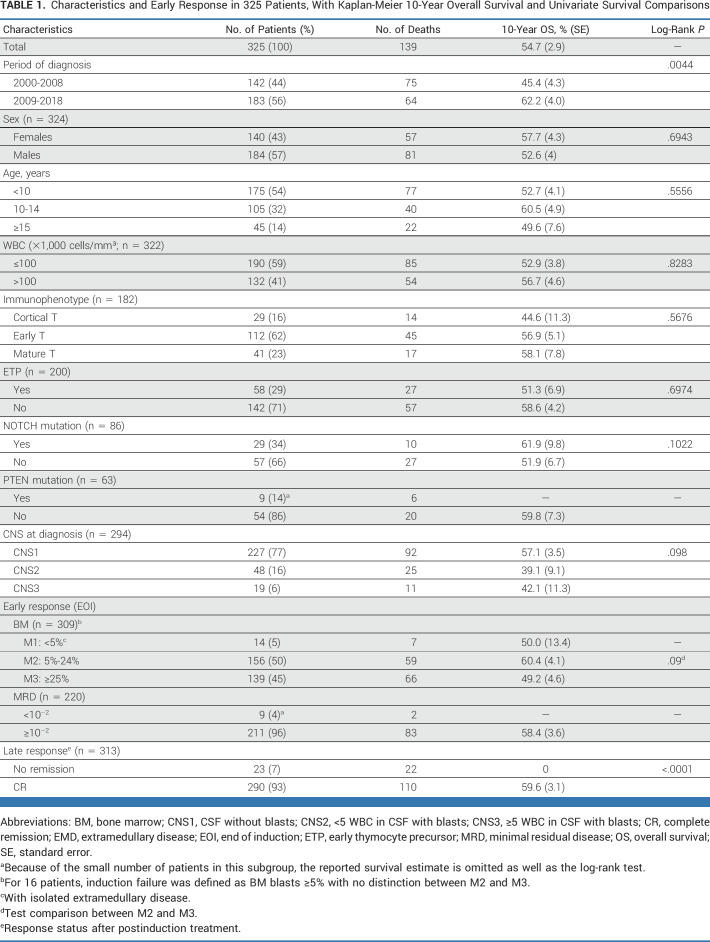
Of the 344 patients assessed, 19 were found ineligible and thus 325 are included in the cohort analyzed (Fig 1). The 5- and 10-year OS were 58.0% (SE = 2.8) and 54.7% (SE = 2.9), respectively, and significantly higher than the 28.5% (SE = 2.9) and 27.6% (SE = 2.9) observed in the historical cohort (N = 241; Fig 2).28 Of note, within the recent cohort, the OS improved even more for patients diagnosed in the period 2009-2018 (n = 183) compared with those diagnosed from 2000 to 2008 (n = 142; OS = 62.2%; SE = 4.0% v 45.4%; SE = 4.3%; P = .0044; Table 1). No significant impact on OS was seen for sex, age, and white blood cell count at diagnosis nor for T-cell immunophenotype maturity (Table 1; Appendix Fig A1, online only). The early thymocyte precursor (ETP) subtype, which represents approximately 15% of T-ALL in children and adolescents, was diagnosed in 58 (29%) of 200 patients with adequate immunophenotypic data, using definitions established at each participating consortium; their 10-year survival was however similar to the non-ETP patients (51.3%; SE = 6.9% v 58.6%; SE = 4.2%, respectively; Appendix Fig A1). Information on NOTCH and PTEN mutations were reported for a minority of patients: NOTCH mutation was detected in 29/86 patients (34%), which is a lower frequency than in unbiased cohorts,34 with no significant difference in survival compared with those with the wild-type; PTEN mutation was present in 9/63 patients (14%) with only three patients surviving (Table 1). Among 294 patients with CNS status data at diagnosis, 227 were CNS1, 48 CNS2, and 19 CNS3, and their survival was not significantly different (Table 1; Appendix Fig A1; P = .098).
FIG 1.
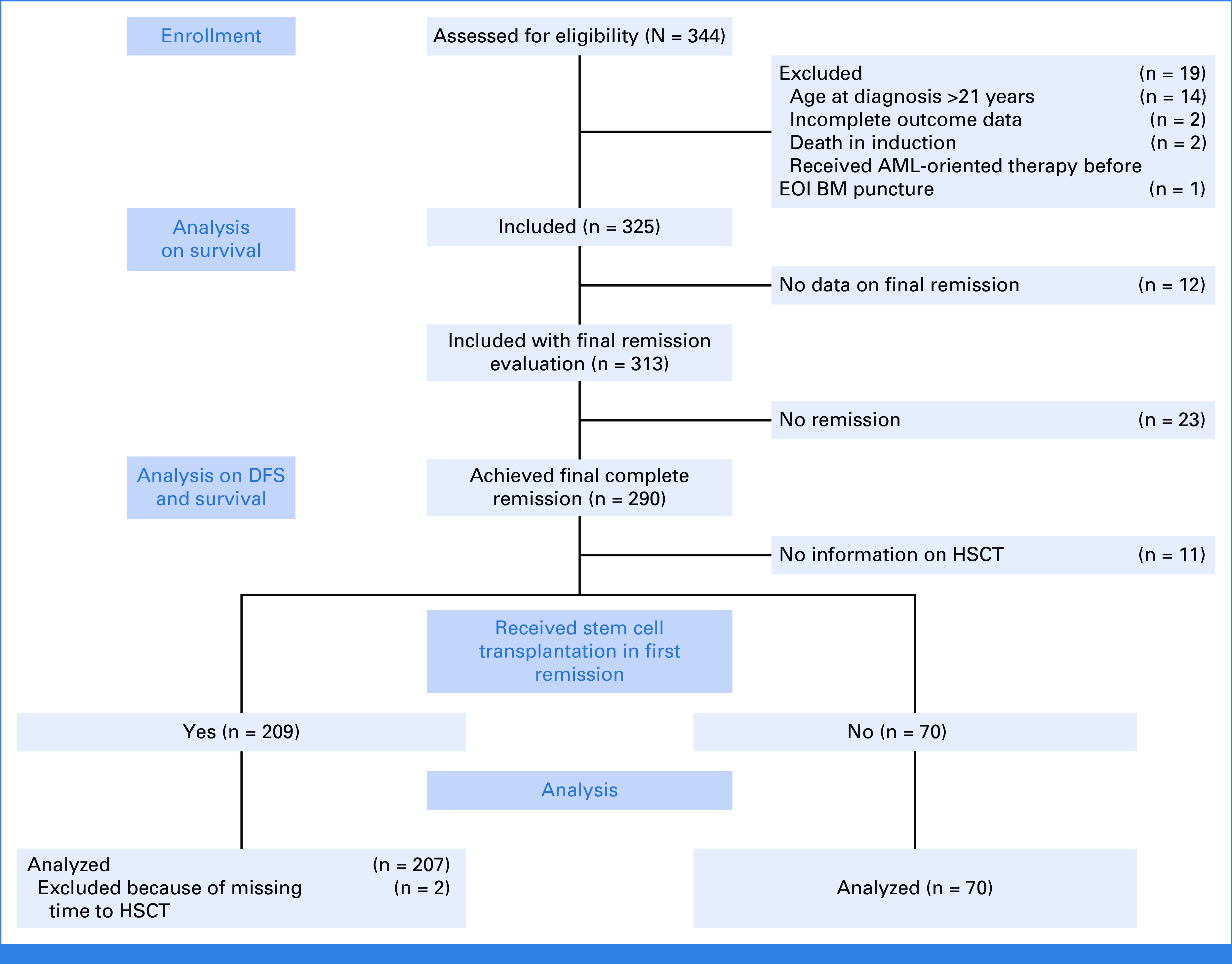
2010 flow diagram. AML, acute myeloid leukemia; BM, bone marrow; DFS, disease-free survival; EOI, end of induction; HSCT, hematopoietic stem cell transplantation.
