Abstract
BACKGROUND
Protein C deficiency is typically associated with venous thromboembolism; however, arterial thrombosis has been reported in several cases. We report the case of a patient with pulmonary thromboembolism and deep vein thrombosis following acute myocardial infarction with high thrombus burden.
CASE SUMMARY
A 40-year-old man was diagnosed with pulmonary thromboembolism and deep vein thrombosis without any provoking factors. The patient was treated with anticoagulants for six months, which were then discontinued. Three months after the discontinuation of anticoagulant therapy, the patient was hospitalized with chest pain and diagnosed with acute myocardial infarction with high thrombus burden. Additional tests revealed protein C deficiency associated with thrombophilia. The patient was treated with anticoagulants combined with dual antiplatelet agents for 1 year after percutaneous coronary intervention, and no recurrent events were reported during a follow-up period of 5 years.
CONCLUSION
Recurrent thromboembolic events including acute myocardial infarction with thrombus should be considered an alarming sign of thrombophilia.
Keywords: Venous thromboembolism, thrombophilia, Protein C deficiency, ST elevation myocardial infarction, Anticoagulation, Case report
Core Tip: This case report outlines a 40-year-old male patient who experienced a sequence of thromboembolic events, including pulmonary thromboembolism, deep vein thrombosis, and acute myocardial infarction, all accompanied by significant thrombus burdens.
INTRODUCTION
Protein C (PC) is present in the blood and plays a role in preventing excessive blood clotting[1]. In the presence of PC deficiency, the thrombin production rate is not regulated and excessive coagulation occurs[2]. PC deficiency is typically associated with venous thromboembolism. Several cases of arterial thrombosis such as myocardial infarction (MI) or aortic mural thrombus have been reported[3,4]. However, recurrent thromboembolic events with combined arterial and venous flows have not been reported. Herein, we report the case of a man who had pulmonary thromboembolism (PTE), deep vein thrombosis (DVT), and acute MI with high thrombus burden in the right coronary artery (RCA).
CASE PRESENTATION
Chief complaints
A 40-year-old man presented with shortness of breath that started five days prior to hospital presentation.
History of present illness
The patient had no previously diagnosed illness, and dyspnea suddenly developed while he was swimming.
History of past illness
The patient had undergone laparoscopic cholecystectomy for cholecystitis caused by gallbladder stones 1 year previously. The patient was not taking any medication at the time of admission.
Personal and family history
The patient was a nonsmoker and social drinker who drank alcohol once per week.
Physical examination
The patient was 173 cm tall, and weighed 81 kg.
Laboratory examinations
Initial arterial blood gas analysis revealed hypoxia, with a pH of 7.44, pCO2 of 38 mmHg, pO2 of 63 mmHg, HCO3 of 26.1 mmol/L, and arterial O2 saturation of 93%.
His complete blood count (hemoglobin 15.3 g/dL; hematocrit 44.9%) and troponin I levels (0.086 ng/mL) (normal range: 0–0.78 ng/mL) were within the normal range; however, his D-dimer level was elevated to 2.17 µg/mL ((normal range: 0–0.22 µg/mL).
Imaging examinations
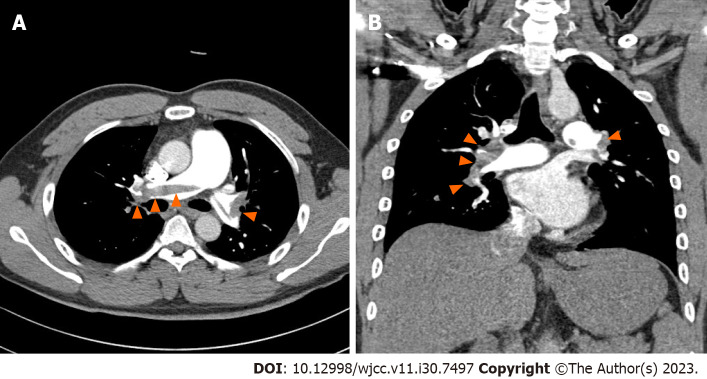
The initial chest radiograph was normal. We then performed chest computed tomography (CT) of the pulmonary artery and suspected acute PTE. Chest CT revealed a large amount of contrast-filling defects in both the main and lobar segmental pulmonary arteries, which was suggestive of acute PTE (Figure 1). Transthoracic echocardiography showed no right ventricular dysfunction (tricuspid regurgitation peak velocity, 3.4 m/sec; mean pulmonary artery pressure calculated using Mahan’s equation, 42 mmHg; right ventricular systolic pressure, 51 mmHg; no inferior vena cava plethora; normal-sized cardiac chambers; and left ventricular ejection fraction, 64%).
