Abstract
Background
Direct-acting oral anticoagulants are first-line agents for prevention of stroke in patients with nonvalvular atrial fibrillation (NVAF), but data are limited for the oldest patients, and with reduced dosing.
Objectives
The purpose of this study was to determine steady-state apixaban peak and trough concentrations during routine care of older adults with NVAF, compare concentrations to clinical trial concentrations, and explore factors associated with concentrations.
Methods
A cross-sectional study of medically stable older adults with NVAF (age ≥75 years or ≥70 years if Black) receiving apixaban. Peak (2.0-4.4 hours post-dose) and trough (before next dose) concentrations were determined by anti-Xa activity calibrated chromogenic assay. Patient characteristics associated with concentrations were determined by multivariate modeling.
Results
The median age of patients (n = 115) was 80 (interquartile range: 77-84) years. The cohort comprised 46 women and 69 men; of which 98 are White, 11 are Black, and 6 are Asian. With 5 mg twice daily per labelling (n = 88), peak concentrations were higher in women: 248 ± 105 vs 174 ± 67 ng/mL in men (P < 0.001) and exceeded expected 95% range in 6 of 30 vs 0 of 55 men (P = 0.002). With 2.5 mg twice daily per label (n = 11), concentrations were <5 mg twice daily (peak: 136 ± 87 vs 201 ± 90 ng/mL, P = 0.026; trough: 65 ± 28 vs 109 ± 56 ng/mL, P < 0.001), but not different than 2.5 mg twice daily without reduction criteria (n = 13; peak: 132 ± 88 ng/mL; trough: 65 ± 31 ng/mL). Covariates associated with concentrations included sex, number of daily medications, and creatinine clearance.
Conclusions
Older women had higher than expected peak apixaban concentrations, and 2.5 mg twice daily produced lower concentrations than standard dosing. Factors not currently included in dosing recommendations affected concentrations. The impact of apixaban concentrations on outcomes needs evaluation.
Key words: anti-Xa activity, anticoagulation, direct-acting oral anticoagulant, geriatric cardiology, novel oral anticoagulant
Central Illustration
Clinical care guidelines currently recommend direct-acting oral anticoagulants (DOACs) as first-line anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF), in part due to improved safety shown in clinical trials when compared to vitamin K antagonists.1, 2, 3 However, clinical trials often underenroll the oldest patients, women, patients with multiple medical conditions, patients receiving multiple medications, patients with moderate renal impairment, and patients with reduced physical function.4, 5, 6, 7, 8 The real-world experience in using DOACs in the decade after marketing approval in the United States has been accompanied by reports of higher rates of major bleeding than the pivotal clinical trials and substantial use of lower-than-labelled dosing for apixaban with potentially worse clinical outcomes for NVAF patients receiving lower-than-recommended doses.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Both increased bleeding rates and reduced doses may have important relationships with DOAC concentrations. Peak concentrations reflect the maximal level of anticoagulation and would be expected to be related to bleeding risk. Trough levels reflect the minimum level of anticoagulation, and some minimum thresholds would be expected to be related to efficacy.
Our goal was to examine peak and trough steady-state concentrations of apixaban, the most commonly prescribed DOAC in older adults with NVAF, and compare concentrations during routine clinical care to reports from the pivotal efficacy trial.1 We also explored whether patient-level factors were related to apixaban concentrations.
Methods
We conducted a cross-sectional prospective study of medically stable older adults with NVAF taking stable doses of apixaban prescribed by health care providers and enrolled non-Black patients aged 75 years or older and self-identified Black patients aged 70 years or older to mirror the age distribution and prevalence of NVAF in clinical populations and the shorter average life expectancy in Blacks. The study was approved by the Institutional Review Board of the University of California, San Francisco (UCSF).
Study population and data collection
Recruitment
Participants were recruited from UCSF Health and the African American Cardiovascular Pharmacogenomics Consortium (ACCOuNT).22 At UCSF Health, we identified patients with NVAF receiving apixaban by searching electronic medical records and research participant registries. Recruitment was via email or postal invitations, and informed consent was obtained. Separately, we established a collaborative data-sharing agreement through Northwestern University Medical School on behalf of the ACCOuNT Consortium. Deidentified data from outpatients enrolled in protocols approved by the Northwestern University Feinberg School of Medicine Institutional Review Board for the Consortium (Northwestern Medicine, University of Chicago Medical Center, University of Illinois Chicago, George Washington University Hospital and Medical Faculty Associates, and Washington DC VA Medical Center) were accessed, and participant data meeting study criteria were included.
Inclusion/exclusion criteria
Subjects were eligible if aged ≥70 years and self-identified as Black, otherwise if aged ≥75 years, taking a stable apixaban dose (≥1 month) for NVAF, and could provide informed consent. Patients were excluded if hospitalized, had medication changes in the past month, or had conditions that were exclusion criteria in the pivotal studies: renal dialysis or renal transplant, moderate or severe hepatic impairment (>Child-Pugh class B) or cirrhosis, or receiving strong combined cytochrome P450 3A4/5 P-glycoprotein (CYP3A4/5 Pg-p) inhibitors or inducers. Moderate or severe cognitive impairment identified by modified telephone-Montreal Cognitive Assessment23 and Telephone-Memory Impairment Screen was also an exclusion criterion.24
Data collection
After informed consent, standardized video, in-person, or telephone interviews were conducted, and data were entered into a REDCap database. Data included demographics, medical conditions, self-reported health status, self-report of activities and instrumental activities of daily living,25 apixaban dosing, and the use of prescription and nonprescription medications. Race and ethnicity was self-reported and categorized as American Indian or Alaska Native, Asian, Black/African American, Hispanic or Latino, Native Hawaiian or Other Pacific Islander, or White based on National Institute of Health Policy on Reporting Race and Ethnicity Data.26 The CHA2DS2-VASc score for atrial fibrillation stroke risk27 and the HAS-BLED score for major bleeding risk 28 were calculated after medical record review. Clinical Frailty Score29 was determined during interviews. Ethnicity, self-reported health status, activities of daily living, and Clinical Frailty Score were not available from the ACCOuNT consortium (n = 11).