FIG 2.
OS since diagnosis of patients with T-ALL and IF in the current study (N = 325) and in the historical cohort (N = 241) reported by Schrappe et al.28 IF, induction failure; OS, overall survival; T-ALL, T-cell acute lymphoblastic leukemia.
TABLE 1.
Characteristics and Early Response in 325 Patients, With Kaplan-Meier 10-Year Overall Survival and Univariate Survival Comparisons
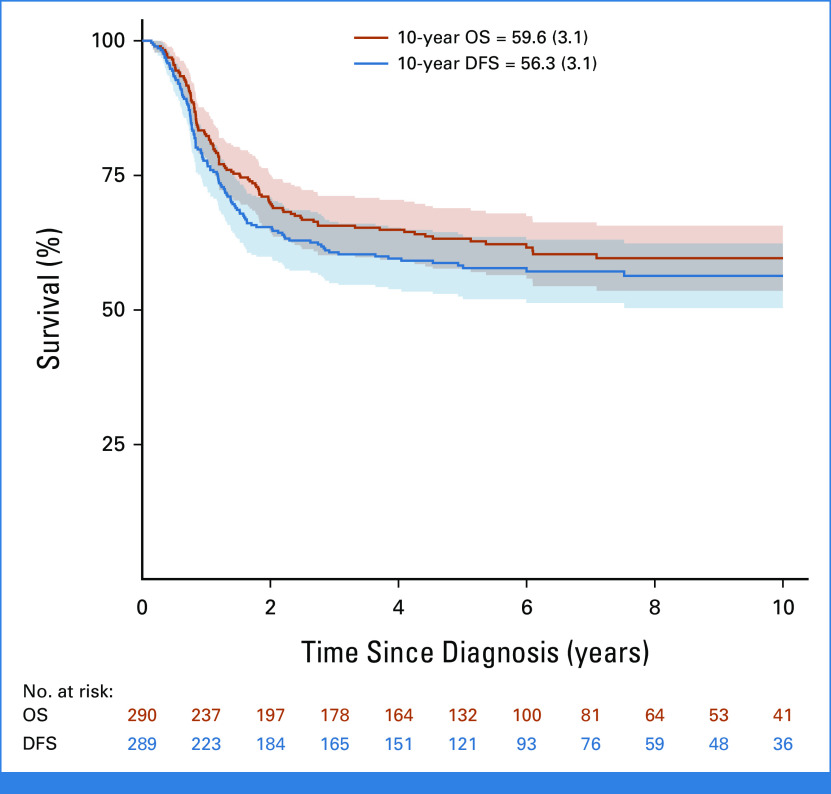
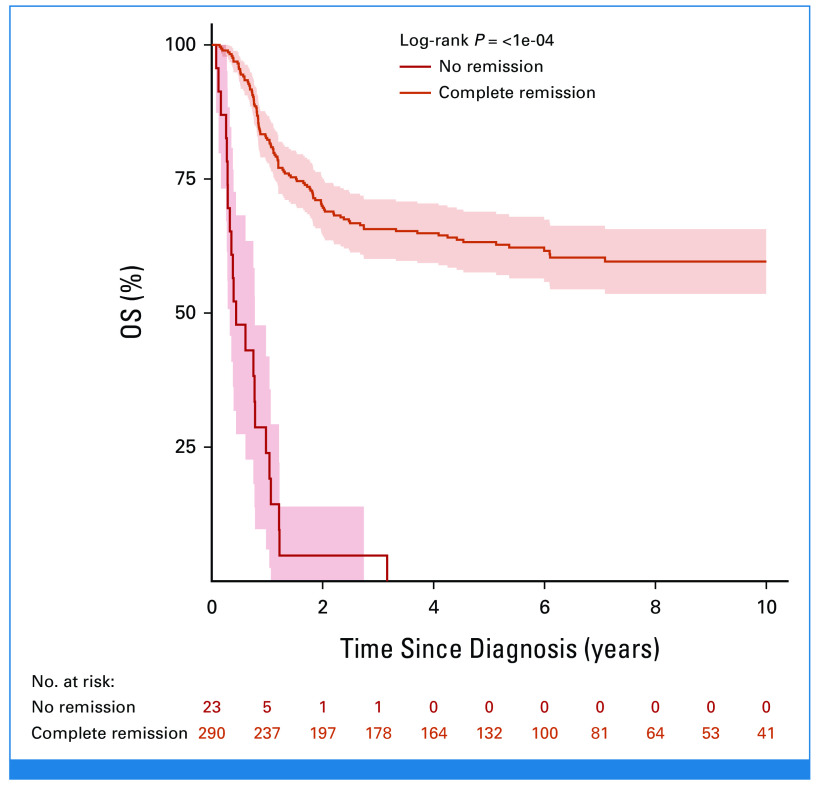
At EOI, 14 patients with CR BM (<5% blasts) had IF because of persistent isolated EMD (one CNS, five mediastinal mass, three lymph nodes, four thymus/liver/spleen/lymph nodes, and one unknown), seven of whom survived. The 10-year OS for the 156 patients with M2 (5%-24% blasts) and the 139 with M3 (≥25% blasts) marrows was 60.4% (SE = 4.1%) and 49.2% (SE = 4.6%; P = .09), respectively. The 211 patients with MRD at EOI ≥10–2 had 10-year OS (58.4%; SE = 3.6%) similar to that of the whole cohort (54.7%; SE = 2.9; Table 1). Of the 313 patients evaluable for CR, 290 patients (93%) achieved a CR (Fig 1) and they had 10-year OS and DFS of 59.6% (SE = 3.1%) and 56.3% (SE = 3.1%), respectively (Fig 3). Among the 290 who achieved CR, 232 had information on the time of remission, reported at a median time of 84 days from diagnosis (IQR, 63-102 days). There was no significant difference in survival, with a 10-year OS of 57.8% (SE = 4.8) in patients who achieved CR by day 84 after diagnosis (n = 118) versus 59.5% (SE = 4.9) in those (n = 114) who obtained CR later (P = .7). Of the 23 patients who did not achieve CR, 22 died at a median of 5 months from diagnosis and one was lost to follow-up (Appendix Fig A2, online only; Table 1).
FIG 3.
OS and DFS of 290 patients with T-ALL resistant to induction therapy who achieved complete remission with postinduction treatment. Date of relapse was not available for one patient, thus it was excluded from DFS analysis. DFS, disease-free survival; OS, overall survival; T-ALL, T-cell acute lymphoblastic leukemia.
As mentioned in the Methods section, we also reanalyzed the historical cohort, which was published in 2012 (period 1985-2000),28 for the data on achievement of CR. Of the 206 with available information on postinduction treatment outcome, 143 (69%) achieved CR, a rate significantly lower than that of the current cohort (P < .001). For those who did achieve CR in the historical cohort, the 10-year OS was 40.1% (SE = 4.1%).
