Abstract
The spatial distribution, movement, and impact of the untreated wastewater outfall from McMurdo Station, Antarctica, were investigated under early austral summer conditions. The benthic environment was examined to determine the distribution of Clostridium perfringens in sediment cores and the intestinal contents of native invertebrates and fish along a transect of stations. These stations extended ca. 411 m south of the outfall. The findings revealed that the concentration of C. perfringens decreased with depth in the sediment and distance from the outfall. High percentages of tunicates and sea urchins were colonized with this bacterium along the transect. Coprostanol concentrations were also measured in sediment samples taken from each of the transect stations, and a similar trend was observed. These results are in agreement with the findings of previous studies performed with the water column and collectively provide evidence that the disposal of domestic wastes deserves special consideration in polar marine environments.
McMurdo Station, Antarctica, which is the largest community on the continent, has released untreated wastewater into the near-shore environment for decades. This practice has the potential to degrade the environment (8, 15, 18, 24, 28, 34), infect indigenous species (25), and contaminate source water for the potable system of the station (19, 27, 37).
The marine environment surrounding McMurdo Station is unique and pristine. Compared to systems in more temperate climates, the low water temperature (ca. −1.8°C) results in prolonged survival of allochthonous bacteria (2, 4, 16, 31) and reduced rates of both bacterivory (26) and degradation of anthropogenic organic materials (20) from the sewage outfall. Previous water column studies have shown that the wastewater plume, as revealed by high densities of coliform bacteria, is distributed by advective influences along the ca. 1-km shoreline and extends at least 300 m seaward (19, 27). These observations suggest that the disposal of sewage and associated problems deserve more attention in this extreme and fragile marine environment. However, there is little information available concerning the spatial distribution of sewage-specific bacterial and chemical signatures or the potential biological impacts of the McMurdo Station outfall on the benthic ecosystem.
Various specific bacterial (12) and chemical (22) indicator systems are widely used to distinguish fecal pollution in marine systems (12). The density of Clostridium perfringens in sediments has been used in studies describing the historical distribution of deep-ocean sludge disposal sites (17, 18, 33), diffuse near-shore marine and estuarine fecal contamination from sewage disposal (11, 28), and nonpoint sources of stream pollution (25). Certain organic biomarker compounds also have been utilized in discriminating fecal water pollution. For example, coprostanol (5β-cholestan-3α-ol) has been used previously as a tracer for human waste in coastal marine sediments (10, 22, 23, 36, 37, 40). Although cats and pigs are the only animals that have fecal sterol profiles similar to those of humans (22), cetacean whales and certain captive seals also have been found to produce some coprostanol (22, 35). However, the significant amounts of coprostanol observed in sediments from the vicinity of the McMurdo Station, originating from the sewage outfalls, are thought to be of human origin (37).
This report describes the distribution of C. perfringens and coprostanol in the benthic near-shore marine environment near McMurdo Station and its sewage outfall. The findings indicate that disposal of untreated sewage into the near-shore marine environment of McMurdo Sound has resulted in contamination of the sediment and some native benthic biota.
MATERIALS AND METHODS
Location.
This study was conducted in McMurdo Sound adjacent to McMurdo Station, Antarctica (166°40′E, 77°51′S), which has a population of ca. 800 to 1,000 people. A transect of six sampling stations was established in the near-shore environment, starting at the McMurdo Station sewage outfall and following the shoreline for ca. 444 m to the south (Table 1) at a depth of 20 to 25 m. A control station was located approximately 3.0 km to the north. The prevailing ocean currents in the area are southward (6). Control samples also were collected from pristine sites that were 10 km (Tent Island) and 65 km (New Harbor) north and west of McMurdo Station.
TABLE 1.