Venous blood was obtained at trough (immediately before next dose) and/or peak apixaban concentrations (2.0-4.4 hours after dosing) using mobile phlebotomy or hospital laboratory phlebotomy sites. Patients completed medication diaries of apixaban intake for 2 days prior to sampling for adherence assessment.
Laboratory measurements included apixaban concentrations by chromogenic anti-Xa assay (STA-Liquid Anti-Xa kit, Diagnostica Stago, Inc) calibrated to apixaban, (analytical measurement range 29-554 ng/mL) at UCSF Clinical laboratories or Northwestern Memorial Hospital Clinical Diagnostic Laboratory. Observed intraday assay coefficient of variation is ≤3.8%; observed interday coefficient of variation is ≤5.1%. Serum creatinine was measured, creatinine clearance (CrCL) estimated with the Cockcroft and Gault formula using total weight,30 and estimated glomerular filtration rate (eGFR) calculated by the CKD-EPI formula.31 Apixaban exposure was summarized as daily area under the concentration vs time curve (AUC) calculated by trapezoidal rule for comparisons to ARISTOTLE (Apixaban for Reduction In Stroke and Other ThromboemboLic Events in Atrial Fibrillation).32
Classifying dosing concentrations and concomitant medications
Apixaban dosing was classified as lower than label recommendation, in agreement, or higher than labelling per US Food and Drug Administration. Label recommendations for use of apixaban in NVAF are 5 mg twice daily, reduced to 2.5 mg twice daily if patients meet 2 of the following criteria (age ≥ 80 years, weight ≤60 kg, serum creatinine ≥1.5 mg/dL) or coadministration of a strong combined Pg-p/CYP3A4 inhibitor if otherwise qualifying for 5 mg twice daily.33 We identified moderate combined Pg-p/CYP3A4 inhibitors or inducers using the Food and Drug Administration-approved label33 and drug interactions tables.34 Expected concentration ranges for apixaban for stroke prevention in patients with NVAF were defined by International Council for Standardization in Hematology recommendations for measurement of DOACs by calibrated anti-Xa activity35 and the literature.36
Statistical methods
We compared baseline characteristics of patients by dosing group and sex using chi-square test for proportions, unpaired 2-sided t-tests for means of normally distributed variables, and Wilcoxon rank sum tests for abnormal continuous variables. Proportions of concentrations outside the expected 5% to 95% ranges were compared using chi-square tests. Simple linear regression was used to assess univariate relationships between patient characteristics and apixaban concentrations. Separate multivariable regression analyses tested for relationships between peak and trough apixaban concentrations and factors expected to influence apixaban pharmacokinetics (age, sex, weight, health status, number daily medications, coadministration of moderate combined CYP3A4/5-Pg-p inhibitors, CrCL, and frailty). Multivariable analyses were confined to the 5 mg twice daily per labelling group due to small numbers receiving 2.5 mg twice daily per label. Backward elimination was used for variable selection, and the significance level at which variables were removed from the model was 0.05. Analyses were conducted using SAS 9.4 and R.37
Results
Population
We enrolled 112 participants from UCSF Health and obtained data from 11 participants from the ACCOuNT consortium. Three withdrew, 4 became ineligible with intercurrent illness or were sampled out-of-target timeframes, and 1 postponed participation (Supplemental Figure 1). Demographic data for the final 115 participants are in Table 1. Median age was 80 years, 40% were women, and 10% self-identified as Black. Ninety-one received apixaban 5 mg twice daily (3 at higher-than-labelling doses). Twenty-four received 2.5 mg twice daily, with 13 not meeting dose-reduction criteria. Participants receiving 2.5 mg twice daily were significantly older, weighed less, were mostly female, had worse health status, lower CrCL, and higher CHA2DS2-VASc scores. Only weight and creatinine differed between men and women. The median number of daily oral medications was 5. Daily medications in >10% included beta-blockers in 78 (68%), statins in 74 (64%), diuretics in 45 (39%), angiotensin-converting enzyme inhibitors in 31 (27%), angiotensin receptor blockers in 26 (23%), thyroid in 26 (23%), dihydropyridine calcium channel blockers in 23 (20%), proton pump inhibitors in 15 (13%), tamsulosin in 14 (12%), aspirin in 16 (14%), antidepressants in 13 (11%), and gabapentin in 13 (11%). Antiarrhythmics were taken by 19 (17%) (flecainide in 8, dofetilide in 5, propafenone in 4, digoxin in 3). Three participants took nonsteroidal anti-inflammatory drugs. A minority (17%) took combined moderate CYP3A4/5-Pg-p inhibitors (9 on amiodarone, 10 on diltiazem, 1 on verapamil, 1 on ciprofloxacin); none took inducers. One hundred and four subjects completed medication diaries (no ACCOuNT participants) and reported 100% apixaban adherence for 2 days prior to sampling.
Table 1.