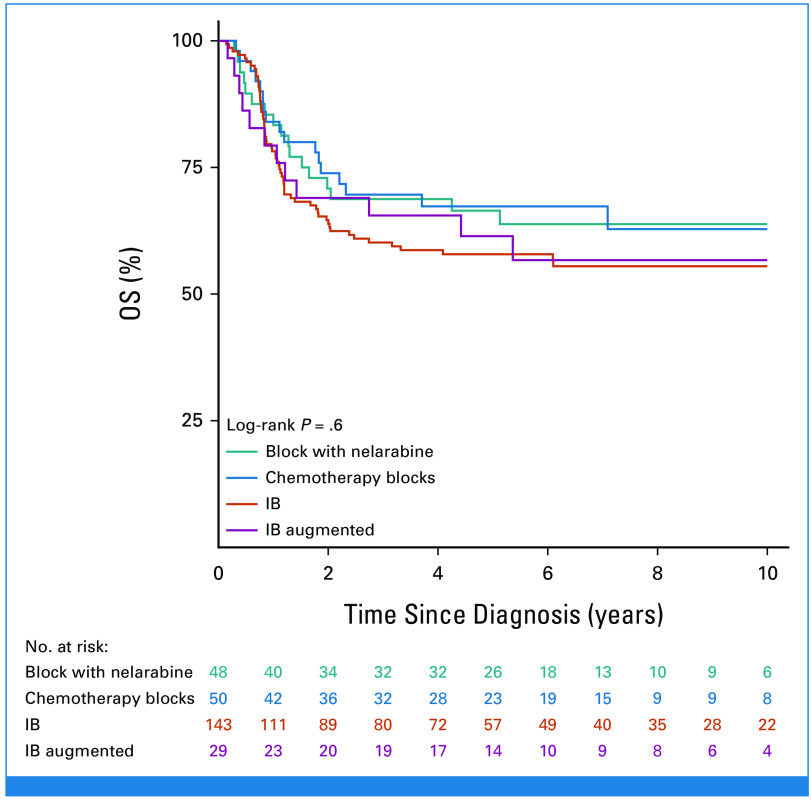
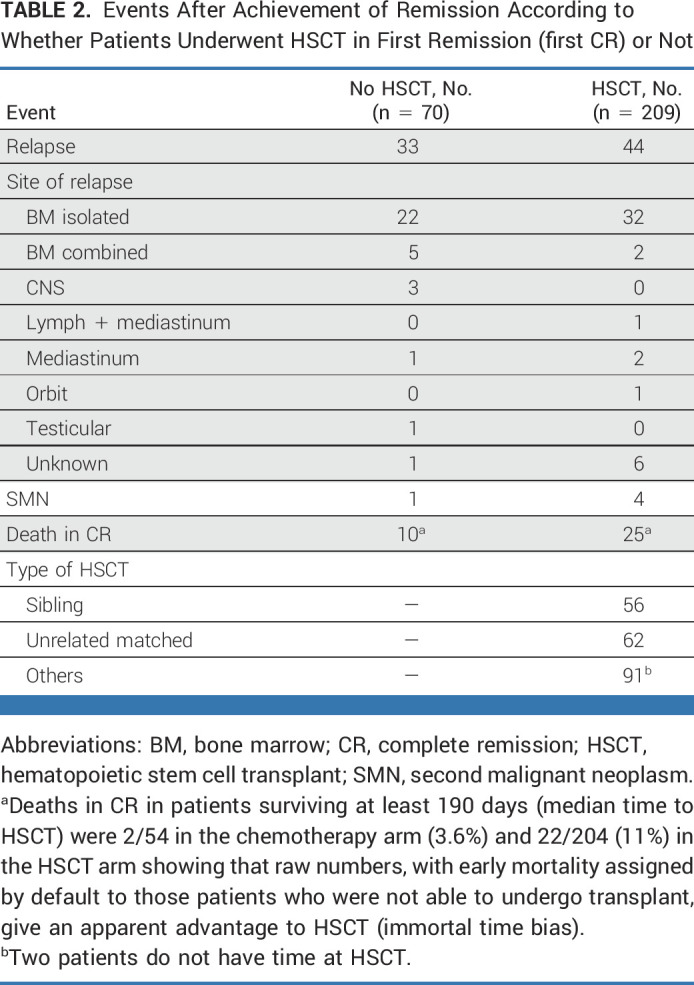
The most commonly used postinduction therapies (in 274 patients with data) were protocol IB (consolidation; n = 143), high-dose chemotherapy blocks (n = 50), nelarabine-containing regimens (n = 48), and augmented IB (n = 29). No significant difference in survival was observed according to treatment received (Appendix Fig A3, online only). Of the 290 patients who achieved CR, 209 (72%) received a transplant and 70 received only chemotherapy (33 relapsed, seven of whom were transplanted in second CR); no data on HSCT were available for 11 patients. In a 190-day landmark analysis (Fig 4), 10-year DFS was significantly better for transplanted patients (63.8% [SE = 3.6] v 45.5% [SE = 7.1]; P = .005), which translated into a nonsignificantly better OS of 66.2% (SE = 3.6) versus 50.8% (SE = 6.8; P = .10). The most frequent adverse event after HSCT was relapse (n = 44) followed by death in CR (n = 25; Table 2). As shown in Appendix Figure A4A (online only), there was an improvement in survival in transplanted patients diagnosed in the period 2009-2018 (5-year OS of 74.4%; SE = 4%) compared with those diagnosed in the period 2000-2008 (5-year OS of 59.8%; SE = 5.4%), although the difference was not statistically significant (P = .08). Small decreases both in the rate of transplant-related mortality (9.5% v 16%) and of post-transplant relapse (19% v 24%) were observed. Of note, compared with patients treated in the early period, those treated in the latter period were more likely to have undergone transplant in CR (78% v 71%) and included more matched unrelated donor HSCTs (33% v 24% of transplanted patients). Survival in transplanted patients by type of donor was higher and similar for sibling (5 years post HSCT 79.8%; SE = 5.5) and matched unrelated (72%; SE = 5.8) donors (P = .3) compared with other types of donors (58.4%; SE = 5.3; P = .03 for the three-way comparison; Appendix Fig A4B).
FIG 4.