Locations of sampling stations near McMurdo Station, Antarctica
| Station | Description | Latitude | Longitude | Distance from outfall (m) |
|---|---|---|---|---|
| A | Outfall | 77°50′ 52.508"S | 166°39′ 12.270"E | 0 |
| B | Transition, north | 77°50′ 56.290"S | 166°39′ 30.810"E | 150 |
| C | Transition, south | 77°51′ 00.720"S | 166°39′ 45.660"E | 332 |
| D | Intake Jetty | 77°51′ 04.020"S | 166°39′ 54.540"E | 444 |
| E | Wall | 77°51′ 10.270"S | 166°39′ 51.700"E | 594 |
| F | Cape Armitage | 77°51′ 18.060"S | 166°39′ 54.440"E | 822 |
| G | Pristine control | 77°50′ 01.313"S | 166°37′ 49.620"E | 3,000 |
Sample collection.
Sediment samples and invertebrates were collected from October to December 1996 by divers using holes drilled through the ca. 2-m-thick sea ice. Sediment samples were obtained with sterile syringes as described by Hill et al. (17) and were transported to the laboratory at 4°C in less than 2 h. Representative benthic invertebrate animals also were collected by divers within a 15-m radius of each station and were transported to the laboratory in less than 2 h for dissection and sampling. Following dissection the gut contents of all invertebrate and fish species were sampled aseptically with sterile cotton swabs. At least 10 individuals of each species were collected at every station.
Microbiological analysis.
The presence of C. perfringens in samples was determined by streaking swabs onto modified m-CP medium (3) that was incubated in an anaerobic atmosphere at 45°C. For enumeration of C. perfringens in sediment cores, 1.0-cm segments of the contents of the syringes were aseptically extruded from the syringes, and each segment was diluted by using 4°C sterile seawater. Random colonies were selected for further characterization with the API 20A anaerobic identification system (API Analytab Products, Plainview, N.J.). Each core segment was dried and weighed following microbiological analysis to allow calculation of the number of CFU per gram (dry weight) in the original sample. Dilutions of each core segment were plated in triplicate. The presence of fecal coliform bacteria was assessed by using the streak plate method on EC agar at 44.5 ± 0.2°C for 24 h. Enterococci were detected on mE agar incubated at 35°C for 24 h. Established methodological guidelines were followed (1).
Fecal sterol analysis.
Forty-gram (wet weight) aliquots from the top ca. 3-cm portions of cores were lyophilized. Samples were later extracted with methylene chloride by using a Virtis Homogenizer, and the sterol fraction was isolated and analyzed by gas chromatography (GC) after derivatization into the silyl ethers by using the method of Venkatesan et al. (39). The identities of compounds were confirmed by GC-mass spectrometry with a Finnigan model 4000 mass spectrometer equipped with a model 9610 gas chromatograph (39). Procedural blanks contained only minor peaks of phthalates and did not interfere with quantitation of the target compounds. The levels of recovery from matrix spike samples of the suite of sterols, including coprostanol and epicoprostanol, ranged from 80 to 94%, and the data were not corrected for the recovery loss.
RESULTS
Distribution of C. perfringens in sediments.
Sediment core samples were collected and analyzed to determine the densities of C. perfringens along a transect of sampling stations extending south ca. 822 m from the McMurdo Station sewage outfall (Table 1). This was done to estimate the movement of this wastewater microbiological signature in the benthic environment since the prevailing currents are toward the south (5, 6). The consistency of the sediment at each of the locations was soft, and satisfactory cores were obtained to a depth of at least 5 cm.
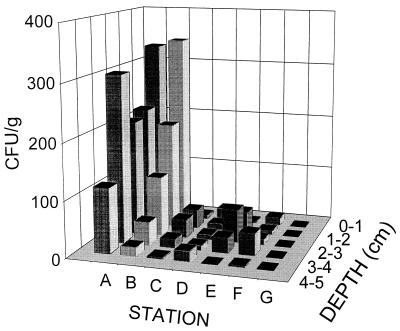
The concentration of C. perfringens was relatively consistent and high (116 to 304 CFU/g) in all strata at the outfall (station A), but the densities of this bacterium were very different at the other stations along the transect (Fig. 1 and Table 2). The 4- to 5-cm stratum of all of the cores except the core collected at station A contained uniformly low concentrations of this bacterium. It is noteworthy that the highest densities observed at stations C, D, and E were at depths between 1 and 4 cm and that lower bacterial levels were found at the tops and bottoms of these cores. The results obtained for the pristine control station (station G) and sediment samples from other pristine locations were uniformly negative for C. perfringens in all strata.