Patient Characteristics and Apixaban Dosing
| Dosing Regimen |
5 mg Twice Daily |
2.5 mg Twice Daily |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 5 mg Twice Daily | 2.5 mg Twice Daily | P Value | Women | Men | P Value | Women | Men | P Value | |
| Total | 115 | 91 (79) | 24 (21) | 31 (34) | 60 (66) | 15 (63) | 9 (38) | |||
| Age (y) | 80 (77-84) | 79 (76-82) | 86 (82-87) | <0.001a | 78 (76-80) | 80 (76-82) | 0.590a | 84 (80-88) | 87 (84-87) | 0.352a |
| Weight (kg) | 77 (67-86) | 80 (72-91) | 58 (54-74) | <0.001a | 70 (62-80) | 81 (77-93) | <0.001a | 56 (51-58) | 76 (73-83) | 0.002a |
| BMI (kg/m2) | 25 (23-28) | 26 (23-29) | 23 (20-25) | 0.002a | 24 (22-28) | 26 (24-29) | 0.233a | 22 (19-24) | 25 (24-28) | 0.008a |
| Male | 69 (60) | 60 (66) | 9 (38) | 0.011b | ||||||
| Race | 0.134b | 0.118b | 0.525b | |||||||
| Asian | 6 (5) | 3 (3) | 3 (13) | 0 (0) | 3 (5) | 3 (20) | 0 (0) | |||
| Black or African American | 11 (10) | 8 (9) | 3 (13) | 5 (16) | 3 (5) | 2 (13) | 1 (11) | |||
| White | 98 (85) | 80 (88) | 18 (75) | 26 (84) | 54 (90) | 10 (67) | 8 (89) | |||
| Ethnicity | 0.696b | 0.116b | 0.533b | |||||||
| Hispanic or Latino | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Not Hispanic or Latino | 104 (90) | 83 (91) | 21 (88) | 26 (84) | 57 (95) | 14 (93) | 7 (78) | |||
| Not reported | 11 (10) | 8 (9) | 3 (13) | 5 (16) | 3 (5) | 1 (7) | 2 (22) | |||
| Self-reported health | 105d | 83 | 22 | 0.042b | 26 | 57 | 0.238b | 14 | 8 | 0.046b |
| Excellent | 8 (8) | 8 (10) | 0 (0) | 3 (12) | 5 (9) | 0 (0) | 0 (0) | |||
| Very good | 45 (43) | 39 (47) | 6 (27) | 8 (31) | 31 (54) | 3 (21) | 3 (38) | |||
| Good | 38 (36) | 28 (34) | 10 (45) | 12 (46) | 16 (28) | 9 (64) | 1 (13) | |||
| Fair | 14 (13) | 8 (10) | 6 (27) | 3 (12) | 5 (9) | 2 (14) | 4 (50) | |||
| ADL | 6 (6-6)d | 6 (6-6) | 6 (6-6) | 0.623a | 6 (6-6) | 6 (6-6) | 0.516a | 6 (6-6) | 6 (6-6) | >0.999a |
| IADL | 8 (8-8)d | 8 (8-8) | 8 (8-8) | 0.727a | 8 (8-8) | 8 (8-8) | 0.387a | 8 (8-8) | 8 (8-8) | 0.188a |
| Creatinine (mg/dL) | 1.00 ± 0.30, 1.00 | 1.00 ± 0.30, 1.00 | 1.00 ± 0.30, 1.00 | 0.835c | 0.90 ± 0.30, 1.00 | 1.10 ± 0.30, 1.00 | 0.005c | 0.90 ± 0.30, 1.00 | 1.20 ± 0.20, 1.00 | 0.010c |
| Range | 0.50-2.10 | 0.50-2.10 | 0.50-1.50 | 0.50-2.10 | 0.70-2.00 | 0.50-1.40 | 0.80-1.50 | |||
| Creatinine clearance (mL/min) | 63 ± 20, 61 | 68 ± 19, 67 | 47 ± 12, 46 | <0.001c | 63 ± 20, 57 | 70 ± 18, 68 | 0.095c | 44 ± 13, 43 | 51 ± 11, 49 | 0.207c |
| Range | 25-122 | 28-122 | 25-75 | 28-106 | 30-122 | 25-75 | 38-69 | |||
| eGFR CKD-EPI (mL/min/1.73 m2) | 69 (55-80) | 70 (58-81) | 60 (50-77) | 0.105a | 70 (52-82) | 70 (58-80) | 0.741a | 66 (52-78) | 57 (46-59) | 0.387a |
| CHA2DS2-VASc score | 4 (3-5) | 4 (3-5) | 5 (3-6) | 0.041a | 4 (4-5) | 4 (3-5) | 0.055a | 5 (4-6) | 4 (3-5) | 0.143a |
| HAS-BLED score | 2 (1-2) | 2 (1-2) | 2 (1-2) | 0.607a | 2 (1-2) | 2 (1-2) | 0.732a | 2 (1-2) | 2 (1-2) | 0.793a |
| Clinical Frailty Scored | 3 (2-3) | 2 (2-3) | 3 (2-3) | 0.400a | 3 (2-4) | 2 (1-3) | 0.106a | 3 (2-3) | 3 (1-4) | 0.619a |
| Charlson Comorbidity Score | 5 (4-6) | 4 (4-6) | 6 (5-7) | 0.012a | 4 (3-6) | 5 (4-6) | 0.100a | 6 (4-6) | 6 (5-7) | 0.624a |
| Coadministration moderate CYP3A4/5-P-gp inhibitors (no inhibitors) | 95 (83) | 76 (84) | 19 (79) | 0.762b | 26 (84) | 50 (83) | 1.00b | 12 (80) | 7 (78) | 1.00b |
| Number of daily medications | 5 (4-7) | 5 (4-7) | 5 (4-7) | 0.953a | 5 (4-8) | 6 (4-7) | 0.889a | 5 (4-6) | 5 (4-8) | 0.832a |
Values are n (%), median (IQR), or mean ± SD, median; percentages may not add to 100 due to rounding.
ADL = activities of daily living; BMI = body mass index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; eGFR = estimated glomerular filtration rate; IADL = independent activities of daily living.
Wilcoxon rank-sum tests.
Chi-square tests.
Unpaired 2-sided t-tests.
Not assessed in 10 subjects included from ACCOuNT Consortium: total (N = 105), 5 mg twice daily (N = 83, women N = 26, men N = 57), 2.5 mg twice daily (N = 22, women N = 14, men N = 8).
Outcomes
Apixaban concentrations
Peak concentrations were measured at a mean of 3 hours after administering apixaban in all groups. Trough concentrations were measured immediately before dosing (at a mean of 12 hours after dosing in participants dosed per labelling, and 11 hours in those taking 2.5 mg lower than the label recommendation). Mean and median apixaban concentrations by dose and sex for patients dosed per labelling are in the Central Illustration and as box plots in Supplemental Figure 2. Peak and trough apixaban concentrations for all dosing regimens are in Figure 1 and Table 2 by dose and sex and in comparison to expected 5% to 95% concentration ranges. Table 3 presents peak and trough data by dose, sex, and patient characteristics.