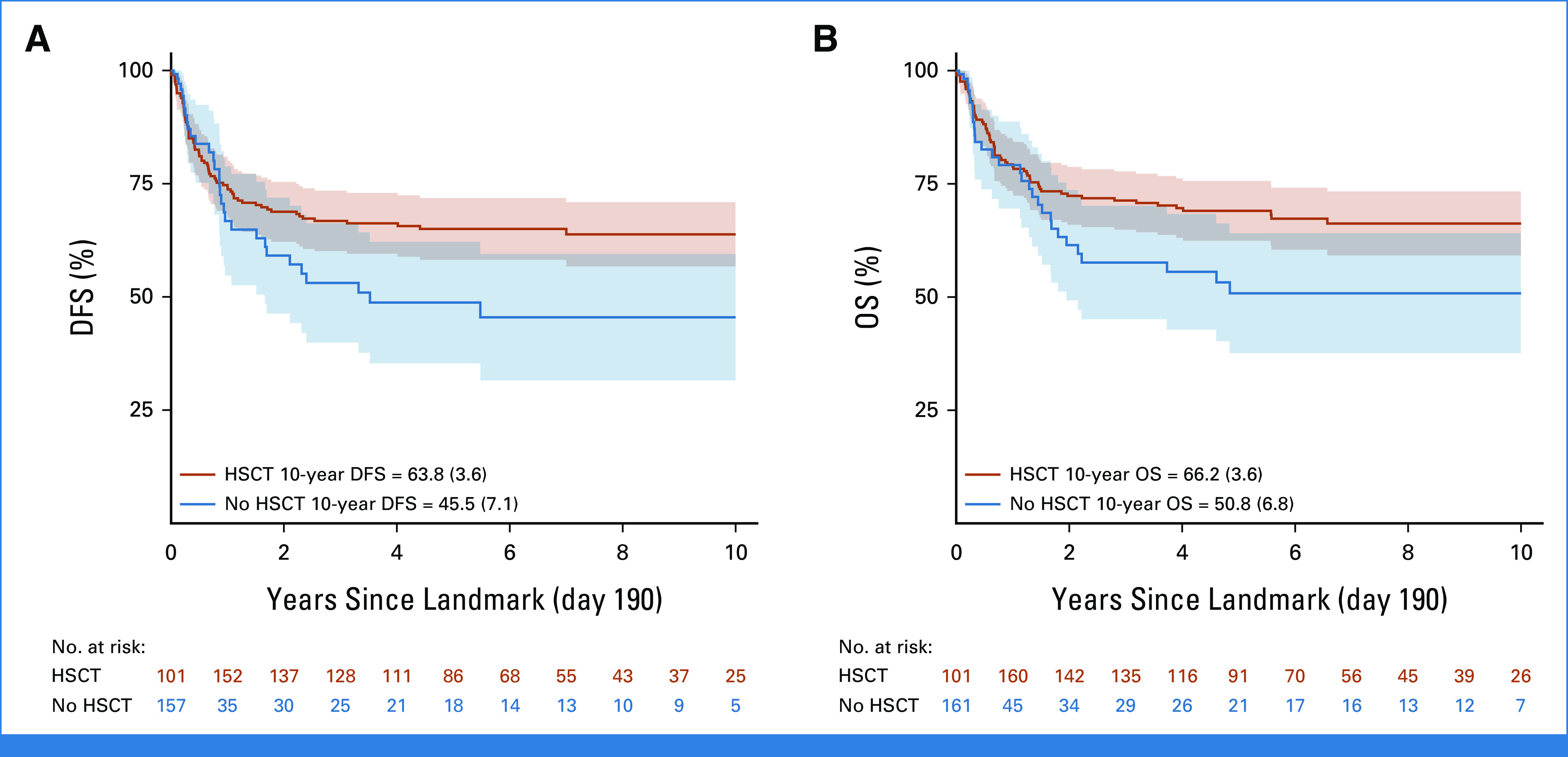
(A) DFS and (B) OS of patients with T-ALL who achieved remission with postinduction treatment according to whether they received HSCT or not in first CR—time since landmark at 190 days (median time from diagnosis to HSCT). DFS comparison: P = .005 (unadjusted Poisson model, likelihood ratio test with five degrees of freedom); OS comparison: P = .1 (unadjusted Poisson model, likelihood ratio test with five degrees of freedom). CR, complete remission; DFS, disease-free survival; HSCT, hematopoietic stem cell transplantation; OS, overall survival; T-ALL, T-cell acute lymphoblastic leukemia.
TABLE 2.
Events After Achievement of Remission According to Whether Patients Underwent HSCT in First Remission (first CR) or Not
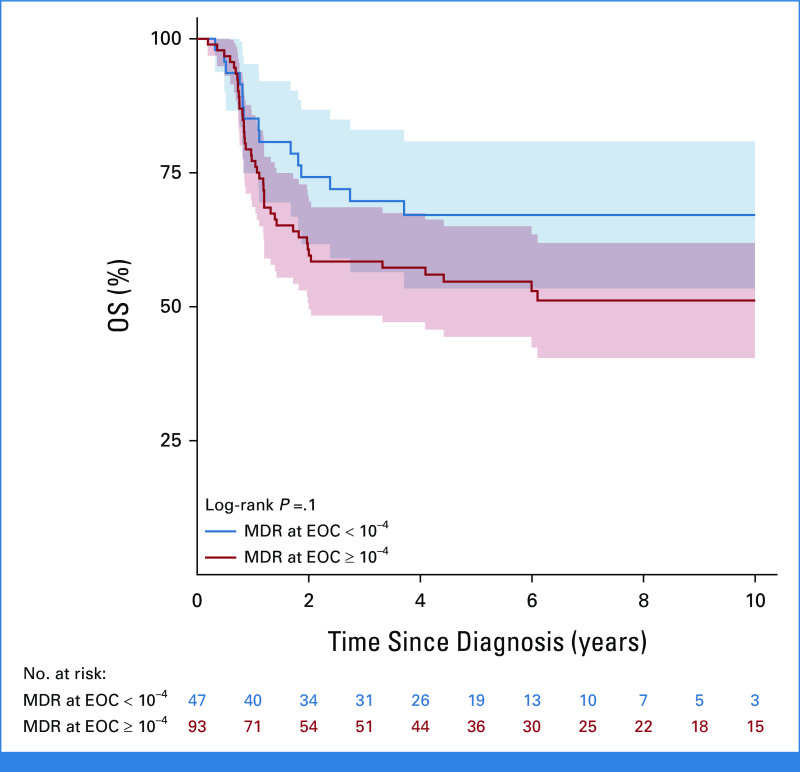
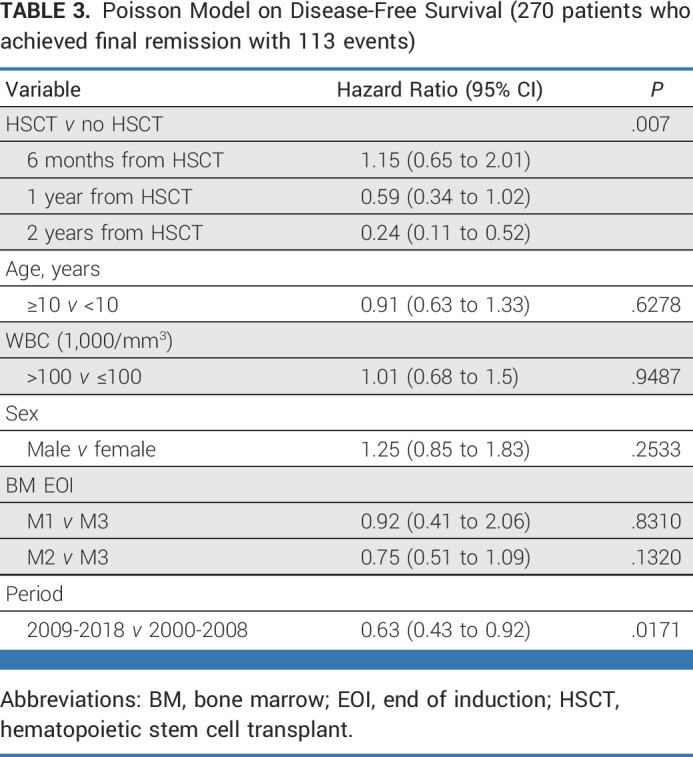
The Poisson model on DFS (Table 3) shows that prognosis was favorably associated with HSCT in CR1 versus no HSCT (P = .007), with a time-dependent effect reporting a significant protection at 2 years post HSCT (HR at 2 years since HSCT = 0.24; 95% CI, 0.11 to 0.52) after adjusting for age, white blood cell count, sex, marrow status at EOI, and period of diagnosis (for this latter variable, the estimated HR was 0.63; 95% CI, 0.43 to 0.92; P = .0171; 2009-2018 v 2000-2008). Although data on MRD level before HSCT were not available, data on MRD at the EOC were available in a subset of patients. Of the 290 patients who achieved CR, 140 had available MRD data at EOC, and there were 47 with EOC MRD <10–4, including 12 patients with PCR MRD that was positive but not quantifiable. The OS of patients with EOC MRD <10–4 was 67.1% (SE = 7%) compared with 51.2% (SE = 5.5%) for ≥10–4 (P = .1; Appendix Fig A5, online only). An exploratory analysis comparing DFS and OS in patients undergoing HSCT or chemotherapy alone showed an advantage for HSCT within both EOC MRD-based subgroups (Appendix Fig A6, online only).