Figure 1.
Large amount of contrast-filling defects observed in both the main and lobar segmental pulmonary arteries on contrast-enhanced chest computed tomography. A: Axial plane; B: Coronal plane.
FINAL DIAGNOSIS
Unprovoked venous thromboembolism.
TREATMENT
After intravenous heparinization for the first 5 d, the treatment was converted to oral dabigatran 150 mg twice daily. The patient was discharged and followed up at the outpatient clinic.
OUTCOME AND FOLLOW-UP
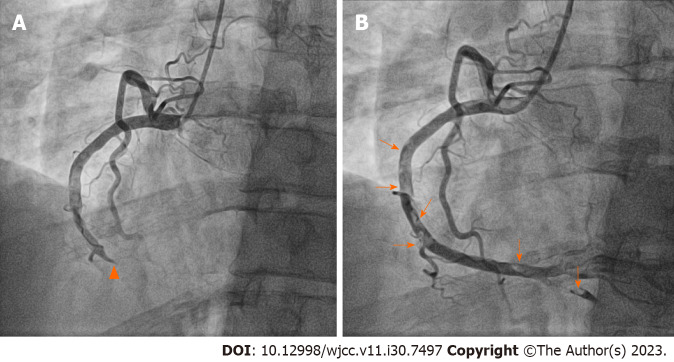
There were no DVT findings on lower extremity Doppler ultrasonography performed 3 mo after treatment initiation. Follow-up chest CT pulmonary arteriography showed a reduced thrombus burden, although a minimal residual contrast-filling defect remained in the pulmonary artery of the right lower lobe. Dabigatran was maintained for an additional 3 mo. After a total of 6 mo of treatment, dabigatran was discontinued and substituted with aspirin. Three months after switching to aspirin, the patient visited the hospital with sudden chest pain that had started 5 h prior. Initial 12-lead electrocardiography revealed ST-segment elevation in the inferior leads and reciprocal changes in the anterior leads. As ST-segment elevation MI was suspected, emergent percutaneous coronary intervention (PCI) was performed. Coronary angiography (CAG) revealed 100% occlusion at the distal RCA without abnormal angiographic lesions in the left coronary artery (Additional file 1: Video 1). A 4.0 mm × 26 mm drug-eluting stent (OsiroTM; Biotronik AG, Bülach, Switzerland) was implanted in the distal RCA lesion after balloon expansion. We administered intracoronary abciximab (10.25 mg of ReoPro®) as there was residual thrombus in the RCA (Figure 2). The thrombus remained in the RCA even on follow-up CAG performed 3 days later (Additional file 2: Video 2). Dual antiplatelet therapy (100 mg of aspirin and 75 mg of clopidogrel daily) and low-molecular-weight heparin (7000 IU of fraxiparine daily) were administered to the patient for the following 7 d. Additional tests for suspected thrombophilia revealed that PC activity had decreased to 49% (reference interval, 70%–130%) and the PC antigen levels had decreased to 39.3% (reference interval, 72%–160%). Therefore, the patient was diagnosed with PC deficiency. Triple antithrombotic therapy (100 mg of aspirin, 75 mg of clopidogrel daily and warfarin) was administered for 1 year after PCI, followed by clopidogrel discontinuation. The patient’s condition remained uneventful for the following 5 years.