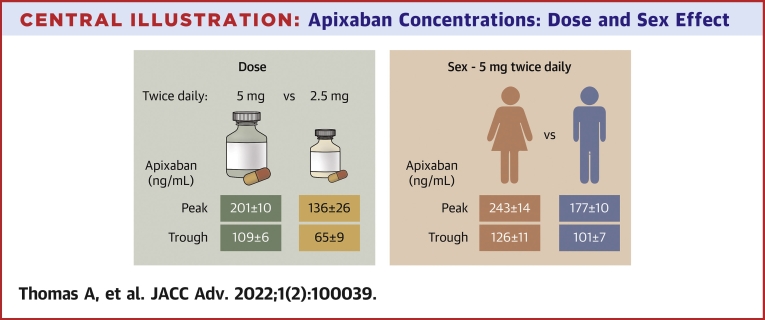
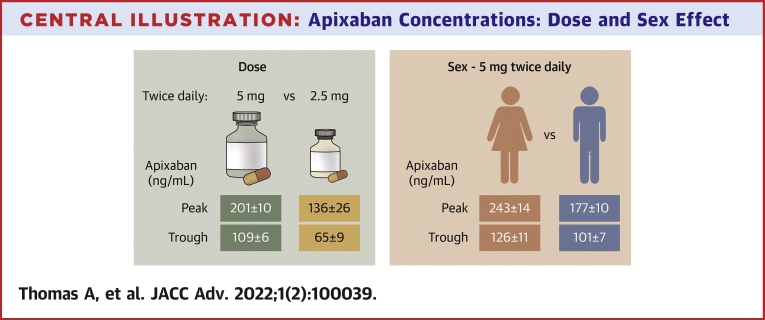
Central Illustration.
Apixaban Concentrations: Dose and Sex Effect
Mean ± SE apixaban concentrations with 5 mg (green) or 2.5 mg (orange) twice daily per labelling are in the left panel and model-derived average concentrations ± SE for women and men receiving 5 mg twice daily per label are on the right.
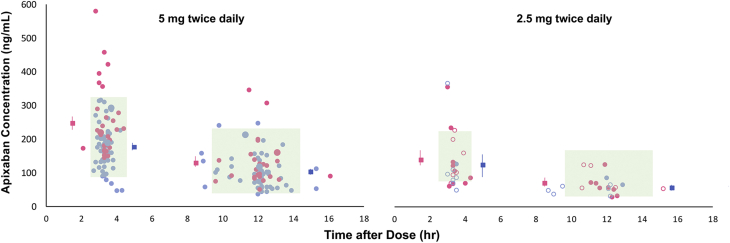
Figure 1.
Peak and Trough Apixaban Concentrations for 5 and 2.5 mg Twice Daily
Apixaban concentrations after dosing in patients receiving 5 mg twice daily (left) or 2.5 mg twice daily (right). Pink indicates data from women, and blue indicates data from men. Squares and vertical lines indicate mean ± SE. Larger closed circles indicate higher-than-labeled dosing, and open circles indicate lower-than-labeled dosing. Shaded rectangles indicate the 5% to 95% range modeled and reported from the pivotal clinical trial (ARISTOTLE) at the time of peak (2.6-4.4 hours) or trough (9.6-14.4 hours) concentrations for 5 mg twice daily, and vertical striped rectangles indicate the reported 5% to 95% range for 2.5 mg twice daily. Reprinted from Cirincione et al.36
Table 2.
Peak and Trough Apixaban Concentrations by Dosing Regimen
| Dosing Regimen |
||||
|---|---|---|---|---|
| 5 mg Twice Daily (n = 91) |
2.5 mg Twice Daily (n = 24) |
|||
| Dosed per Label | Dosed Higher Than Label | Dosed per Label | Dosed Lower Than Label | |
| Total | 88 (97) | 3 (3) | 11 (46) | 13 (54) |
| Men, women | 58, 30 | 2, 1 | 2, 9 | 7, 6 |
| Peak concentrations (ng/mL) | ||||
| 83 (94) | 3 (100) | 11 (100) | 13 (100) | |
| 201 ± 90 | 233 ± 53 | 136 ± 87 | 132 ± 88 | |
| Compared to range for 5 mg twice dailya | ||||
| Above 95% range | 6 (7) | 0 (0) | 1 (9) | 1 (8) |
| Women | 6 | 0 | 1 | 0 |
| Below 5% range | 5 (6) | 0 (0) | 4 (36) | 5 (38) |
| Men | 5 | 0 | 0 | 4 |
| Compared to range for 2.5 mg twice dailyb | ||||
| Above 95% range | - | - | 2 (18) | 2 (15) |
| Women | 2 | 1 | ||
| Below 5% range | - | - | 1 (9) | 1 (8) |
| Men | 0 | 1 | ||
| Trough concentrations (ng/mL) | ||||
| 80 (91) | 3 (100) | 10 (91) | 11 (85) | |
| 109 ± 56 | 163 ± 48 | 65 ± 28 | 65 ± 31 | |
| Compared to range for 5 mg twice dailya | ||||
| Above 95% range | 4 (5) | 0 (0) | 0 (0) | 0 (0) |
| Women | 2 | 0 | - | - |
| Below 5% range | 1 (1) | 0 (0) | 2 (20) | 2 (18) |
| Men | 1 | 0 | 0 | 2 |
| Compared to range for 2.5 mg twice dailyb | ||||
| Above 95% range | - | - | 0 (0) | 0 (0) |
| Below 5% range | - | - | 2 (20) | 1 (9) |
| Men | 2 | 1 | ||
| Daily AUC (ng∗h/mL)c | ||||
| 75 (85) | 3 (100) | 10 (91) | 11 (85) | |
| 3,643 ± 1,540 | 4,784 ± 1,015 | 2,138 ± 905 | 2,056 ± 1,072 | |
Values are n (%) or mean ± SD. Percentages may not add to 100% due to rounding.
AUC = area under the concentration vs time curve.
Compared to estimated 5% to 95% range for apixaban 5 mg twice daily.35
Italics are compared to estimated 5% to 95% range for apixaban 2.5 mg twice daily per labelling.35
Participants with both peak and trough concentrations.
Table 3.