TABLE 3.
Poisson Model on Disease-Free Survival (270 patients who achieved final remission with 113 events)
DISCUSSION
T-ALL with IF occurs in approximately 8% of patients,9,35 representing about 1% of all cases of childhood ALL. Although survival rates for pediatric patients with newly diagnosed T-ALL without IF have steadily improved and now tend to approximate those achieved in B-ALL, T-ALL with IF remains challenging to treat.13 Such an uncommon subgroup can best be investigated in a large intergroup collaboration, such as that of the PDL Group. A previous PDL study of IF reported a 10-year OS of 27.6% (SE = 2.9%) in 241 patients with T-ALL and IF diagnosed between 1985 and 2000.28 Seventy-seven (54%) of the 143 patients who achieved CR underwent HSCT and the 10-year OS was 40% in patients who received a matched related donor and 45.8% in the 55% patients who received HSCT from other donors.28
We report an improvement in 10-year OS to 54.7% (SE = 2.9%; P < .0001) for 325 patients with T-ALL and IF treated in a subsequent era from 2000 to 2018. The improved outcome might be attributable to a higher proportion of patients achieving CR after subsequent treatment (93% v 69%; P < .0001) and proceeding to CR1 HSCT (72% v 54%), including increased use of unrelated and haploidentical donors. A variety of postinduction treatments were used to achieve CR but most included standard/augmented IB with (17%) or without nelarabine. CR was achieved in 97.2% (139 of 143) after IB-based therapy, 89.6% (26 of 29) after augmented IB, and 85.4% after nelarabine followed by augmented IB (41 of 48). There was no significant difference in outcome on the basis of postinduction treatment given, and EOC MRD was only available in 30% of subjects reported here. Thus, no recommendation can be made on the optimal regimen on the basis of our data. Attainment of an MRD-negative remission before HSCT could have affected outcomes as well; however, these data were not routinely available and/or reported in this study. As expected, all patients who did not achieve CR had a fatal outcome.
Although we cannot exclude selection biases, the outcome of transplanted patients in CR1 (adjusted by landmark analysis at 190 days) was significantly better than those not transplanted, in regard to DFS (63.8%; SE = 3.6 v 45.5%; SE = 7.1) with a tendency for improved OS (66.2%; SE = 3.6 v 50.8%; SE = 6.8). Patients transplanted from sibling and unrelated donors had superior outcomes compared with alternative donor transplants. Patients diagnosed in the later half of the study period had a better outcome (10-year survival estimate of 62.2% v 45.4% in 2009-2018 and 2000-2008, respectively). Although the proportion achieving CR1 was similar, a slightly higher proportion of patients were transplanted in the later period (78% v 71%) which, along with better post-transplant outcomes, might partly explain the improved overall outcome.
We had limited data on immunophenotype, cytogenetic, and molecular profiles. Several studies have reported a higher incidence of IF in the ETP subgroup11,36 and our data confirm that observation with an enrichment of the ETP subtype (29%) compared with T-ALL at diagnosis (15%). Similar to the previous reports of patients with ETP ALL without IF,11,36 ETP patients with IF had no worse outcome than other patients with T-ALL and IF. In this study, IF was firmly established by MRD in 96% of patients with M2/3 BM. Of the nine patients with MRD <1 × 10–2, seven remain in continuous CR, of whom three received HSCT and four chemotherapy only. The relatively favorable outcome of these patients may suggest an incorrect morphologic classification of the BM and emphasizes the importance of MRD in establishing IF in future cases.
Although our study is limited by its retrospective nature, heterogeneity of chemotherapy regimens used to achieve CR after IF, and the use of different types of transplantation procedures, we can report a significant improvement in outcome compared with a historical cohort. The use of nelarabine as salvage therapy did not affect treatment outcomes in our study. Notably, attainment of a CR after IF is paramount as there were no survivors among patients with refractory disease, highlighting the need for effective salvage regimens. Our study suggests transplantation should be considered in patients with T-ALL IF who subsequently attain a CR with conventional chemotherapy, regardless of MRD status at the EOC. Despite the reported improvement in this more recent treatment era, the outcome of patients with T-ALL and IF remains considerably worse than those who achieve CR after induction therapy and they should be candidates for early-phase studies of new T-cell–targeted therapy including cellular approaches.
ACKNOWLEDGMENT
The authors would like to thank those who prepared the data for this study, namely Michal Kicinski (EORTC Headquarters), Hester de Groot (Princess Máxima Center), Yael Flamand (DFCI), Atsushi Sato (Department of Hematology/Oncology, Miyagi Children's Hospital, Sendai, Japan), Yasuhiro Okamoto (Department of Pediatrics, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan), Mitsuteru Hiwatari (Department of Pediatrics, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan), Daniela Silvestri (AIEOP) and Martin Zimmermann (BFM, Hannover, Germany), and Zhiguo Chen, Department of Biostatistics, University of Florida, Gainesville, FL (COG).
APPENDIX
TABLE A1.
Participating Cooperative Study Groups
FIG A1.
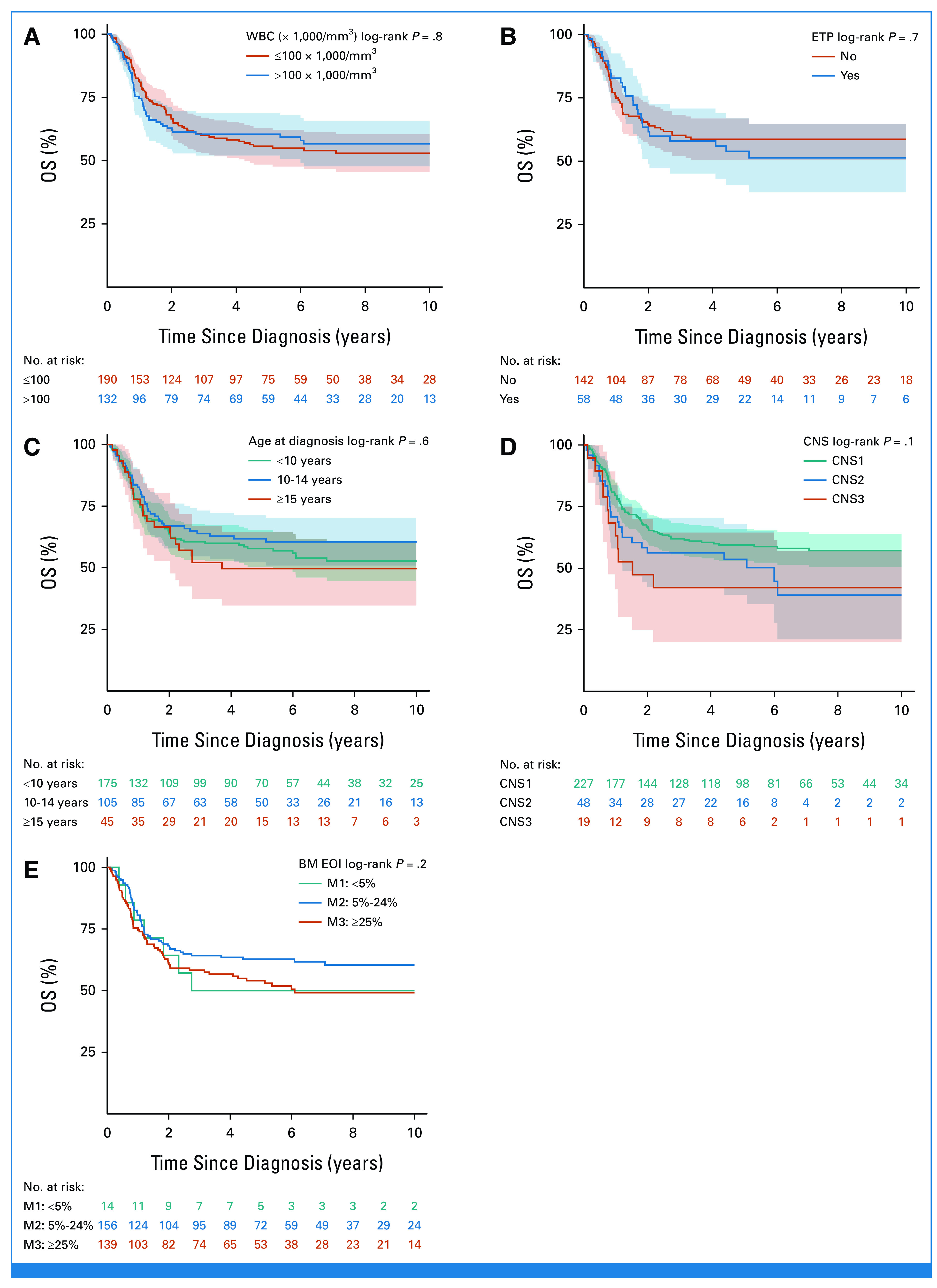
Kaplan-Meier OS estimate by baseline characteristics: (A) by WBC, (B) by ETP status, (C) by age, (D) by CNS status at diagnosis, and (E) by marrow at EOI (M1 with isolated extramedullary disease; the test for comparison between M2 and M3 gives P = 0.09). BM, bone marrow; EOI, end of induction; ETP, early thymocyte precursor; OS, overall survival.