FIG. 1.
Population densities of C. perfringens with depth in sediment cores taken along the sampling transect near McMurdo Station, Antarctica. Station G is a distant pristine control location.
TABLE 2.
C. perfringens densities at different depths in sediment at stations near McMurdo Station, Antarctica
| Sediment depth (cm) |
C. perfringens density (CFU/g) at:
|
||||||
|---|---|---|---|---|---|---|---|
| Station A | Station B | Station C | Station D | Station E | Station F | Station G | |
| 0-1 | 331.8 ± 92.5a | 343.8 ± 97.0 | 6.5 ± 1.8 | 22.5 ± 9.9 | 2.0 ± 1.1 | 13.9 ± 5.7 | 0 |
| 1-2 | 219.9 ± 114 | 192.9 ± 38.6 | 33.8 ± 8.5 | 11.6 ± 1.4 | 37.3 ± 7.9 | 6.9 ± 2.7 | 0 |
| 2-3 | 208.3 ± 43.1 | 107.4 ± 14.3 | 33.0 ± 9.4 | 9.0 ± 1.1 | 59.0 ± 29.7 | 9.9 ± 1.0 | 0 |
| 3-4 | 303.9 ± 110.5 | 42.0 ± 14.0 | 15.5 ± 0.5 | 8.7 ± 1.4 | 27.1 ± 13.9 | 39.1 ± 6.1 | 0 |
| 4-5 | 116.4 ± 12.3 | 16.2 ± 1.7 | 2.9 ± 1.6 | 18.6 ± 1.1 | 0 | 2.8 ± 1.4 | 0 |
Average ± standard error of the mean.
Distribution of C. perfringens in invertebrates and fish.
Representatives of the most abundant ambient benthic invertebrates were initially collected at the outfall (station A) to establish which species contained the test bacterium at that contaminated location. The invertebrate species collected included sea urchins (Sterechinus neumayeri), tunicates (Cnemidocarpa verrucosa), starfish (Odontaster validus), clams (Laternula elliptica), and nemertean worms (Parborlasia corrugatus). The most abundant fish (Trematomus spp.) were also collected. The intestinal contents from these species near the outfall contained C. perfringens, and the following percentages of samples were positive: tunicates, 100%; sea urchins, 83%; starfish, 32%; and clams, 90%. Samples of representatives of all of these species collected at the control location (station G) and at other more distant pristine sites did not contain this bacterium. The intestinal contents of fish and nemertean worms collected at station A also did not contain C. perfringens.
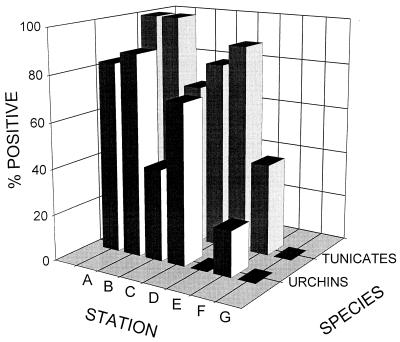
Sea urchins and tunicates were selected as the representative benthic invertebrate species which were used for further examination of the distribution of C. perfringens-colonized invertebrates along the sampling transect (Fig. 2). High percentages (>70%) of the tunicates contained this bacterium at all of the transect stations except the location most distant from the outfall (station F), where 40% of the tunicates were positive. The results for the sea urchins showed a similar pattern, although the levels of positive tunicates (72 to 100%) were consistently higher the levels of positive sea urchins (45 to 50%) when animals from the same location were compared.
FIG. 2.
Distribution of C. perfringens-positive tunicates and sea urchins along the sample transect.
Fecal sterols.