Univariate Analyses of Apixaban Concentration Data for 5 mg Twice Daily Per Label
| 5 mg Twice Daily Dosing Regimen |
||||||||
|---|---|---|---|---|---|---|---|---|
| Peak Concentration (ng/mL) |
Trough Concentration (ng/mL) |
|||||||
| Difference in Means (95% CI) | P Valuea | Difference in Means (95% CI) | P Valuea | |||||
| Categorical | ||||||||
| Sex | ||||||||
| Male | 53 (64) | 174 ± 67 | Reference group | <0.001 | 55 (69) | 100 ± 47 | Reference group | 0.041 |
| Female | 30 (36) | 248 ± 105 | 74.9 (37.3 to 112.4) | 25 (31) | 128 ± 70 | 27.5 (1.1 to 54.0) | ||
| Race | ||||||||
| White | 74 (89) | 196 ± 78 | Reference group | 0.330 | 76 (95) | 107 ± 57 | Reference group | 0.489 |
| Black or African American | 7 (8) | 222 ± 182 | 26.0 (−44.5 to 96.5) | 1 (1) | 109 ± NA | 1.8 (−111.1 to 114.8) | ||
| Asian | 2 (2) | 282 ± 45 | 86.1 (−41.6 to 213.8) | 3 (4) | 147 ± 47 | 39.8 (−26.2 to 105.9) | ||
| Self-reported health status | ||||||||
| Very good or excellent | 43 (57) | 192 ± 67 | Reference group | 0.698 | 44 (56) | 102 ± 46 | Reference group | 0.522 |
| Good | 25 (33) | 209 ± 81 | 16.6 (−22.9 to 56.1) | 27 (34) | 117 ± 63 | 15.1 (−12.6 to 42.7) | ||
| Fair or poor | 8 (11) | 203 ± 123 | 10.5 (−50.0 to 71.0) | 8 (10) | 116 ± 85 | 13.5 (−30.0 to 56.9) | ||
| Clinical Frailty Score | ||||||||
| Very fit or Well | 38 (50) | 196 ± 75 | Reference group | 0.492 | 40 (51) | 104 ± 51 | Reference group | 0.147 |
| Managing well | 22 (29) | 189 ± 66 | −7.7 (−49.5 to 34.2) | 23 (29) | 100 ± 36 | −4.6 (−33.7 to 24.5) | ||
| Vulnerable or mildly frail | 16 (21) | 219 ± 100 | 22.4 (−24.2 to 68.9) | 16 (20) | 133 ± 85 | 28.9 (−4.0 to 61.8) | ||
| Combined CYP3A4/5-P-gp moderate inhibitors | ||||||||
| No inhibitors | 69 (83) | 191 ± 83 | Reference group | 0.032 | 69 (86) | 107 ± 57 | Reference group | 0.600 |
| Inhibitors | 14 (17) | 247 ± 110 | 56.0 (4.9 to 107.2) | 11 (14) | 117 ± 49 | 9.7 (−26.8 to 46.1) | ||
| Continuous | ||||||||
| 1 Additional daily medication | 11.1 (3.4 to 18.7) | 0.005 | 5.5 (0.4 to 10.6) | 0.036 | ||||
| Age (per 5 y) | 5.8 (−17.6 to 29.2) | 0.624 | 7.7 (−7.3 to 22.7) | 0.310 | ||||
| 10 mL/min decrease in CrCL | 17.7 (7.9 to 27.6) | <0.001 | 7.5 (0.5 to 14.5) | 0.035 | ||||
| Weight (per 5 kg) | −1.4 (−7.4 to 4.7) | 0.654 | −0.3 (−4.0 to 3.4) | 0.886 | ||||
| Charlson Comorbidity Score | 0.4 (−11.0 to 11.8) | 0.949 | 3.5 (−3.6 to 10.6) | 0.332 | ||||
Values are n (%) or mean ± SD.
CI = confidence interval; CrCL = creatinine clearance.
P value is based on overall F-test of univariate linear regression of concentration on selected characteristic.
Patients taking apixaban 5 mg twice daily
Mean peak concentrations were 248 ± 105 ng/mL in women vs 174 ± 67 ng/mL in men (P < 0.001); trough concentrations were 128 ± 70 ng/mL in women vs 100 ± 47 ng/mL in men receiving recommended doses (P < 0.041). Six participants (7%) had peak concentrations > expected 95% range (>321 ng/mL) with labelled dosing; all were women (6 of 30; P = 0.002). One weighed <60 kg, 1 received a combined CYP3A4/5 P-glycoprotein moderate inhibitors, and 3 had CrCL <50 mL/min (and eGFR <60 mL/min/1.73 m2). Trough concentration was in the <5% range in 1 man. The 3 subjects taking 5 mg twice daily at higher than labelling had peak and trough concentrations within the expected 5% to 95% range.
Patients taking apixaban 2.5 mg twice daily
Patients receiving 2.5 mg twice daily per label had concentrations similar to the expected ranges. Compared to expected ranges for 5 mg twice daily, peak concentrations were >95% for 1 woman dosed per label (CrCL of 38 mL/min; eGFR of 56 mL/min/1.73 m2) and for 1 man dosed per label (CrCL 30 mL/min, eGFR 52 mL/min/1.73 m2). Trough concentrations were in the <5% range in 2 women (dosed per label) and 1 man (dosed lower than label). No differences in peak or trough concentrations were detected between patients receiving 2.5 mg twice daily per labelling and those receiving lower-than-label dosing. Supplemental Figure 3 presents peak and trough data for individuals for all dosing regimens.
Dosing regimen comparisons
Both mean peak and trough concentrations, and daily exposure as AUC, were 32% to 41% lower in patients receiving recommended 2.5 mg twice daily than those in patients receiving recommended 5 mg twice daily (P = 0.026 for peak, P < 0.001 for trough, and P = 0.003 for AUC).
Characteristics associated with apixaban concentrations
On univariate testing for dosing with 5 mg twice daily, significant associations between apixaban concentrations and sex, number of daily medications, or CrCL were identified (Table 3). Associations were also seen with coadministration of moderate CYP3A4/5 inhibitors. No associations were detected for age, weight, race, self-reported health status, or Clinical Frailty Score. Figure 2 presents estimated mean and 95% CI for concentrations defined by variables in multivariate analysis. The final multivariable model of peak concentrations included sex, number of daily medications, and CrCL (R2 = 0.34) (Table 4). Adjusted average peak apixaban concentrations were 65.9 ng/mL (95% CI: 31.5-100.2 ng/mL) higher in women than in men, increased by 11.0 ng/mL (95% CI: 4.4-17.6 ng/mL) for each additional daily medication, and increased by 13.8 ng/mL (95% CI: 5.0-22.7 ng/mL) for every 10 mL/min decrease in CrCL. Estimated peak concentrations for patients with CrCL of 40 mL/min were 217.0 ± 17.4 ng/mL for a man and 283.0 ± 17.3 ng/mL for a woman.