FIG A2.
Kaplan-Meier OS estimate by remission status. OS, overall survival.
FIG A3.
Kaplan-Meier OS estimate by postinduction treatment. OS, overall survival.
FIG A4.
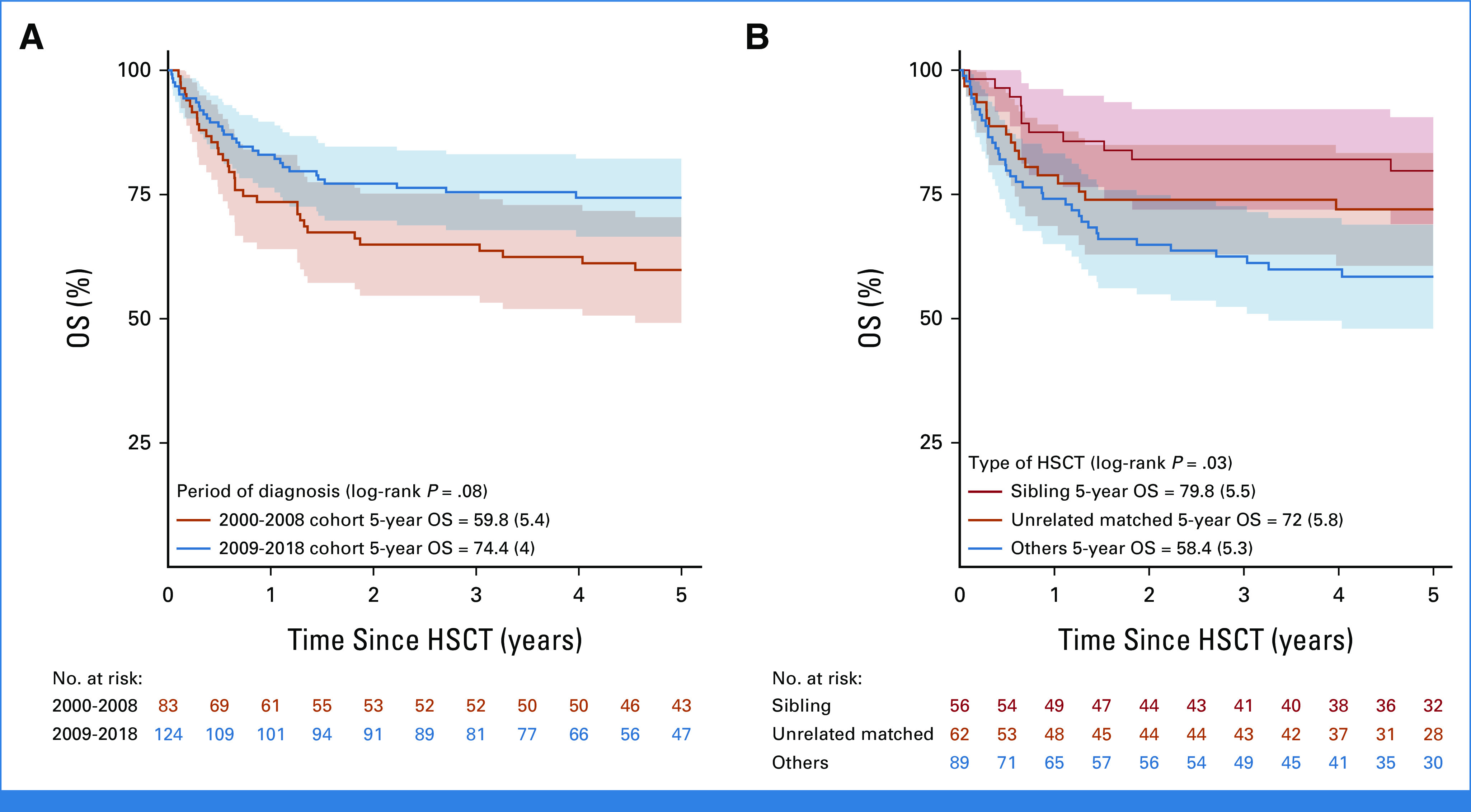
OS after hematopoietic stem cell transplant (HSCT in 207 patients) by (A) period of diagnosis and (B) type of donor. Two HSCT patients were excluded as date of transplant was missing. HSCT, hematopoietic stem cell transplant; OS, overall survival.
FIG A5.
OS according to MRD status at the EOC. EOC, end of consolidation; MRD, minimal residual disease; OS, overall survival.
FIG A6.