Concentrations of coprostanol and other sterols were measured in surface sediment samples collected at each of the stations on the transect. The highest concentration (199.4 ng/g) was observed at the sewage outfall, and the levels decreased along the transect, as follows: station B, 44.5 ng/g; station C, 20.3 ng/g; and station D, 34.4 ng/g. Only traces of coprostanol were detected at the most distant stations (stations E and F), and none was detected at the pristine control station (station G). None of the samples contained detectable levels of the coprostanol epimer, epicoprostanol. However, the station E and F samples did contain other biogenic sterols, such as cholesterol, campesterol, and β-sitosterol, in small amounts. The levels of these compounds were 1 order of magnitude higher at stations A, B, and C than at the more distant sampling locations. Only a C32 triterpanol was present in the sediment sample from station G, while none of the sterols was detected in that sample. The distribution of normal alkanes in the samples was consistent with sterol content (data not shown). Stations A, B, and C contained a pronounced unresolved complex hump underlying carbon numbers from 25 to 31. Odd-carbon-number alkanes having high molecular weights predominated, and C23–33 n-alkanes occurred at concentrations ranging from 4 to 6 μg/g of dry sediment. These values were about 10 to 100 times higher than those observed at the other stations.
DISCUSSION
Classical indicator bacteria have proved to be useful in studies describing the environmental distribution of wastewater discharges (11, 12, 15, 19, 27, 28, 32, 33). C. perfringens has been used successfully as an indicator of fecal contamination in aquatic systems (11, 12, 17, 18, 28, 32, 33). Concentrations of C. perfringens have also been shown to correlate with the occurrence of waterborne pathogens in an estuarine system (14), and this bacterium has been shown to be an ideal indicator organism under conditions under which extended survival is critical (9). In work done previously at McMurdo Station, Antarctica, the workers used coliform bacteria to determine the dimensions and movement of the plume from the sewage outfall in the water column of the near-shore environment (19, 27). The results reported here supplemented the findings of this work by extending the study to the benthic environment.
The observation that the sediment from the two sampling stations nearest the outfall (stations A and B) had the highest levels of C. perfringens (Fig. 1) and the high bacterial density-versus-depth pattern are not surprising because of the proximity of these stations to the outfall. These observations are compatible with the results of previous studies in which the water column microbiology (19, 27) and water current movements (6, 19, 27) at the same location and time of year were described. The current patterns in the near-shore area between Winter Quarters Bay and the Intake Jetty are dominated by a counterclockwise gyre with occasional flow reversals to the south. Southward beyond station D, however, the prevailing near-shore advection is toward the south (5, 6). The finding that higher concentrations of the indicator bacterium followed the sampling transect only as far as station B (Fig. 1) indicates that sedimentary bacterial deposition from the plume was largely limited to a radius of less than 200 m to the south of the outfall. These results are compatible with previous observations which revealed the periodic movement of high concentrations of bacteria from the outfall 400 m south, with some coliform bacteria entering the intake ca. 444 m south of the outfall (19, 27). The uniformly negative bacterial results for sediment samples from the control station (station G) and other pristine locations support the validity of the hypothesis that C. perfringens is a reliable indicator of fecal contamination originating from the McMurdo Station outfall.
The presence of C. perfringens in the intestinal contents of the ambient benthic biota was used as another index reflecting the microbiological distribution and potential biological impact of the sewage outfall from McMurdo Station in the near-shore environment. The high percentages of the tunicates (100%) and sea urchins (83%) sampled at the outfall station (station A) that were positive for C. perfringens, as well as the abundance of these invertebrate species, justified their selection as organisms which were used for further sampling and examination along the transect. The consistently higher level of tunicates than of sea urchins that were positive for the test bacterium might be explained by the feeding behavior of the former organisms, which may be similar to that of sea urchins (26a), which typically display necrophagous behavior (26). However, the gut contents of tunicates in the study area consisted of diatoms, detritus, and sediment (10a). This observation is supported by reports that organic material (20) and bacteria (4, 16, 31) originating from the sewage outfall have extended persistence in the ambient polar marine environment. The low ambient temperature (−1.8°C) (2, 4, 16) and reduced bacterivory (29) have been suggested as the primary factors responsible for prolonged bacterial persistence in this environment. A comparison of the data in Fig. 1 and 2 suggests that intestinal colonization of tunicates by C. perfringens appears to be a more sensitive index of sewage contamination than the density of C. perfringens in the sediment in this benthic system.