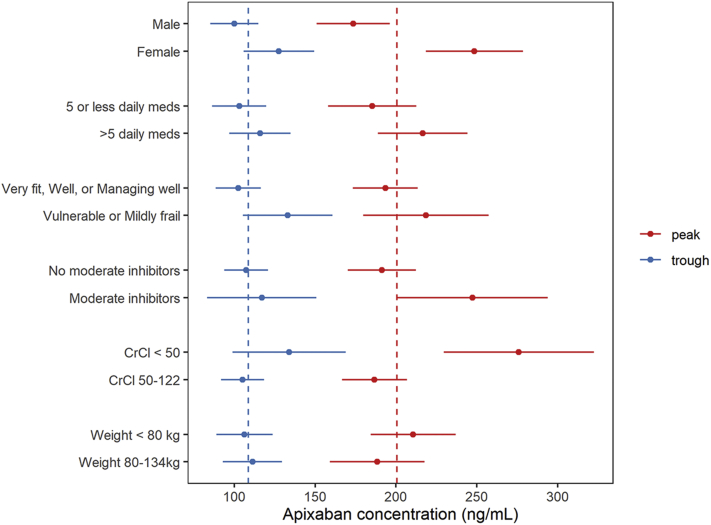
Figure 2.
Forest Plot of Multivariate Results for 5 mg Apixaban Twice Daily per Label
The estimated mean and 95% confidence interval for peak and trough apixaban concentrations defined by variables in the multivariate analysis: sex, number of daily medications, clinical frailty category, coadministrations of moderate CYP3A-Pgp inhibitors, and dichotomized for creatinine clearance, weight, and number of daily medications. The dashed vertical lines represent the average for 5 mg twice daily per label. CrCL = creatinine clearance.
Table 4.
Multivariate Analyses of Apixaban Concentration Data for 5 mg Twice Daily Per Label
| Peak Concentration |
Trough Concentration |
|||||
|---|---|---|---|---|---|---|
| Estimate (ng/mL) | (95% CI) | R2 | Estimate (ng/mL) | (95% CI) | R2 | |
| Female vs male | 65.9 | (31.5–100.2) | 0.34 | 25.9 | (0.2–51.5) | 0.17 |
| 1 Additional daily medication | 11.0 | (4.4–17.6) | 6.4 | (1.5–11.3) | ||
| Per 10 mL/min decrease creatinine clearance | 13.8 | (5.0–22.7) | 7.0 | (0.2–13.8) | ||
The final multivariable model of trough concentrations also included sex, number of daily medications, and CrCL (R2 = 0.17). The adjusted average trough concentrations were 25.9 ng/mL (95% CI: 0.2-51.5 ng/mL) higher in women than in men, increased by 6.4 ng/mL (95% CI: 1.5-11.3 ng/mL) for each additional daily medication, and increased by 7.0 ng/mL (95% CI: 0.2-13.8 ng/mL) for every 10 mL/min decrease in CrCL. Estimated trough concentrations for patients with CrCL of 40 mL/min were 121 ± 12.9 ng/mL for a man and 147 ± 13.5 ng/mL for a woman.
Discussion
Our findings challenge the current consensus regarding apixaban dosing in older patients with NVAF in several important ways. We found that women on average had higher peak and trough concentrations than men. Mean peak concentrations were 43% higher in older women with NVAF receiving recommended doses of 5 mg twice daily than those in older men receiving recommended doses of 5 mg twice daily. This was associated with daily apixaban exposure, or daily AUC, that was 36% higher in women than in men. These differences are larger than the 15% higher daily apixaban exposure seen in women than in men in the pivotal clinical trial (ARISTOTLE)1 that informed labelling recommendations.32 Perhaps more important than average differences between women and men was that 20% of these older women receiving 5 mg twice daily according to labelling had peak concentrations above the upper 95% range estimated from ARISTOTLE.36 Apixaban has a slow absorption rate, producing broad peaks, suggesting these women had high concentrations for a significant fraction of a dosing interval. Concentrations observed in these women and the apixaban daily exposure as AUC were associated with the highest major bleeding rates in analyses from ARISTOTLE.32 Observational studies have reported higher-than-expected single apixaban concentrations at varying timepoints after dosing in older Caucasian patients with NVAF,38, 39, 40, 41 have related higher concentrations to bleeding,42,43 and reported that trough concentrations that are high on 1 measurement are high on repeated measurements.44 The aggregate data suggest that higher apixaban concentrations are a contributing and potentially modifiable factor to higher bleeding rates in older patients,45 especially older women.
The second finding with important clinical implications is the lower concentrations with reduced dosing of apixaban. Even with label-recommended dosing, 2.5 mg twice daily produced 32% to 41% lower apixaban exposure than 5 mg twice daily. This is a larger difference than the 25% lower exposure observed in ARISTOTLE.1 Only 4.7% of those randomized to apixaban in ARISTOTLE received 2.5 mg twice daily (with 15% not meeting study-defined reduction criteria). Thus, conclusions regarding the efficacy of apixaban for stroke prevention in NVAF are based on the 95% of NVAF patients receiving 5 mg twice daily and concentrations associated with those doses. Curvilinear relationships have been modeled for stroke prevention as well as bleeding, for dabigatran and edoxaban.46,47 Across the range of apixaban concentrations analyzed in subgroups of ARISTOTLE and AVERROES (Apixaban VERsus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed Or Are Unsuitable for Vitamin K Antagonist Treatment), relationships were detected for bleeding and concentrations, but not for stroke, although events were low in the limited number of patients with concentrations measured.1,48 One observational study examining DOAC concentrations and stroke/thrombotic events in 565 atrial fibrillation patients (208 on apixaban) found thromboembolic complications only in patients with concentrations in the lowest quartile for each drug.49 Estimates are that 25% to 40% of NVAF patients are prescribed 2.5 mg twice daily, and outcomes may be worse when dosed lower than label recommendations.11,12 Until the stroke-prevention efficacy of 2.5 mg of apixaban twice daily is demonstrated, it would seem logical that evidence-based goals in patients with NVAF would be to approximate concentrations shown to be efficacious for stroke prevention with 5 mg twice daily dosing.