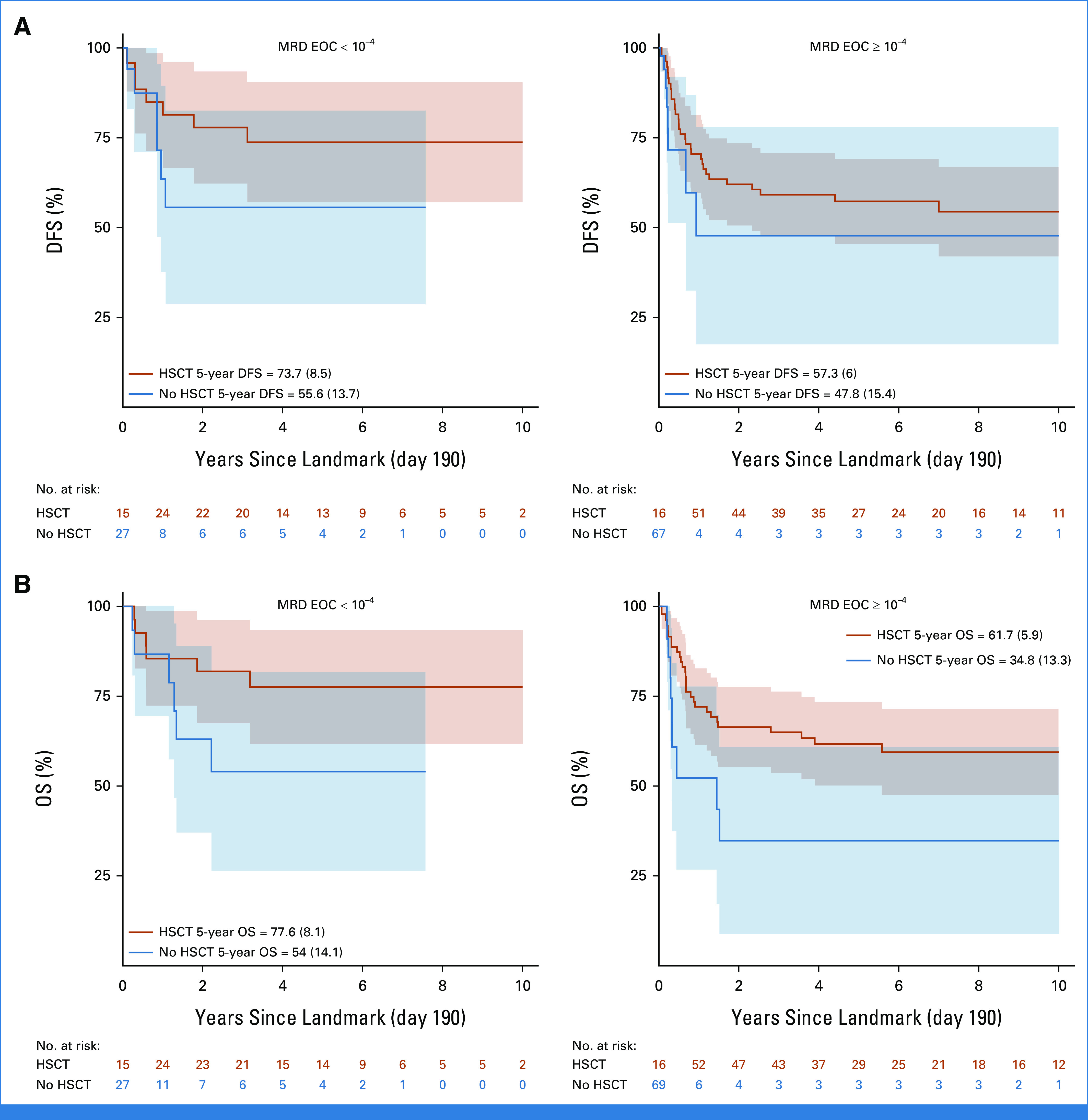
(A) DFS and (B) OS of patients with T-ALL who achieved remission with postinduction treatment according to whether they received HSCT or not in first CR according to MRD status at the EOC. Time originates at landmark (190 days, median time from diagnosis to HSCT). DFS, disease-free survival; EOC, end of consolidation; HSCT, hematopoietic stem cell transplant; MRD, minimal residual disease; OS, overall survival; T-ALL, T-cell acute lymphoblastic leukemia.
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: BMS
Valentino Conter
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Martin Schrappe
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals
Speakers' Bureau: Jazz Pharmaceuticals
Research Funding: Servier (Inst)
Meenakshi Devidas
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Novartis, Merck
Chihaya Imai
Stock and Other Ownership Interests: Cured Inc
Consulting or Advisory Role: Cured Inc
Research Funding: Cured Inc
Patents, Royalties, Other Intellectual Property: Chimeric receptor with 4-1BB signaling
Barbara De Moerloose
Honoraria: Daiichi Sankyo (Inst), Pfizer (Inst), Gilead Sciences (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Kjeld Schmiegelow
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Illumina, Jazz Pharmaceuticals, Servier
Speakers' Bureau: Medscape
Research Funding: Novo Nordisk
Melissa A. Burns
Consulting or Advisory Role: Branch Bioscience, Ensoma, Cellarity, Novartis
Sarah Elitzur
Honoraria: Novartis, Medison
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Novartis, Takeda Science Foundation
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Allen Yeoh
Consulting or Advisory Role: Amgen
Other Relationship: Amgen (Inst)
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis, Amgen
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Travel, Accommodations, Expenses: Amgen
Gunnar Cario
Honoraria: Amgen (Inst)
Consulting or Advisory Role: Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals (Inst)
Anthony V. Moorman
Honoraria: Amgen
Travel, Accommodations, Expenses: Amgen
Barbara Buldini
Speakers' Bureau: Beckman Coulter, Becton Dickinson, Amgen
Travel, Accommodations, Expenses: Beckman Coulter, Becton Dickinson, Amgen
Ajay Vora
Consulting or Advisory Role: Janssen Oncology, Novartis Pharmaceuticals UK Ltd
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by Children's Oncology Group grants U10 CA98543, U10 CA98413, U10 CA180886, and U10 CA180899; CA21765; American Lebanese Syrian Associated Charities; Deutsche Krebshilfe No. 108106, 108588, Project PRIN 2017(14041, PI MGV).This work is also part of the Danish nationwide research program Childhood Oncology Network Targeting Research, Organisation & Life expectancy (CONTROL) and supported by the Danish Cancer Society (R-257-A14720) and the Danish Childhood Cancer Foundation (2019-5934 and 2020-5769).
E.A.R. and P.R. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth A. Raetz, Valentino Conter, Martin Schrappe, Kjeld Schmiegelow, Rob Pieters, Andishe Attarbaschi, Ajay Vora, Maria Grazia Valsecchi
Provision of study materials or patients: Elizabeth A. Raetz, Meenakshi Devidas, Gabriele Escherich, Barbara De Moerloose, Kjeld Schmiegelow, Sarah Elitzur, Rob Pieters, Andishe Attarbaschi, Ching-Hon Pui, Jan Stary, Gunnar Cario, Anthony V. Moorman
Collection and assembly of data: Elizabeth A. Raetz, Paola Rebora, Valentino Conter, Martin Schrappe, Meenakshi Devidas, Gabriele Escherich, Chihaya Imai, Barbara De Moerloose, Melissa A. Burns, Sarah Elitzur, Andishe Attarbaschi, Ching-Hon Pui, Jan Stary, Gunnar Cario, Nicole Bodmer, Anthony V. Moorman, Barbara Buldini, Ajay Vora
Data analysis and interpretation: Elizabeth A. Raetz, Paola Rebora, Valentino Conter, Martin Schrappe, Gabriele Escherich, Andishe Attarbaschi, Ching-Hon Pui, Gunnar Cario, Barbara Buldini, Ajay Vora, Maria Grazia Valsecchi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcome for Children and Young Adults With T-Cell ALL and Induction Failure in Contemporary Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: BMS
Valentino Conter
Consulting or Advisory Role: Amgen
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Martin Schrappe
Consulting or Advisory Role: Servier, Jazz Pharmaceuticals
Speakers' Bureau: Jazz Pharmaceuticals
Research Funding: Servier (Inst)
Meenakshi Devidas
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Novartis, Merck
Chihaya Imai
Stock and Other Ownership Interests: Cured Inc
Consulting or Advisory Role: Cured Inc
Research Funding: Cured Inc
Patents, Royalties, Other Intellectual Property: Chimeric receptor with 4-1BB signaling
Barbara De Moerloose
Honoraria: Daiichi Sankyo (Inst), Pfizer (Inst), Gilead Sciences (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Kjeld Schmiegelow
Stock and Other Ownership Interests: Novo Nordisk
Consulting or Advisory Role: Illumina, Jazz Pharmaceuticals, Servier
Speakers' Bureau: Medscape
Research Funding: Novo Nordisk
Melissa A. Burns
Consulting or Advisory Role: Branch Bioscience, Ensoma, Cellarity, Novartis
Sarah Elitzur
Honoraria: Novartis, Medison
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals
Rob Pieters
Honoraria: Jazz Pharmaceuticals, Kite, a Gilead company, Novartis, Servier/Pfizer
Consulting or Advisory Role: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: Kite, a Gilead company, Jazz Pharmaceuticals, Servier
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Novartis, Takeda Science Foundation
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Allen Yeoh
Consulting or Advisory Role: Amgen
Other Relationship: Amgen (Inst)
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis, Amgen
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Travel, Accommodations, Expenses: Amgen
Gunnar Cario
Honoraria: Amgen (Inst)
Consulting or Advisory Role: Jazz Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Jazz Pharmaceuticals (Inst)
Anthony V. Moorman
Honoraria: Amgen
Travel, Accommodations, Expenses: Amgen
Barbara Buldini
Speakers' Bureau: Beckman Coulter, Becton Dickinson, Amgen
Travel, Accommodations, Expenses: Beckman Coulter, Becton Dickinson, Amgen
Ajay Vora
Consulting or Advisory Role: Janssen Oncology, Novartis Pharmaceuticals UK Ltd
No other potential conflicts of interest were reported.