Figure 2.
Each figure shows the left-anterior-oblique 30° view on right coronary angiography before and after percutaneous coronary intervention. A: Coronary angiography revealed a contrast-filling defect lesion in the mid right coronary artery (RCA) without angiographic stenosis (arrowheads) and complete occlusion of the distal RCA; B: Multifocal diffuse thrombi remained in the RCA after percutaneous coronary intervention (arrows).
DISCUSSION
Venous thromboembolism (VTE) can be classified into unprovoked or provoked, depending on whether a predisposing factor is present. If VTE has a specific cause, the recurrence risk is relatively low when the cause is addressed. However, in the case of unprovoked VTE, the recurrence risk increases after the discontinuation of anticoagulant therapy[5]. Therefore, both treatment and identification of cause are equally important when VTE is first diagnosed. Unfortunately, the patient in this report stopped taking direct oral anticoagulants for 6 mo without the cause of VTE being clearly identified at the time of initial diagnosis. Subsequently, the patient developed acute MI due to arterial thrombosis. Therefore, when diagnosing VTE, possible predisposing factors should be assessed, and in the case of unprovoked VTE, a more careful decision should be made before discontinuing anticoagulation therapy. Careful history-taking is required to confirm the predisposing factors for VTE. Predisposing factors for VTE are classified into weak, moderate, and strong risk factors, such as bed rest, obesity, cancer, thrombophilia, fracture, and major trauma[6]. Identifying the predisposing factors and performing additional tests are necessary for the diagnosis of VTE. The patient in our study was overweight, had a body mass index of 27 kg/m2 and was swimming regularly when VTE was first diagnosed. Therefore, bed rest or obesity could not be considered predisposing factors for this patient, and the possibility of cancer was excluded from the tests performed during the initial hospitalization. Thrombophilia is a major cause of VTE; however, there are no clear guidelines regarding when thrombophilia testing should be performed. Patients with a first episode of VTE are not routinely recommended to undergo thrombophilia testing owing to its high cost. According to several studies, a positive thrombophilia test does not reduce the risk of VTE recurrence[7-9]. A thrombophilia test was not performed for this patient, and other predisposing factors were not identified during the initial VTE episode. Furthermore, thrombophilia was not considered before the discontinuation of anticoagulant therapy in this young nonsmoking patient with no other risk factors. PC deficiency is a heritable or acquired risk factor for thrombophilia and causes of VTE. PC deficiency can be diagnosed when PC activity and antigen assays are performed as laboratory tests and the values are lower than the standard values[2]. Although PC deficiency is rare, the risk of developing recurrent VTE is high. According to previous studies, the incidence of PC deficiency in healthy people is estimated to be 0.2%–0.5%, and that of clinically significant PC deficiency is known to be approximately 0.005%[1]. The annual incidence of recurrent VTE in patients with PC deficiency during a follow-up period of 4.6 years is approximately 6.0%[10]. Whether evaluation for PC deficiency should be performed at the time of the initial episode of VTE is controversial; however, the evaluation of thrombophilia, including PC deficiency, must be considered in patients with recurrent VTE. The development of acute MI in this patient was probably due to PC deficiency. According to Virchow’s triad (venous stasis, hypercoagulability, and endothelial injury), thrombi are more likely to occur in veins with slower blood flow than in arteries. If acute MI occurs due to an embolism from the venous side, there should be a right-to-left shunt, such as an atrial septal defect, patent foramen ovale, or ventricular septal defect. If an embolism originating from the left side of the heart causes MI, a left ventricular (LV) thrombus with a decreased LV ejection fraction or a thrombus in the left atrial appendage is possible. However, this patient had no structural defects or previously observed thrombi in the heart, as confirmed by transthoracic echocardiography, and had no history of atrial fibrillation, which could have caused the formation a thrombus in the atrial appendage. Therefore, acute MI in this patient was most likely caused by a thrombus originating from the coronary artery. A contrast-filling defect lesion was observed in the mid-RCA without angiographic stenosis or abrupt occlusion from the distal part on the initial coronary angiography. Diffuse thromboses were observed after passing the guidewire through the distal RCA lesion. MI that occurs without angiographic stenosis, such as that in the mid-RCA in this patient, is called MI with nonobstructive coronary arteries (MINOCA)[11]. Approximately 40% of patients diagnosed with MINOCA experience plaque rupture[12]. It can be considered that arterial thrombosis in this patient was caused by hypercoagulability due to PC deficiency, blood flow stasis and endothelial injury due to atherosclerosis. Previously published large cohort studies have concluded that atherosclerosis induces VTE or that the two diseases share the same risk factors, although the mechanism is unclear[13,14].