Important differences exist between clinical trial populations and patients receiving apixaban during routine clinical care. Patients receiving DOACs during routine clinical care are on average older with 25% to 40% over the age of 80 years compared to the mean age of 70 years in the pivotal clinical trial. Renal clearance on average is lower, with only 15% with moderate renal impairment enrolled in ARISTOTLE compared to 26% in this study, and the number of comorbidities and comedications are higher in real-world patients with NVAF treated with DOACs. Routine monitoring of DOACs or apixaban concentrations or anti-Xa activity is not currently recommended, and a number of experts advocate for strict adherence to the doses used in ARISTOTLE.50, 51, 52 We found significant associations of apixaban concentrations with the number of daily medications and with CrCL in this study focused exclusively on older adults. To date, polypharmacy has not been a consideration in dosing guidelines. Most DOAC doses are adjusted for estimated renal clearance, but criteria for apixaban dosage reduction are based on age, body weight, and serum creatinine (or comedications). Measurement of creatinine alone to estimate renal clearance is recognized as suboptimal in older adults. Our data suggest CrCL may have a role in estimating apixaban dosing as recommended by at least 1 European society.53
Study limitations
Our study has limitations as a prospective observational study with dosing prescribed by health care providers. The sample was moderately sized yet may not reflect the entire target patient population. However, the age and sex distribution and proportion of patients receiving each dosage match real-world data from large databases,9,13,14,19 unlike the pivotal ARISTOTLE trial.1 We did not assess outcomes. We sampled at the time of estimated peak concentrations, and our results may be underestimates. Fewer patients were prescribed apixaban 2.5 mg twice daily, limiting our analyses for this regimen. Finally, the study was conducted during the COVID-19 pandemic that limited a broader or more diverse population sample.
Conclusions
In this prospective cross-sectional study of older adults with NVAF, we found female sex, number of daily medications, and CrCL to be associated with apixaban concentrations. Our data suggest that current dosing recommendations may not provide concentrations shown to be associated with safety or efficacy in patients with atrial fibrillation. While data on using monitored DOAC concentrations to improve clinical outcomes are currently lacking, our findings support a practice of ensuring that apixaban concentrations in older women and those on 2.5 mg twice daily are within expected ranges. The role of concentration measurements to improve clinical outcomes with apixaban warrants evaluation.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Routine monitoring of DOAC concentrations is not currently recommended during care of patients with NVAF, but current dosing recommendations may not support apixaban concentrations previously shown to be safe and effective. Concentrations may lie outside expected ranges in older women, patients with reduced CrCL, and those receiving polypharmacy or moderate CYP3A4/5 inhibitors.
TRANSLATIONAL OUTLOOK: Higher-than-expected peak concentrations of apixaban in older women may be a modifiable risk factor for bleeding that warrants consideration and investigation. Reduced dosing with apixaban was infrequent in randomized trials, and the lower concentrations seen with reduced dosing suggests that reduced-dosing regimens need further evaluation.
Funding support and author disclosures
This project was supported by R21AG067463 and U54MD010723 and in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR001872, and NIH/NHGRI/NICHD grant U24 HG010615. Study data were collected and managed using REDCap electronic data capture tools hosted at University of California, San Francisco and Northwestern University.54,55 The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr Schwartz has received grant funding from BMS/Pfizer and Advisory Board reimbursement from Pfizer, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Supplementary data
References
- 1.Granger C.B., Alexander J.H., McMurray J.J.V., et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 2.Connolly S.F., Ezekowitz M.D., Yusuf S., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Giugliano R., Ruff C., Braunwald E., et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 4.Dhruva S., Redberg R. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168(2):136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 5.Chen A., Wright H., Itana H., et al. Representation of women and minorities in clinical trials for new molecular entities and original therapeutic biologics approved by FDA CDER from 2013 to 2015. J Womens Health (Larchmt) 2018;27(4):418–429. doi: 10.1089/jwh.2016.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon R., Khanijow K., Umarjee S., et al. Participation of women and sex analyses in late-phase clinical trials of new molecular entity drugs and biologics approved by the FDA in 2007-2009. J Womens Health (Larchmt) 2013;22(7):604–616. doi: 10.1089/jwh.2012.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffel du Vaure C., Dechartres A., Battin C., Ravaud P., Boutron I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials.gov: a systematic review of registration details. BMJ Open. 2016;6(9):e012265. doi: 10.1136/bmjopen-2016-012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academies of Sciences, Engineering, and Medicine. 2021. Drug Research and Development for Adults Across the Older Age Span: Proceedings of a Workshop. The National Academies Press. Accessed May 2022. 10.17226/25998 [DOI] [PubMed]
- 9.Lip G.Y., Keshishian A., Li X., et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. The ARISTOPHANES Study. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STOKEAHA.1118.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barra M.E., Fanikos J., Connors J.M., Sylvester K.W., Piazza G., Goldhaber S.Z. Evaulation of dose-reduced direct oral anticoagulant therapy. Am J Med. 2016;129(11):1198–1204. doi: 10.1016/j.amjmed.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg B.A., Shrader P., Pieper K., et al. Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (the outcomes registry for better informed treatment of atrial fibrillation II) J Am Heart Assoc. 2018;7(4) doi: 10.1161/JAHA.117.007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg B.A., Shrader P., Thomas L., et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes. The ORBIT-AF II registry. J Am Coll Cardiol. 2016;68(24):2597–2604. doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 13.Graham D.J., Baro E., Zhang R., et al. Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.e11. doi: 10.1016/j.amjmed.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Graham D.J., Reichman M.E., Wernecke M., et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with abigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176(11):1662–1671. doi: 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