REFERENCES
- 1. Pui CH, Behm FG, Crist WM. Clinical and biologic relevance of immunologic marker studies in childhood acute lymphoblastic leukemia. Blood. 1993;82:343–362. [PubMed] [Google Scholar]
- 2. Ludwig WD, Harbott J, Bartram CR, et al. Incidence and prognostic significance of immunophenotypic subgroups in childhood acute lymphoblastic leukemia: Experience of the BFM study 86. Recent Results Cancer Res. 1993;131:269–282. doi: 10.1007/978-3-642-84895-7_24. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg JM, Silverman LB, Levy DE, et al. Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 4. Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 5. Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 6. Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118:2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 7. Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raetz E, Lu X, Devidas M, et al. Continued improvements in overall survival (OS) in children with newly diagnosed acute lymphoblastic leukemia (ALL): A Children’s Oncology Group (COG) report. Pediatr Blood Cancer. 2018;65:S222–S223. [Google Scholar]
- 9. Dunsmore KP, Winter SS, Devidas M, et al. Children’s Oncology Group AALL0434: A phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38:3282–3293. doi: 10.1200/JCO.20.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–2112. doi: 10.1182/blood-2015-09-670729. [DOI] [PubMed] [Google Scholar]
- 11. Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166:421–424. doi: 10.1111/bjh.12882. [DOI] [PubMed] [Google Scholar]
- 12. Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli l-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 13. Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology. 2016;2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teachey DT, Devidas M, Wood BL, et al. Children’s Oncology Group trial AALL1231: A phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2022;40:2106–2118. doi: 10.1200/JCO.21.02678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2014;15:809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 16. Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: Results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–2934. doi: 10.1200/JCO.2018.77.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rheingold SR, Ji L, Xu X, et al. Prognostic factors for survival after relapsed acute lymphoblastic leukemia (ALL): A Children’s Oncology Group (COG) study. J Clin Oncol. 2019;37 suppl 15; abstr 10008. [Google Scholar]
- 18. Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34:2591–2601. doi: 10.1200/JCO.2015.64.6364. [DOI] [PubMed] [Google Scholar]
- 19. Quist-Paulsen P, Toft N, Heyman M, et al. T-cell acute lymphoblastic leukemia in patients 1-45 years treated with the pediatric NOPHO ALL2008 protocol. Leukemia. 2020;34:347–357. doi: 10.1038/s41375-019-0598-2. [DOI] [PubMed] [Google Scholar]
- 20. Takahashi H, Kajiwara R, Kato M, et al. Treatment outcome of children with acute lymphoblastic leukemia: The Tokyo Children’s Cancer Study Group (TCCSG) Study L04-16. Int J Hematol. 2018;108:98–108. doi: 10.1007/s12185-018-2440-4. [DOI] [PubMed] [Google Scholar]
- 21. Hasegawa D, Imamura T, Yumura-Yagi K, et al. Risk-adjusted therapy for pediatric non-T cell ALL improves outcomes for standard risk patients: Results of JACLS ALL-02. Blood Cancer J. 2020;10:23. doi: 10.1038/s41408-020-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hofmans M, Suciu S, Ferster A, et al. Results of successive EORTC‐CLG 58 881 and 58 951 trials in paediatric T‐cell acute lymphoblastic leukaemia (ALL) Br J Haematol. 2019;186:741–753. doi: 10.1111/bjh.15983. [DOI] [PubMed] [Google Scholar]
- 23. Escherich G, zur Stadt U, Zimmermann M, et al. Clofarabine in combination with pegylated asparaginase in the frontline treatment of childhood acute lymphoblastic leukaemia: A feasibility report from the CoALL 08-09 trial. Br J Haematol. 2013;163:240–247. doi: 10.1111/bjh.12520. [DOI] [PubMed] [Google Scholar]
- 24. Schramm F, Zur Stadt U, Zimmermann M, et al. Results of CoALL 07-03 study childhood ALL based on combined risk assessment by in vivo and in vitro pharmacosensitivity. Blood Adv. 2019;3:3688–3699. doi: 10.1182/bloodadvances.2019000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thastrup M, Marquart HV, Levinsen M, et al. Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: A Nordic Society of Pediatric Hematology and Oncology study. Leukemia. 2020;34:336–346. doi: 10.1038/s41375-019-0570-1. [DOI] [PubMed] [Google Scholar]
- 26. Silverman LB, Gelber RD, Young ML, et al. Induction failure in acute lymphoblastic leukemia of childhood. Cancer. 1999;85:1395–1404. doi: 10.1002/(sici)1097-0142(19990315)85:6<1395::aid-cncr25>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27. Oudot C, Auclerc MF, Levy V, et al. Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: The FRALLE 93 study. J Clin Oncol. 2008;26:1496–1503. doi: 10.1200/JCO.2007.12.2820. [DOI] [PubMed] [Google Scholar]
- 28. Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 30. Schrauder A, Reiter A, Gadner H, et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: Results from ALL-BFM 90 and 95. J Clin Oncol. 2006;24:5742–5749. doi: 10.1200/JCO.2006.06.2679. [DOI] [PubMed] [Google Scholar]
- 31. Balduzzi A, Valsecchi MG, Uderzo C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: Comparison by genetic randomisation in an international prospective study. Lancet. 2005;366:635–642. doi: 10.1016/S0140-6736(05)66998-X. [DOI] [PubMed] [Google Scholar]
- 32. Buchmann S, Schrappe M, Baruchel A, et al. Remission, treatment failure, and relapse in pediatric ALL: An international consensus of the Ponte-di-Legno consortium. Blood. 2022;139:1785–1793. doi: 10.1182/blood.2021012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rebora P, Galimberti S, Valsecchi MG. Using multiple timescale models for the evaluation of a time-dependent treatment. Stat Med. 2015;34:3648–3660. doi: 10.1002/sim.6597. [DOI] [PubMed] [Google Scholar]
- 34. Liu Y, Easton J, Shao Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211–1218. doi: 10.1038/ng.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Connor D, Moorman AV, Wade R, et al. Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2017;35:660–667. doi: 10.1200/JCO.2016.69.6278. [DOI] [PubMed] [Google Scholar]
- 36. Conter V, Valsecchi MG, Buldini B, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: A retrospective analysis. Lancet Haematol. 2016;3:e80–e86. doi: 10.1016/S2352-3026(15)00254-9. [DOI] [PubMed] [Google Scholar]