CONCLUSION
Recurrent thromboembolic events, including acute MI after unprovoked venous thromboembolism, should be considered an alarming sign of thrombophilia. In the present case, the patient was diagnosed with PC deficiency and required treatment to prevent atherosclerosis as well as anticoagulation therapy. In addition, thrombophilia, such as PC deficiency, should be considered the cause of MINOCA.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and accompanying images.
Conflict-of-interest statement: All the authors declare that they have no conflict of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 23, 2023
First decision: September 26, 2023
Article in press: October 16, 2023
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tan X, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Jeongduk Seo, Department of Cardiology, Gachon University Gil Medical Center, Incheon 405-760, South Korea.
Joonpyo Lee, Department of Cardiology, Gachon University Gil Medical Center, Incheon 405-760, South Korea.
Yong Hoon Shin, Department of Cardiology, Gachon University Gil Medical Center, Incheon 405-760, South Korea.
Albert Youngwoo Jang, Department of Cardiology, Gachon University Gil Medical Center, Incheon 405-760, South Korea.
Soon Yong Suh, Department of Cardiology, Gachon University Gil Medical Center, Incheon 405-760, South Korea. ssy@gilhospital.com.
References
- 1.Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14:1214–1221. doi: 10.1111/j.1365-2516.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 2.Dinarvand P, Moser KA. Protein C Deficiency. Arch Pathol Lab Med. 2019;143:1281–1285. doi: 10.5858/arpa.2017-0403-RS. [DOI] [PubMed] [Google Scholar]
- 3.Ueda K, Morishita E, Shiraki H, Matsuoka S, Imashuku S. Aortic Mural Thrombus Associated with Congenital Protein C Deficiency in an Elderly Patient. J Atheroscler Thromb. 2020;27:100–103. doi: 10.5551/jat.48819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiong IY, Alkotob ML, Ghaffari S. Protein C deficiency manifesting as an acute myocardial infarction and ischaemic stroke. Heart. 2003;89:E7. doi: 10.1136/heart.89.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121:1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Áinle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54 doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 7.Middeldorp S. Is thrombophilia testing useful? Hematology Am Soc Hematol Educ Program. 2011;2011:150–155. doi: 10.1182/asheducation-2011.1.150. [DOI] [PubMed] [Google Scholar]
- 8.Connors JM. Thrombophilia Testing and Venous Thrombosis. N Engl J Med. 2017;377:1177–1187. doi: 10.1056/NEJMra1700365. [DOI] [PubMed] [Google Scholar]
- 9.Stern RM, Al-Samkari H, Connors JM. Thrombophilia evaluation in pulmonary embolism. Curr Opin Cardiol. 2019;34:603–609. doi: 10.1097/HCO.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 10.Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93–99. [PubMed] [Google Scholar]
- 11.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 12.Abdu FA, Mohammed AQ, Liu L, Xu Y, Che W. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): A Review of the Current Position. Cardiology. 2020;145:543–552. doi: 10.1159/000509100. [DOI] [PubMed] [Google Scholar]
- 13.Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, Prins MH, Girolami A. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–1441. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 14.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]