The absence of C. perfringens in the intestines of bottom-dwelling fish and nemertean worms during the study period indicates that these species probably do not become colonized, at least in early spring. These findings are not in agreement with the results of a previous study which revealed intestinal colonization of fish with C. perfringens in Puget Sound, Wash. (25). This previous report also demonstrated that the number of C. perfringens recovered from the guts of deliberately infected sculpins decreased to the level of detection in 144 h. One possible mechanistic explanation for the observed absence of the test bacterium in the gut contents of some species reported here might be related to the previous work of Sieburth on the gastrointestinal bacterial flora of certain penguins in Antarctica (30). This author demonstrated that acrylic acid, derived from marine krill, can act as a broad-spectrum antimicrobial agent within the gut. Something similar could be responsible for the findings reported here if the gut contents of fish and invertebrates that lack C. perfringens, and possibly other bacterial species, are antibacterial.
The sediment near the outfall (station A) contained the highest level of coprostanol in the suite of samples studied. However, this level is 1 order of magnitude less than the level reported for the station near the outfall in the 1989-1990 austral summer (37). The decrease in fecal sterol concentration might be explained by the installation (in ca. 1990) of a masticator and the relocation of the outfall from the shoreline to a depth of 20 to 25 m ca. 75 m offshore. The increased bioavailability of organic matter in the sewage caused by these changes could have resulted in enhanced biodegradation in the water column (21, 23), resulting in reduced levels of fecal sterols that reached the sediment, where they became more recalcitrant (7, 23). Despite the uniformly low levels of coprostanol in the latest samples examined, the trend was consistent with the results of our prior study in that the sample near the outfall contained the maximal level of sewage-derived coprostanol compared to the other samples. The distribution of normal alkanes in the sediments further corroborates the fecal sterol content results and the spatial distribution of sewage carbon in the study area. Analogous to the coprostanol distribution, the alkane content also was 1 order of magnitude less in the current samples than in our 1990 study (37). The unresolved complex hump in the GC profiles of the alkanes was most probably derived from weathered petroleum residues (13, 24, 38). The other alkanes found in the sediments, which were predominantly high-molecular-weight, odd-carbon-number compounds (especially C25 to C33 compounds), are characteristic of land plants. The most probable source of these compounds was kitchen waste from McMurdo Station. Thus, the hydrocarbons in the samples were probably from the sewage originating from the various operations at McMurdo Station. The absence of epicoprostanol in the samples rules out any contribution from cetaceans, such as blue and fin whales (35).
The results reported here demonstrate that C. perfringens and coprostanol are useful biological and chemical indicators of human sewage in polar marine environments. The similarity in the spatial distributions of these signatures in the benthic environment and the relatively high concentrations of coliforms in the water column (as high as 105 bacteria/100 ml), determined previously at the same time of year, is striking. It is also noteworthy that the qualitative presence of C. perfringens in the intestines of benthic invertebrates appears to be a more sensitive sewage indicator than the density of this bacterium in the sediment. Collectively, these tools provide sensitive measurements of the distribution of the sewage plume and its potential biological impact in the benthic, antarctic marine environment.
ACKNOWLEDGMENTS
This study was supported by a grant from the Division of Polar Programs of the National Science Foundation.
We acknowledge administrative supported provided by Rikk Kvitek and Kathy Conlan, the technical and diving assistance of Kathy Conlan, Carrie Bretz, Stewart Lamerdin, Jonna Engel, Chris Malzone, Lance Horn, Pat Iampietro, Tom Gelatt, and T. Northrup, and the numerous employees of Antarctic Support Associates who provided essential assistance. Advice concerning methods from Rita Colwell, Jim McClintock, Russell Hill, and Peter Nichols is also acknowledged.