- 15.Graham D.J., Reichman M.E., Wernecke M., et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz J., Merrill S., de Leon N., Thompson A., Fang M. Dosing accuracy of direct oral anticoagulants in an academic medical center. J Hosp Med. 2017;12(7):544–550. doi: 10.12788/jhm.2769. [DOI] [PubMed] [Google Scholar]
- 17.Bhagirath V.C., Chan N., Hirsh J., Ginsberg J., de Vries T.A.C., Eikelboom J. Plasma apixaban levels in patients treated off label with the lower dose. J Am Coll Cardiol. 2020;76(24):2906–2907. doi: 10.1016/j.jacc.2020.09.615. [DOI] [PubMed] [Google Scholar]
- 18.Yao X., Shah N.D., Sangaralingham L.R., Gersh B.J. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. doi: 10.1016/j.jacc.2017.03.600. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen E., White C.M., Patel M.R., et al. Doses of apixaban and rivaroxaban prescribed in real-world United States cardiology practices compared to registration trials. Curr Med Res Opin. 2016;32(7):1277–1279. doi: 10.1185/03007995.2016.1170672. [DOI] [PubMed] [Google Scholar]
- 20.Camm A.J., Cools F., Virdone S., et al. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. 2020;76(12):1425–1436. doi: 10.1016/j.jacc.2020.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer J.K., Errickson J., Li Y., et al. Adverse events associated with the addition of aspirin to direct oral anticoagulant therapy without a clear indication. JAMA Intern Med. 2021;181(6):817–824. doi: 10.1001/jamainternmed.2021.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman P.N., Shaazuddin M., Gong L., et al. The ACCOuNT consortium: a model for the discovery, translation, and implementation of precision medicine in African Americans. Clin Transl Sci. 2019;12(3):209–217. doi: 10.1111/cts.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz M.J., Wang C., Nester C.O., et al. T-MoCA: a valid phone screen for cognitive impairment in diverse community samples. Alzheimers Dement (Amst) 2021;13(1) doi: 10.1002/dad2.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton R.B., Katz M.J., Kuslansky G., et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 25.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 26.Not-OD-01-053: NIH Policy on reporting race and ethnicity data: Subjects in clinical research. National Institutes of Health. Published August 1, 2001. Accessed May 16, 2022. https://grants.nih.gov/grants/guide/notice-files/not-od-01-053.html
- 27.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 28.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 29.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Levey A., Stevens L., Schmid C., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drugs@FDA: FDA approved drugs. Eliquis. Reviews for NDA 202155. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202155
- 33.Eliquis prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf
- 34.Table of substrates, inhibitors and inducers. U.S. Food and Drug Administration. Published March 10, 2020. Accessed May 1, 2020. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers
- 35.Gosselin R.C., Adcock D.M., Bates S.M., et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118(3):437–450. doi: 10.1055/s-0038-1627480. [DOI] [PubMed] [Google Scholar]
- 36.Cirincione B., Kowalski K., Nielsen J., et al. Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst Pharmacol. 2018;7(11):728–738. doi: 10.1002/psp4.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R: a language and environment for statistical computing. R Foundation for Statistical Computing. Published 2021. Accessed April 1, 2022. https://www.R-project.org/
- 38.Gulilat M., Tang A., Gryn S.E., et al. Interpatient variation in rivaroxaban and apixaban plasma concentrations in routine care. Can J Cardiol. 2017;33:1036–1043. doi: 10.1016/j.cjca.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Sukumar S., Gulilat M., Linton B., et al. Apixaban concentrations with lower than recommended dosing in older adults with atrial fibrillation. J Am Geriatr Soc. 2019;67(9):1902–1906. doi: 10.1111/jgs.15982. [DOI] [PubMed] [Google Scholar]
- 40.Testa S., Tripodi A., Legnani C., et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–183. doi: 10.1016/j.thromres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Nissan R., Spectre G., Hershkovitz A., et al. Apixaban levels in octogenarian patients with non-valvular atrial fibrillation. Drugs Aging. 2019;36:165–177. doi: 10.1007/s40266-018-0613-8. [DOI] [PubMed] [Google Scholar]
- 42.Testa S., Legnani C., Antonucci E., et al. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17(7):1064–1072. doi: 10.1111/jth.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skornova I., Samos M., Bolek T., et al. Direct oral anticoagulants plasma levels in patients with atrial fibrillation at the time of bleeding: a pilot prospective study. J Cardiovasc Pharmacol. 2021;78(1):e122–e127. doi: 10.1097/FJC.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 44.de Vries T., Hirsh J., Bhagirath V., et al. Can a single measurement of apixaban levels identify patients at risk of overexposure? A prospective cohort study. TH Open. 2022;6(1):e10–e17. doi: 10.1055/s-0041-1740492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hylek E.M., Held C., Alexander J.H., et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE trial (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol. 2014;63(20):2141–2147. doi: 10.1016/j.jacc.2014.02.549. [DOI] [PubMed] [Google Scholar]
- 46.Ruff C.T., Giugliano R.P., Braunwald E., et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015;385(9984):2288–2295. doi: 10.1016/S0140-6736(14)61943-7. [DOI] [PubMed] [Google Scholar]
- 47.Yin O.Q.P., Antman E.M., Braunwald E., et al. Linking endogenous factor Xa activity, a biologically relevant pharmacodynamic marker, to edoxaban plasma concentrations and clinical outcomes in the ENGAGE AF-TIMI 48 trial. Circulation. 2018;138(18):1963–1973. doi: 10.1161/CIRCULATIONAHA.118.033933. [DOI] [PubMed] [Google Scholar]
- 48.Connolly S.J., Eikelboom J., Joyner C., et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 49.Testa S., Paoletti O., Legnani C., et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16(5):842–848. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 50.Alexander K.P., Alexander J.H. Safe and effective anticoagulation: what does drug concentration add? J Am Geriatr Soc. 2019;67(9):1772–1773. doi: 10.1111/jgs.15981. [DOI] [PubMed] [Google Scholar]
- 51.Weitz J.I., Eikelboom J.W. Appropriate apixaban dosing: prescribers take note. JAMA Cardiol. 2016;1(6):635–636. doi: 10.1001/jamacardio.2016.1841. [DOI] [PubMed] [Google Scholar]
- 52.Naccarelli G.V. Direct oral anticoagulant dosing. J Am Coll Cardiol. 2020;76(12):1437–1439. doi: 10.1016/j.jacc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Steffel J., Verhamme P., Potpara T.S., et al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 54.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez M., Conde J.G. Research electronic data capture (REDCap). A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris P., Taylor R., Minor B., et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.