Footnotes
Publication 5069 of the Institute of Geophysics and Planetary Physics, University of California, Los Angeles.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C: American Public Health Association; 1995. [Google Scholar]
- 2.Anderson I C, Rhodes M W, Kantor H I. Seasonal variation in survival of Escherichia coli exposed in in situ membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983;45:1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armon R, Payment P. A modified m-CP medium for enumerating C. perfringens from water samples. Can J Microbiol. 1988;34:78–79. doi: 10.1139/m88-014. [DOI] [PubMed] [Google Scholar]
- 4.Baros J A, Hanus F J, Morita R Y. Survival of human enteric and other sewage microorganisms under simulated deep-sea conditions. Appl Environ Microbiol. 1975;30:309–318. doi: 10.1128/am.30.2.309-318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry J P. Hydrographic patterns in McMurdo Sound, Antarctica and their relationship to local benthic communities. Polar Biol. 1988;8:377–391. [Google Scholar]
- 6.Barry J P, Dayton P K. Current patterns in McMurdo Sound, Antarctica and their relationship to local biotic communities. Polar Biol. 1988;8:367–376. [Google Scholar]
- 7.Bartlett P D. Degradation of coprostanol in an experimental system. Mar Pollut Bull. 1978;18:27–29. [Google Scholar]
- 8.Biamon E J, Hazen T C. Survival and distribution of A. hyrophila in near-shore coastal Puerto Rico. Water Res. 1983;17:319–326. [Google Scholar]
- 9.Bisson J W, Cabelli V J. Membrane filter enumeration method for Clostridium perfringens. Appl Environ Microbiol. 1979;37:55–66. doi: 10.1128/aem.37.1.55-66.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan K-H, Lam M H W, Poon K-F, Yeung H-Y, Chiu T K T. Application of sedimentary fecal stanols and sterols in tracing sewage pollution in coastal water. Water Res. 1998;32:225–235. [Google Scholar]
- 10a.Conlan, K. Personal communication.
- 11.Davies C M, Long J A H, Donald M, Ashbolt N J. Survival of fecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol. 1995;61:1888–1896. doi: 10.1128/aem.61.5.1888-1896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott E L, Colwell R R. Indicator organisms for estuarine and marine waters. FEMS Microbiol Rev. 1985;32:61–79. [Google Scholar]
- 13.Farrington J W, Quinn J G. Petroleum hydrocarbons in Narragansett Bay. I. Survey of hydrocarbons in sediments and clams (Mercenaria mercenaria) Estuarine Coastal Mar Sci. 1973;1:71–79. [Google Scholar]
- 14.Ferguson C M, Cotte B G, Ashbolt N J, Stevenson I M. Relationships between indicators, pathogens and water quality in an estuarine system. Water Res. 1996;30:2045–2054. [Google Scholar]
- 15.Grimes D J, Singleton F L, Stemmler J, Palmer L, Brayton P, Colwell R R. Microbiological effects of wastewater effluent discharged into coastal waters of Puerto Rico. Water Res. 1984;18:613–619. [Google Scholar]
- 16.Halton J E, Nehlsen W R. Survival of E. coli in zero degree centigrade sea water. J Water Pollut Control Fed. 1968;40:865–868. [PubMed] [Google Scholar]
- 17.Hill R T, Knight I T, Anikis M S, Colwell R R. Benthic distribution of sewage sludge indicated by Clostridium perfringens at a deep-ocean dump site. Appl Environ Microbiol. 1993;59:47–51. doi: 10.1128/aem.59.1.47-51.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill R T, Straube W L, Palmisano A C, Gibson S L, Colwell R R. Distribution of sewage indicated by Clostridium perfringens at a deep-water disposal site after cessation of sewage disposal. Appl Environ Microbiol. 1996;62:1741–1746. doi: 10.1128/aem.62.5.1741-1746.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howington J P, McFeters G A, Barry J P, Smith J J. Distribution of the McMurdo Station sewage plume. Mar Pollut Bull. 1992;25:324–327. [Google Scholar]
- 20.Howington J P, McFeters G A, Jones W L, Smith J J. The effect of low temperature on BOD in antarctic sea water. Water Res. 1994;28:2585–2587. [Google Scholar]
- 21.Kirchmer C J. 5β-Cholestan-3α-ol: an indicator of fecal pollution. Ph.D. thesis. Gainesville: The University of Florida; 1971. [Google Scholar]
- 22.Leeming R, Ball A, Ashbolt N, Nichols P. Using faecal sterols from humans to distinguish faecal pollution in receiving waters. Water Res. 1996;30:2893–2900. [Google Scholar]
- 23.Leeming R, Nichols P. Concentrations of coprostanol that correspond to existing bacterial indicator guidelines. Water Res. 1996;30:2997–3006. [Google Scholar]
- 24.Lenihan H S, Oliver J S, Oakden M D, Stephenson M D. Intense and localized pollution around McMurdo Station, Antarctica. Mar Pollut Bull. 1990;21:422–430. [Google Scholar]
- 25.Matches J R, Liston J, Curran D. Clostridium perfringens in the environment. Appl Microbiol. 1974;28:655–660. doi: 10.1128/am.28.4.655-660.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClintock J B. Trophic biology of antarctic shallow-water echinoderms. Mar Ecol Prog Ser. 1994;111:191–202. [Google Scholar]
- 26a.McClintock, J. B. Personal communication.
- 27.McFeters G A, Barry J P, Howington J P. Distribution of enteric bacteria in antarctic seawater surrounding a sewage outfall. Water Res. 1993;27:645–650. doi: 10.1016/0043-1354(93)90174-g. [DOI] [PubMed] [Google Scholar]
- 28.Paul J H, Rose J B, Jaing S, Kellogg C, Shinn E A. Occurrence of fecal indicator bacteria in surface waters and the subsurface aquifer in Key Largo, Florida. Appl Environ Microbiol. 1995;61:2235–2241. doi: 10.1128/aem.61.6.2235-2241.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putt M, Stoecker D, Alstatt J. Bacterivory in McMurdo Sound. I. Grazing by heterotrophic nanoflagellates. Antarct J U S. 1991;26:139–140. [Google Scholar]
- 30.Sieburth J M. Antibiotic properties of acrylic acid, a factor in the gastrointestinal antibiosis of polar marine animals. J Bacteriol. 1961;82:72–79. doi: 10.1128/jb.82.1.72-79.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith J J, Howington J P, McFeters G A. Survival, physiological response and recovery of enteric bacteria exposed to a polar marine environment. Appl Environ Microbiol. 1994;60:2977–2984. doi: 10.1128/aem.60.8.2977-2984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen D L, Eberl S G, Dicksa R A. Clostridium perfringens as a point source in non-point polluted streams. Water Res. 1989;23:191–197. [Google Scholar]
- 33.Takizawa M, Straube W L, Hill R T, Colwell R R. Near-bottom pelagic bacteria at deep-water sewage sludge disposal site. Appl Environ Microbiol. 1993;59:3406–3410. doi: 10.1128/aem.59.10.3406-3410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Congress Office of Technology Assessment. Wastes in marine environments. Publication OTA-344. U.S. Washington, D.C: Government Printing Office; 1987. [Google Scholar]
- 35.Venkatesan M I, Santiago C A. Sterols in ocean sediments: novel tracers to examine habitats of cetacaeans, pinnipeds, penguins and humans. Mar Biol. 1989;102:431–437. [Google Scholar]
- 36.Venkatesan M I, Kaplan I R. Sedimentary coprostanol as an index of sewage addition in Santa Monica Basin, southern California. Environ Sci Technol. 1990;24:208–214. [Google Scholar]
- 37.Venkatesan M I, Mirsadeghi F H. Coprostanol as sewage tracer in McMurdo Sound, Antarctica. Mar Pollut Bull. 1992;25:9–12. [Google Scholar]
- 38.Venkatesan M I, Brenner S, Ruth E, Bonilla J, Kaplan I R. Hydrocarbons in age-dated sediment cores from two basins in the Southern California Bight. Geochim Cosmochim Acta. 1980;44:789–802. [Google Scholar]
- 39.Venkatesan M I, Ruth E, Kaplan I R. Coprostanols in Antarctic marine sediments: a biomarker for marine mammals and not human pollution. Mar Pollut Bull. 1986;17:554–557. [Google Scholar]
- 40.Walker R W, Wun C K, Litsky W. Coprostanol as an indicator of fecal pollution. Crit Rev Environ Control. 1982;12:91–112. [Google Scholar]